Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of SBP and Heart Rate
2.3. Aortic Samples Preparation
2.4. Alizarin Red Staining
2.5. Measurement of Plasma Insulin and Triglyceride
2.6. Cell Culture and Treatment
2.7. Cell Viability Assay
2.8. DHE Fluorescence Staining for ROS Level Assay
2.9. Determination of ROS Level and NADPH Oxidase Activity
2.10. Measurement of Calcium Content
2.11. Measurement of Alkaline Phosphatase (ALP) Activity
2.12. Western Blot Analysis
2.13. Reagents and Antibodies
2.14. Statistics
3. Results
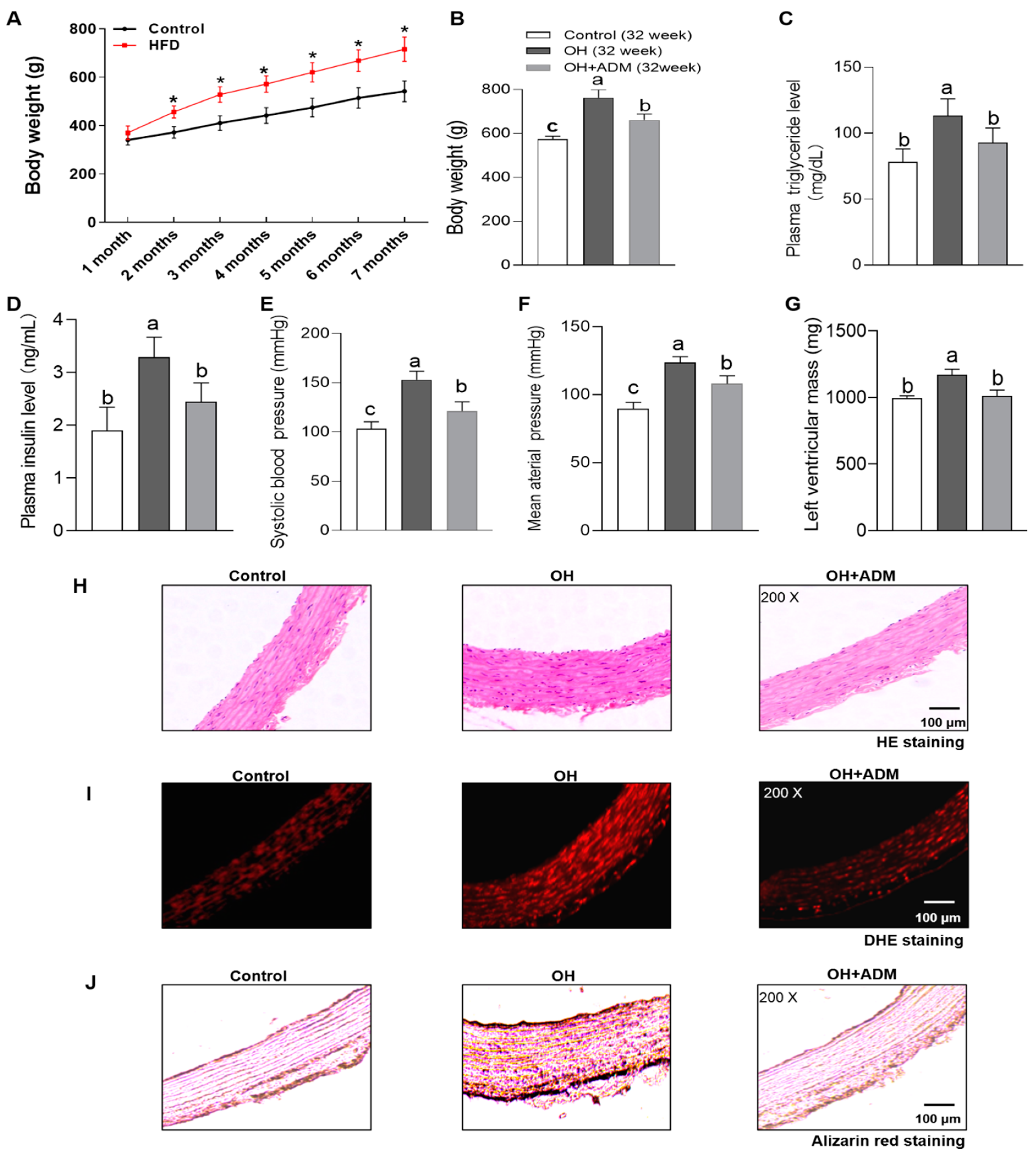
3.1. The Effects of ADM on Body Weight, Metabolic Parameters, Blood Pressure and Left Ventricular Mass in HFD-Induced OH Rats
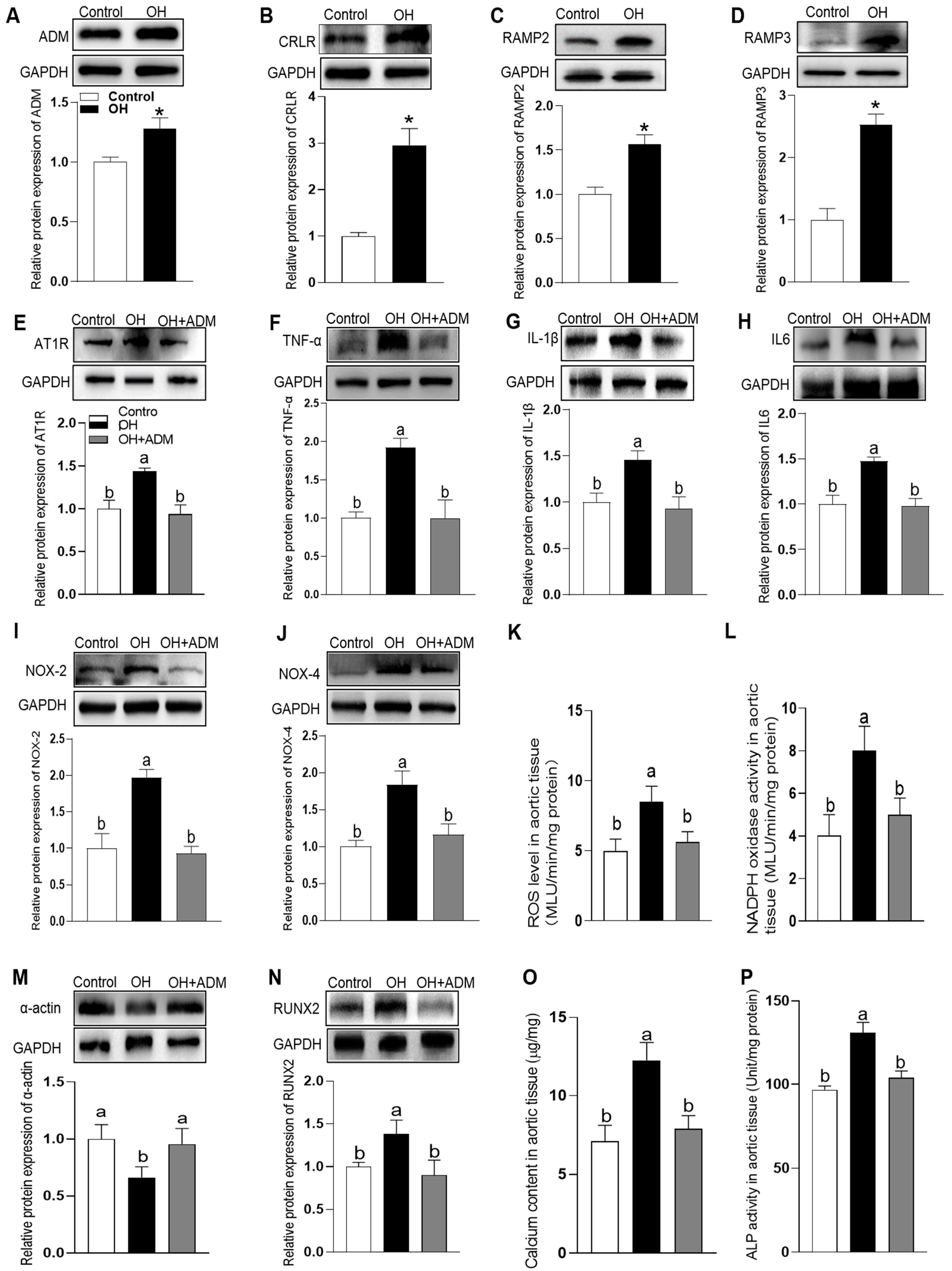
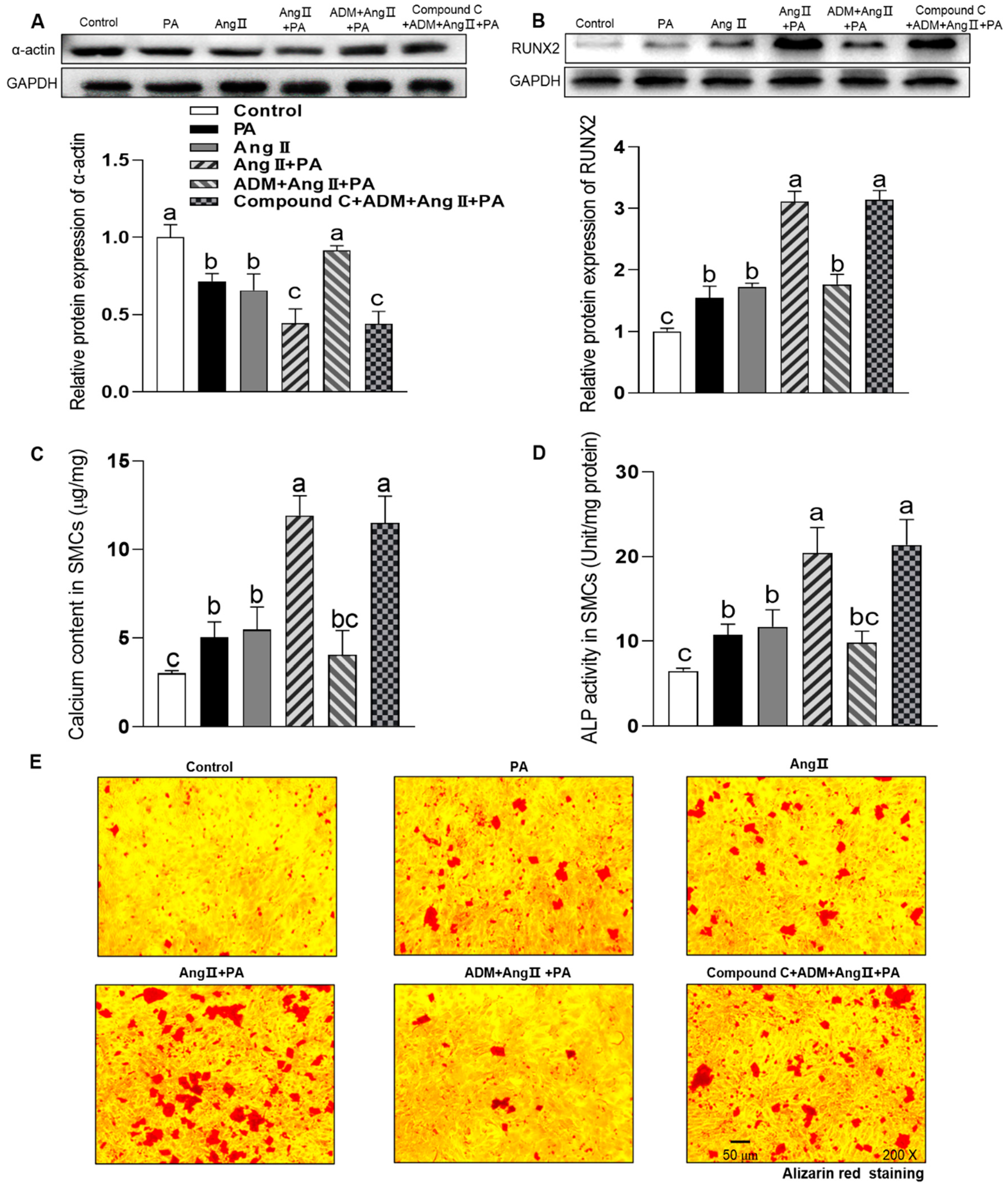
3.2. The Endogenous Expression of ADM and Its Receptor System and The Effects of ADM on Aortic Inflammation, Oxidative Stress and Calcification in HFD-Induced OH Rats
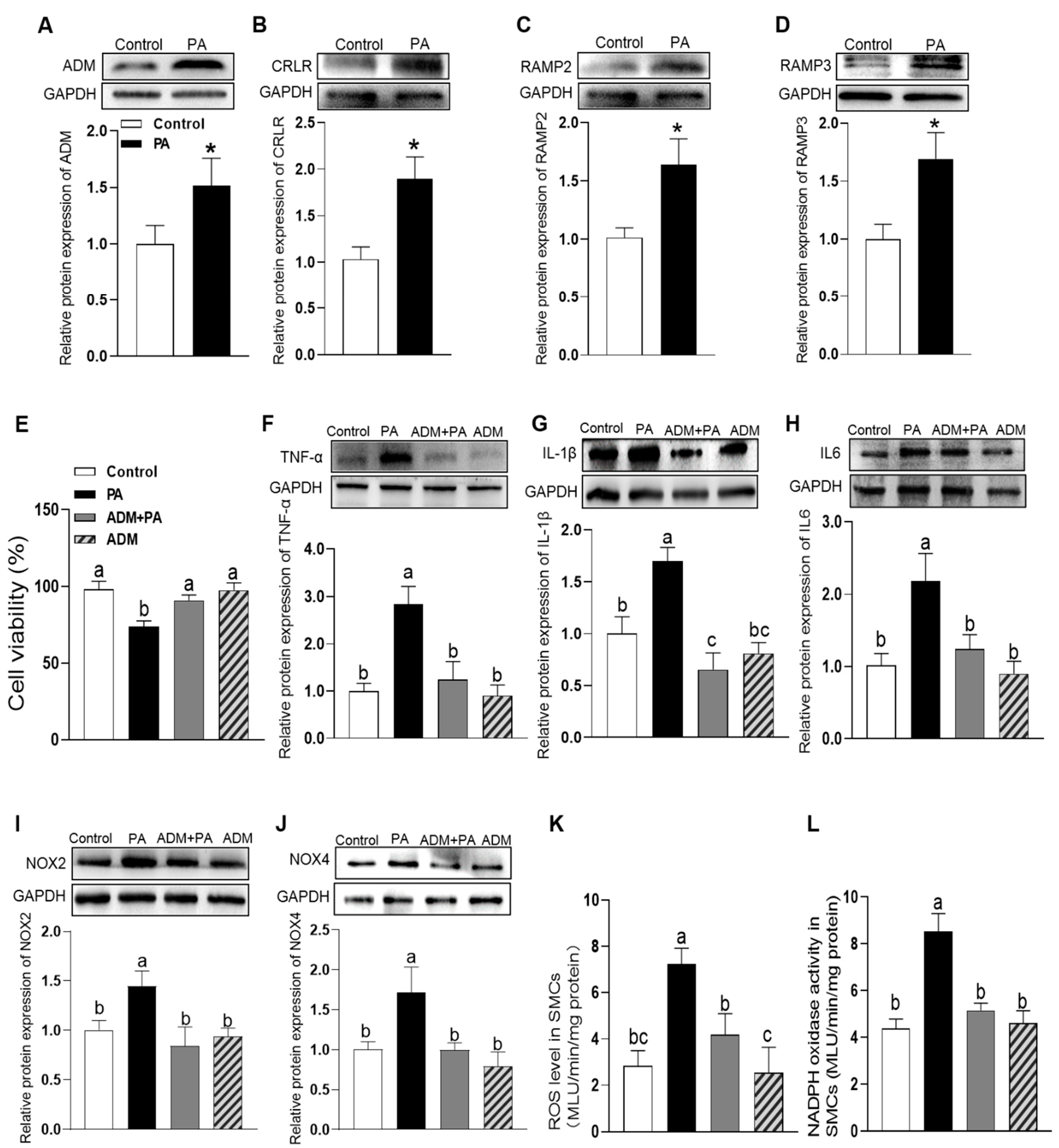
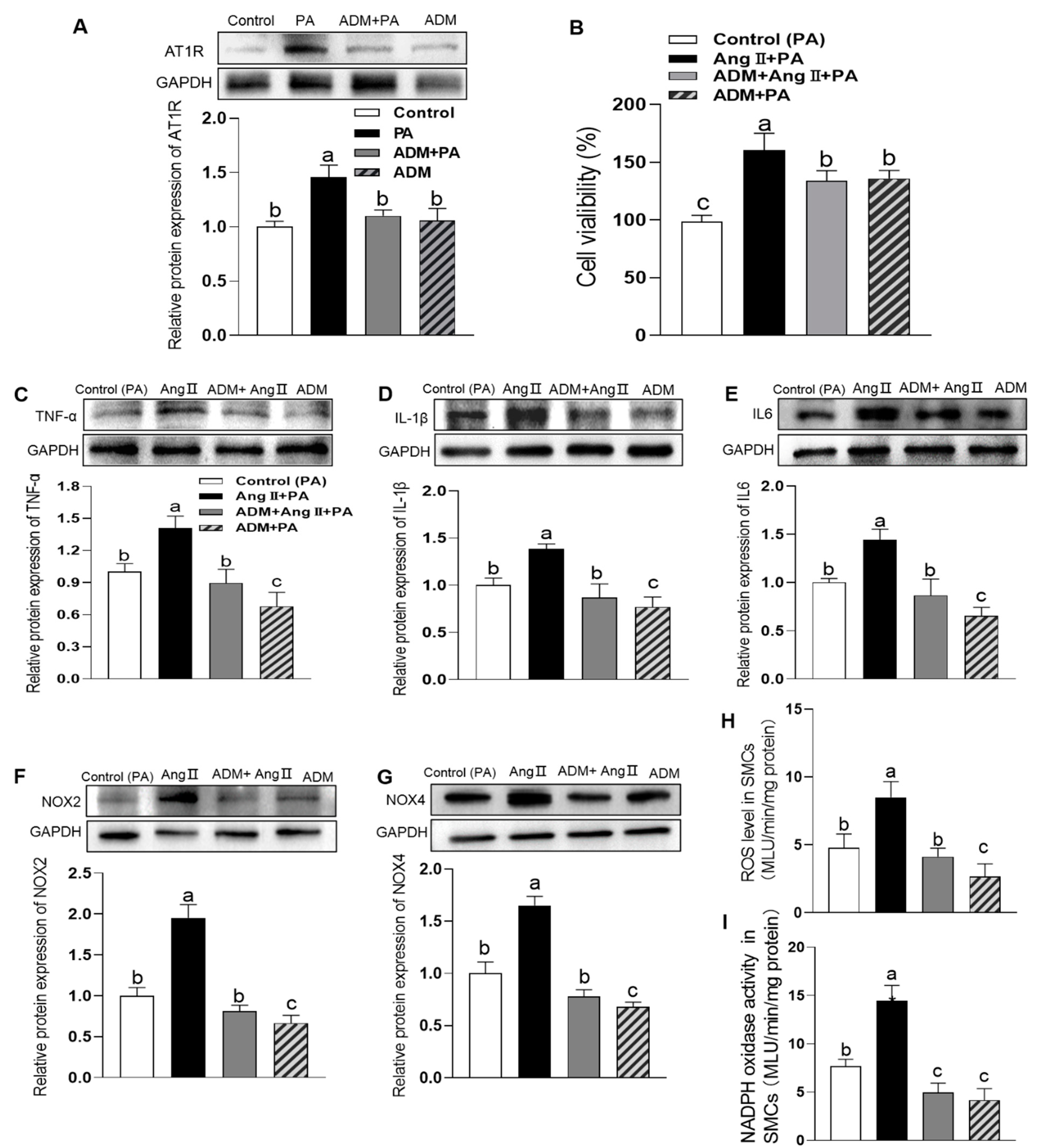
3.3. The Effects of Exogenous ADM Pretreatment on PA-Induced Cell Viability, Inflammation and Oxidative Stress in A7r5 Cells and Its Possible Mechanisms
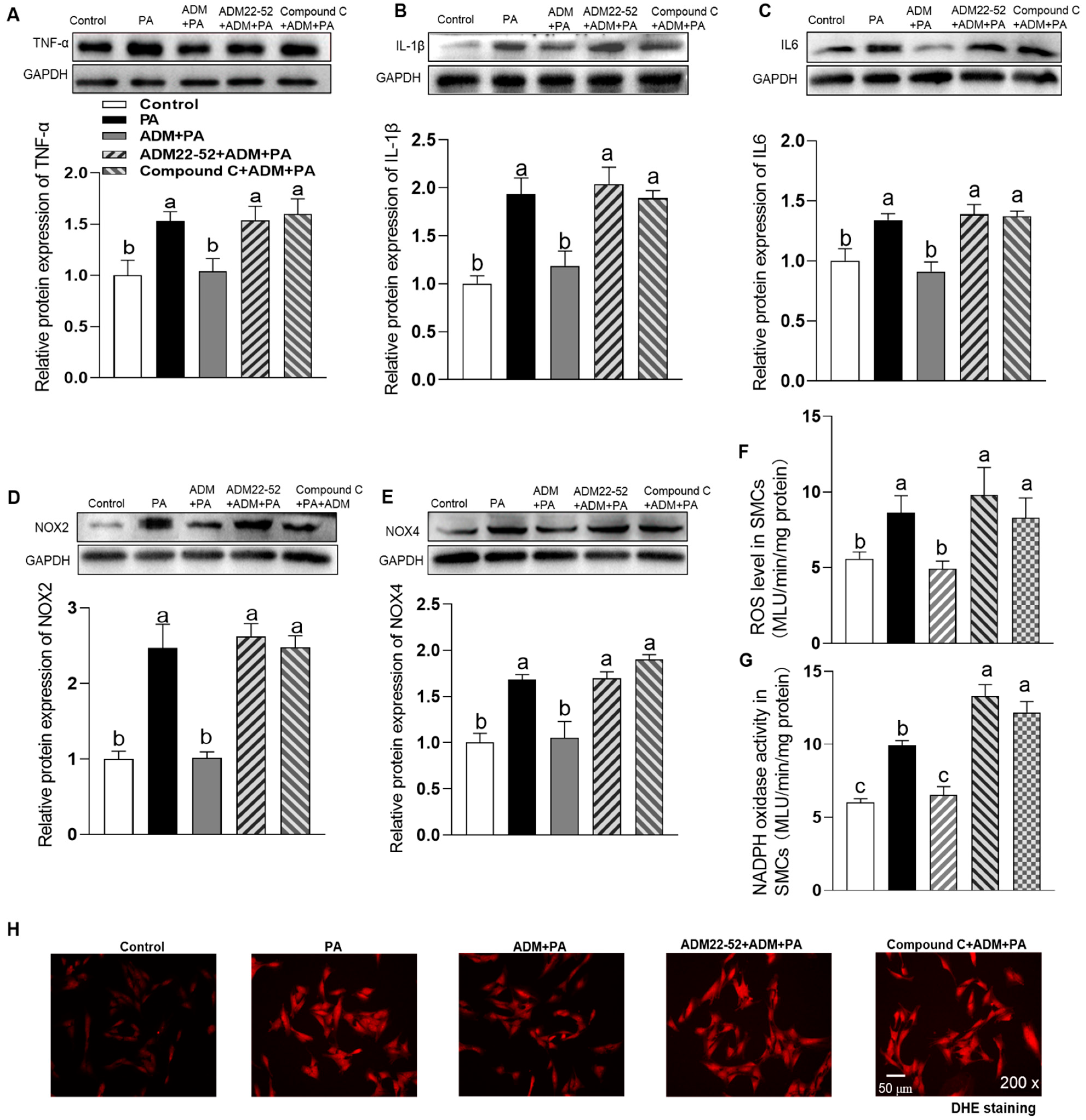
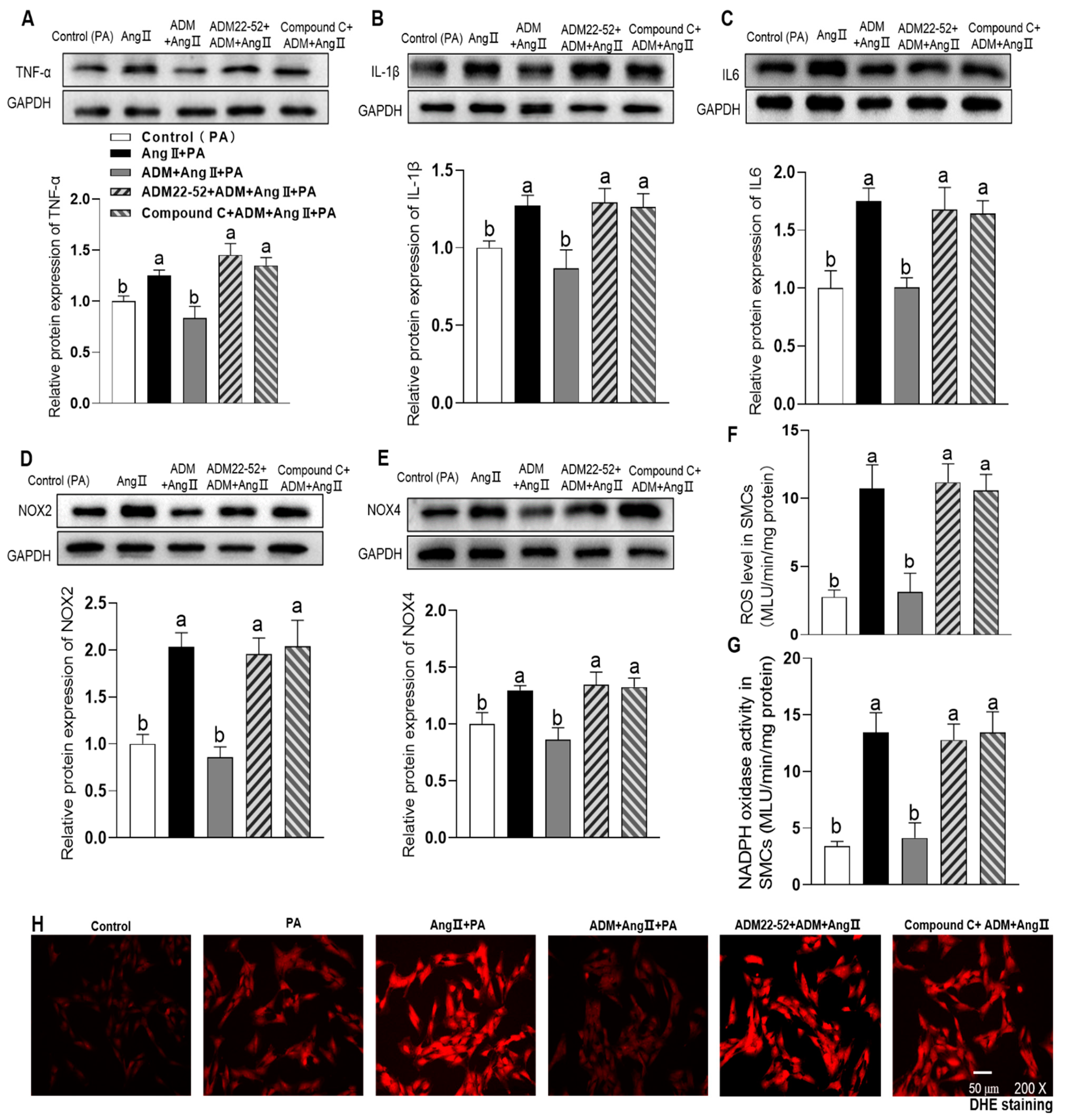
3.4. The Effects of Exogenous ADM Pretreatment on PA Plus Ang II-Induced Inflammation and Oxidative Stress in A7r5 Cells and Its Possible Mechanisms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Brown, I.A.; Diederich, L.; Good, M.; DeLalio, L.; Murphy, S.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arter. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, H.-H.; Chiang, C.-L.; Liu, C.-H.; Yeh, J.-I.; Chen, M.-F.; Chen, P.-Y.; Kuo, J.-S.; Lee, T.J. Role of Perivascular Adipose Tissue–Derived Methyl Palmitate in Vascular Tone Regulation and Pathogenesis of Hypertension. Circulation 2011, 124, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Wang, L.; Espinosa-Diez, C.; Wang, B.; Hahn, S.A.; Noda, K.; Rochon, E.R.; Dent, M.R.; Levine, A.R.; Baust, J.J.; et al. Metabolic Syndrome Mediates ROS-miR-193b-NFYA-Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise-Induced Pulmonary Hypertension in Heart Failure with Preserved Ejection Fraction. Circulation 2021, 144, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A Novel Angiotensin II-Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ. Res. 2018, 123, 1298–1312. [Google Scholar] [CrossRef]
- Huang, S.; You, S.; Qian, J.; Dai, C.; Shen, S.; Wang, J.; Huang, W.; Liang, G.; Wu, G. Myeloid differentiation 2 deficiency attenuates AngII-induced arterial vascular oxidative stress, inflammation, and remodeling. Aging 2021, 13, 4409–4427. [Google Scholar] [CrossRef]
- Wortmann, M.; Arshad, M.; Hakimi, M.; Böckler, D.; Dihlmann, S. Deficiency in Aim2 affects viability and calcification of vascular smooth muscle cells from murine aortas and angiotensin-II induced aortic aneurysms. Mol. Med. 2020, 26, 87. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Bouvet, C.; Barrette, M.; Moreau, P. Palmitic Acid Increases Medial Calcification by Inducing Oxidative Stress. J. Vasc. Res. 2013, 50, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Donis, N.; Jiang, Z.; D’Emal, C.; Hulin, A.; Debuisson, M.; Dulgheru, R.; Nguyen, M.-L.; Postolache, A.; Lallemand, F.; Coucke, P.; et al. Differential Biological Effects of Dietary Lipids and Irradiation on the Aorta, Aortic Valve, and the Mitral Valve. Front. Cardiovasc. Med. 2022, 9, 839720. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrero, S.; Martínez, A. Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Cheung, T.T.; Cheung, B.M.Y. Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc. Dis. 2012, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.-D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Koyama, T.; Kuriyama, N.; Suzuki, Y.; Saito, S.; Tanaka, R.; Iwao, M.; Tanaka, M.; Maki, T.; Itoh, H.; Ihara, M.; et al. Mid-regional pro-adrenomedullin is a novel biomarker for arterial stiffness as the criterion for vascular failure in a cross-sectional study. Sci. Rep. 2021, 11, 305. [Google Scholar] [CrossRef]
- Metwalley, K.A.; Farghaly, H.S.; Sherief, T. Plasma adrenomedullin level in children with obesity: Relationship to left ventricular function. World J. Pediatr. 2018, 14, 84–91. [Google Scholar] [CrossRef]
- Nomura, I.; Kato, J.; Tokashiki, M.; Kitamura, K. Increased plasma levels of the mature and intermediate forms of adrenomedullin in obesity. Regul. Pept. 2009, 158, 127–131. [Google Scholar] [CrossRef]
- Qian, P.; Wang, Q.; Wang, F.-Z.; Dai, H.-B.; Wang, H.-Y.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin Improves Cardiac Remodeling and Function in Obese Rats with Hypertension. Pharmaceuticals 2022, 15, 719. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Vannacci, A.; Rastogi, S. Oxidative Stress and Inflammation in Obesity, Diabetes, Hypertension, and Related Cardiometabolic Complications. Oxidative Med. Cell. Longev. 2014, 2014, 506948. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Improving obesity and blood pressure. Hypertens. Res. 2020, 43, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Kitamura, K. Translational studies of adrenomedullin and related peptides regarding cardiovascular diseases. Hypertens. Res. 2022, 45, 389–400. [Google Scholar] [CrossRef]
- Cheung, B.M.; Tang, F. Adrenomedullin: Exciting new horizons. Recent Pat. Endocr. Metab. Immune Drug. Discov. 2012, 6, 4–17. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Fukai, N.; Sato, R.; Sugiyama, T.; Ozawa, N.; Shichiri, M.; Hirata, Y. Antioxidant Effect of Adrenomedullin on Angiotensin II-Induced Reactive Oxygen Species Generation in Vascular Smooth Muscle Cells. Endocrinology 2004, 145, 3331–3337. [Google Scholar] [CrossRef]
- Zhou, Y.-B.; Gao, Q.; Li, P.; Han, Y.; Zhang, F.; Qi, Y.-F.; Tang, C.-S.; Gao, X.-Y.; Zhu, G.-Q. Adrenomedullin attenuates vascular calcification in fructose-induced insulin resistance rats. Acta Physiol. 2013, 207, 437–446. [Google Scholar] [CrossRef]
- Tsuruda, T.; Kato, J.; Kuwasako, K.; Kitamura, K. Adrenomedullin: Continuing to explore cardioprotection. Peptides 2019, 111, 47–54. [Google Scholar] [CrossRef]
- Dai, H.-B.; Wang, H.-Y.; Wang, F.-Z.; Qian, P.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin ameliorates palmitic acid-induced insulin resistance through PI3K/Akt pathway in adipocytes. Acta Diabetol. 2022, 59, 661–673. [Google Scholar] [CrossRef]
- Chakravarty, P.; Suthar, T.P.; Coppock, H.A.; Nicholl, C.G.; Bloom, S.R.; Legon, S.; Smith, D.M. CGRP and adrenomedullin binding correlates with transcript levels for Calcitonin Receptor-Like Receptor (CRLR) and Receptor Activity Modifying Proteins (RAMPs) in rat tissues. Br. J. Pharmacol. 2000, 130, 189–195. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, M.Q.; Ni, S.H.; Wang, M.; Liu, L.Y.; You, H.Y.; Wu, X.H.; Wang, Y.J.; Lu, L.; Wei, L.B. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020, 867, 172797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R.; Zou, J.; Ying, Y.; Luo, Z. Dual Roles of the AMP-Activated Protein Kinase Pathway in Angiogenesis. Cells 2019, 8, 752. [Google Scholar] [CrossRef]
- Meng, T.; Qin, W.; Liu, B. SIRT1 Antagonizes Oxidative Stress in Diabetic Vascular Complication. Front. Endocrinol. 2020, 11, 568861. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Sánchez, A.; Sáenz-Medina, J.; Muñoz, M.; Hernández, M.; López, M.; Rivera, L.; Contreras, C.; Prieto, D. Activation of AMP kinase ameliorates kidney vascular dysfunction, oxidative stress and inflammation in rodent models of obesity. Br. J. Pharmacol. 2021, 178, 4085–4103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Qiu, Y.; Wu, N.; Chen, A.-D.; Zhou, H.; Chen, Q.; Kang, Y.-M.; Li, Y.-H.; Zhu, G.-Q. FNDC5 Attenuates Oxidative Stress and NLRP3 Inflammasome Activation in Vascular Smooth Muscle Cells via Activating the AMPK-SIRT1 Signal Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 6384803. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, C.; Cho, K.; Jang, W. Policosanol attenuates Pi-induced calcification via AMPK-mediated INSIGs expression in rat VSMCs. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1336–1345. [Google Scholar] [CrossRef]
- Hong, Y.; Hay, D.L.; Quirion, R.; Poyner, D.R. The pharmacology of adrenomedullin 2/intermedin. Br. J. Pharmacol. 2012, 166, 110–120. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Y.; Lv, Y.; Sun, L.; Zhang, S.; Li, Y.; Wang, Y.; Liu, G.; Xu, M.-J.; Wang, X.; et al. Intermedin Restores Hyperhomocysteinemia-induced Macrophage Polarization and Improves Insulin Resistance in Mice. J. Biol. Chem. 2016, 291, 12336–12345. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.-S.; Ren, J.-L.; Zhang, Y.-R.; Wu, N.; Jia, M.-Z.; Yu, Y.-R.; Ning, Z.-P.; Tang, C.-S.; Qi, Y.-F. Intermedin1–53 attenuates aging-associated vascular calcification in rats by upregulating sirtuin. Aging 2020, 12, 5651–5674. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-W.; Zhao, L.; Zhang, J.-S.; Hou, Y.-L.; Yu, Y.-R.; Jia, M.-Z.; Tang, C.-S.; Qi, Y.-F. Intermedin1–53 protects against cardiac hypertrophy by inhibiting endoplasmic reticulum stress via activating AMP-activated protein kinase. J. Hypertens. 2015, 33, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Y.; Wang, F.-Z.; Chang, R.; Wang, Q.; Liu, S.-Y.; Cheng, Z.-X.; Gao, Q.; Zhou, H.; Zhou, Y.-B. Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension. Int. J. Mol. Sci. 2023, 24, 3943. https://doi.org/10.3390/ijms24043943
Wang H-Y, Wang F-Z, Chang R, Wang Q, Liu S-Y, Cheng Z-X, Gao Q, Zhou H, Zhou Y-B. Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension. International Journal of Molecular Sciences. 2023; 24(4):3943. https://doi.org/10.3390/ijms24043943
Chicago/Turabian StyleWang, Hong-Yu, Fang-Zheng Wang, Rui Chang, Qian Wang, Si-Yu Liu, Ze-Xiong Cheng, Qing Gao, Hong Zhou, and Ye-Bo Zhou. 2023. "Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension" International Journal of Molecular Sciences 24, no. 4: 3943. https://doi.org/10.3390/ijms24043943
APA StyleWang, H.-Y., Wang, F.-Z., Chang, R., Wang, Q., Liu, S.-Y., Cheng, Z.-X., Gao, Q., Zhou, H., & Zhou, Y.-B. (2023). Adrenomedullin Improves Hypertension and Vascular Remodeling partly through the Receptor-Mediated AMPK Pathway in Rats with Obesity-Related Hypertension. International Journal of Molecular Sciences, 24(4), 3943. https://doi.org/10.3390/ijms24043943






