Genome-Wide Identification, Characterization, and Expression Analysis of SPIRAL1 Family Genes in Legume Species
Abstract
1. Introduction
2. Results
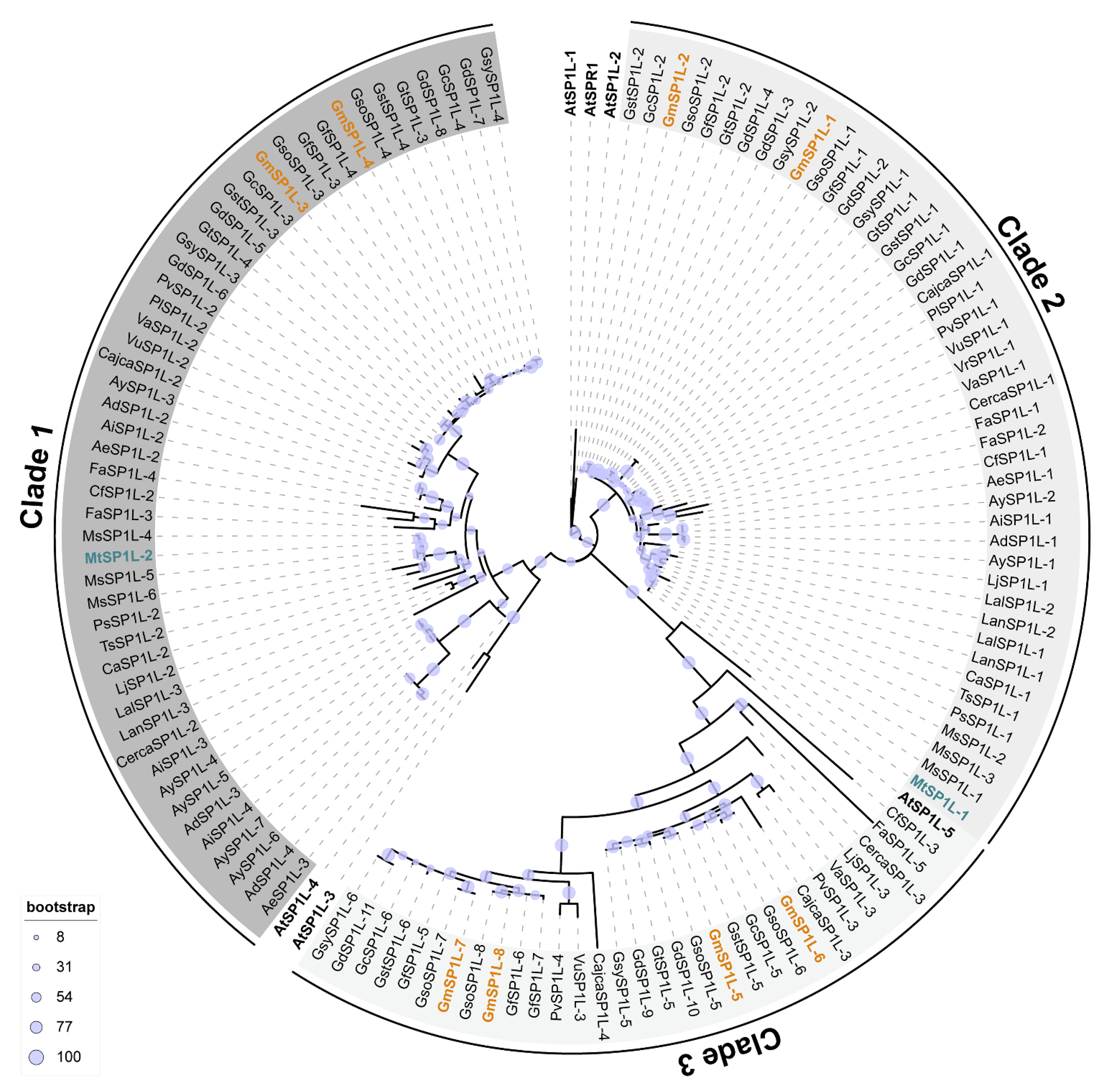
2.1. Identification and Phylogenetic Analysis of SPIRAL1-like (SP1L) Genes in Legume Species
2.2. Multiple Sequence Alignment of SP1L Genes in Medicago, Soybean, and Arabidopsis
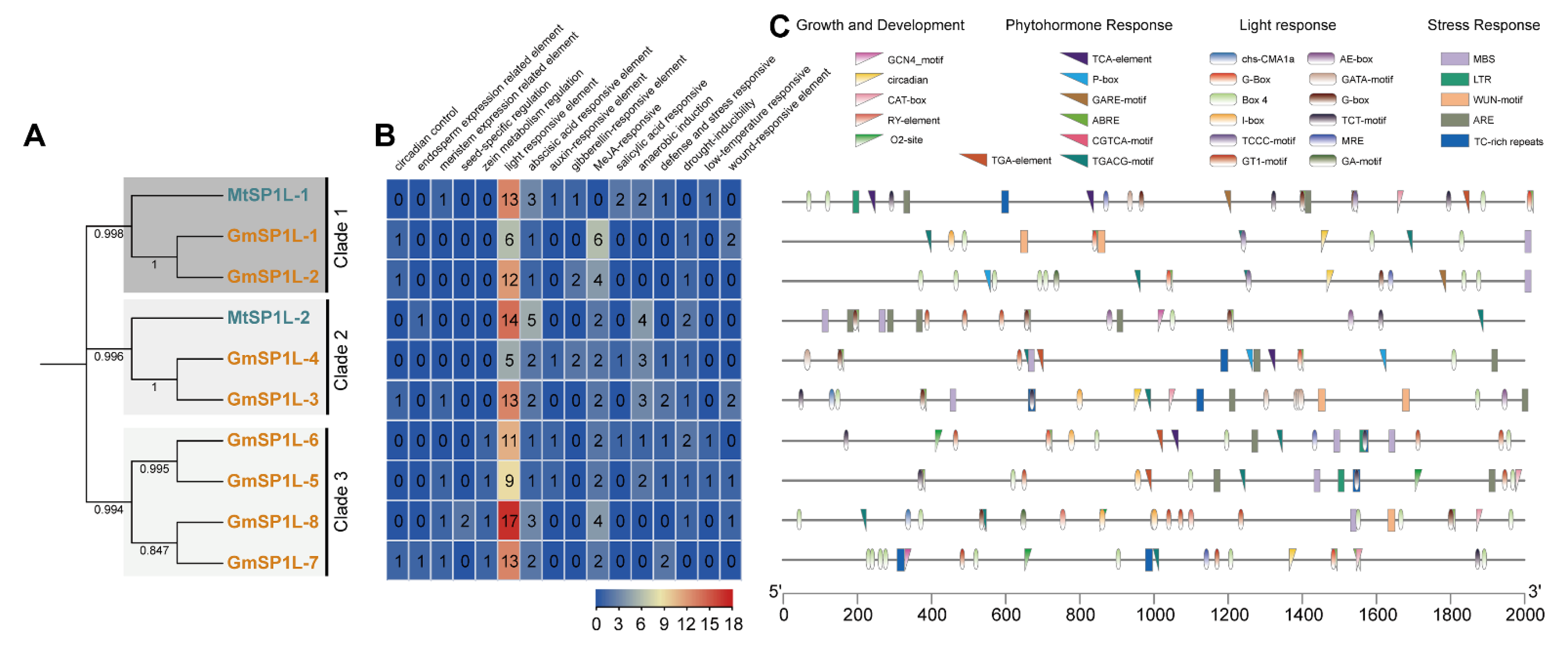
2.3. Analysis of Gene Structure and Conserved Motifs in SP1L Genes
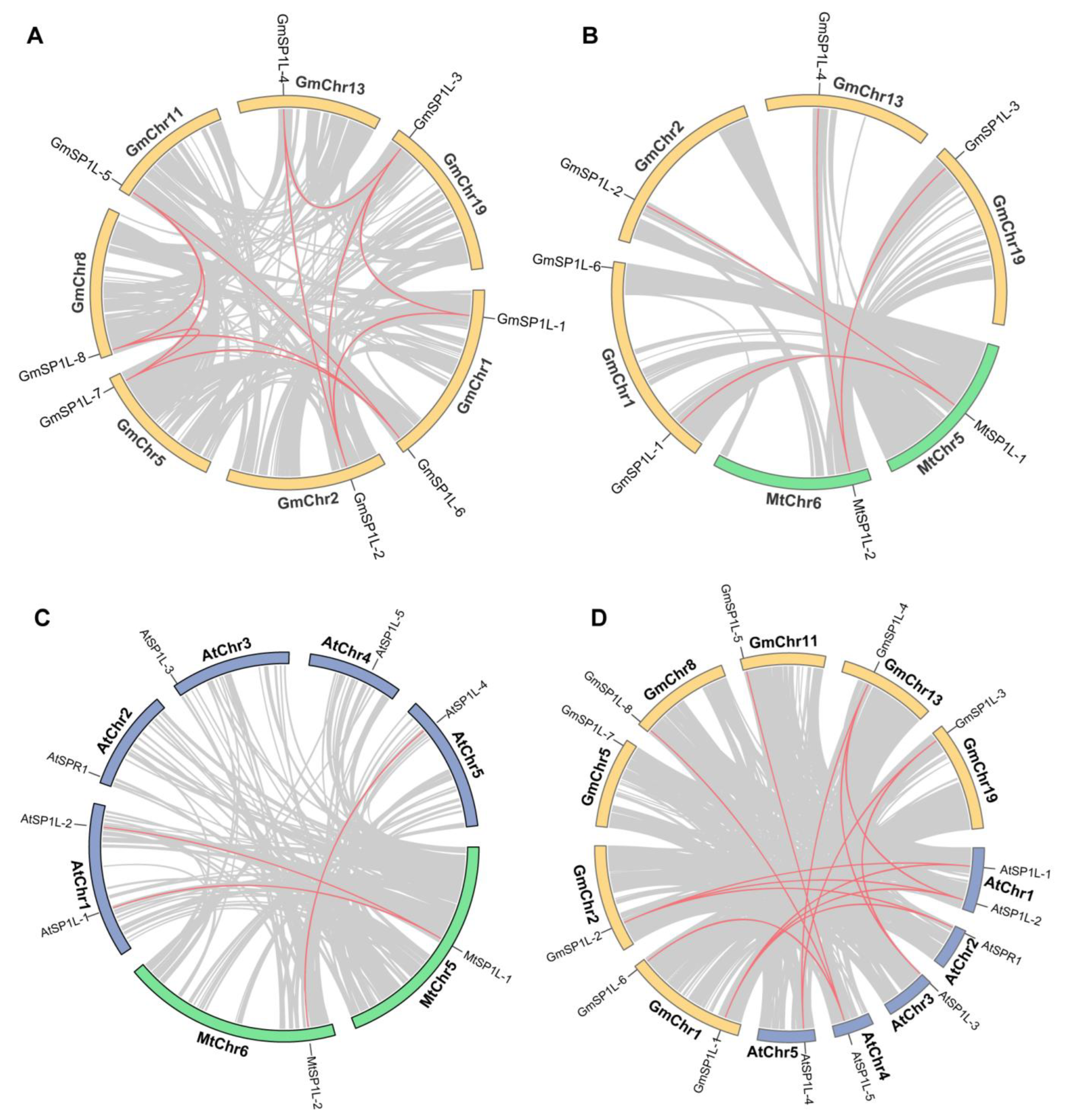
2.4. Analyses of the Chromosomal Distribution and Synteny in the SP1L Genes in Medicago and Soybean
2.5. Analysis of the Cis-Elements in the Promoter Sequences of the SP1L Genes in Medicago and Soybean
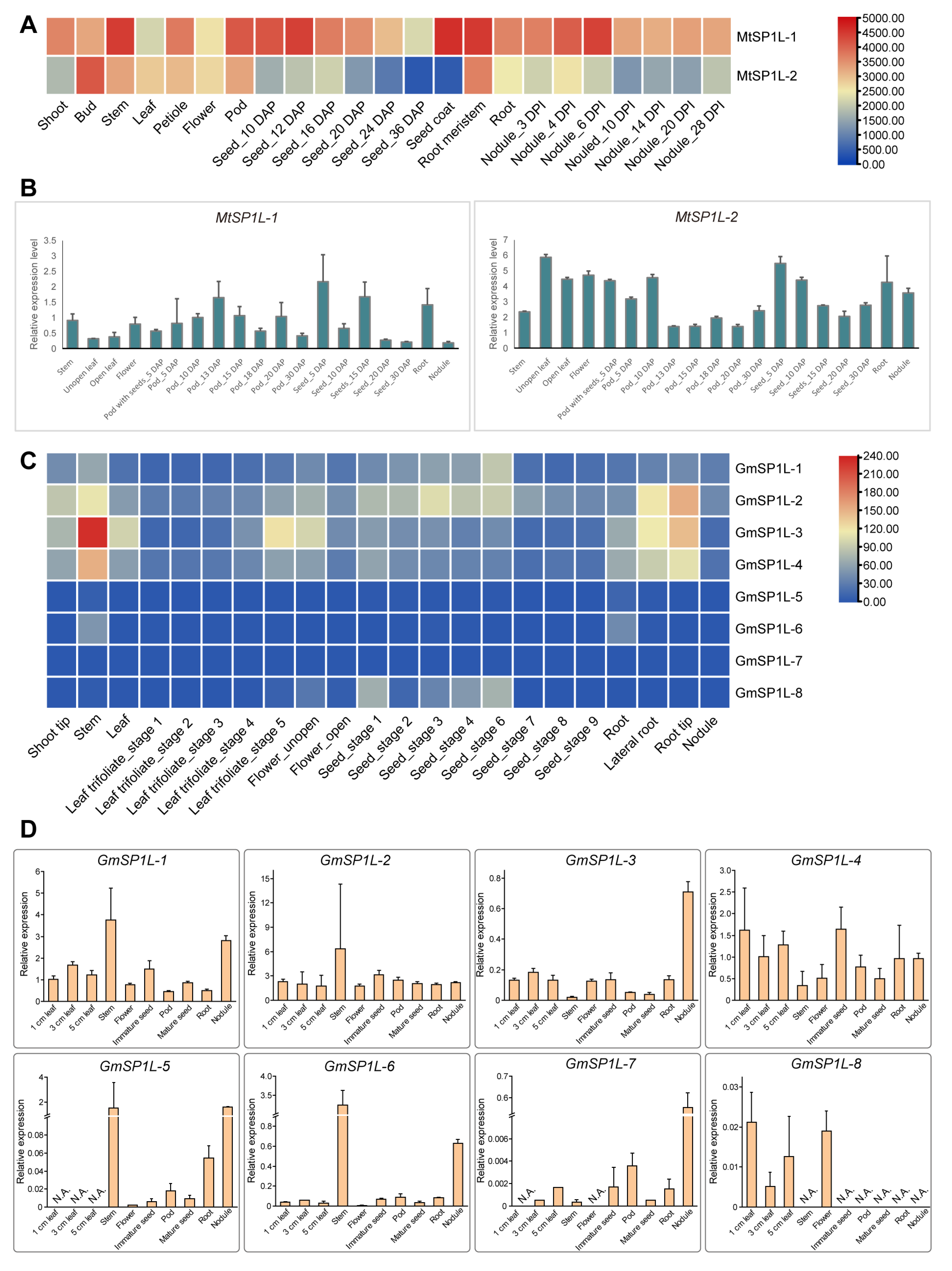
2.6. Expression Patterns of SP1L Genes in Various Tissues of M. truncatula and G. max
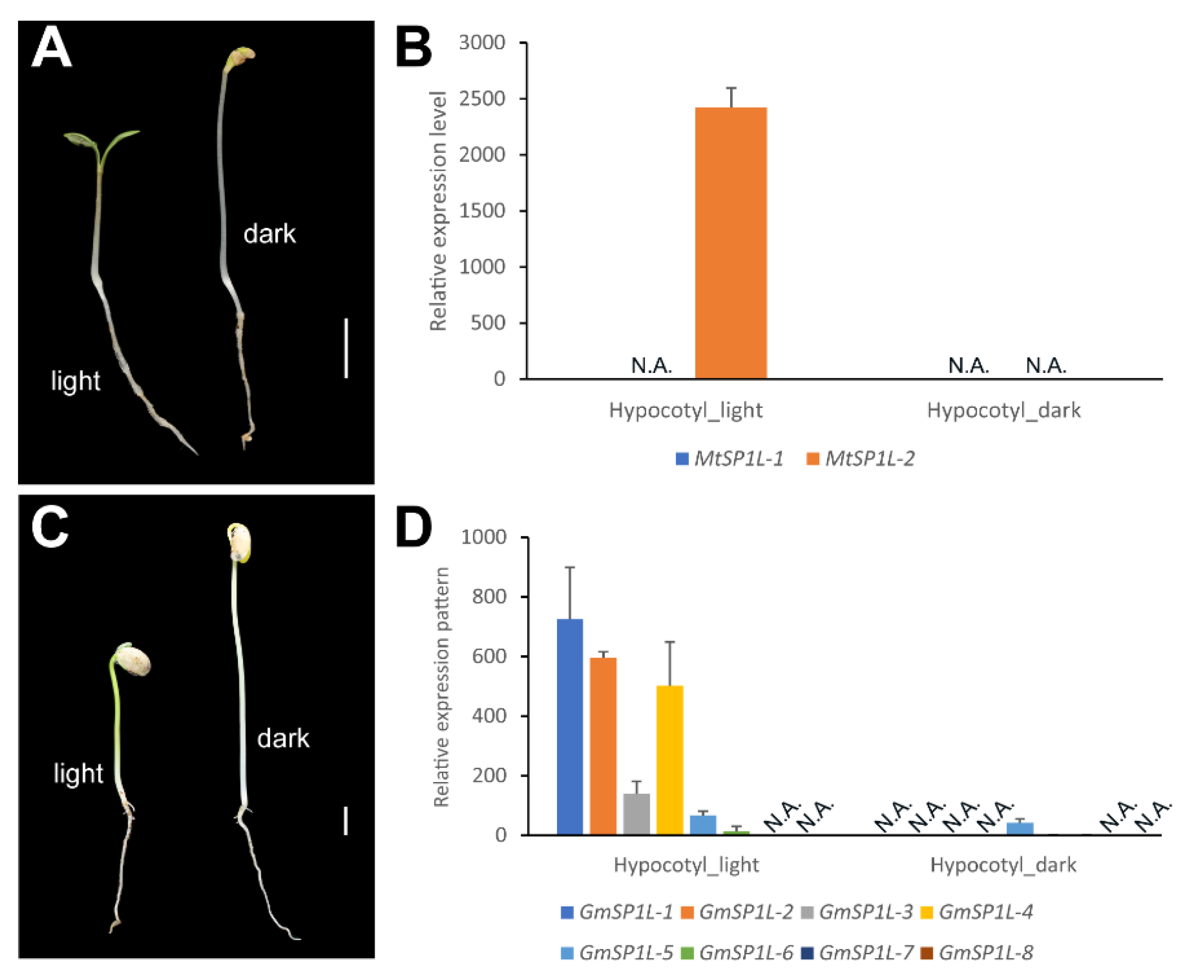
2.7. Comparison of SP1Ls’ Expression Patterns in the Light- and Dark-Grown Hypocotyls in M. truncatula and G. max
2.8. The Expression of SP1Ls under Saline Stress
3. Discussion
4. Materials and Methods
4.1. Identification of the SP1L Proteins in Legume Genomes
4.2. Phylogenetic Analysis and Multiple Sequence Alignment
4.3. Gene Structure and Motif Analysis
4.4. 3D Structural Analysis of the SP1L Proteins
4.5. Analyses of the Chromosome Locations and Collinearity of the AtSP1L, MtSP1L, and GmSP1L Genes
4.6. Cis-Acting Elements Analysis
4.7. Plant Materials
4.8. Analysis of the Expression Levels of the SP1L Genes in the Different Organs and Tissues of M. truncatula and G. max
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ledbetter, M.C.; Porter, K.R. Morphology of microtubules of plant cell. Science 1964, 144, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Shaw, P.J.; Warn, R.M.; Lloyd, C.W. Dynamic reorientation of cortical microtubules, from transverse to longitudinal, in living plant cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6050–6053. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Mattsson, O.; Mundy, J. Plants flex their skeletons. Trends Plant Sci. 2003, 8, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Himmelspach, R.; Williamson, R.E.; Wasteneys, G.O. Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 2003, 15, 1414–1429. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kato, T. Cortical control of plant microtubules. Curr. Opin. Plant Biol. 2006, 9, 5–11. [Google Scholar] [CrossRef]
- Bringmann, M.; Li, E.; Sampathkumar, A.; Kocabek, T.; Hauser, M.T.; Persson, S. POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 2012, 24, 163–177. [Google Scholar] [CrossRef]
- Kesten, C.; Wallmann, A.; Schneider, R.; McFarlane, H.E.; Diehl, A.; Khan, G.A.; van Rossum, B.J.; Lampugnani, E.R.; Szymanski, W.G.; Cremer, N.; et al. The companion of cellulose synthase 1 confers salt tolerance through a Tau-like mechanism in plants. Nat. Commun. 2019, 10, 857. [Google Scholar] [CrossRef]
- Buschmann, H.; Borchers, A. Handedness in plant cell expansion: A mutant perspective on helical growth. New Phytol. 2020, 225, 53–69. [Google Scholar] [CrossRef]
- Kumar, S.; Jeevaraj, T.; Yunus, M.H.; Chakraborty, S.; Chakraborty, N. The plant cytoskeleton takes center stage in abiotic stress responses and resilience. Plant Cell Environ. 2023, 46, 5–22. [Google Scholar] [CrossRef]
- Wang, P.; Qi, S.; Wang, X.; Dou, L.; Jia, M.A.; Mao, T.; Guo, Y.; Wang, X. The OPEN STOMATA1-SPIRAL1 module regulates microtubule stability during abscisic acid-induced stomatal closure in Arabidopsis. Plant Cell 2022, 35, 260–278. [Google Scholar] [CrossRef]
- Wang, S.; Kurepa, J.; Hashimoto, T.; Smalle, J.A. Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. Plant Cell 2011, 23, 3412–3427. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Pike, S.; Gassmann, W.; Baskin, T.I. Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol. 2003, 44, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Abdrakhamanova, A.; Wang, Q.Y.; Khokhlova, L.; Nick, P. Is microtubule disassembly a trigger for cold acclimation? Plant Cell Physiol. 2003, 44, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, D.; Hardham, A.R. The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol. 2004, 136, 3864–3876. [Google Scholar] [CrossRef]
- Nick, P. Signaling to the microtubular cytoskeleton in plants. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1998; Volume 184, pp. 33–80. [Google Scholar]
- Komis, G.; Apostolakos, P.; Galatis, B. Hyperosmotic stress induces formation of tubulin macrotubules in root-tip cells of Triticum turgidum: Their probable involvement in protoplast volume control. Plant Cell Physiol. 2002, 43, 911–922. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Yuan, M. Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol. 2007, 48, 1534–1547. [Google Scholar] [CrossRef]
- Nakajima, K.; Kawamura, T.; Hashimoto, T. Role of the SPIRAL1 gene family in anisotropic growth of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 513–522. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Ehrhardt, D.W.; Fisher, S.E.; Scheible, W.R.; Somerville, C.R. The Arabidopsis sku6/spiral1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell 2004, 16, 1506–1520. [Google Scholar] [CrossRef]
- Nakajima, K.; Furutani, I.; Tachimoto, H.; Matsubara, H.; Hashimoto, T. SPIRAL1 encodes a plant-specific microtubule-localized protein required for directional control of rapidly expanding Arabidopsis cells. Plant Cell 2004, 16, 1178–1190. [Google Scholar] [CrossRef]
- Furutani, I. The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 2000, 127, 4443–4453. [Google Scholar] [CrossRef]
- Galva, C.; Kirik, V.; Lindeboom, J.J.; Kaloriti, D.; Rancour, D.M.; Hussey, P.J.; Bednarek, S.Y.; Ehrhardt, D.W.; Sedbrook, J.C. The microtubule plus-end tracking proteins SPR1 and EB1b interact to maintain polar cell elongation and directional organ growth in Arabidopsis. Plant Cell 2014, 26, 4409–4425. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.; Pinto, R.B.; Boatwright, J.; Borges, L.; Brown, G.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny—The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Lewis, G.; Schrire, B.; Mackinder, B.; Loch, M. Legumes of the World; Royal Botanic Gardens, Kew: Richmond, UK, 2005. [Google Scholar]
- Hyams, J.S.; Lloyd, C.W. Microtubules; Wiley-Liss: New York, NY, USA, 1994; Volume 9. [Google Scholar]
- Ruff, K.M.; Pappu, R.V. AlphaFold and implications for intrinsically disordered proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [PubMed]
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef]
- Demuth, J.P.; Hahn, M.W. The life and death of gene families. Bioessays 2009, 31, 29–39. [Google Scholar] [CrossRef]
- Jia, B.; Wang, Y.; Zhang, D.; Li, W.; Cui, H.; Jin, J.; Cai, X.; Shen, Y.; Wu, S.; Guo, Y.; et al. Genome-wide identification, characterization and expression analysis of soybean CHYR gene family. Int. J. Mol. Sci. 2021, 22, 12192. [Google Scholar] [CrossRef]
- Meng, J.; Peng, M.; Yang, J.; Zhao, Y.; Hu, J.; Zhu, Y.; He, H. Genome-wide analysis of the Cyclin gene family and their expression profile in Medicago truncatula. Int. J. Mol. Sci. 2020, 21, 9430. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, L.; Du, H.; Wang, Y.; Wang, W.; Liu, J.; Huang, J.; Huang, W.; Ge, L. Genome-wide study of C2H2 zinc finger gene family in Medicago truncatula. BMC Plant Biol. 2020, 20, 401. [Google Scholar] [CrossRef]
- Cai, Z.; Xian, P.; Lin, R.; Cheng, Y.; Lian, T.; Ma, Q.; Nian, H. Characterization of the soybean GmIREG family genes and the function of GmIREG3 in conferring tolerance to Aluminum stress. Int. J. Mol. Sci. 2020, 21, 497. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Sui, J.; Luo, Y.; Rao, G. The Salix SmSPR1 involved in light-regulated cell expansion by modulating microtubule arrangement. Front. Cell Dev. Biol. 2019, 7, 309. [Google Scholar] [CrossRef]
- Carter, D.L. Problems of salinity in agriculture. In Plants in Saline Environments; Springer: Berlin/Heidelberg, Germany, 1975; pp. 25–35. [Google Scholar]
- Duzan, H.M.; Zhou, X.; Souleimanov, A.; Smith, D.L. Perception of Bradyrhizobium japonicum Nod factor by soybean [Glycine max (L.) Merr.] root hairs under abiotic stress conditions. J. Exp. Bot. 2004, 55, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, E.M.; Baranova, E.N.; Smirnova, E.A. Reorganization of interphase microtubules in root cells of Medicago sativa L. during acclimation to osmotic and salt stress. Cell Tissue Biol. 2017, 11, 324–334. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B.; Deerfield, D.W. GeneDoc: Analysis and visualization of genetic variation. EMBnet News 1997, 4, 1–14. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Limami, A.M.; Ricoult, C.; Planchet, E.; González, E.M.; Ladrera, R.; Larrainzar, E.; Arrese-Igor, C.; Merchan, F.; Crespi, M.; Frugier, F. Response of Medicago truncatula to abiotic stress. In Medicago Truncatula Handbook; Samuel Roberts Noble Foundation: Ardmore, PA, USA, 2007. [Google Scholar]
- Carrere, S.; Verdier, J.; Gamas, P. MtExpress, a comprehensive and curated RNAseq-based gene expression atlas for the model legume Medicago truncatula. Plant Cell Physiol. 2021, 62, 1494–1500. [Google Scholar] [CrossRef]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene Name | TIGR Locus | Chr | Start Site | End Site | Strand | CDS (bp) | Size (aa) | MWs (Da) | pI |
|---|---|---|---|---|---|---|---|---|---|
| MtSP1L-1 | MtrunA17_Chr5g0419471 | Medtr_chr05 | 19,809,242 | 19,812,567 | - | 315 | 104 | 10,102.97 | 9.16 |
| MtSP1L-2 | MtrunA17_Chr6g0454801 | Medtr_chr06 | 5,010,040 | 5,013,939 | + | 420 | 139 | 13,634.85 | 9.26 |
| GmSP1L-1 | Glyma.01G060900 | Glyma_chr01 | 8,375,261 | 8,378,172 | + | 327 | 108 | 10,681.55 | 8.08 |
| GmSP1L-2 | Glyma.02G119200 | Glyma_chr02 | 11,459,648 | 11,462,099 | + | 327 | 108 | 10,575.38 | 9.16 |
| GmSP1L-3 | Glyma.19G032000 | Glyma_chr19 | 4,032,554 | 4,035,979 | + | 393 | 130 | 12,443.56 | 9.36 |
| GmSP1L-4 | Glyma.13G055400 | Glyma_chr13 | 14,317,887 | 14,320,766 | - | 366 | 121 | 11,834.82 | 9.65 |
| GmSP1L-5 | Glyma.11G021900 | Glyma_chr11 | 1,550,669 | 1,551,650 | - | 300 | 99 | 10,278.25 | 8.11 |
| GmSP1L-6 | Glyma.01G221900 | Glyma_chr01 | 56,191,552 | 56,192,480 | + | 291 | 96 | 10,170.11 | 7.97 |
| GmSP1L-7 | Glyma.05G210800 | Glyma_chr05 | 39,307,017 | 39,308,083 | + | 264 | 87 | 9177.07 | 9.10 |
| GmSP1L-8 | Glyma.08G017200 | Glyma_chr08 | 1,395,764 | 1,396,925 | + | 264 | 87 | 9161.93 | 6.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Liu, J.; Jiang, J.; Liu, F.; Zhang, Z.; Yu, X.; Li, M.; Alam, I.; Ge, L. Genome-Wide Identification, Characterization, and Expression Analysis of SPIRAL1 Family Genes in Legume Species. Int. J. Mol. Sci. 2023, 24, 3958. https://doi.org/10.3390/ijms24043958
Yu Q, Liu J, Jiang J, Liu F, Zhang Z, Yu X, Li M, Alam I, Ge L. Genome-Wide Identification, Characterization, and Expression Analysis of SPIRAL1 Family Genes in Legume Species. International Journal of Molecular Sciences. 2023; 24(4):3958. https://doi.org/10.3390/ijms24043958
Chicago/Turabian StyleYu, Qianxia, Junjie Liu, Jiayu Jiang, Fudong Liu, Zhen Zhang, Xiaoye Yu, Mengru Li, Intikhab Alam, and Liangfa Ge. 2023. "Genome-Wide Identification, Characterization, and Expression Analysis of SPIRAL1 Family Genes in Legume Species" International Journal of Molecular Sciences 24, no. 4: 3958. https://doi.org/10.3390/ijms24043958
APA StyleYu, Q., Liu, J., Jiang, J., Liu, F., Zhang, Z., Yu, X., Li, M., Alam, I., & Ge, L. (2023). Genome-Wide Identification, Characterization, and Expression Analysis of SPIRAL1 Family Genes in Legume Species. International Journal of Molecular Sciences, 24(4), 3958. https://doi.org/10.3390/ijms24043958




