Cardamom (Elettaria cardamomum (L.) Maton) Seeds Intake Increases Energy Expenditure and Reduces Fat Mass in Mice by Modulating Neural Circuits That Regulate Adipose Tissue Lipolysis and Mitochondrial Oxidative Metabolism in Liver and Skeletal Muscle
Abstract
1. Introduction
2. Results
2.1. Phenolics and Terpenoids Profiling by LC-MS and GC-MS
2.2. Cardamom Intake Reduces Fat Mass Accretion in Mice
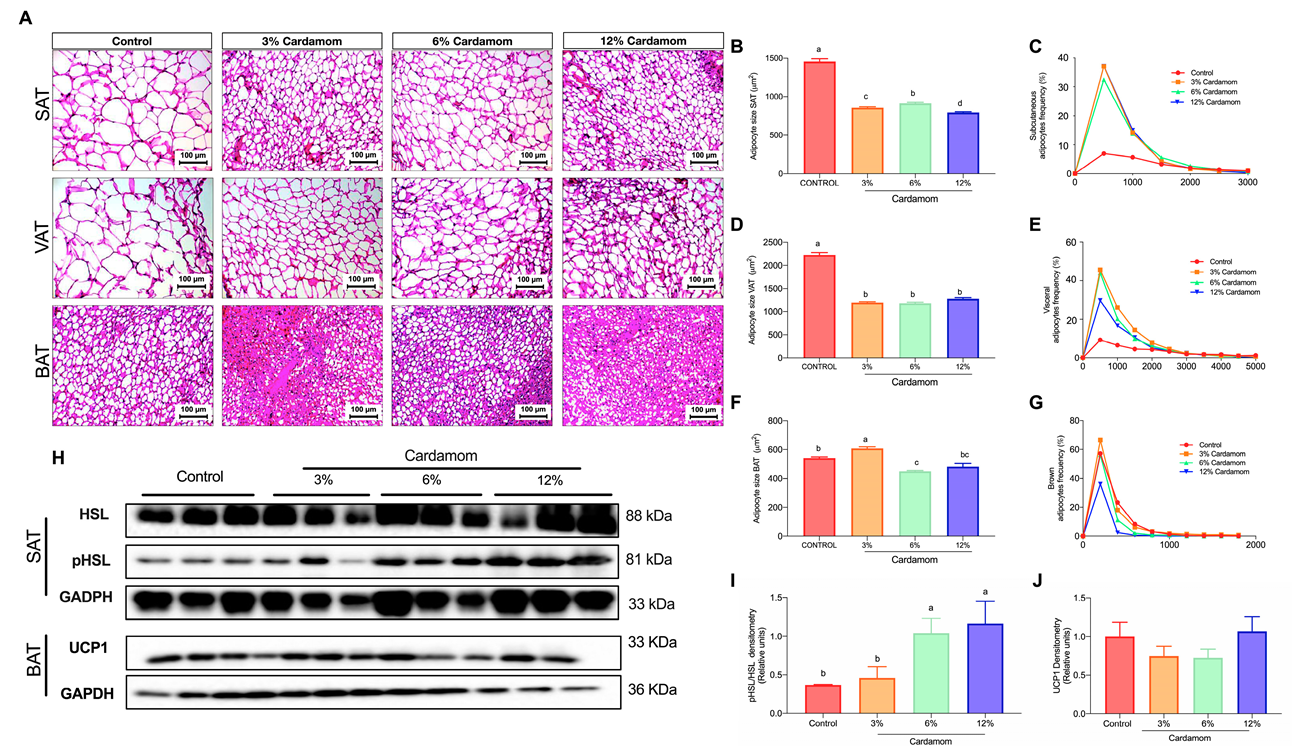
2.3. Mice Fed with Diets Containing Cardamom Seeds Display Reduced Adipocytes Size Associated with Increased Lipolysis
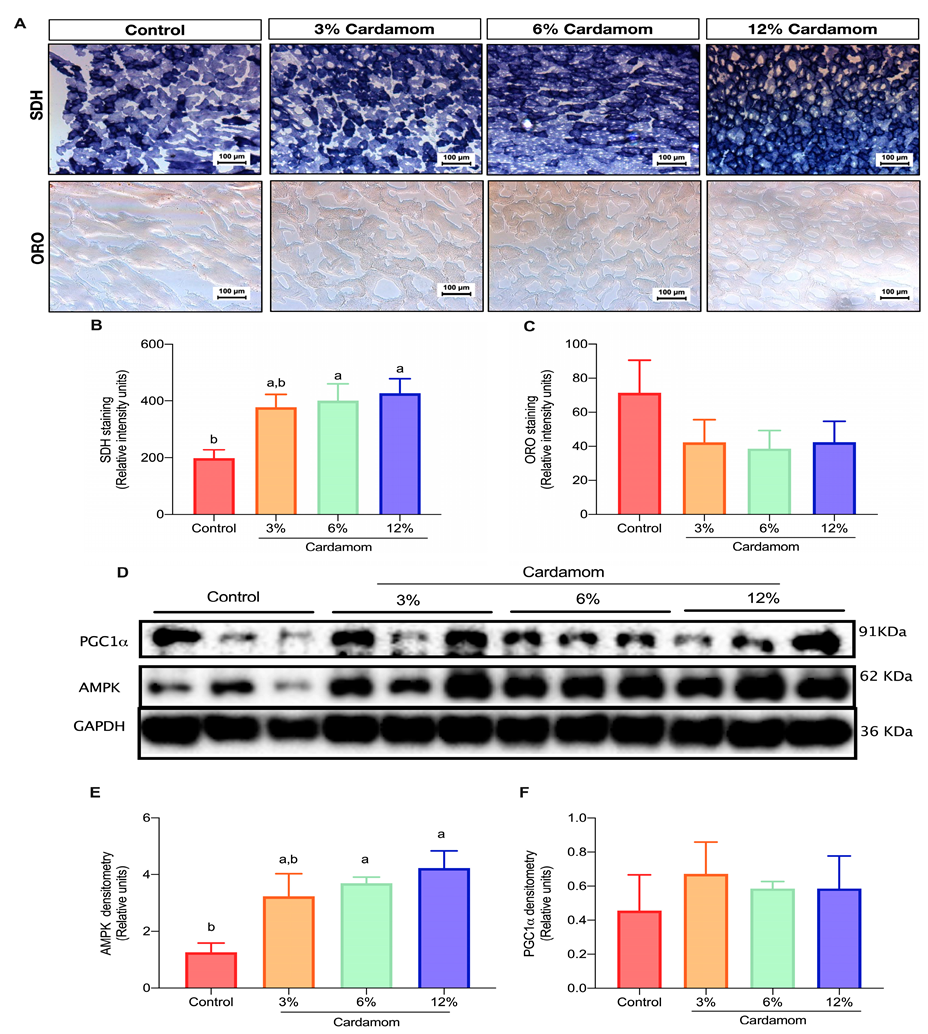
2.4. Cardamom Seeds Intake Increases Mitochondrial Activity and AMPK Content in Skeletal Muscle
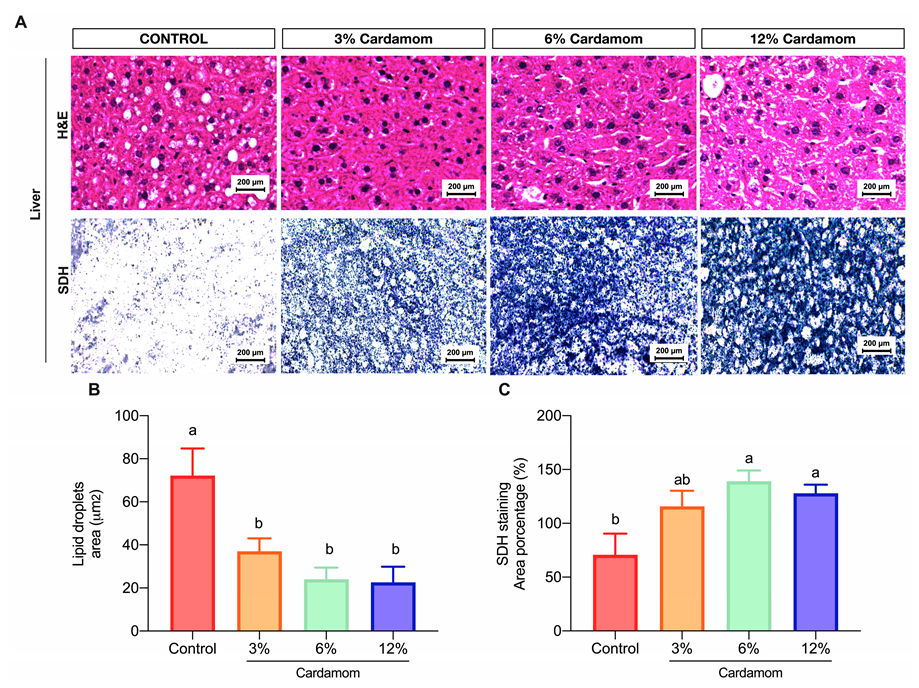
2.5. Mice Fed with Diets Containing Cardamom Seeds Showed High Hepatic Mitochondrial Activity
2.6. Cardamom Intake Increased Oxygen Consumption and Metabolic Flexibility in Mice
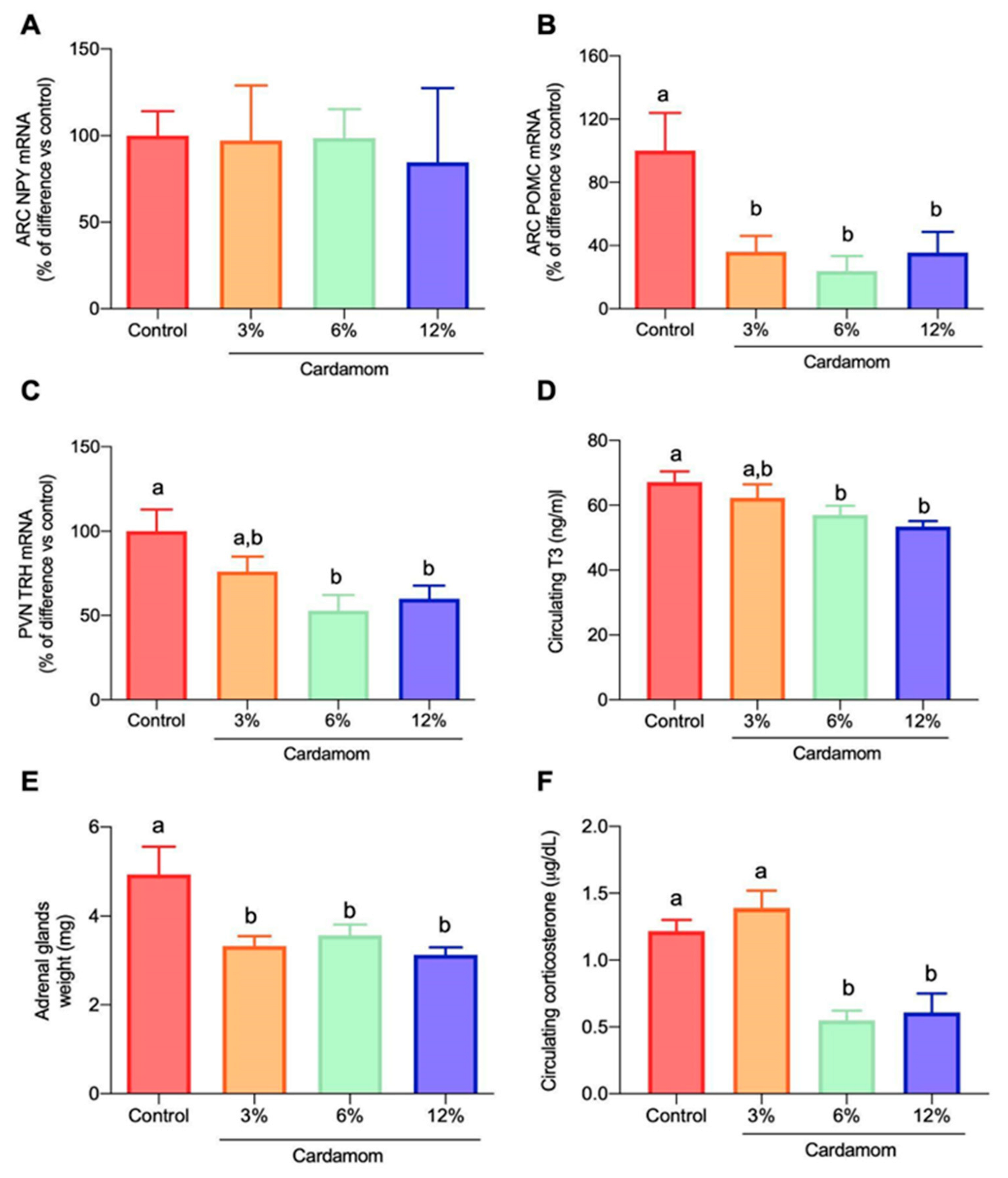
2.7. Cardamom Intake Modulated the Expression of Hypothalamic Peptides and the Circulating Levels of Triiodothyronine (T3) and Corticosterone
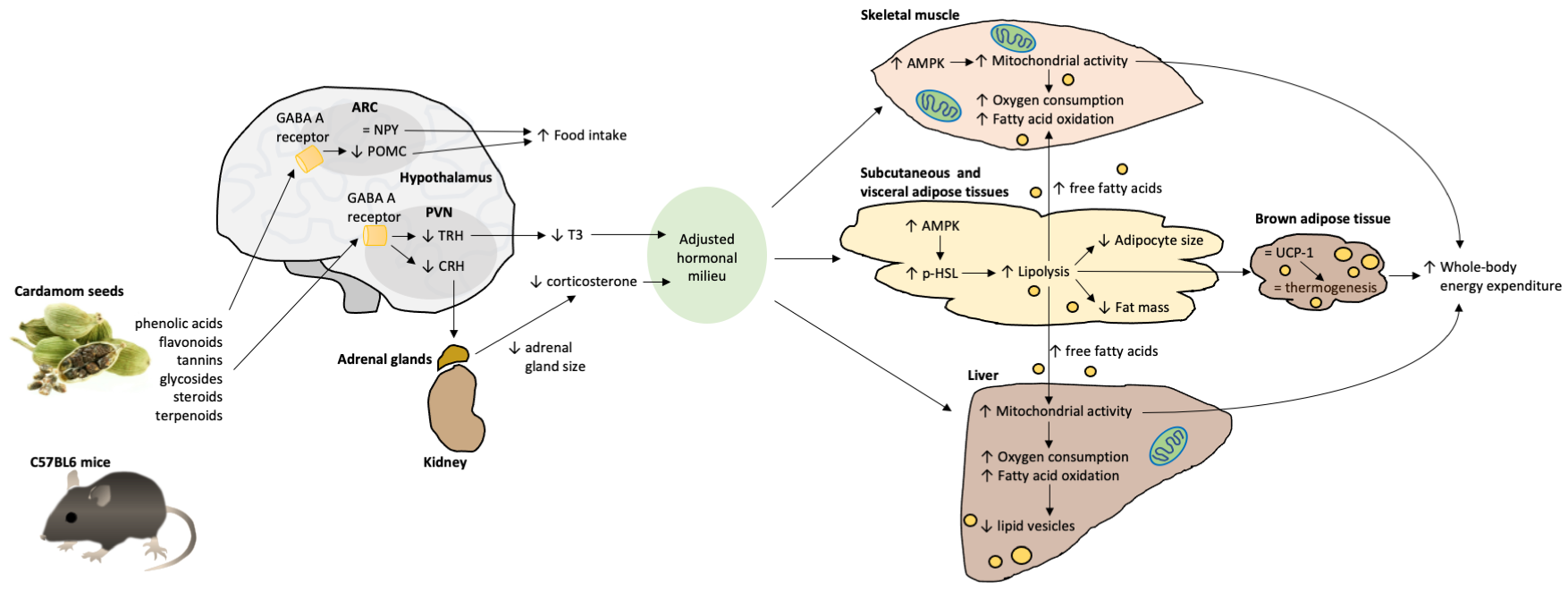
3. Discussion
4. Materials and Methods
4.1. Cardamom Samples
4.2. Sample Preparation for Polyphenol Analysis and LC-MS Polyphenol Profiling
4.3. Sample Preparation for Terpenoid Analysis and GC-MS Profiling
4.4. Animals
4.5. Experimental Diets
4.6. Body Composition and Energy Expenditure Measurement
4.7. Histological Analysis of Liver and Adipose Tissue
4.8. Determination of Corticosterone and Triiodothyronine (T3) in Plasma
4.9. Determination of Mitochondrial Activity and Lipid Content in Skeletal Muscle and Liver
4.10. Immunoblotting
4.11. Gene Expression of Hypothalamic Peptides
4.12. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singletary, K. Cardamom Potential Health Benefits. Nutr. Today 2022, 57, 38–49. [Google Scholar] [CrossRef]
- Aboelnaga, S.M.H. Effect of Some Levels of Cardamom, Clove and Anise on Hepatotoxicity in Rats Caused by CCL4. World Appl. Sci. J. 2015, 33, 854–865. [Google Scholar]
- Asbaghi, O.; Nazarian, B.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Chamani, M.; Asemi, Z. The Effects of Grape Seed Extract on Glycemic Control, Serum Lipoproteins, Inflammation, and Body Weight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phyther. Res. 2020, 34, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Alam, M.N.; Ulla, A.; Sumi, F.A.; Subhan, N.; Khan, T.; Sikder, B.; Hossain, H.; Reza, H.M.; Alam, M.A. Cardamom Powder Supplementation Prevents Obesity, Improves Glucose Intolerance, Inflammation and Oxidative Stress in Liver of High Carbohydrate High Fat Diet Induced Obese Rats. Lipids Health Dis. 2017, 16, 151. [Google Scholar] [CrossRef]
- Paul, K.; Bhattacharjee, P.; Chatterjee, N.; Pal, T.K. Nanoliposomes of Supercritical Carbon Dioxide Extract of Small Cardamom Seeds Redresses Type 2 Diabetes and Hypercholesterolemia. Recent Pat. Biotechnol. 2019, 13, 284–303. [Google Scholar] [CrossRef]
- Nitasha Bhat, G.M.; Nayak, N.; Vinodraj, K.; Chandralekha, N.; Mathai, P.; Cherian, J. Comparison of the Efficacy of Cardamom (Elettaria Cardamomum) with Pioglitazone on Dexamethasone-Induced Hepatic Steatosis, Dyslipidemia, and Hyperglycemia in Albino Rats. J. Adv. Pharm. Technol. Res. 2015, 6, 136–140. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; El-Mokhtar, M.A.; Abdel-Rahman, E.A.; Ahmed, I.A.; Nicola, M.A. Terpenoid-Rich Elettaria Cardamomum Extract Prevents Alzheimer-like Alterations Induced in Diabetic Rats via Inhibition of GSK3β Activity, Oxidative Stress and pro-Inflammatory Cytokines. Cytokine 2019, 113, 2167–2185. [Google Scholar] [CrossRef]
- Gilani, A.H.; Jabeen, Q.; Khan, A.U.; Shah, A.J. Gut Modulatory, Blood Pressure Lowering, Diuretic and Sedative Activities of Cardamom. J. Ethnopharmacol. 2007, 115, 463–472. [Google Scholar] [CrossRef]
- Sabino, C.K.B.; Ferreira-Filho, E.S.; Mendes, M.B.; da Silva-Filho, J.C.; Ponte, M.P.T.R.; Moura, L.H.P.; Oliveira, E.C.A.; Quintans-Junior, L.J.; dos Santos, M.R.V.; de Cássia Meneses Oliveira, R.; et al. Cardiovascular Effects Induced by α-Terpineol in Hypertensive Rats. Flavour Fragr. J. 2013, 28, 333–339. [Google Scholar] [CrossRef]
- Nagashree, S.; Archana, K.K.; Srinivas, P.; Srinivasan, K.; Sowbhagya, H.B. Anti-Hypercholesterolemic Influence of the Spice Cardamom (Elettaria cardamomum) in Experimental Rats. J. Sci. Food Agric. 2017, 97, 3204–3210. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; de Sousa, D.P.; Bonjardim, L.R.; Quintans, L.J. Cardiovascular Effects of Monoterpenes: A Review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Auti, S.T.; Kulkarni, Y.A. Neuroprotective Effect of Cardamom Oil against Aluminum Induced Neurotoxicity in Rats. Front. Neurol. 2019, 10, 399. [Google Scholar] [CrossRef]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic Control of Energy Expenditure and Thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef]
- Variyar, P.S.; Bandyopadhyay, C. Estimation of Phenolic Acids in Cinnamon, Clove, Cardamom, Nutmeg and Mace by High Performance Liquid Chromatography. J. Spices Aromat. Crop. 1995, 4, 129–134. [Google Scholar]
- Pura Naik, J.; Jagan Mohan Rao, L.; Mohan Kumar, T.; Sampathu, S. Chemical composition of the volatile oil from the pericarp (husk) of large cardamom (Amomum subulatum Roxb.). Flavour Fragr. J. 2004, 19, 441–444. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States—Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Blond, E.; Maitrepierre, C.; Normand, S.; Sothier, M.; Roth, H.; Goudable, J.; Laville, M. A New Indirect Calorimeter Is Accurate and Reliable for Measuring Basal Energy Expenditure, Thermic Effect of Food and Substrate Oxidation in Obese and Healthy Subjects. e-SPEN 2011, 6, e7–e15. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Metabolic Flexibility and Its Impact on Health Outcomes. Mayo Clin. Proc. 2022, 97, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Abazari, A.R.; Rameshrad, M.; Hosseinzadeh, H. Carnosic acid prevented olanzapine-induced metabolic disorders through AMPK activation. Mol. Biol. Rep. 2020, 47, 7583–7592. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lee, H.; Jung, E.S.; Seyedian, R.; Jo, M.; Kim, J.; Kim, J.S.; Kim, E. Saffron (Crocus sativus L.) Increases Glucose Uptake and Insulin Sensitivity in Muscle Cells via Multipathway Mechanisms. Food Chem. 2012, 135, 2350–2358. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein Stimulates Insulin Sensitivity through Gut Microbiota Reshaping and Skeletal Muscle AMPK Activation in Obese Subjects. BMJ Open Diabetes Res. Care 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; de Oliveira, A.M.; Tostes, R.C. Hypotensive Action of Naturally Occurring Diterpenes: A Therapeutic Promise for the Treatment of Hypertension. Fitoterapia 2010, 81, 690–702. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Agnihothri, R.V.; Courville, A.B.; Linderman, J.D.; Smith, S.; Brychta, R.; Remaley, A.; Chen, K.Y.; Simchowitz, L.; Celi, F.S. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid 2014, 24, 19–26. [Google Scholar] [CrossRef]
- Diano, S.; Horvath, T.L. Type 3 Deiodinase in Hypoxia: To Cool or to Kill? Cell Metab. 2008, 7, 363–364. [Google Scholar] [CrossRef]
- Lanni, A.; De Felice, M.; Lombardi, A.; Moreno, M.; Fleury, C.; Ricquier, D.; Goglia, F. Induction of UCP2 mRNA by Thyroid Hormones in Rat Heart. FEBS Lett. 1997, 418, 171–174. [Google Scholar] [CrossRef]
- Lanni, A.; Beneduce, L.; Lombardi, A.; Moreno, M.; Boss, O.; Muzzin, P.; Giacobino, J.P.; Goglia, F. Expression of Uncoupling Protein-3 and Mitochondrial Activity in the Transition from Hypothyroid to Hyperthyroid State in Rat Skeletal Muscle. FEBS Lett. 1999, 444, 250–254. [Google Scholar] [CrossRef] [PubMed]
- De Lange, P.; Lanni, A.; Beneduce, L.; Moreno, M.; Lombardi, A.; Silvestri, E.; Goglia, F. Uncoupling Protein-3 Is a Molecular Determinant for the Regulation of Resting Metabolic Rate by Thyroid Hormone. Endocrinology 2001, 142, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Hartzell, D.; Azain, M.J.; Baile, C.A. TRH Decreases Food Intake and Increases Water Intake and Body Temperature in Rats. Physiol. Behav. 2002, 77, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, E.; McCann, S.M. Suppression of Feeding and Drinking Activity in Rats Following Intraventricular Injection of Thyrotropin Releasing Hormone (TRH). Endocrinology 1977, 100, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Légrádi, G.; Mihály, E.; Huang, Q.H.; Tatro, J.B.; Rand, W.M.; Emerson, C.H.; Lechan, R.M. α-Melanocyte-Stimulating Hormone Is Contained in Nerve Terminals Innervating Thyrotropin-Releasing Hormone-Synthesizing Neurons in the Hypothalamic Paraventricular Nucleus and Prevents Fasting-Induced Suppression of Prothyrotropin-Releasing Hormone Gene E. J. Neurosci. 2000, 20, 1550–1558. [Google Scholar] [CrossRef]
- Masoumi-Ardakani, Y.; Mahmoudvand, H.; Mirzaei, A.; Esmaeilpour, K.; Ghazvini, H.; Khalifeh, S.; Sepehri, G. The Effect of Elettaria Cardamomum Extract on Anxiety-like Behavior in a Rat Model of Post-Traumatic Stress Disorder. Biomed. Pharmacother. 2017, 87, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Arima, H.; Watanabe, M.; Goto, M.; Banno, R.; Sato, I.; Ozaki, N.; Nagasaki, H.; Oiso, Y. Glucocorticoids Increase Neuropeptide Y and Agouti-Related Peptide Gene Expression via Adenosine Monophosphate-Activated Protein Kinase Signaling in the Arcuate Nucleus of Rats. Endocrinology 2008, 149, 4544–4553. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by Quercetin of Cortico trophin Releasing Factor Induced Anxiety- and Depression-like Effect in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef]
- Filho, A.W.; Filho, V.C.; Olinger, L.; De Souza, M.M. Quercetin: Further Investigation of Its Antinociceptive Properties and Mechanisms of Action. Arch. Pharmacal. Res. 2008, 31, 713–721. [Google Scholar] [CrossRef]
- Horvath, T.L.; Naftolin, F.; Leranth, C. GABAergic and Catecholaminergic Innervation of Mediobasal Hypothalamic β-Endorphin Cells Projecting to the Medial Preoptic Area. Neuroscience 1992, 51, 391–399. [Google Scholar] [CrossRef]
- Atasoy, D.; Nicholas Betley, J.; Su, H.H.; Sternson, S.M. Deconstruction of a Neural Circuit for Hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, X.; Zhao, Z.; Feng, Q.; Lin, R.; Sun, Y.; Ding, T.; Xu, F.; Luo, M.; Zhan, C. Whole-Brain Mapping of the Direct Inputs and Axonal Projections of POMC and AgRP Neurons. Front. Neuroanat. 2015, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Bäckberg, M.; Ultenius, C.; Fritschy, J.M.; Meister, B. Cellular Localization of GABAA Receptor α Subunit Immunoreactivity in the Rat Hypothalamus: Relationship with Neurones Containing Orexigenic or Anorexigenic Peptides. J. Neuroendocrinol. 2004, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Bagamasbad, P.D.; Espina, J.E.C.; Knoedler, J.R.; Subramani, A.; Harden, A.J.; Denver, R.J. Coordinated Transcriptional Regulation by Thyroid Hormone and Glucocorticoid Interaction in Adult Mouse Hippocampus-Derived Neuronal Cells. PLoS ONE 2019, 14, e220378. [Google Scholar] [CrossRef]
- Gil-Ibáñez, P.; Bernal, J.; Morte, B. Thyroid Hormone Regulation of Gene Expression in Primary Cerebrocortical Cells: Role of Thyroid Hormone Receptor Subtypes and Interactions with Retinoic Acid and Glucocorticoids. PLoS ONE 2014, 9, e91692. [Google Scholar] [CrossRef]
- Espina, J.E.C.; Bagamasbad, P.D. Synergistic Gene Regulation by Thyroid Hormone and Glucocorticoid in the Hippocampus. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2022; Volume 118, pp. 35–81. [Google Scholar]
- Lapp, H.E.; Bartlett, A.A.; Hunter, R.G. Stress and Glucocorticoid Receptor Regulation of Mitochondrial Gene Expression. J. Mol. Endocrinol. 2019, 62, R121–R128. [Google Scholar] [CrossRef]
- Scheller, K.; Seibel, P.; Sekeris, C.E. Glucocorticoid and Thyroid Hormone Receptors in Mitochondria of Animal. Cells. Int. Rev. Cytol. 2003, 222, 1–61. [Google Scholar] [CrossRef]
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive Effect of Quercetin on Acute Stress-Induced Hypothalamic-Pituitary-Adrenal Axis Response in Wistar Rats. J. Nutr. Biochem. 2010, 21, 374–380. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s Milk Intake Prevents Obesity, Hepatic Steatosis and Insulin Resistance in Mice Fed a High-Fat Diet by Reducing Inflammatory Markers and Increasing Energy Expenditure and Mitochondrial Content in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel Concentrate from Agave salmiana and its Extracted Saponins Attenuated Obesity and Hepatic Steatosis and Increased Akkermansia muciniphila in C57BL6 Mice. Sci Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Food and Drug Administration: Rockville, MD, USA, 2005. [Google Scholar]
- Zambrano, E.; Rodríguez-González, G.L.; Reyes-Castro, L.A.; Bautista, C.J.; Castro-Rodríguez, D.C.; Juárez-Pilares, G.; Ibáñez, C.A.; Hernández-Rojas, A.; Nathanielsz, P.W.; Montaño, S.; et al. DHA Supplementation of Obese Rats throughout Pregnancy and Lactation Modifies Milk Composition and Anxiety Behavior of Offspring. Nutrients 2021, 13, 4243. [Google Scholar] [CrossRef] [PubMed]
- Waterborg, J.H.; Matthews, H.R. The Lowry Method for Protein Quantitation. Methods Mol. Biol. 1984, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
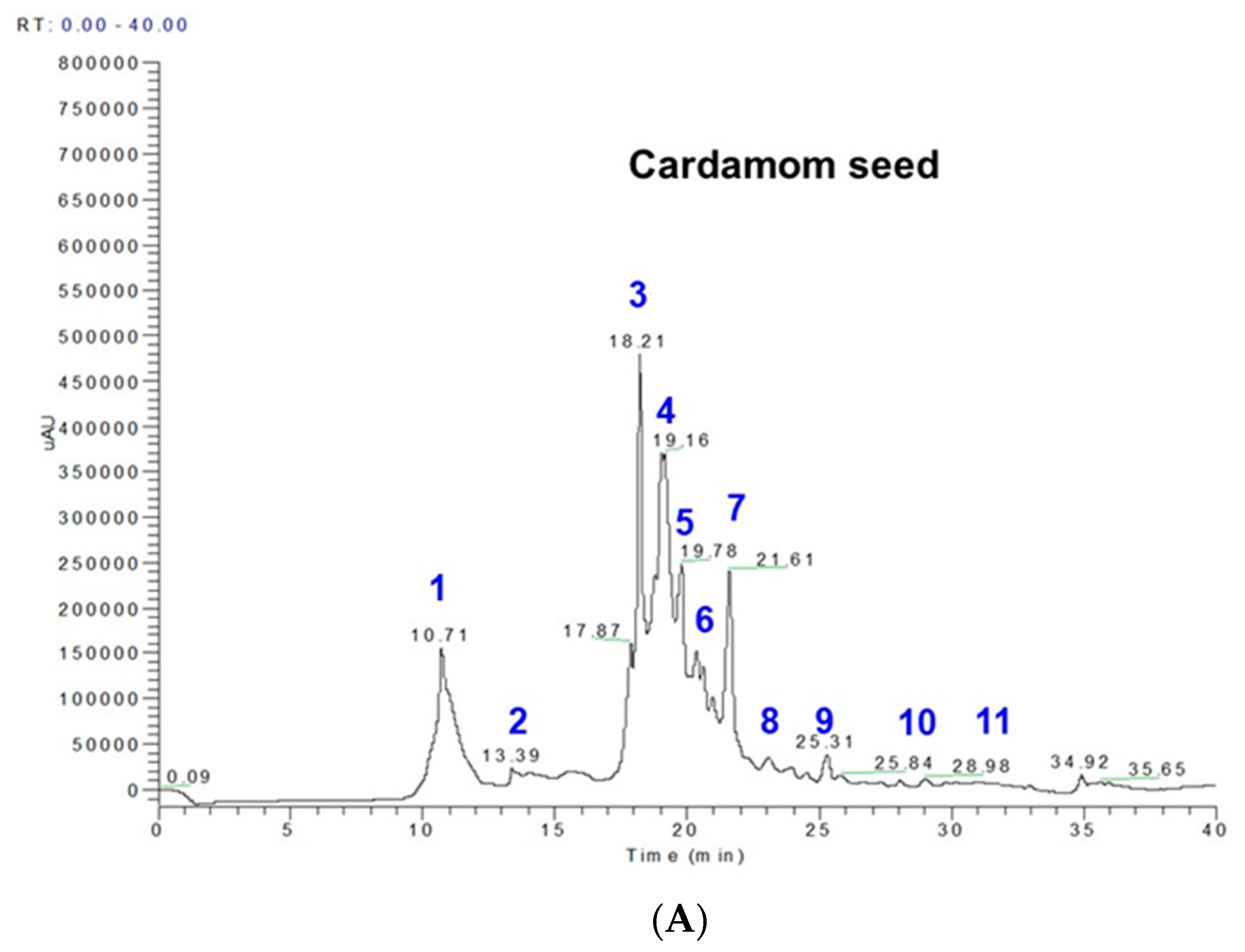
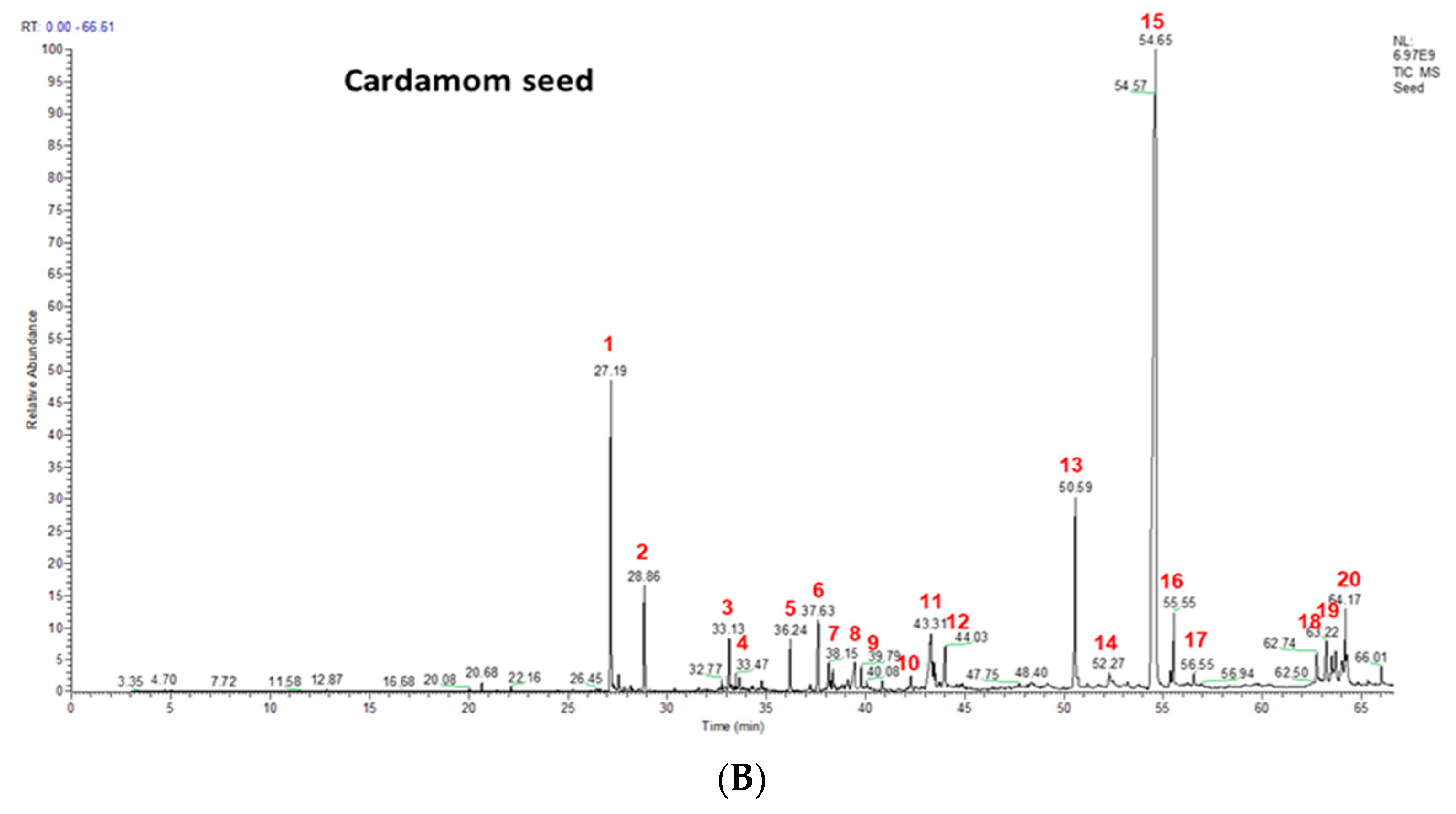



| Peak No. | RT | M-H | MS Fragments * | Compound Name and Molecular formula | EC Seeds (mg/100 g) |
|---|---|---|---|---|---|
| 1 | 10.70–10.78 | 153 | 109 | protocatechuic acid; 3,4-dihydroxybenzoic acid; (HO)2C6H3CO2H | 29.69 |
| 2 | 14.00–14.33 | 153 | 109, 103 | gentisic acid; 2,5-dihydroxybenzoic acid; C7H6O4 | 1.98 |
| 3 | 18.20–18.21 | 179 | 161, 135 | caffeic acid; 3,4-dihydroxybenzeneacrylic acid; (HO)2C6H3CH=CHCO2H | 26.23 |
| 4 | 18.25–18.35 | 197 | 191, 173 | syringic acid; 3,5-dimethoxy-4-hydroxybenzoic acid; HOC6H2(OCH3)2CO2H | 36.43 |
| 5 | 19.16–19.22 | 193 | 179 | ferulic acid; 4-hydroxy-3-methoxycinnamic acid; C10H10O4 | 6.68 |
| 6 | 19.78–18.02 | 167 | 135, 121 | vanillic acid; 4-hydroxy-3-methoxybenzoic acid; HOC6H3(OCH3)CO2H | 4.28 |
| 7 | 20.8–21.19 | 353 | 190, 179 | 5-O-caffeoylquinic acid | 14.6 |
| 8 | 22.6–22.8 | 163 | 143, 135, 121 | p-coumaric acid; trans-4-hydroxycinnamic acid; HOC6H4CH=CHCO2H | 0.44 |
| 9 | 23.08–24.8 | 397 | 191, 173 | sinapoylquinic acid | 1.46 |
| 10 | 25.31–27.31 | 367 | 173, 161 | feruloylquinicacid; 3-O-feruloylquinic acid; C17H20O9 | 0.054 |
| 11 | 31.3–31.34 | 609 | 447, 301 | rutin; rutoside; C27H30O16 | 2.69 |
| Peak No. | Compound Name and Molecular formula | Mass | Retention Time | EC Seeds (% Area) |
|---|---|---|---|---|
| 1 | terpineol cis-α-terpineol; C10H18O | 154 | 27.17 | 7.99 |
| 2 | beta-terpineol; cis-β-terpineol; C10H18O | 154 | 27.57 | 1.43 |
| 3 | linalool; 3,7-dimethylocta-1,6-dien-3-ol; C10H18O | 154 | 33.66 | 0.84 |
| 4 | terpinen-4-ol; (1R)-4-methyl-1-propan-2-ylcyclohex-3-en-1-ol; C10H18O | 154 | 34.94 | 0.23 |
| 5 | α-terpineol; alpha-terpineol; C10H18O | 154 | 36.22 | 0.92 |
| 6 | geraniol; lemanol; C10H18O | 154 | 37.57 | 1.05 |
| 7 | trans-nerolidol; (6E)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol; C15H16O | 222 | 38.15 | 0.47 |
| 8 | α-acorenol; acorenol; C15H26O | 222 | 39.39 | 0.52 |
| 9 | cubebol; (1R,4S,5R,6R,7S,10R)-4,10-dimethyl-7-propan-2-yltricyclo[4.4.0.01,5]decan-4-ol; C15H26O | 222 | 39.78 | 0.26 |
| 10 | isospathulenol; 1aR,7S,7aS,7bR)-1,1,4,7-tetramethyl-2,3,5,6,7a,7b-hexahydro-1aH-cyclopropa[h]azulen-7-ol; C15H24O | 220 | 41.64 | 0.26 |
| 11 | globulol; (1aR,4R,4aR,7R,7aS,7bS)-1,1,4,7-tetramethyl-2,3,4a,5,6,7,7a,7b-octahydro-1aH-cyclopropa[e]azulen-4-oL; C15H26O | 222 | 42.24 | 0.75 |
| 12 | trans-fFarnesol; (2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-ol; C15H26O | 222 | 43.91 | 0.62 |
| 13 | ambrial; 2-(5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl)acetaldehyde; C16H26O | 234 | 50.49 | 5.3 |
| 14 | eicosane; icosane; C20H42 | 282 | 51.21 | 0.22 |
| 15 | costunolide; (3aS,6E,10E,11aR)-6,10-dimethyl-3-methylidene-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-2-one; C15H20O2 | 232 | 54.43 | 68.11 |
| 16 | coronarin; 4-[2-[(1S,4aS,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethyl]-2-methoxy-2H-furan-5-one; C21H23O3 | 300 | 54.85 | 1.12 |
| 17 | β-mono palmitin | 330 | 50.59 | 0.23 |
| 18 | β-mono stearin | 358 | 56.65 | 0.39 |
| 19 | erucylamide; (Z)-docos-13-enamide; C22H43NO | 337 | 62.71 | 0.41 |
| 20 | β-sitosterol; 17-(5-Ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,11,12,14, 15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; C29H50O | 414 | 64.17 | 0.84 |
| Elettaria cardamomum Seed | ||||
|---|---|---|---|---|
| % | Control | 3% | 6% | 12% |
| Proteins | 20.30 | 20.40 | 20.60 | 20.90 |
| Carbohydrates | 63.70 | 63.50 | 63.20 | 62.70 |
| Fat | 16.0 | 16.10 | 16.20 | 16.40 |
| Ingredients (g/kg): | ||||
| Casein a | 200 | 196.10 | 192.20 | 184.40 |
| Sucrose | 100 | 100 | 100 | 100 |
| Maltodextrin | 132 | 132 | 132 | 132 |
| Corn starch | 397.50 | 377.58 | 357.66 | 317.82 |
| Soy oil | 70 | 67.42 | 64.84 | 59.68 |
| Cellulose | 50 | 46.40 | 42.80 | 35.60 |
| Vitamin mix b | 10 | 10 | 10 | 10 |
| Mineral mix c | 35 | 35 | 35 | 35 |
| L-Cysteine d | 3 | 3 | 3 | 3 |
| Choline d | 2.50 | 2.5 | 2.5 | 2.5 |
| EC seeds | 30 | 60 | 120 | |
| Total | 1000 | 1000 | 1000 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgadillo-Puga, C.; Torre-Villalvazo, I.; Cariño-Cervantes, Y.Y.; García-Luna, C.; Soberanes-Chávez, P.; de Gortari, P.; Noriega, L.G.; Bautista, C.J.; Cisneros-Zevallos, L. Cardamom (Elettaria cardamomum (L.) Maton) Seeds Intake Increases Energy Expenditure and Reduces Fat Mass in Mice by Modulating Neural Circuits That Regulate Adipose Tissue Lipolysis and Mitochondrial Oxidative Metabolism in Liver and Skeletal Muscle. Int. J. Mol. Sci. 2023, 24, 3909. https://doi.org/10.3390/ijms24043909
Delgadillo-Puga C, Torre-Villalvazo I, Cariño-Cervantes YY, García-Luna C, Soberanes-Chávez P, de Gortari P, Noriega LG, Bautista CJ, Cisneros-Zevallos L. Cardamom (Elettaria cardamomum (L.) Maton) Seeds Intake Increases Energy Expenditure and Reduces Fat Mass in Mice by Modulating Neural Circuits That Regulate Adipose Tissue Lipolysis and Mitochondrial Oxidative Metabolism in Liver and Skeletal Muscle. International Journal of Molecular Sciences. 2023; 24(4):3909. https://doi.org/10.3390/ijms24043909
Chicago/Turabian StyleDelgadillo-Puga, Claudia, Ivan Torre-Villalvazo, Yonatan Y. Cariño-Cervantes, Cinthia García-Luna, Paulina Soberanes-Chávez, Patricia de Gortari, Lilia G. Noriega, Claudia J. Bautista, and Luis Cisneros-Zevallos. 2023. "Cardamom (Elettaria cardamomum (L.) Maton) Seeds Intake Increases Energy Expenditure and Reduces Fat Mass in Mice by Modulating Neural Circuits That Regulate Adipose Tissue Lipolysis and Mitochondrial Oxidative Metabolism in Liver and Skeletal Muscle" International Journal of Molecular Sciences 24, no. 4: 3909. https://doi.org/10.3390/ijms24043909
APA StyleDelgadillo-Puga, C., Torre-Villalvazo, I., Cariño-Cervantes, Y. Y., García-Luna, C., Soberanes-Chávez, P., de Gortari, P., Noriega, L. G., Bautista, C. J., & Cisneros-Zevallos, L. (2023). Cardamom (Elettaria cardamomum (L.) Maton) Seeds Intake Increases Energy Expenditure and Reduces Fat Mass in Mice by Modulating Neural Circuits That Regulate Adipose Tissue Lipolysis and Mitochondrial Oxidative Metabolism in Liver and Skeletal Muscle. International Journal of Molecular Sciences, 24(4), 3909. https://doi.org/10.3390/ijms24043909








