Voglibose Regulates the Secretion of GLP-1 Accompanied by Amelioration of Ileal Inflammatory Damage and Endoplasmic Reticulum Stress in Diabetic KKAy Mice
Abstract
1. Introduction
2. Results
2.1. Voglibose Ameliorates Glucose Metabolism in Diabetic KKAy Mice
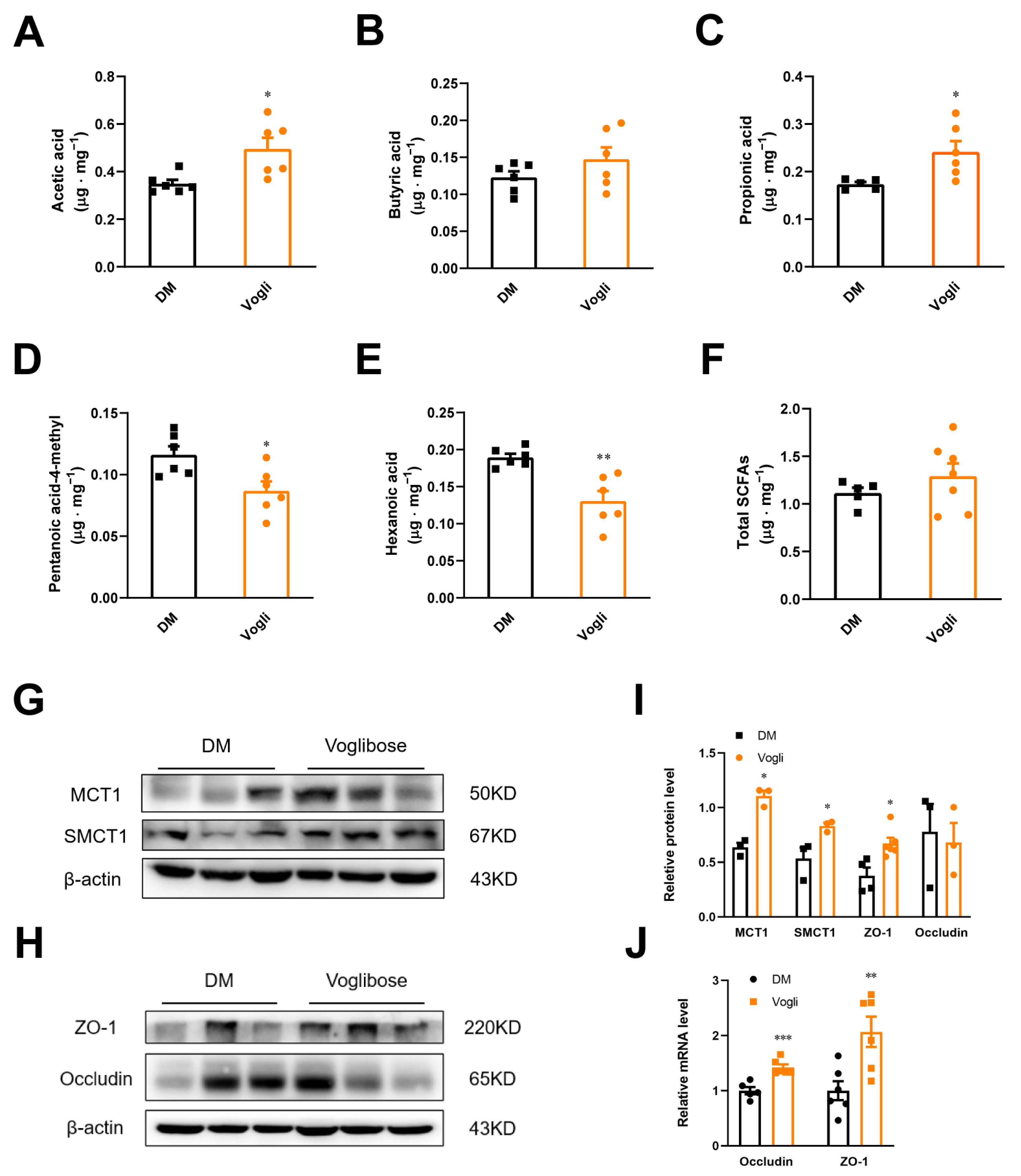
2.2. Voglibose Modulates the Composition and Transposition of SCFAs
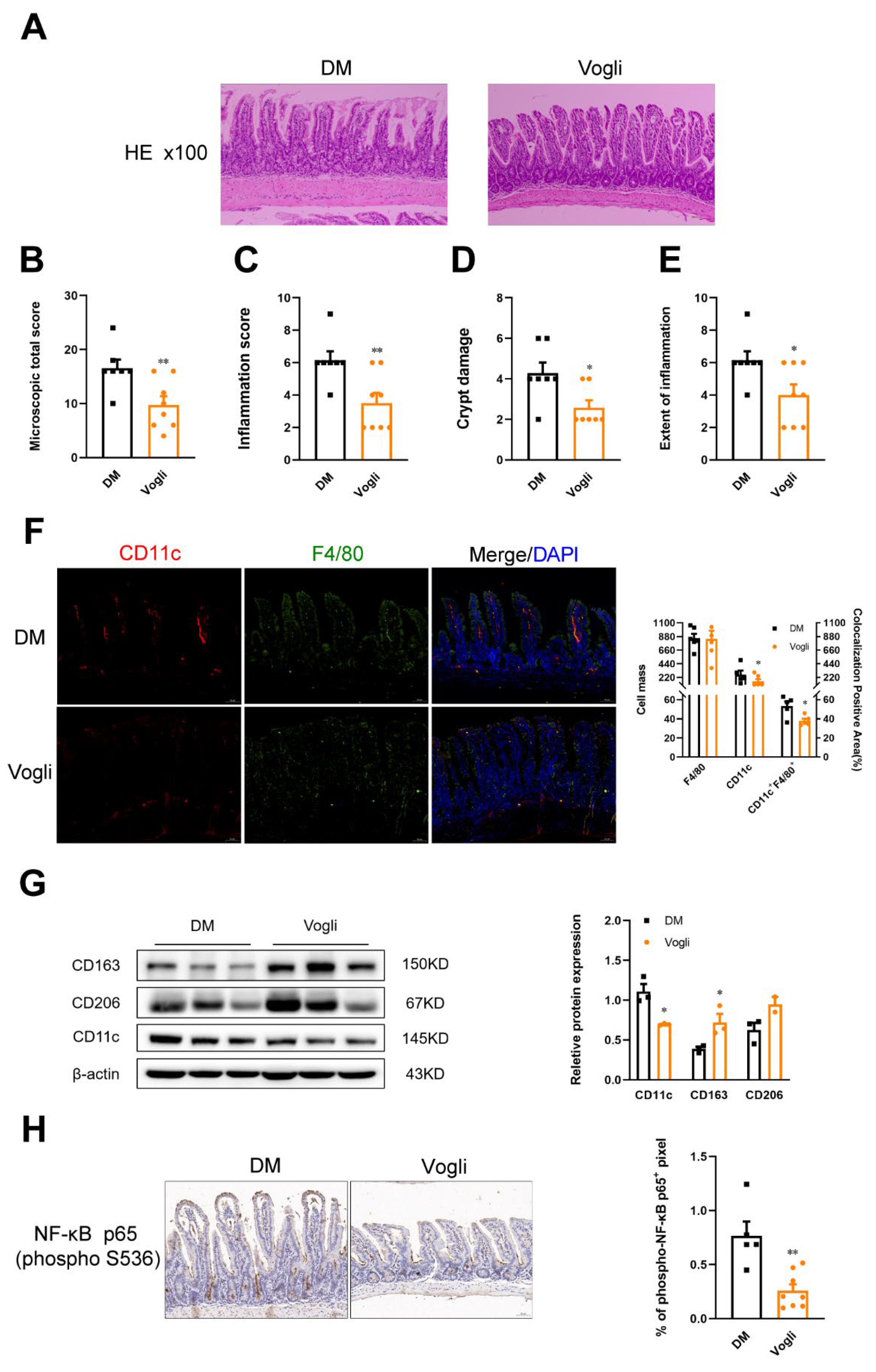
2.3. Voglibose Alleviates Ileal Inflammation and Maintains Intestinal Integrity in Diabetic KKAy Mice
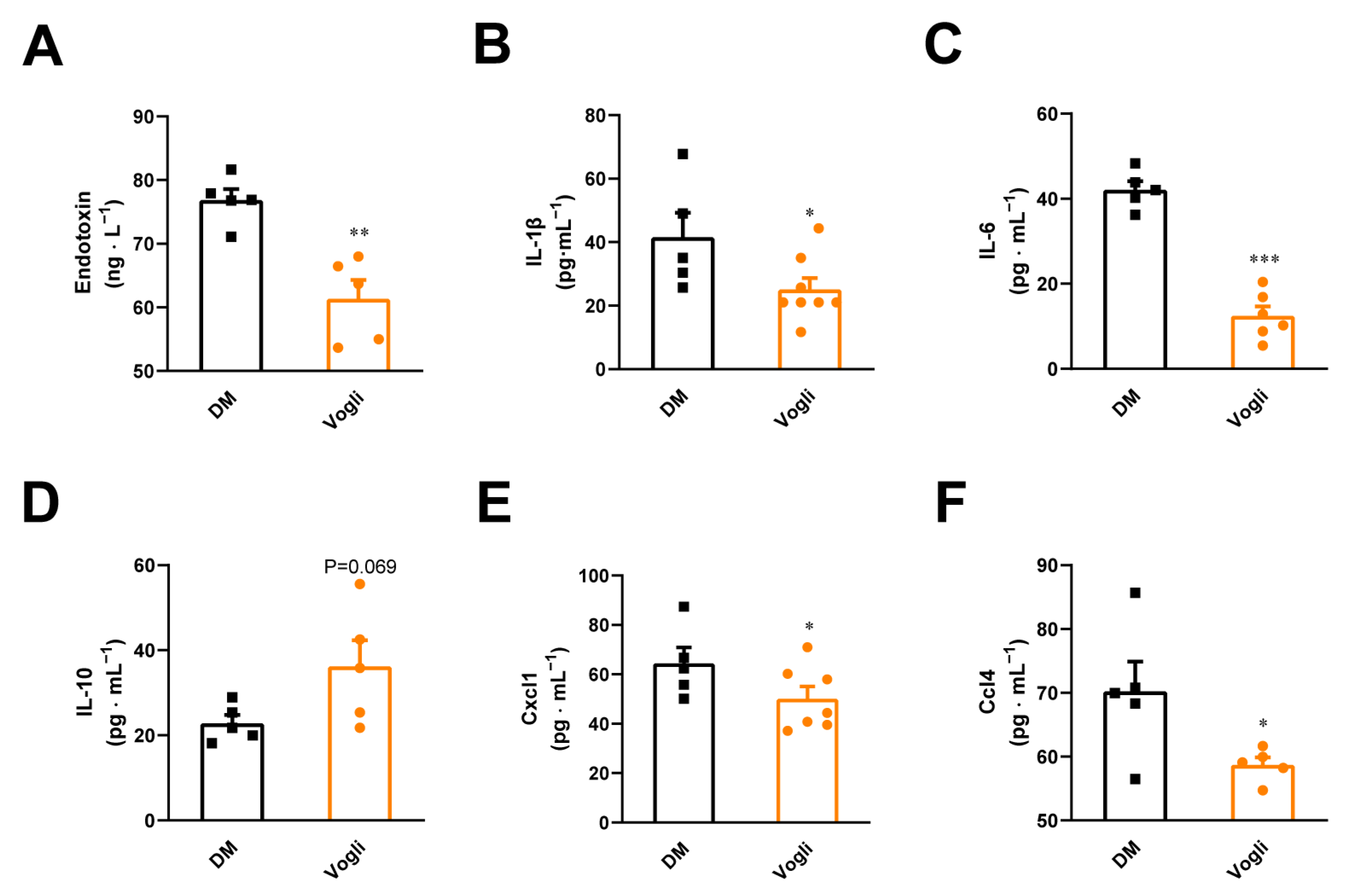
2.4. Voglibose Attenuates Endotoxin Content, Pro-Inflammatory Cytokines and Chemokine Levels in the Serum of Diabetic KKAy Mice
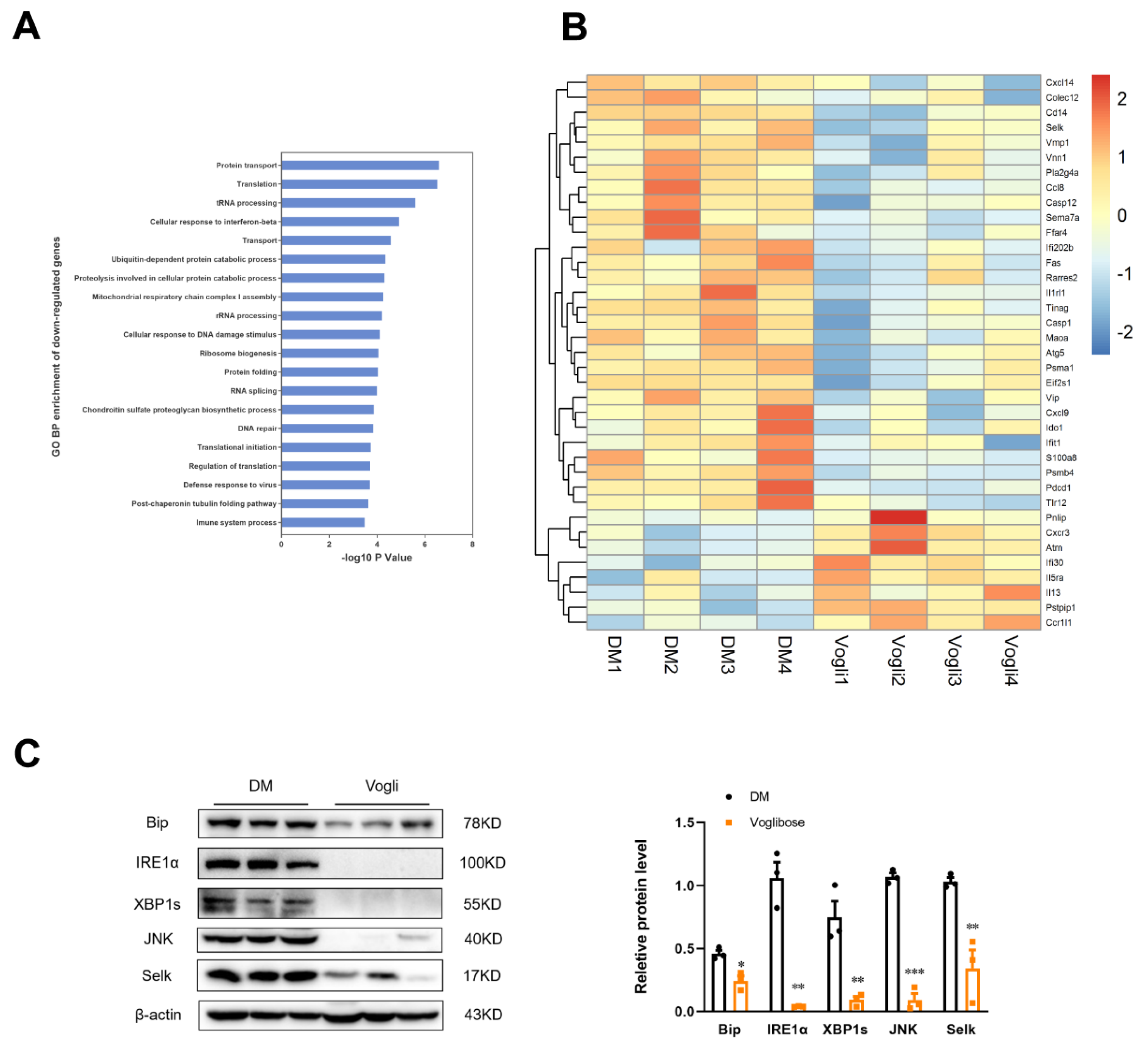
2.5. Voglibose Mitigates the Ileal Expression of ERS-Related Factors in Diabetic KKAy Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experimental Design
4.2. Blood Glucose and Glycated Hemoglobin Measurements
4.3. Oral Glucose-Stimulated Insulin and Glucagon-like Peptide 1 (GLP-1) Secretion Test
4.4. Histopathological Evaluation, Immunofluorescence, and Immunohistochemistry Assay of the Ileum
4.5. Fecal Short-Chain Fatty Acids Analysis
4.6. Serum Cytokines and Chemokines Assay
4.7. Western Blotting and Quantitative Real-Time Polymerase Chain Reaction
4.8. GeneChip Micoarray Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Koehler, J.A.; Baggio, L.L.; Powers, A.C.; Sandoval, D.A.; Drucker, D.J. Gut-Proglucagon-Derived Peptides Are Essential for Regulating Glucose Homeostasis in Mice. Cell Metab. 2019, 30, 976–986.e3. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Lin, R.; Li, D.; Xu, Y.; Wei, M.; Chen, Q.; Deng, Y.; Wen, J. Chronic cereulide exposure causes intestinal inflammation and gut microbiota dysbiosis in mice. Environ. Pollut. 2021, 288, 117814. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Rohm, T.V.; Fuchs, R.; Muller, R.L.; Keller, L.; Baumann, Z.; Bosch, A.J.T.; Schneider, R.; Labes, D.; Langer, I.; Pilz, J.B.; et al. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front. Immunol. 2021, 12, 668654. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Kawano, Y.; Nakae, J.; Watanabe, N.; Kikuchi, T.; Tateya, S.; Tamori, Y.; Kaneko, M.; Abe, T.; Onodera, M.; Itoh, H. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016, 24, 295–310. [Google Scholar] [CrossRef]
- Rohm, T.V.; Keller, L.; Bosch, A.J.T.; AlAsfoor, S.; Baumann, Z.; Thomas, A.; Wiedemann, S.J.; Steiger, L.; Dalmas, E.; Wehner, J.; et al. Targeting colonic macrophages improves glycemic control in high-fat diet-induced obesity. Commun. Biol. 2022, 5, 370. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.L.; Dotti, I.; Pascoal, L.B.; Morari, J.; Esteller, M.; Coope, A.; Ayrizono, M.L.S.; Salas, A.; Leal, R.F. Endoplasmic Reticulum Stress in Colonic Mucosa of Ulcerative Colitis Patients Is Mediated by PERK and IRE1 Pathway Activation. Mediat. Inflamm. 2022, 2022, 6049500. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Yamada, R.; Das, S.S.; Sato, T.; Takahashi, A.; Hiratsuka, M.; Hirasawa, N. Glucagon-like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress. Metabolism 2014, 63, 800–811. [Google Scholar] [CrossRef]
- Moritoh, Y.; Takeuchi, K.; Hazama, M. Chronic administration of voglibose, an alpha-glucosidase inhibitor, increases active glucagon-like peptide-1 levels by increasing its secretion and decreasing dipeptidyl peptidase-4 activity in ob/ob mice. J. Pharmacol. Exp. Ther. 2009, 329, 669–676. [Google Scholar] [CrossRef]
- Kato, J.; Shirakami, Y.; Mizutani, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Shimizu, M. Alpha-Glucosidase Inhibitor Voglibose Suppresses Azoxymethane-Induced Colonic Preneoplastic Lesions in Diabetic and Obese Mice. Int. J. Mol. Sci. 2020, 21, 2226. [Google Scholar] [CrossRef]
- Ma, R.C.W. Epidemiology of diabetes and diabetic complications in China. Diabetologia 2018, 61, 1249–1260. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Li, C.N.; Wang, X.; Lei, L.; Liu, M.Z.; Li, R.C.; Sun, S.J.; Liu, S.N.; Huan, Y.; Zhou, T.; Liu, Q.; et al. Berberine combined with stachyose induces better glycometabolism than berberine alone through modulating gut microbiota and fecal metabolomics in diabetic mice. Phytother. Res. 2020, 34, 1166–1174. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Cao, H.; Ji, W.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; et al. Ramulus Mori (Sangzhi) Alkaloids (SZ-A) Ameliorate Glucose Metabolism Accompanied by the Modulation of Gut Microbiota and Ileal Inflammatory Damage in Type 2 Diabetic KKAy Mice. Front. Pharmacol. 2021, 12, 642400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Huang, H.; Xue, Y.; Zhang, D.; Zhang, Y.; Li, W.; Li, X. Fucoidan ameliorates glucose metabolism by the improvement of intestinal barrier and inflammatory damage in type 2 diabetic rats. Int. J. Biol. Macromol. 2022, 201, 616–629. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Xia, W.; Khan, I.; Li, X.A.; Huang, G.; Yu, Z.; Leong, W.K.; Han, R.; Ho, L.T.; Wendy Hsiao, W.L. Adaptogenic flower buds exert cancer preventive effects by enhancing the SCFA-producers, strengthening the epithelial tight junction complex and immune responses. Pharmacol. Res. 2020, 159, 104809. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- de Kort, S.; Keszthelyi, D.; Masclee, A.A. Leaky gut and diabetes mellitus: What is the link? Obes. Rev. 2011, 12, 449–458. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Niederreiter, L.; Blumberg, R.S.; Kaser, A. Endoplasmic reticulum stress and inflammation. Dig. Dis. 2012, 30, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic Reticulum Stress and Intestinal Inflammation: A Perilous Union. Front. Immunol. 2020, 11, 543022. [Google Scholar] [CrossRef]
- Cao, H.; Li, C.N.; Lei, L.; Wang, X.; Liu, S.N.; Liu, Q.; Huan, Y.; Sun, S.J.; Shen, Z.F. Stachyose Improves the Effects of Berberine on Glucose Metabolism by Regulating Intestinal Microbiota and Short-Chain Fatty Acids in Spontaneous Type 2 Diabetic KKAy Mice. Front Pharmacol. 2020, 11, 578943. [Google Scholar] [CrossRef]
- Wang, X.; Huan, Y.; Li, C.; Cao, H.; Sun, S.; Lei, L.; Liu, Q.; Liu, S.; Ji, W.; Liu, H.; et al. Diphenyl diselenide alleviates diabetic peripheral neuropathy in rats with streptozotocin-induced diabetes by modulating oxidative stress. Biochem. Pharmacol. 2020, 182, 114221. [Google Scholar] [CrossRef]

| Gene | Primer sequence (5′—3′) |
|---|---|
| Occludin | Forward: ATGTCCGGCCGATGCTCTC Reverse: TTTGGCTGCTCTTGGGTCTGTAT |
| ZO-1 | Forward: ACCCGAAACTGATGCTGTGGATAG Reverse: AAATGGCCGGGCAGAACTTGTGTA |
| β-actin | Forward: ACTCTTCCAGCCTTCCTTC Reverse: ATCTCCTTCTGCATCCTGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Ji, W.; Liu, Q.; Zhang, L.; Li, C.; Huan, Y.; Lei, L.; Gao, X.; Chen, L.; Feng, C.; et al. Voglibose Regulates the Secretion of GLP-1 Accompanied by Amelioration of Ileal Inflammatory Damage and Endoplasmic Reticulum Stress in Diabetic KKAy Mice. Int. J. Mol. Sci. 2022, 23, 15938. https://doi.org/10.3390/ijms232415938
Fu Y, Ji W, Liu Q, Zhang L, Li C, Huan Y, Lei L, Gao X, Chen L, Feng C, et al. Voglibose Regulates the Secretion of GLP-1 Accompanied by Amelioration of Ileal Inflammatory Damage and Endoplasmic Reticulum Stress in Diabetic KKAy Mice. International Journal of Molecular Sciences. 2022; 23(24):15938. https://doi.org/10.3390/ijms232415938
Chicago/Turabian StyleFu, Yaxin, Wenming Ji, Quan Liu, Lin Zhang, Caina Li, Yi Huan, Lei Lei, Xuefeng Gao, Leilei Chen, Cunyu Feng, and et al. 2022. "Voglibose Regulates the Secretion of GLP-1 Accompanied by Amelioration of Ileal Inflammatory Damage and Endoplasmic Reticulum Stress in Diabetic KKAy Mice" International Journal of Molecular Sciences 23, no. 24: 15938. https://doi.org/10.3390/ijms232415938
APA StyleFu, Y., Ji, W., Liu, Q., Zhang, L., Li, C., Huan, Y., Lei, L., Gao, X., Chen, L., Feng, C., Lei, L., Zhai, J., Li, P., Cao, H., Liu, S., & Shen, Z. (2022). Voglibose Regulates the Secretion of GLP-1 Accompanied by Amelioration of Ileal Inflammatory Damage and Endoplasmic Reticulum Stress in Diabetic KKAy Mice. International Journal of Molecular Sciences, 23(24), 15938. https://doi.org/10.3390/ijms232415938






