Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions
Abstract
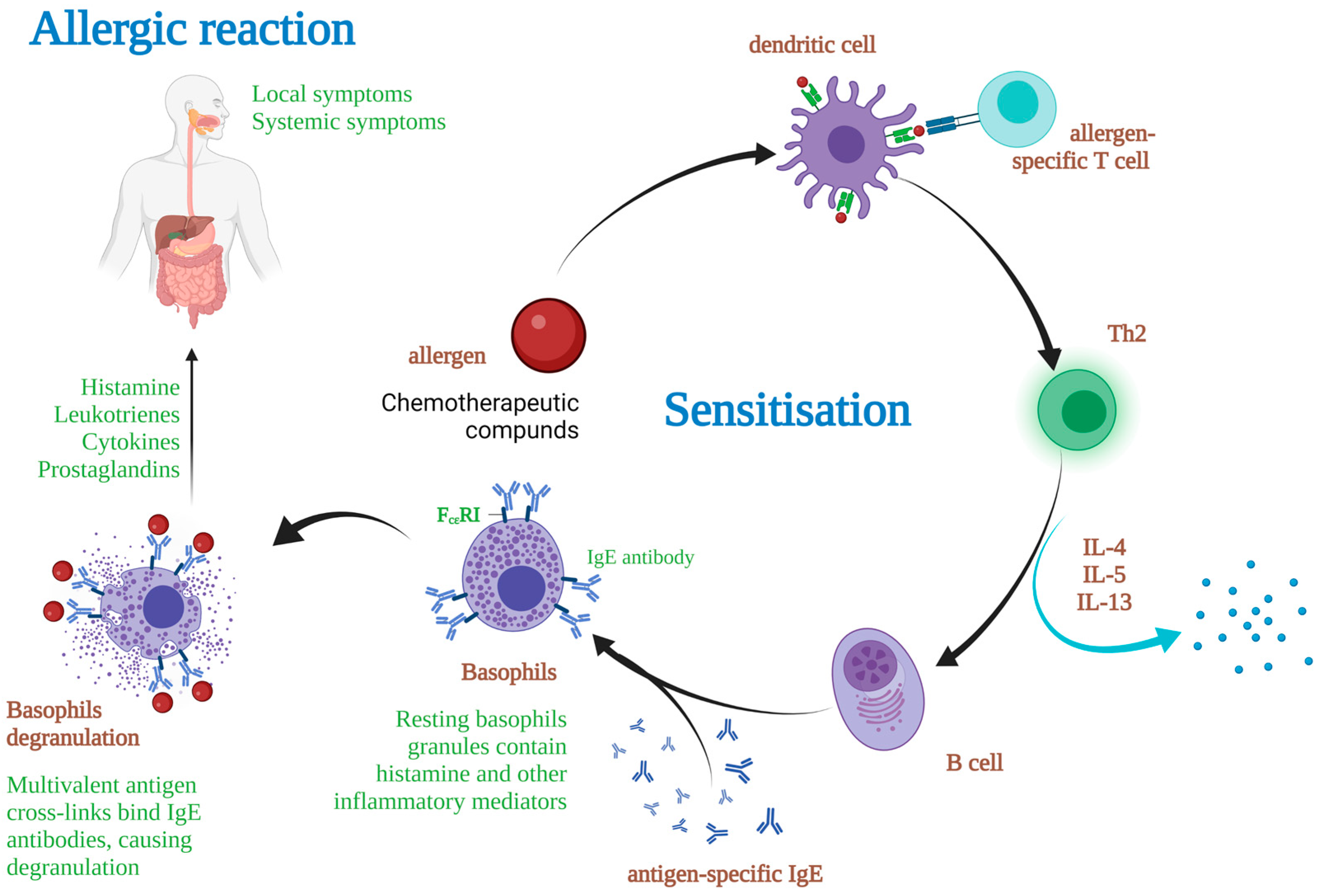
1. Introduction
2. Antineoplastic Therapy Involved in Hypersensitivity Reactions
2.1. Platinum Compounds
2.1.1. Cisplatin
2.1.2. Carboplatin
2.1.3. Oxaliplatin
2.1.4. Cross-Reactivity among Platinum Agents
2.2. Taxanes
2.2.1. Paclitaxel
2.2.2. Docetaxel
2.2.3. Cabazitaxel
2.2.4. Nab-Paclitaxel
2.2.5. Cross-Reactivity between Taxanes
2.3. Disruption of Protein Synthesis
2.4. Bleomycin
2.5. Topoisomerase II Inhibitors
2.5.1. Epipodophyllotoxins
2.5.2. Anthracyclines and Other Related Agents
2.6. Alkylating Agents—Cyclophosphamide/Ifosfamide
2.7. Antimetabolites—Pyrimidine Analogues
2.8. Monoclonal Antibodies
2.8.1. Rituximab
2.8.2. Trastuzumab
2.8.3. Cetuximab
3. Clinical Presentation and Diagnostic Methods in HSRs
3.1. Clinical Presentation of HSRs
3.2. Diagnostic Methods
3.2.1. Skin Tests (STs)
3.2.2. Drug Provocation Tests (DPTs)
3.2.3. In Vitro Tests
Preclinical Stages of Drug Development
Clinical Stages of Drug Development
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The Pathophysiology of Anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Roselló, S.; Blasco, I.; Fabregat, L.G.; Cervantes, A.; Jordan, K. Management of Infusion Reactions to Systemic Anticancer Therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28, iv100–iv118. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M. The Complex Clinical Picture of Presumably Allergic Side Effects to Cytostatic Drugs: Symptoms, Pathomechanism, Reexposure, and Desensitization. Med. Clin. 2010, 94, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, A.A.J.; Vashisht, R.; Zito, P.M. Immediate Hypersensitivity Reactions. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R. Hypersensitivity Reactions to Chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef]
- Castells, M.C.; Matulonis, U.A.; Horton, T.M.; Adkinson, N.F., Jr.; Feldweg, A.M. Infusion Reactions to Systemic Chemotherapy; UpToDate: Waltham, MA, USA, 2015. [Google Scholar]
- Brunton, L.L.; Lazo, J.S.; Parker, K.L. The Pharmacological Basis of Therapeutics; Gilmans, G., Ed.; McGraw-Hill: New York, NY, USA, 2006; Volume 11. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology; Elsevier Health Sciences: Philadelphia, PA, USA, 2011; ISBN 0-7020-4504-7. [Google Scholar]
- Dillman, R.O.; Hendrix, C.S. Unique Aspects of Supportive Care Using Monoclonal Antibodies in Cancer Treatment. Support. Cancer Ther. 2003, 1, 38–48. [Google Scholar] [CrossRef]
- Koda-Kimble, M.A. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 1-60913-713-2. [Google Scholar]
- Baldo, B.A.; Pham, N.H. Adverse Reactions to Targeted and Non-Targeted Chemotherapeutic Drugs with Emphasis on Hypersensitivity Responses and the Invasive Metastatic Switch. Cancer Metastasis Rev. 2013, 32, 723–761. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H. Kinase-Targeted Cancer Therapies: Progress, Challenges and Future Directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Boross, P.; Leusen, J.H. Mechanisms of Action of CD20 Antibodies. Am. J. Cancer Res. 2012, 2, 676. [Google Scholar]
- Jelinek, T.; Hajek, R. Monoclonal Antibodies—A New Era in the Treatment of Multiple Myeloma. Blood Rev. 2016, 30, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kimby, E. Tolerability and Safety of Rituximab (MabThera®). Cancer Treat. Rev. 2005, 31, 456–473. [Google Scholar] [CrossRef] [PubMed]
- Caiado, J.; Venemalm, L.; Pereira-Santos, M.C.; Costa, L.; Barbosa, M.P.; Castells, M. Carboplatin-, Oxaliplatin-, and Cisplatin–Specific IgE: Cross-Reactivity and Value in the Diagnosis of Carboplatin and Oxaliplatin Allergy. J. Allergy Clin. Immunol. Pract. 2013, 1, 494–500. [Google Scholar] [CrossRef]
- Wong, M.; Grossman, J.; Hahn, B.H.; La Cava, A. Cutaneous Vasculitis in Breast Cancer Treated with Chemotherapy. Clin. Immunol. 2008, 129, 3–9. [Google Scholar] [CrossRef]
- Chabner, B.A.; Longon, D.L. Cancer Chemotherapy, Immunotherapy and Biotherapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018; ISBN 1-4963-7515-7. [Google Scholar]
- Tsao, L.R.; Young, F.D.; Otani, I.M.; Castells, M.C. Hypersensitivity Reactions to Platinum Agents and Taxanes. Clin. Rev. Allergy Immunol. 2022, 62, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Makrilia, N.; Syrigou, E.; Kaklamanos, I.; Manolopoulos, L.; Saif, M.W. Hypersensitivity Reactions Associated with Platinum Antineoplastic Agents: A Systematic Review. Met.-Based Drugs 2010, 2010, 207084. [Google Scholar] [CrossRef]
- Caiado, J.; Castells, M. Presentation and Diagnosis of Hypersensitivity to Platinum Drugs. Curr. Allergy Asthma Rep. 2015, 15, 15. [Google Scholar] [CrossRef]
- Otani, I.M.; Wong, J.; Banerji, A. Platinum Chemotherapy Hypersensitivity: Prevalence and Management. Immunol. Allergy Clin. 2017, 37, 663–677. [Google Scholar]
- Zanotti, K.; Rybicki, L.; Kennedy, A.; Belinson, J.; Webster, K.; Kulp, B.; Peterson, G.; Markman, M. Carboplatin Skin Testing: A Skin-Testing Protocol for Predicting Hypersensitivity to Carboplatin Chemotherapy. J. Clin. Oncol. 2001, 19, 3126–3129. [Google Scholar] [CrossRef]
- Puxeddu, I.; Petrelli, F.; Guerrieri, M.E.; Cosio, S.; Del Corso, I.; Rocchi, V.; Manca, M.L.; Migliorini, P.; Gadducci, A. The Role of Skin Tests in the Prevention and Diagnosis of Hypersensitivity Reactions to Platinum Agents in Gynecological Cancer: A Single-Center Italian Retrospective Study. Cancers 2021, 13, 5468. [Google Scholar] [CrossRef]
- Gadducci, A.; Tana, R.; Teti, G.; Zanca, G.; Fanucchi, A.; Genazzani, A. Analysis of the Pattern of Hypersensitivity Reactions in Patients Receiving Carboplatin Retreatment for Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2008, 18, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Ray-Coquard, I.; Fabbro, M.; Donoghoe, M.; Boman, K.; Sugimoto, A.; Vaughan, M.; Reinthaller, A.; Vergote, I.; Ferrandina, G. Decreased Hypersensitivity Reactions with Carboplatin-Pegylated Liposomal Doxorubicin Compared to Carboplatin-Paclitaxel Combination: Analysis from the GCIG CALYPSO Relapsing Ovarian Cancer Trial. Gynecol. Oncol. 2011, 122, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; Ling, M.; Patil, S.; Banerji, A.; Long, A. Oxaliplatin Hypersensitivity: Evaluation, Implications of Skin Testing, and Desensitization. J. Allergy Clin. Immunol. Pract. 2014, 2, 40–45. [Google Scholar] [CrossRef]
- Silver, J.; Garcia-Neuer, M.; Lynch, D.-M.; Pasaoglu, G.; Sloane, D.E.; Castells, M. Endophenotyping Oxaliplatin Hypersensitivity: Personalizing Desensitization to the Atypical Platin. J. Allergy Clin. Immunol. Pract. 2020, 8, 1668–1680. [Google Scholar] [CrossRef]
- Kim, B.H.; Bradley, T.; Tai, J.; Budman, D.R. Hypersensitivity to Oxaliplatin: An Investigation of Incidence and Risk Factors, and Literature Review. Oncology 2009, 76, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.; Chan, R.; Au, G. Hypersensitivity Reactions to Oxaliplatin: Experience in a Single Institute. Ann. Oncol. 2006, 17, 259–261. [Google Scholar] [CrossRef]
- Koren, C.; Yerushalmi, R.; Katz, A.; Malik, H.; Sulkes, A.; Fenig, E. Hypersensitivity Reaction to Cisplatin during Chemoradiation Therapy for Gynecologic Malignancy. Am. J. Clin. Oncol. 2002, 25, 625–626. [Google Scholar] [CrossRef]
- Pradelli, J.; Verdoire, P.; Boutros, J.; Frin, A.-C.; Follana, P.; Duquesne, J.; Marquette, C.-H.; Benzaquen, J.; Hayoun, M.B.; Leroy, S. Allergy Evaluation of Hypersensitivity to Platinum Salts and Taxanes: A Six-Year Experience. J. Allergy Clin. Immunol. Pract. 2020, 8, 1658–1664. [Google Scholar] [CrossRef]
- Pasteur, J.; Favier, L.; Pernot, C.; Guerriaud, M.; Bernigaud, C.; Lepage, C.; Jouve, J.-L.; Isambert, N.; Collet, E. Low Cross-Reactivity between Cisplatin and Other Platinum Salts. J. Allergy Clin. Immunol. Pract. 2019, 7, 1894–1900. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Bandera, C.; Bradley, A.; Brard, L.; Legare, R.; Granai, C.; Dizon, D.S. Does the Platinum-Free Interval Predict the Incidence or Severity of Hypersensitivity Reactions to Carboplatin? The Experience from Women and Infants’ Hospital. Gynecol. Oncol. 2007, 105, 81–83. [Google Scholar] [CrossRef]
- Pandey, A.; Bhosale, B.; Pandita, V.; Singh, A.; Ghosh, J.; Ghosh, J.; Bajpai, J. Carboplatin Hypersensitivity in Relapsed Ovarian Carcinoma: A Therapeutic Challenge. Indian J. Med. Paediatr. Oncol. 2014, 35, 17–20. [Google Scholar] [CrossRef]
- Markman, M.; Kennedy, A.; Webster, K.; Elson, P.; Peterson, G.; Kulp, B.; Belinson, J. Clinical Features of Hypersensitivity Reactions to Carboplatin. J. Clin. Oncol. 1999, 17, 1141. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Kelly, M.G.; O’malley, D.M.; Azodi, M.; Coombe, K.; Schwartz, P.E.; Rutherford, T.J. Atypical Presentations of Carboplatin Hypersensitivity Reactions: Characterization and Management in Patients with Gynecologic Malignancies. Gynecol. Oncol. 2006, 103, 288–292. [Google Scholar] [CrossRef]
- Hesterberg, P.E.; Banerji, A.; Oren, E.; Penson, R.T.; Krasner, C.N.; Seiden, M.V.; Wong, J.T. Risk Stratification for Desensitization of Patients with Carboplatin Hypersensitivity: Clinical Presentation and Management. J. Allergy Clin. Immunol. 2009, 123, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Høydahl, Ø.; Edna, T.-H.; Xanthoulis, A.; Lydersen, S.; Endreseth, B.H. Long-Term Trends in Colorectal Cancer: Incidence, Localization, and Presentation. BMC Cancer 2020, 20, 1077. [Google Scholar] [CrossRef]
- Masse, M.; Caimmi, D.; Demoly, P. A Delayed Reaction to Oxaliplatin. J. Investig. Allergol. Clin. Immunol. 2012, 22, 372–373. [Google Scholar]
- Polyzos, A.; Tsavaris, N.; Gogas, H.; Souglakos, J.; Vambakas, L.; Vardakas, N.; Polyzos, K.; Tsigris, C.; Mantas, D.; Papachristodoulou, A. Clinical Features of Hypersensitivity Reactions to Oxaliplatin: A 10-Year Experience. Oncology 2009, 76, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; King, T.-M.; Chang, M.-C.; Hsu, C.-W. Oxaliplatin-Induced Severe Anaphylactic Reactions in Metastatic Colorectal Cancer: Case Series Analysis. World J. Gastroenterol. WJG 2012, 18, 5427. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, M.-H.; Liu, J.-H.; Yen, C.-C.; Lin, P.-C.; Teng, H.-W.; Wang, W.-S.; Chiou, T.-J.; Chen, P.-M. Severe Anaphylactic Reactions in Patients Receiving Oxaliplatin Therapy: A Rare but Potentially Fatal Complication. Support. Care Cancer 2007, 15, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Maindrault-Goebel, F.; André, T.; Tournigand, C.; Louvet, C.; Perez-Staub, N.; Zeghib, N.; De Gramont, A. Allergic-Type Reactions to Oxaliplatin: Retrospective Analysis of 42 Patients. Eur. J. Cancer 2005, 41, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Garufi, C.; Cristaudo, A.; Vanni, B.; Bria, E.; Aschelter, A.; Santucci, B.; Terzoli, E. Skin Testing and Hypersensitivity Reactions to Oxaliplatin. Ann. Oncol. 2003, 14, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.S.; Lee, K.H.; Yoon, P.H.; Kim, S.J.; Park, I.; Kim, Y.S.; Ahn, H.K.; Hong, J.; Shin, D.B.; Sym, S.J. Oxaliplatin-Induced Immune-Mediated Thrombocytopenia: A Case Report. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2015, 47, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Bautista, M.A.; Stevens, W.T.; Chen, C.-S.; Curtis, B.R.; Aster, R.H.; Hsueh, C.-T. Hypersensitivity Reaction and Acute Immune-Mediated Thrombocytopenia from Oxaliplatin: Two Case Reports and a Review of the Literature. J. Hematol. Oncol. 2010, 3, 12. [Google Scholar] [CrossRef]
- Teng, C.-J.; Hsieh, Y.-Y.; Chen, K.-W.; Chao, T.-C.; Tzeng, C.-H.; Wang, W.-S. Sudden-Onset Pancytopenia with Intracranial Hemorrhage after Oxaliplatin Treatment: A Case Report and Literature Review. Jpn. J. Clin. Oncol. 2011, 41, 125–129. [Google Scholar] [CrossRef]
- Shao, Y.-Y.; Hong, R.-L. Fatal Thrombocytopenia after Oxaliplatin-Based Chemotherapy. Anticancer Res. 2008, 28, 3115–3117. [Google Scholar] [PubMed]
- Ureña-Tavera, A.; Zamora-Verduga, M.; Madrigal-Burgaleta, R.; Angel-Pereira, D.; Berges-Gimeno, M.P.; Alvarez-Cuesta, E. Hypersensitivity Reactions to Racemic Calcium Folinate (Leucovorin) during FOLFOX and FOLFIRI Chemotherapy Administrations. J. Allergy Clin. Immunol. 2015, 135, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Damaske, A.; Ma, N.; Williams, R. Leucovorin-Induced Hypersensitivity Reaction. J. Oncol. Pharm. Pract. 2012, 18, 136–139. [Google Scholar] [CrossRef]
- Dieu, C.T.N.; Nguyen, G.H.; Mai, Q.T.H. Anaphylaxis to Cisplatin after Carboplatin Hypersensitivity Reaction in Advanced Non-Small Cell Lung Cancer. Case Rep. Clin. Med. 2021, 10, 295–302. [Google Scholar] [CrossRef]
- Chikazawa, K.; Netsu, S.; Imai, K.; Ishiguro, A.; Kimura, A.; Wang, L.; Kuwata, T.; Konno, R. Nedaplatin Use in Patients with Hypersensitivity Reaction Episodes to Carboplatin. Taiwan. J. Obstet. Gynecol. 2020, 59, 546–550. [Google Scholar] [CrossRef]
- Picard, M.; Castells, M.C. Re-Visiting Hypersensitivity Reactions to Taxanes: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The Drawbacks and Advantages of Vehicle Selection for Drug Formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- A Fatal Anaphylactic Reaction to Paclitaxel Is Described, Which Was Preceded by a Possible Delayed Reaction to the Initial Infusion. Allergy Asthma Proc. 2011, 32, 79. [CrossRef]
- Syrigou, E.; Dannos, I.; Kotteas, E.; Makrilia, N.; Tourkantonis, I.; Dilana, K.; Gkiozos, I.; Saif, M.W.; Syrigos, K.N. Hypersensitivity Reactions to Docetaxel: Retrospective Evaluation and Development of a Desensitization Protocol. Int. Arch. Allergy Immunol. 2011, 156, 320–324. [Google Scholar] [CrossRef]
- Marchetti, M.A.; Noland, M.-M.; Dillon, P.M.; Greer, K.E. Taxane Associated Subacute Cutaneous Lupus Erythematosus. Dermatol. Online J. 2013, 19, 19259. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, A.; Aoe, K.; Murakami, T.; Maeda, T.; Eda, R.; Takeyama, H. Stevens-Johnson Syndrome Induced by Paclitaxel in a Patient with Squamous Cell Carcinoma of the Lung: A Case Report. Anticancer Res. 2004, 24, 1135–1138. [Google Scholar] [PubMed]
- Schrijvers, D.; Wanders, J.; Dirix, L.; Prove, A.; Vonck, I.; Van Oosterom, A.; Kaye, S. Coping with Toxicities of Docetaxel (TaxotereTM). Ann. Oncol. 1993, 4, 610–611. [Google Scholar] [CrossRef]
- Pazdur, R.; Lassere, Y.; Soh, L.; Ajani, J.; Bready, B.; Soo, E.; Sugarman, S.; Patt, Y.; Abbruzzese, J.; Levin, B. Phase II Trial of Docetaxel (Taxotere®) in Metastatic Colorectal Carcinoma. Ann. Oncol. 1994, 5, 468–470. [Google Scholar] [CrossRef]
- Wong, N.Y.; Parsons, L.M.; Trotter, M.J.; Tsang, R.Y. Drug-Induced Subacute Cutaneous Lupus Erythematosus Associated with Docetaxel Chemotherapy: A Case Report. BMC Res. Notes 2014, 7, 785. [Google Scholar] [CrossRef]
- Taj, A. Docetaxel-Induced Hypersensitivity Pneumonitis Mimicking Lymphangitic Carcinomatosis in a Patient with Metastatic Adenocarcinoma of the Lung. Hematol. Oncol. Stem Cell Ther. 2013, 6, 117–119. [Google Scholar] [CrossRef]
- Ardavanis, A.; Tryfonopoulos, D.; Yiotis, I.; Gerasimidis, G.; Baziotis, N.; Rigatos, G. Non-Allergic Nature of Docetaxel-Induced Acute Hypersensitivity Reactions. Anticancer Drugs 2004, 15, 581–585. [Google Scholar] [CrossRef]
- Weiszhár, Z.; Czúcz, J.; Révész, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement Activation by Polyethoxylated Pharmaceutical Surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.I.; Madrigal-Burgaleta, R.; Banerji, A.; Castells, M.; Alvarez-Cuesta, E. Controversies in Allergy: Chemotherapy Reactions, Desensitize, or Delabel? J. Allergy Clin. Immunol. Pract. 2020, 8, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kalish, R.S.; Askenase, P.W. Molecular Mechanisms of CD8+ T Cell–Mediated Delayed Hypersensitivity: Implications for Allergies, Asthma, and Autoimmunity. J. Allergy Clin. Immunol. 1999, 103, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Koralewski, P.; Hidalgo, J.; Chan, A.; Goncalves, A.; Schwartsmann, G.; Assadourian, S.; Lotz, J. A Multicenter Phase II Study of XRP6258 Administered as a 1-h Iv Infusion Every 3 Weeks in Taxane-Resistant Metastatic Breast Cancer Patients. Ann. Oncol. 2008, 19, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.C.; Denis, L.J.; Rowinsky, E.K.; DeBono, J.S.; Goetz, A.D.; Ochoa, L.; Forouzesh, B.; Beeram, M.; Patnaik, A.; Molpus, K. Phase I and Pharmacokinetic Study of XRP6258 (RPR 116258A), a Novel Taxane, Administered as a 1-Hour Infusion Every 3 Weeks in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2009, 15, 723–730. [Google Scholar] [CrossRef]
- Nightingale, G.; Ryu, J. Cabazitaxel (Jevtana): A Novel Agent for Metastatic Castration-Resistant Prostate Cancer. Pharm. Ther. 2012, 37, 440. [Google Scholar]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L. Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- He, F.; Liu, J.; Shen, X.; Wang, Z.; Li, Q.; Li, G. Adverse Event Profile for Nanoparticle Albumin-Bound Paclitaxel Compared with Solvent-Based Taxanes in Solid-Organ Tumors: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Ann. Pharmacother. 2022, 56, 898–909. [Google Scholar] [CrossRef]
- Vishnu, P.; Roy, V. Safety and Efficacy of Nab-Paclitaxel in the Treatment of Patients with Breast Cancer. Breast Cancer Basic Clin. Res. 2011, 5, BCBCR-S5857. [Google Scholar] [CrossRef]
- Dizon, D.; Rojan, A.; Miller, J.; Schwartz, J.; Gordinier, M.; Pires, L.; Disilvestro, P.; Moore, R.; Granai, C.; Gass, J. Cross-Sensitivity between Paclitaxel and Docetaxel in a Women’s Cancers Program. J. Clin. Oncol. 2005, 23, 2052. [Google Scholar] [CrossRef]
- de Leon, M.C.; Bolla, S.; Greene, B.; Hutchinson, L.; Del Priore, G. Successful Treatment with Nab-Paclitaxel after Hypersensitivity Reaction to Paclitaxel and Docetaxel. Gynecol. Oncol. Case Rep. 2013, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Carrera, J. Hypersensitivity Reactions to Taxanes and Subsequent Treatment with Nab-Paclitaxel: Case Reports of 2 Women with Early-Stage Breast Cancer. J. Hematol. Oncol. Pharm. 2021, 11, 329–334. [Google Scholar]
- Pellegrino, B.; Boggiani, D.; Tommasi, C.; Palli, D.; Musolino, A. Nab-Paclitaxel after Docetaxel Hypersensitivity Reaction: Case Report and Literature Review. Acta Bio Medica Atenei Parm. 2017, 88, 329. [Google Scholar]
- Burke, M.J.; Zalewska-Szewczyk, B. Hypersensitivity Reactions to Asparaginase Therapy in Acute Lymphoblastic Leukemia: Immunology and Clinical Consequences. Future Oncol. 2022, 18, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, H.; Sengupta, S.; Purohit, V.; Kotagere, A.; Moulik, N.R.; Prasad, M.; Dhamne, C.; Narula, G.; Banavali, S.; Gota, V. A Comparison of Asparaginase Activity in Generic Formulations of E. Coli Derived L-asparaginase: In-vitro Study and Retrospective Analysis of Asparaginase Monitoring in Pediatric Patients with Leukemia. Br. J. Clin. Pharmacol. 2020, 86, 1081–1088. [Google Scholar] [CrossRef]
- Ovalle, P.; Azócar, M.; Nicklas, C.; Villarroel, M.; Morales, J. Reacciones de Hipersensibilidad Asociadas al Uso de Asparaginasa En Niños Con Leucemia Linfoblástica Aguda. Andes Pediatr. 2021, 92, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Eschalier, A.; Lavarenne, J.; Burtin, C.; Renoux, M.; Chapuy, E.; Rodriguez, M. Study of Histamine Release Induced by Acute Administration of Antitumor Agents in Dogs. Cancer Chemother. Pharmacol. 1988, 21, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lwin, Z.T.; Yong, Z. Bleomycin Induced Drug Allergy Mimicking Herpes Skin Infection: A Case Report. Open J. Blood Dis. 2020, 10, 55–60. [Google Scholar] [CrossRef]
- Khansur, T.; Little, D.; Tavassoli, M. Fulminant and Fatal Angioedema Caused by Bleomycin Treatment. Arch. Intern. Med. 1984, 144, 2267. [Google Scholar] [CrossRef]
- Roberts, L.; Braswell, L.; Maves, G.; Stumpf, K.; Redmond, M.; Tobias, J.D. Intraoperative Anaphylaxis Following Injection of a Bleomycin-Gelatin Solution for Sclerotherapy. J. Med. Cases 2022, 13, 159. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, S.-H.; Yang, W.-K.; Park, Y.-C.; Kim, H.K. Bleomycin Aggravates Atopic Dermatitis via Lung Inflammation in 2, 4-Dinitrochlorobenzene-Induced NC/Nga Mice. Front. Pharmacol. 2018, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Lazović, B.; Milenković, V.; Đelić, M.; Mazić, S.; Jeremic, K.; Hrgović, Z. Hypersensitivity to Etoposide in Case of Metastatic Gestational Choriocarcinoma. Case Rep. Oncol. 2013, 6, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Siderov, J.; Prasad, P.; De Boer, R.; Desai, J. Safe Administration of Etoposide Phosphate after Hypersensitivity Reaction to Intravenous Etoposide. Br. J. Cancer 2002, 86, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Cotteret, C.; Rousseau, J.; Zribi, K.; Schlatter, J. Severe Hypersensitivity Reaction to Etoposide Phosphate: A Case Report. Clin. Case Rep. 2020, 8, 1821–1823. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, P.J.; King, S.A.; Fortner, C.L.; Leyland-Jones, B. Hypersensitivity Reactions to Teniposide (VM-26): An Analysis. J. Clin. Oncol. 1986, 4, 1262–1269. [Google Scholar] [CrossRef]
- Solimando, D.A., Jr.; Wilson, J.P. Doxorubicin-Induced Hypersensitivity Reactions. Drug Intell. Clin. Pharm. 1984, 18, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Treat, J.; Roh, J.-K.; Potkul, L.A.; Alvord, W.G.; Forst, D.; Woolley, P.V. A Phase I Clinical Trial and Pharmacokinetic Evaluation of Liposome-Encapsulated Doxorubicin. J. Clin. Oncol. 1990, 8, 1093–1100. [Google Scholar] [CrossRef]
- Chanan-Khan, A.; Szebeni, J.; Savay, S.; Liebes, L.; Rafique, N.; Alving, C.; Muggia, F. Complement Activation Following First Exposure to Pegylated Liposomal Doxorubicin (Doxil®): Possible Role in Hypersensitivity Reactions. Ann. Oncol. 2003, 14, 1430–1437. [Google Scholar] [CrossRef]
- Sharma, L.; Subedi, A.; Shah, B. Anaphylaxis to Pegylated Liposomal Doxorubicin: A Case Report. West Indian Med. J. 2014, 63, 376. [Google Scholar]
- Rosas-Vargas, M.A.; Casas-Becerra, B.; Velázquez-Armenta, Y.; Sienra-Monge, J.J.; Del Río-Navarro, B.E. Cyclophosphamide Hypersensitivity in a Leukemic Child. Ther. Drug Monit. 2005, 27, 263–264. [Google Scholar] [CrossRef]
- Popescu, N.A.; Sheehan, M.G.; Kouides, P.A.; Loughner, J.E.; Condemi, J.J.; Looney, R.J.; Leddy, J.P. Allergic Reactions to Cyclophosphamide: Delayed Clinical Expression Associated with Positive Immediate Skin Tests to Drug Metabolites in Five Patients. J. Allergy Clin. Immunol. 1996, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Vial, T.; Archimbaud, E. Anaphylactoid Reaction with Bronchospasm Following Intravenous Cyclophosphamide Administration. Ann. Hematol. 1991, 62, 74–75. [Google Scholar] [CrossRef]
- Muñoz-Osores, E.; Wietstruck, A.; Hoyos-Bachiloglu, R. Mesna, an Unusual Agent Causing Hypersensitivity Reactions during Chemotherapy. Ann. Allergy. Asthma. Immunol. 2022, 129, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Prada, A.; Borrás, J.; Farzanegan, R.; Torres Gorriz, M.C.; Germán-Sánchez, A.; Cervera Aznar, R.; Raducan, I.; Castelló, J.V.; Sanchez-Hernandez, A.; Enrique, E. Cutaneous Reaction to Ifosfamide plus Mesna Treated with Desensitization Challenge: A Case Report. Clin. Mol. Allergy 2022, 20, 7. [Google Scholar] [CrossRef]
- Shimogori, K.; Araki, M.; Shibazaki, S.; Tuda, K.; Miura, K. Nonimmediate Allergic Reactions Induced by Mesna. J. Gen. Fam. Med. 2017, 18, 285–287. [Google Scholar] [CrossRef]
- Jirasek, M.A.; Herrington, J.D. Cytarabine Syndrome despite Corticosteroid Premedication in an Adult Undergoing Induction Treatment for Acute Myelogenous Leukemia. J. Oncol. Pharm. Pract. 2016, 22, 795–800. [Google Scholar] [CrossRef]
- Albanesi, M.; Carluccio, P.; Nico, A.; Giliberti, L.; Di Bona, D.; Caiaffa, M.F.; Specchia, G.; Macchia, L. A Desensitization Protocol for Delayed Allergy to Cytarabine: Analysis of Two Cases. Adv. Dermatol. Allergol. Dermatol. Alergol. 2018, 35, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Isabwe, G.A.C.; Neuer, M.G.; de las Vecillas Sanchez, L.; Lynch, D.-M.; Marquis, K.; Castells, M. Hypersensitivity Reactions to Therapeutic Monoclonal Antibodies: Phenotypes and Endotypes. J. Allergy Clin. Immunol. 2018, 142, 159–170. [Google Scholar] [CrossRef]
- Picard, M.; Galvão, V.R. Current Knowledge and Management of Hypersensitivity Reactions to Monoclonal Antibodies. J. Allergy Clin. Immunol. Pract. 2017, 5, 600–609. [Google Scholar] [CrossRef]
- Rombouts, M.D.; Swart, E.L.; Van Den Eertwegh, A.J.; Crul, M. Systematic Review on Infusion Reactions to and Infusion Rate of Monoclonal Antibodies Used in Cancer Treatment. Anticancer Res. 2020, 40, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Fouda, G.E.; Bavbek, S. Rituximab Hypersensitivity: From Clinical Presentation to Management. Front. Pharmacol. 2020, 11, 572863. [Google Scholar] [CrossRef]
- Paul, F.; Cartron, G. Infusion-Related Reactions to Rituximab: Frequency, Mechanisms and Predictors. Expert Rev. Clin. Immunol. 2019, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, S.; Darby, A.; Mead, G.; Lister, A. Stevens–Johnson Syndrome after Treatment with Rituximab. Ann. Oncol. 2002, 13, 1948–1950. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.M.; Eckmann, K.; Boster, B.L.; Hess, K.R.; Michaud, L.B.; Esteva, F.J.; Hortobágyi, G.N.; Barnett, C.M. Incidence, Risk Factors, and Management of Infusion-Related Reactions in Breast Cancer Patients Receiving Trastuzumab. Oncologist 2014, 19, 228–234. [Google Scholar] [CrossRef]
- LaCasce, A.S.; Castells, M.C.; Burstein, H.J.; Meyerhardt, J.A.; Adkinson, N.F., Jr.; Feldweg, A.M. Infusion-Related Reactions to Therapeutic Monoclonal Antibodies Used for Cancer Therapy; UpToDate: Waltham, MA, USA, 2019. [Google Scholar]
- Price, L.; Brunt, A. Trastuzumab Infusion Reactions in Breast Cancer. Should We Routinely Observe after the First Dose? Eur. J. Hosp. Pharm. 2018, 25, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Bouza, T.R.; Hsu, F.I.; Sloane, D.E.; Castells, M.C. Hypersensitivity Reactions to MAbs: 105 Desensitizations in 23 Patients, from Evaluation to Treatment. J. Allergy Clin. Immunol. 2009, 124, 1259–1266. [Google Scholar] [CrossRef]
- Begum, P.; Ajauskaite, K.; Ring, A. 244P The Incidence of Infusion Related Reactions with Trastuzumab-Emtansine. Ann. Oncol. 2022, 33, S235. [Google Scholar] [CrossRef]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.-T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1, 3-Galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Peccerillo, J.; Potter, V. Successful Re-Challenge with Panitumumab in Patients Who Developed Hypersensitivity Reactions to Cetuximab: Report of Three Cases and Review of Literature. Cancer Chemother. Pharmacol. 2009, 63, 1017–1022. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Allen, R.; Spigel, D.R.; Stinchcombe, T.E.; Moore, D.T.; Berlin, J.D.; Goldberg, R.M. High Incidence of Cetuximab-Related Infusion Reactions in Tennessee and North Carolina and the Association with Atopic History. J. Clin. Oncol. 2007, 25, 3644–3648. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, J.; Beom, S.H.; Shin, S.J.; Ahn, J.B.; Kim, S.-R.; Lee, J.-H.; Park, J.-W. Nationwide Pharmacovigilance Data for Cetuximab-Induced Anaphylaxis and Predictive Model Validation Using Prospective Specific IgE Detection. World Allergy Organ. J. 2021, 14, 100553. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Saltoun, C. Skin Testing in Allergy. Allergy Asthma Proc. 2019, 40, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Lax, T.; Long, A.; Banerji, A. Skin Testing in the Evaluation and Management of Carboplatin-Related Hypersensitivity Reactions. J. Allergy Clin. Immunol. Pract. 2015, 3, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Zanotti, K.; Peterson, G.; Kulp, B.; Webster, K.; Belinson, J. Expanded Experience with an Intradermal Skin Test to Predict for the Presence or Absence of Carboplatin Hypersensitivity. J. Clin. Oncol. 2003, 21, 4611–4614. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cuesta, E.; Madrigal-Burgaleta, R.; Angel-Pereira, D.; Ureña-Tavera, A.; Zamora-Verduga, M.; Lopez-Gonzalez, P.; Berges-Gimeno, M. Delving into Cornerstones of Hypersensitivity to Antineoplastic and Biological Agents: Value of Diagnostic Tools Prior to Desensitization. Allergy 2015, 70, 784–794. [Google Scholar] [CrossRef]
- Patil, S.U.; Long, A.A.; Ling, M.; Wilson, M.T.; Hesterberg, P.; Wong, J.T.; Banerji, A. A Protocol for Risk Stratification of Patients with Carboplatin-Induced Hypersensitivity Reactions. J. Allergy Clin. Immunol. 2012, 129, 443–447. [Google Scholar] [CrossRef]
- Callahan, M.B.; Lachance, J.A.; Stone, R.L.; Kelsey, J.; Rice, L.W.; Jazaeri, A.A. Use of Cisplatin without Desensitization after Carboplatin Hypersensitivity Reaction in Epithelial Ovarian and Primary Peritoneal Cancer. Am. J. Obstet. Gynecol. 2007, 197, 199-e1. [Google Scholar] [CrossRef]
- Dizon, D.S.; Sabbatini, P.J.; Aghajanian, C.; Hensley, M.L.; Spriggs, D.R. Analysis of Patients with Epithelial Ovarian Cancer or Fallopian Tube Carcinoma Retreated with Cisplatin after the Development of a Carboplatin Allergy. Gynecol. Oncol. 2002, 84, 378–382. [Google Scholar] [CrossRef]
- Aberer, W.; Bircher, A.; Romano, A.; Blanca, M.; Campi, P.; Fernandez, J.; Brockow, K.; Pichler, W.J.; Demoly, P. ENDA Drug Provocation Testing in the Diagnosis of Drug Hypersensitivity Reactions: General Considerations. Allergy 2003, 58, 854–863. [Google Scholar] [CrossRef]
- Soyer, O.; Sahiner, U.M.; Sekerel, B.E. Pro and Contra: Provocation Tests in Drug Hypersensitivity. Int. J. Mol. Sci. 2017, 18, 1437. [Google Scholar] [CrossRef]
- Madrigal-Burgaleta, R.; Bernal-Rubio, L.; Berges-Gimeno, M.P.; Carpio-Escalona, L.V.; Gehlhaar, P.; Alvarez-Cuesta, E. A Large Single-Hospital Experience Using Drug Provocation Testing and Rapid Drug Desensitization in Hypersensitivity to Antineoplastic and Biological Agents. J. Allergy Clin. Immunol. Pract. 2019, 7, 618–632. [Google Scholar] [CrossRef]
- Madrigal-Burgaleta, R.; Berges-Gimeno, M.; Angel-Pereira, D.; Ferreiro-Monteagudo, R.; Guillen-Ponce, C.; Pueyo, C.; Gomez de Salazar, E.; Alvarez-Cuesta, E. Hypersensitivity and Desensitization to Antineoplastic Agents: Outcomes of 189 Procedures with a New Short Protocol and Novel Diagnostic Tools Assessment. Allergy 2013, 68, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.; Pichler, W. International Con Sensus on Drug Allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; McKinnon, D.; Chu, K. Emergency Department Anaphylaxis: A Review of 142 Patients in a Single Year. J. Allergy Clin. Immunol. 2001, 108, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Guitart, M.C. Rapid Drug Desensitization for Hypersensitivity Reactions to Chemotherapy and Monoclonal Antibodies in the 21st Century. J. Investig. Allergol. Clin. Immunol. 2014, 24, 72–79. [Google Scholar]
- Cho, S.; Choi, M.; Park, S.; An, S.; Park, J. Application of Spectro-DPRA, KeratinoSensTM and H-CLAT to Estimation of the Skin Sensitization Potential of Cosmetics Ingredients. J. Appl. Toxicol. 2020, 40, 300–312. [Google Scholar] [CrossRef]
- Bauch, C.; Kolle, S.N.; Fabian, E.; Pachel, C.; Ramirez, T.; Wiench, B.; Wruck, C.J.; van Ravenzwaay, B.; Landsiedel, R. Intralaboratory Validation of Four in Vitro Assays for the Prediction of the Skin Sensitizing Potential of Chemicals. Toxicol. In Vitro 2011, 25, 1162–1168. [Google Scholar] [CrossRef]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F. Assessing Skin Sensitization Hazard in Mice and Men Using Non-Animal Test Methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef]
- Galbiati, V.; Papale, A.; Kummer, E.; Corsini, E. In Vitro Models to Evaluate Drug-Induced Hypersensitivity: Potential Test Based on Activation of Dendritic Cells. Front. Pharmacol. 2016, 7, 204. [Google Scholar] [CrossRef]
- OECD The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins 2014. Available online: https://www.oecd.org/env/the-adverse-outcome-pathway-for-skin-sensitisation-initiated-by-covalent-binding-to-proteins-9789264221444-en.htm (accessed on 14 October 2022).
- OECD Test No. 442D: In Vitro Skin Sensitisation 2022. Available online: https://www.oecd.org/env/ehs/testing/test-no-442d-in-vitro-skin-sensitisation-9789264229822-en.htm (accessed on 14 October 2022).
- OECD Test No. 442C: In Chemico Skin Sensitisation 2022. Available online: https://www.oecd.org/env/ehs/testing/Test%20No.442C-English.pdf (accessed on 14 October 2022).
- OECD Test No. 442E: In Vitro Skin Sensitisation 2022. Available online: https://www.oecd.org/env/test-no-442e-in-vitro-skin-sensitisation-9789264264359-en.htm (accessed on 14 October 2022).
- United Nations. Economic Commission for Europe. In Secretariat Globally Harmonized System of Classification and Labelling of Chemicals (GHS); Copyright Law of the United St; United Nations: New York, NY, USA, 2015; ISBN 92-1-117087-7. [Google Scholar]
- Sousa, N.; Martínez-Aranguren, R.; Fernández-Benitez, M.; Ribeiro, F.; Sanz, M. Comparison of Basophil Activation Test Results in Blood Preserved in Acid Citrate Dextrose and EDTA. J. Investig. Allergol. Clin. Immunol. 2010, 20, 535–536. [Google Scholar]
- Newton, H.S.; Dobrovolskaia, M.A. Immunophenotyping: Analytical Approaches and Role in Preclinical Development of Nanomedicines. Adv. Drug Deliv. Rev. 2022, 185, 114281. [Google Scholar] [CrossRef] [PubMed]
- Holl, E.K.; Frazier, V.N.; Landa, K.; Beasley, G.M.; Hwang, E.S.; Nair, S.K. Examining Peripheral and Tumor Cellular Immunome in Patients with Cancer. Front. Immunol. 2019, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.P.; de la Losa, F.P. Immunoglobulin E-Mediated Severe Anaphylaxis to Paclitaxel. J. Investig. Allergol. Clin. Immunol. 2010, 20, 170–171. [Google Scholar]
- Caiado, J.; Brás, R.; Paulino, M.; Costa, L.; Castells, M. Rapid Desensitization to Antineoplastic Drugs in an Outpatient Immunoallergology Clinic: Outcomes and Risk Factors. Ann. Allergy. Asthma. Immunol. 2020, 125, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Burgaleta, R.; Vazquez-Revuelta, P.; Marti-Garrido, J.; Lleonart, R.; Ali, F.; Alvarez-Cuesta, E. Importance of Diagnostics Prior to Desensitization in New Drug Hypersensitivity: Chemotherapeutics and Biologicals. Curr. Treat. Options Allergy 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.; Makowska, J. In Vitro Tests for Drug Hypersensitivity Reactions: An ENDA/EAACI Drug Allergy Interest Group Position Paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Sugimoto, H.; Tabata, T.; Okuda, M. Clinical Utility of Basophil CD203c as a Biomarker for Predicting the Timing of Hypersensitivity Reaction in Carboplatin Rechallenge: Three Case Reports. Clin. Ther. 2016, 38, 1537–1541. [Google Scholar] [CrossRef]
- Castells, M.C.; Tennant, N.M.; Sloane, D.E.; Hsu, F.I.; Barrett, N.A.; Hong, D.I.; Laidlaw, T.M.; Legere, H.J.; Nallamshetty, S.N.; Palis, R.I. Hypersensitivity Reactions to Chemotherapy: Outcomes and Safety of Rapid Desensitization in 413 Cases. J. Allergy Clin. Immunol. 2008, 122, 574–580. [Google Scholar] [CrossRef]
- Banerji, A.; Lax, T.; Guyer, A.; Hurwitz, S.; Camargo, C.A., Jr.; Long, A.A. Management of Hypersensitivity Reactions to Carboplatin and Paclitaxel in an Outpatient Oncology Infusion Center: A 5-Year Review. J. Allergy Clin. Immunol. Pract. 2014, 2, 428–433. [Google Scholar] [CrossRef]
- Pichler, W.; Tilch, J. The Lymphocyte Transformation Test in the Diagnosis of Drug Hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Patil, S.U.; Banerji, A. Immediate Hypersensitivity Reaction to Chemotherapeutic Agents. J. Allergy Clin. Immunol. Pract. 2017, 5, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.L.; Chapdelaine, J.M.; Descotes, J.; Germolec, D.; Holsapple, M.; House, R.; Lebrec, H.; Meade, J.; Pieters, R.; Hastings, K.L. Evaluation of a Lymph Node Proliferation Assay for Its Ability to Detect Pharmaceuticals with Potential to Cause Immune-Mediated Drug Reactions. J. Immunotoxicol. 2005, 2, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Casula, M.; Tragni, E.; Galbiati, V.; Pallardy, M. Tools to Investigate and Avoid Drug-Hypersensitivity in Drug Development. Expert Opin. Drug Discov. 2018, 13, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ginghină, O.; Hudiță, A.; Zaharia, C.; Tsatsakis, A.; Mezhuev, Y.; Costache, M.; Gălățeanu, B. Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients. Materials 2021, 14, 2440. [Google Scholar] [CrossRef] [PubMed]
- Hudiță, A.; Radu, I.C.; Zaharia, C.; Ion, A.C.; Ginghină, O.; Gălățeanu, B.; Măruțescu, L.; Grama, F.; Tsatsakis, A.; Gurevich, L. Bio-and Hemo-Compatible Silk Fibroin PEGylated Nanocarriers for 5-Fluorouracil Chemotherapy in Colorectal Cancer: In Vitro Studies. Pharmaceutics 2021, 13, 755. [Google Scholar] [CrossRef]
| Type I | IgE antibody-mediated reactions, e.g., anaphylaxis |
| Type II | Antibody-mediated cytotoxic reactions, e.g., hemolytic anemia, thrombocytopenia, blood transfusion reactions |
| Type III | Immune complex-mediated hypersensitivity, e.g., serum sickness, vasculitis |
| Type IV | Delayed T cell-mediated responses, e.g., allergic contact dermatitis, maculopapular exanthema, erythema multiforme, toxic epidermal necrolysis, Drug-induced hypersensitivity syndrome (DiHS), hypersensitivity pneumonitis |
| CLASS | Representatives | Examples | Administration Route | Indications | Hypersensitivity | Adverse Reactions | |
|---|---|---|---|---|---|---|---|
| Cutaneous | Systemic | ||||||
| ALKYLATING AGENTS [10,11] | Nitrogen mustard derivatives | Cyclophosphamide | Oral, IV | Hodgkin or non-Hodgkin acute and chronic lymphatic or myeloid leukemia, malignant lymphomas, OC, BC, LC, neuroblastoma | Anaphylaxis | Urticaria, angioedema, acral erythema, erythema multiformae, nail disorders, stomatitis, vasculitis, SJS, TEN, RRR, pruritus | Pulmonary toxicity, BMS hemorrhagic cystitis, HVOD, EFT, cardiac toxicity, UTRT |
| Melphalan | oral | polycythemia vera, multiple myeloma, advanced breast carcinoma, breast adenocarcinoma | Anaphylaxis | Rash, urticaria, angioedema, pruritus, stomatitis, scleroderma, erythema, alopecia | Interstitial pneumonitis pulmonary fibrosis, BMS | ||
| Mechlorethamine | IV | CML, CLL, CTCL, HL, NHL, brain | Anaphylaxis | Urticaria, angioedema ACD, hyper pigmentation, pruritus, erythema multiformae, SJS | Lymphocytopenia | ||
| Triazines | Dacarbazine | IV | malignant melanoma, sarcoma, islet cells, PC, neuroblastoma | Anaphylaxis | Rash, photosensitivity, ISR, urticarial | fever, hypereosinophilia, allergic hepatitis, BMS | |
| Temozolomide | oral | brain cancer, melanoma | Anaphylaxis | Rash, pruritus, photosensitivity, acral erythema, urticaria, pigmentation, MPR, SJS, TEN, alopecia | Fever, peripheral edema, pneumonitis, thrombocytopenia, neutropenia | ||
| Ethylenimines | ThioTEPA | IV, intrathecal, intravesicular | OC, BC, bladder cancer leukemia, multiple myeloma | Anaphylaxis | Rash, urticaria, angioedema, pruritus, pigmentation, ISR, alopecia, dermatitis | Fever, wheezing, BMS, thrombocytopenia, anemia, leukopenia | |
| ANTIMETABOLITES [10,11] | Folate antagonists | Methotrexate | Oral, IV | gestational choriocarcinoma, choriocarcinoma and hydatidiform mole, BC, head, and neck cancer, LC (scaly and with small cells), NHL | Anaphylaxis | Acral erythema, rash, pruritis, urticaria, vasculitis, erythema multiformae, photosensitivity, stomatitis, psoriasis, pigmentation, SJS, TEN, alopecia | Bronchospasm, pulmonary infiltrates, hemolytic anemia, agranulocytosis, myelosuppression, hepatotoxicity |
| Pemetrexed | IV | malignant pleural mesothelioma, NSCLC | - | Rash, pruritus, vasculitis, mucositis, stomatitis, TEN, alopecia | Neutropenia, thrombocytopenia, anemia, interstitial pneumonitis, dyspnea, mucositis | ||
| Pyrimidine analogues | Capecitabine | oral | metastatic CRC | - | Acral erythema, erythema, exfoliative dermatitis, PPK, stomatitis, photosensitivity, hyperpigmentation, RRR, IRAK | Lymphopenia, thrombocytopenia, neutropenia, anemia | |
| Cytarabine | IV | acute nonlymphocyticleukemia, ALL, chronic myelocytic leukemia | Anaphylaxis | Urticaria, angioedema, acral erythema, pruritus, TEN, vasculitis, AGEP, NEH, MPR | Thrombocytopenia, leucopenia, fever, dyspnea, ARDS, hepatotoxicity, bleeding | ||
| 5′-Fluorouracil | IV | CRC, BC, GC, PC, liver, uterine, OC, bladder cancer | Anaphylaxis | Acral erythema, MPR, photosensitivity, stomatitis, contact dermatitis, ISR, alopecia | Myelosuppression, mucositis | ||
| Gemcitabine | IV | NSCLC, PC, OC, BC, bladder cancer | - | Cutaneous: Pruritus, MPR, acral erythema, bullous dermatitis, vasculitis, stomatitis, SJS, TEN, RRR, | Fever, pneumonitis, leukopenia, neutropenia, anemia, thrombocytopenia | ||
| PLATINUM COMPOUNDS [17] | DNA cross-linkers | Carboplatin | IV | OC, LC, BC, head and neck, testicular, brain (children) cancers | Anaphylaxis | Rash, pruritus, urticaria, angioedema, erythema, edema | BMS, bronchospasm, dyspnea, peripheral neuropathy |
| Cisplatin | IV | sarcomas, OC, lymphomas, LC, germ cell cancers | Anaphylaxis | flushing, rash, urticaria, pruritus | Bronchospasm, dyspnea, hemolytic anemia, renal toxicity | ||
| Oxaliplatin | IV | Metastatic CRC, OC, GC | Anaphylaxis | Flushing, erythema, urticaria, angioedema, pruritus, rash, acralerythema, RRR | Fever, dyspnea, wheezing, types II and III hypersensitivity | ||
| MITOTIC INHIBITORS [3] | Vinca alkaloids | (a) Vinblastine (b) Vincristine (c) Vindesine (d) Vinorelbine | IV | (a) HL, NSCLC, head and neck, NHL, BC, Kaposi syndrome. (b) HL, WT, leukemia, Ewing sarcoma, rhabdomyosarcoma, neuroblastoma, nephroblastoma, embrionary child tumors, osteosarcoma. (c) Melanoma, LC, BC, leukemia. (d) BC, bone, NSCLC, | Anaphylaxis | Acral erythema, rash, phlebitis, cellulitis, stomatitis, nail lesions, alopecia, Raynaud’s phenomenon | Fever, bronchospasm, ARF, pulmonary edema, pleural effusion, interstitial pneumonitis |
| Taxanes | Docetaxel | IV | OC, BC, LC, AIDS-related Kaposi sarcoma | - | Acral erythema, scleroderma-like, CLE, stomatitis, RRR, ISR, nail abnormalities, TEN | Anemia, neutropenia, leukopenia, bronchospasm, dyspnea, back pain | |
| Paclitaxel | IV | BC, NSCLC, metastatic hormone-resistant prostate cancer, GC, squamous cell of head and neck cancers | - | Acral erythema, ISR, erythema multiformae, RRR, AGEP-like, SIS, alopecia | Neutropenia, BMS, hypersens pneumonitis, dyspnea, back pain | ||
| Cabazitaxel | IV | hormone-resistant metastatic prostate cancer | - | Rash | Myelosuppression, nausea, vomiting, constipation, peripheral -neuropathy, neuromuscular pain | ||
| TOPOISOMERASE INHIBITORS [8] | Topoisomerase I inhibitors | Irinotecan | IV | CRC, squamous cell carcinoma of the cervix | Anaphylaxis | Rash, alopecia | Neutropenia, anemia, dyspnea |
| Topoisomerase II inhibitors | Daunorubicin | IV | AML, ALL, neuroblastoma | Anaphylaxis | Rash, urticaria, angioedema, hyperpigmentation | BMS, fever, cardiac toxicity | |
| Doxorubicin | IV | HL, hematologic, OC, BC, LC, bladder cancer, Kaposi’s | Anaphylaxis, | Rash, acral erythema, ISR, pruritus, urticaria, angioedema, NEH, RRR | Myelosuppression, bronchospasm, cardiotoxicity | ||
| Mitoxantrone | IV | BC, AML, NHL, ALL | Anaphylaxis | Rash, ISR, purpura, nail discoloration, stomatitis, alopecia | Myelosuppression, | ||
| DISRUPTION OF PROTEIN SYNTHESIS [12] | L-Asparaginase | IV | ALL, AML | Anaphylaxis | Urticaria, angioedema, rash, pruritus, stomatitis, TEN | Laryngospasm, BMS, pancreatitis | |
| MONOCLONAL ANTIBODY [15] | Rituximab | IV | follicular lymphoma stage III-IV, diffuse big B cell NHL, LLC | Anaphylaxis | Paraneoplastic pemphigus, lichenoid dermatitis, vesiculobullous dermatitis, SJS, TEN | Pulmonary events, renal toxicity, neutropenia, serum sickness, fever, lymphopenia, chills, asthenia | |
| Brentuximab vedotin | IV | HL, Systemic anaplastic large cell lymphoma, Cutaneous T-cell lymphoma | Anaphylaxis | Rash, pruritus, SJS, alopecia | Cytopenia, immunogenicity, URTI, pyrexia, nausea, vomiting, fatigue, cough | ||
| Cetuximab | IV | CRC, Squamous cell cancer of the head and neck | Anaphylaxis | Acneiform rash, nail changes, xeroderma, paronychial inflammation, pruritus | Electrolyte imbalance, infection, headache, diarrhea | ||
| Trastuzumab | Anaphylaxis/Angioedema | Rash, nail disorders, pruritus | Neutropenia, anemia, thrombocytopenia, pulmonary events, LVD, chills, fever, URTI, headache, cough, stomatitis, mucosal inflammation | ||||
| SIGNAL TRANSDUCTION INHIBITORS [13] | Non-receptor tyrosine kinase inhibitors | Bosutinib | IV | Ph+CML | Anaphylaxis | Rash, pruritus | Neutron, anemia, edema, hepatotoxic, pneumonia, pyrexia, cough, renal toxicity, EFT |
| Receptor tyrosine kinase inhibitors | Lapatinib | IV | Metastatic BC | Anaphylaxis | Rash, HFSR, pruritus, xerosis, paronychia, nail disorders, alopecia | Cardiac toxicity, hepatotoxicity, ILD, diarrhea | |
| HORMONES, HORMONE ANALOGS, AND HORMONE ANTAGONISTS [18] | Aromatase inhibitors | Exemestane | oral | BC in Postmenopausal women | Anaphylaxis | Rash, urticarial, pruritus, cutaneous vasculitis, erythema multiformae, AGEP, alopecia, dermatitis | Hot flushes, arthralgia, dyspnea, decreased bone density, EFT |
| Letrozole | oral | BC in Postmenopausal women | Anaphylaxis | Angioedema, rash, erythema multiformae, TEN, alopecia | Flushing, dyspnea, EFT, diaphoresis, arthralgia, hypertension, peripheral edema, decreased bone density | ||
| Gonadotropin-releasing hormone analogs (GnRH agonists) | Goserelin | IV | Prostate cancer, BC | Anaphylaxis | Rash, itching, RPCS, acne, seborrhea, alopecia | Hot flushes, DIDF, anemia, osteoporosis, vaginitis | |
| Leuprolide | Prostate cancer, BC | Anaphylaxis | Rash, injection site granuloma, pruritus, xerosis, ecchymosis, photosens, pigmentation | Hot fushes, DIDF, thrombo, anemia, peripheral edema | |||
| Carboplatin [6,20,21,22,23,24,25,26,27] | Oxaliplatin [6,20,22,23,28,29,30,31] | Cisplatin [6,20,22] | |
|---|---|---|---|
| Risk factors | |||
| Previous multiple exposures | √ | √ | √ |
| Previous treatment with another platinum drug | √ | √ | √/x |
| High cumulative dose | √ | √ | NA |
| Long platinum-free interval | √ | √ | NA |
| Positive ST result after a previous reaction | √ | √ | NA |
| Female gender | √ | √ | √ |
| Young age | √/x | √/x | NA |
| Atopy history | √/x | √/x | NA |
| Cardiovascular involvement | √/x | √/x | NA |
| Others | Certain chemotherapy regimens (higher with carboplatin/paclitaxel) | Palliative second-line setting | Concomitant radiation |
| BRCA 1/2 mutation | |||
| Weekly infusions in children with high-grade glioma |
| Immune-Mediated Reactions | Pseudo-Anaphylactic Reactions | Substance Responsible for HSR | Observations | References | ||
|---|---|---|---|---|---|---|
| Immediate | Delayed | |||||
| Platinum analogs | Ig-E mediated in most cases. Direct mast cell degranulation in other cases. | |||||
| Cisplatin | √ | x | x | Chimiotherapeutic agent | [32,33,34] | |
| Carboplatin | √ | √ | √ | Chimiotherapeutic agent | [21,22,26,36,37] | |
| Oxaliplatin | √ | √ | x | Chimiotherapeutic agent | [17,23,28,41,43,46] | |
| Taxanes | Direct mast cell degranulation in most cases. IgE sometimes reported. | |||||
| Paclitaxel | √ | √ | √ | Both chemotherapeutic agent and solvent (ChremophorEl) | [6,7,20,55,57] | |
| Docetaxel | √(rare) | √ | √ | Both chemotherapeutic agent and solvent (Polysorbate 80) | [58,61,62,63,64] | |
| Cabazitaxel | x | x | √ | Both chemotherapeutic agent and solvent (Polysorbate 80) | [69,70,71,72] | |
| Nab-paclitaxel | √ | x | x | NK | [73,76,78] | |
| L-asparaginase | √ | √ | √ | Mostly induced by E. coli-derived asparaginase Erwinia 332 asparaginase | Mostly specific anti-drug antibodies | [6,7,79] |
| Bleomycin | √(rare) | √ | √(rare) | NK | Presumably direct mast cell activation | [83,84,85] |
| Topoisomerase inhibitors | ||||||
| Etoposide/teniposide | x | x | √ | Solvent polysorbate-80 | Direct mast cell activation | [6,87,88] |
| Doxorubicin | √(rare) | x | x | NK | ||
| pegylated liposomal doxorubicin | x | x | √ | surface component of the liposome | Direct complement activation | [7,91,94] |
| Alkylating agents | ||||||
| cyclophosphamide | √(rare) | x | x | metabolites phosphoramide mustard, and acrolein | Presumed IgE-mediated reactions to the two main metabolites | [95,96,97] |
| ifosfamide | √(rare) | x | x | most ifosfamide-related reactions are considered to be due to MESNA | [98,99,100] | |
| Antimetabolites | ||||||
| Citarabine | √(rare) | √(rare) | √ | NK | Infusion reactions are usually described as “flu-like cytarabine syndrome” | [101,102] |
| Monoclonal antibodies | most of the infusion reactions associated coincide with the cytokine-mediated pattern/IgE-mediated reactions also described | |||||
| Rituximab | √(rare) | √(rare) | √ | NK | [103,104,105,106] | |
| Trastuzumab | √(rare) | x | √ | NK | [111,112,113] | |
| Cetuximab | √ | x | √ | galactose-alpha-1,3-galactose, oligosaccharide presents on the cetuximab mouse-derived heavy chain | [115,116,117] | |
| Drug | Prick Test Dilutions (mg/mL) | Intradermal Test Dilutions (mg/mL) |
|---|---|---|
| Carboplatin | 1/1 (10) | 1/100 (0.1) |
| 1/10 (1) | ||
| Cisplatin | 1/1 (10) | 1/100 (0.01) |
| 1/10 (0.1) | ||
| 1/1 (1) | ||
| Oxaliplatin | 1/1 (5) | 1/100 (0.05) |
| 1/10 (0.5) | ||
| 1/1 (5) | ||
| Paclitaxel | 1/10 1 (6) | 1/1000 (0.001 [0.006]) |
| 1/100 (0.001 [0.006]) | ||
| 1/10 (0.6) | ||
| Docetaxel | 1/1 4 (1) | 1/100 (0.04 [0.01]) |
| 1/10 0.4 [0.1] | ||
| Procarbazine | 1/1 (5) | 1/100 (0.05) |
| Gemcitabine | 1/1 (38) | 1/1000 (0.0038) |
| 1/100 (0.038) | ||
| 1/10 and 1/1 | ||
| Methotrexate | 1/1 (10) | 1/100 (0.1) |
| 1/10 (1) | ||
| 1/1 (10) | ||
| L-Asparaginase | A drop of reconstitute 5000 KU | 0.01 mL of reconstitute 5000 KU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galateanu, B.; Pușcașu, A.I.; Tircol, S.A.; Tanase, B.C.; Hudita, A.; Negrei, C.; Burcea-Dragomiroiu, G.-T.-A.; Negreanu, L.; Vacaroiu, I.A.; Ginghină, O. Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions. Int. J. Mol. Sci. 2023, 24, 3886. https://doi.org/10.3390/ijms24043886
Galateanu B, Pușcașu AI, Tircol SA, Tanase BC, Hudita A, Negrei C, Burcea-Dragomiroiu G-T-A, Negreanu L, Vacaroiu IA, Ginghină O. Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions. International Journal of Molecular Sciences. 2023; 24(4):3886. https://doi.org/10.3390/ijms24043886
Chicago/Turabian StyleGalateanu, Bianca, Alexandra Ioana Pușcașu, Simona Andreea Tircol, Bogdan Cosmin Tanase, Ariana Hudita, Carolina Negrei, George-Traian-Alexandru Burcea-Dragomiroiu, Lucian Negreanu, Ileana Adela Vacaroiu, and Octav Ginghină. 2023. "Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions" International Journal of Molecular Sciences 24, no. 4: 3886. https://doi.org/10.3390/ijms24043886
APA StyleGalateanu, B., Pușcașu, A. I., Tircol, S. A., Tanase, B. C., Hudita, A., Negrei, C., Burcea-Dragomiroiu, G.-T.-A., Negreanu, L., Vacaroiu, I. A., & Ginghină, O. (2023). Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions. International Journal of Molecular Sciences, 24(4), 3886. https://doi.org/10.3390/ijms24043886











