Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine
Abstract
1. Introduction
2. Results
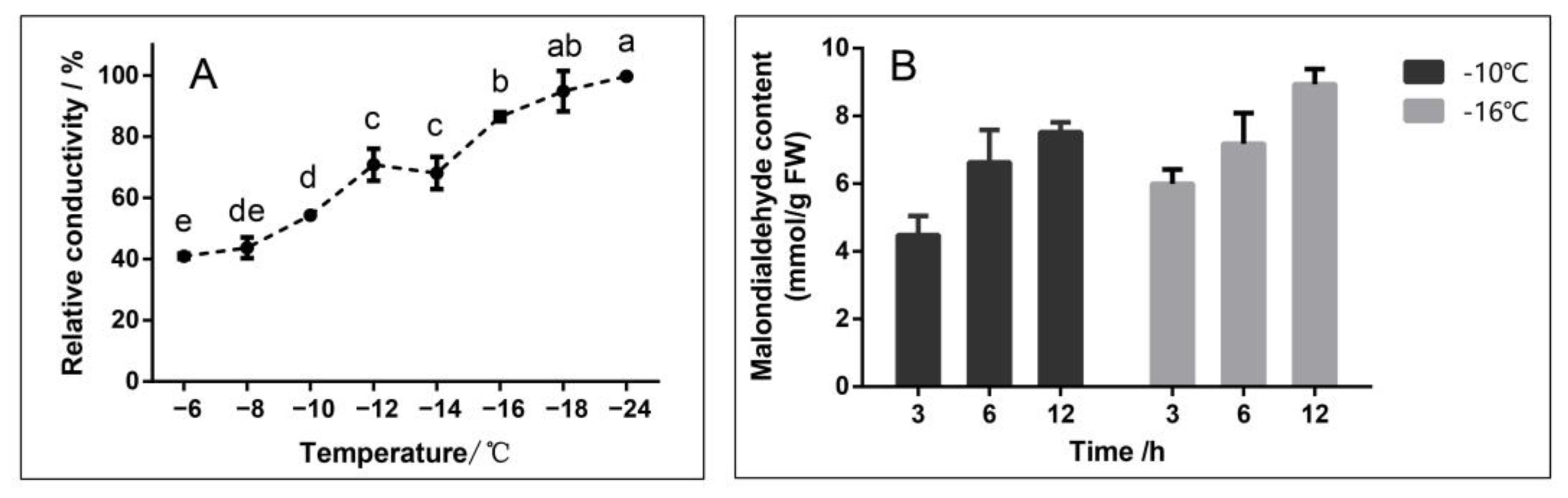
2.1. Selection of Experimental Conditions
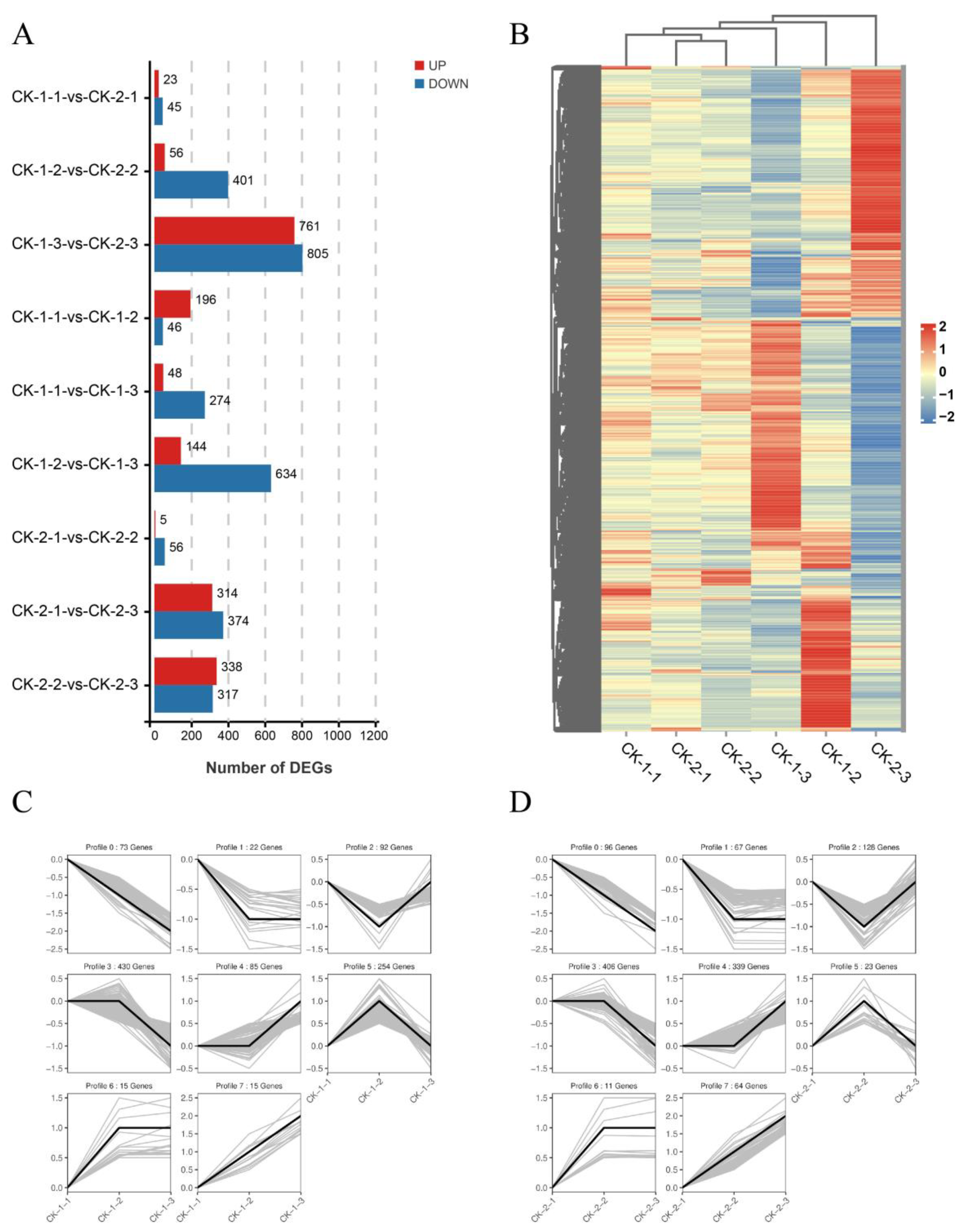
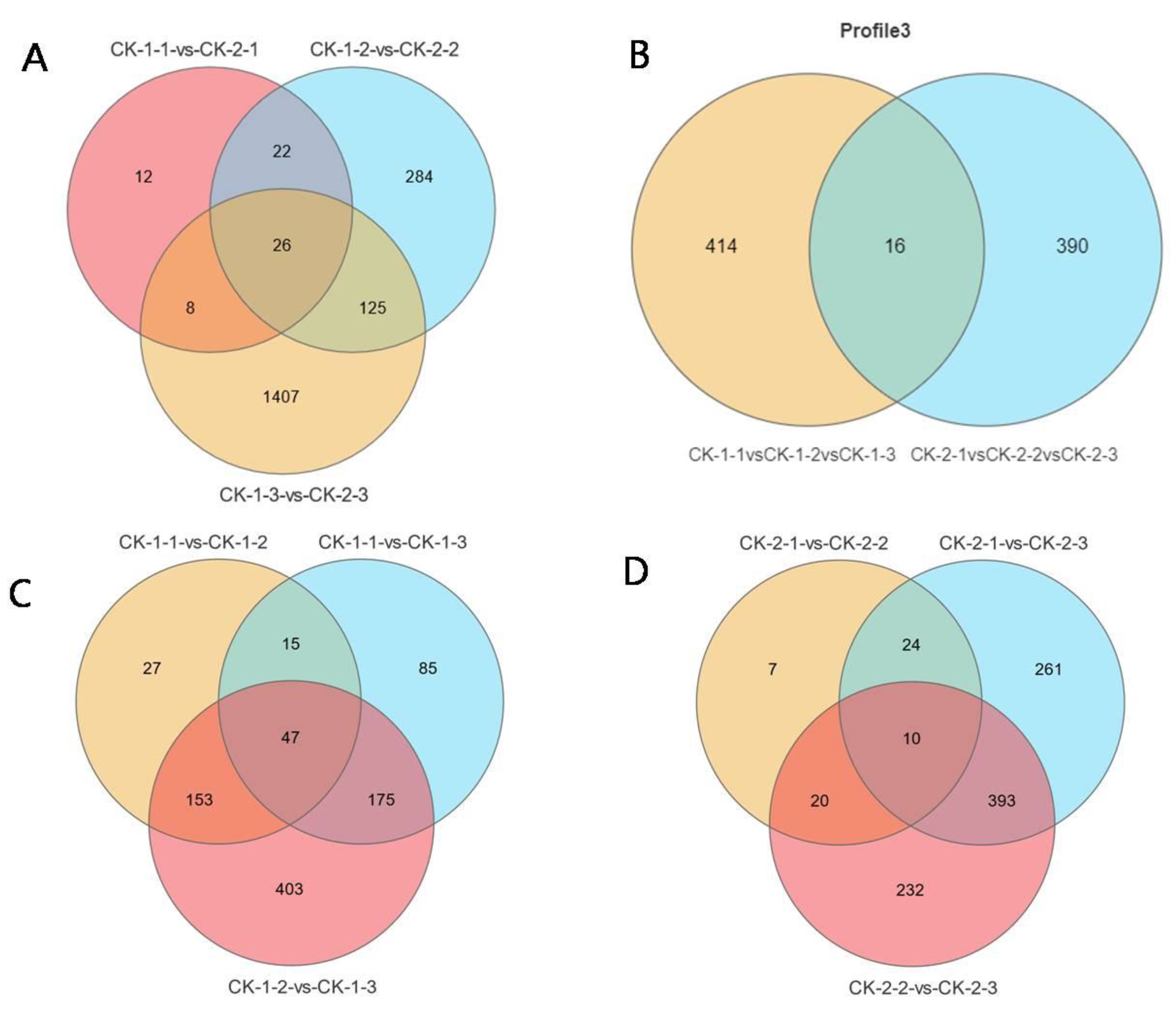
2.2. Expression Analysis of the Data Set
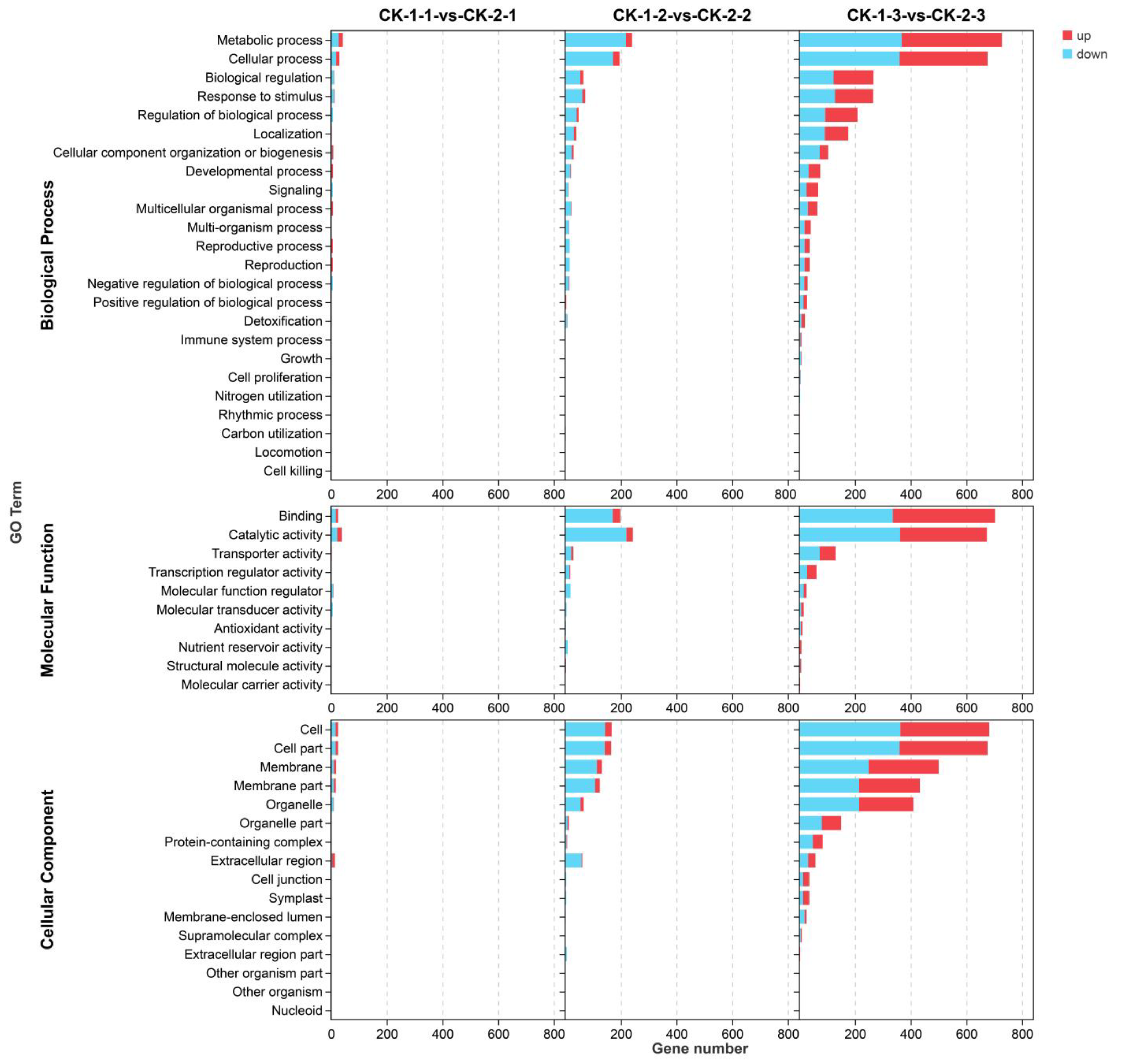
2.3. Functional Analyses of the Transcriptome Changes in Response to Different Freezing Treatments
2.4. Response of Cabernet Sauvignon to Low Temperatures
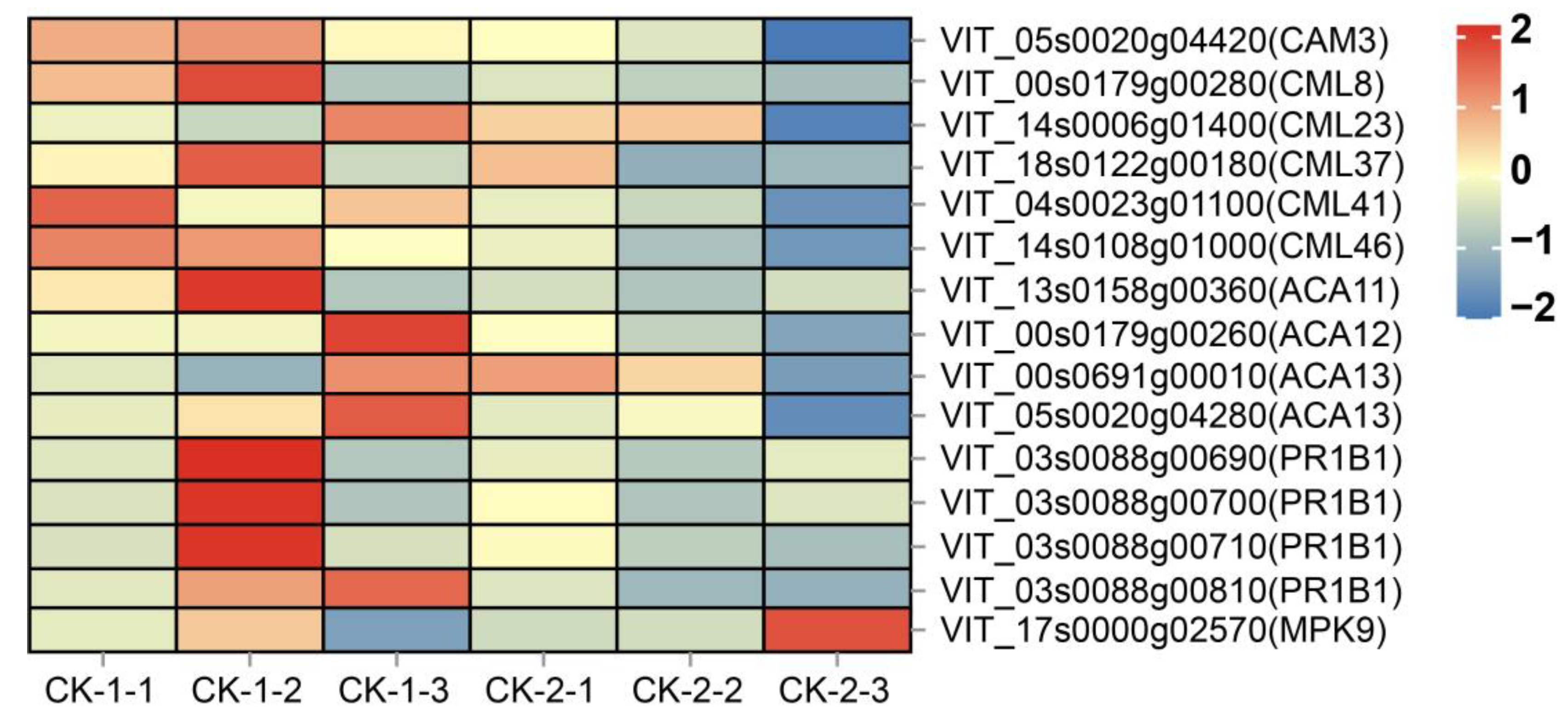
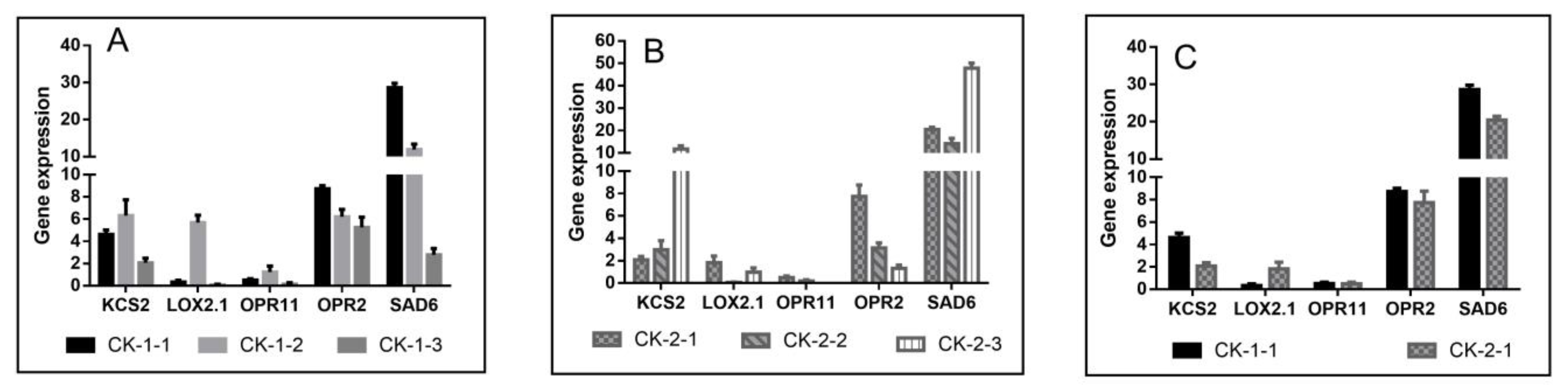
2.4.1. Effect of Freezing Stress on Signal Transduction
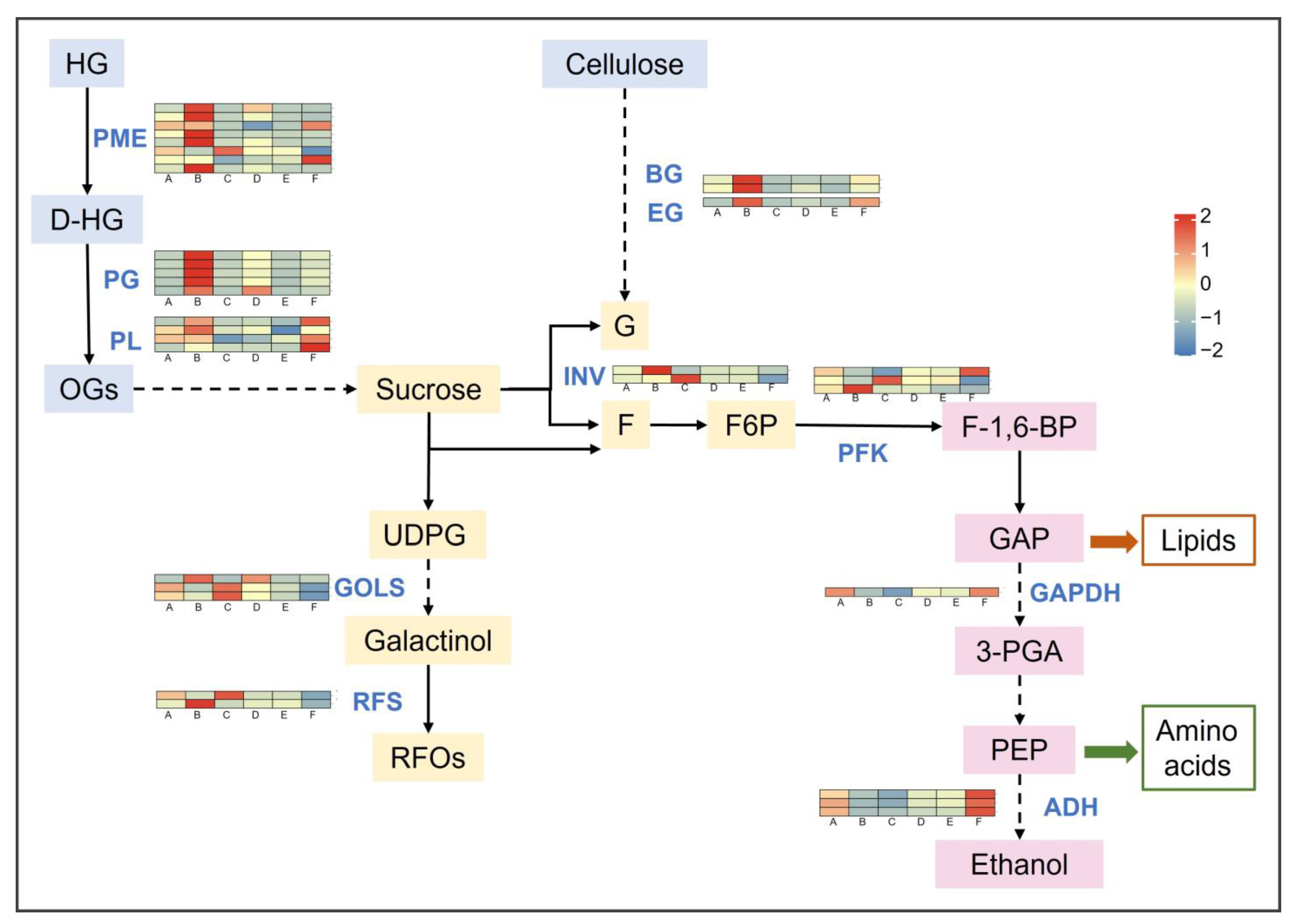
2.4.2. Effect of Freezing Stress on Sugar Metabolism
2.4.3. Effect of Freezing Stress on Lipid Metabolism
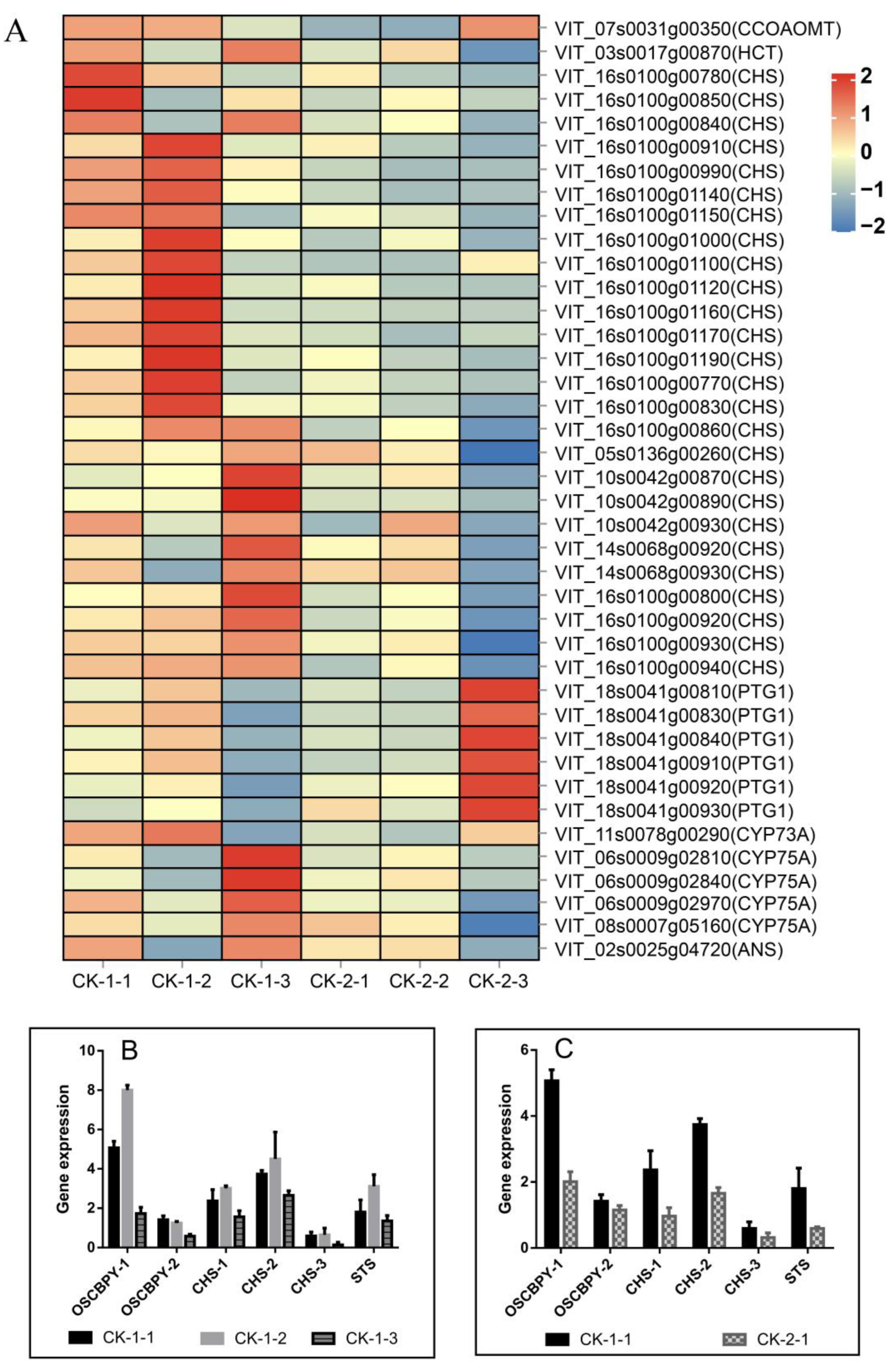
2.4.4. Effect of Freezing Stress on Secondary Metabolite Synthesis
3. Discussion
3.1. Low-Temperature Signal Transduction
3.2. Carbohydrate Metabolism
3.3. Membrane Damage and the Metabolism of Unsaturated Fatty Acids
3.4. Flavonoids and Stilbenes
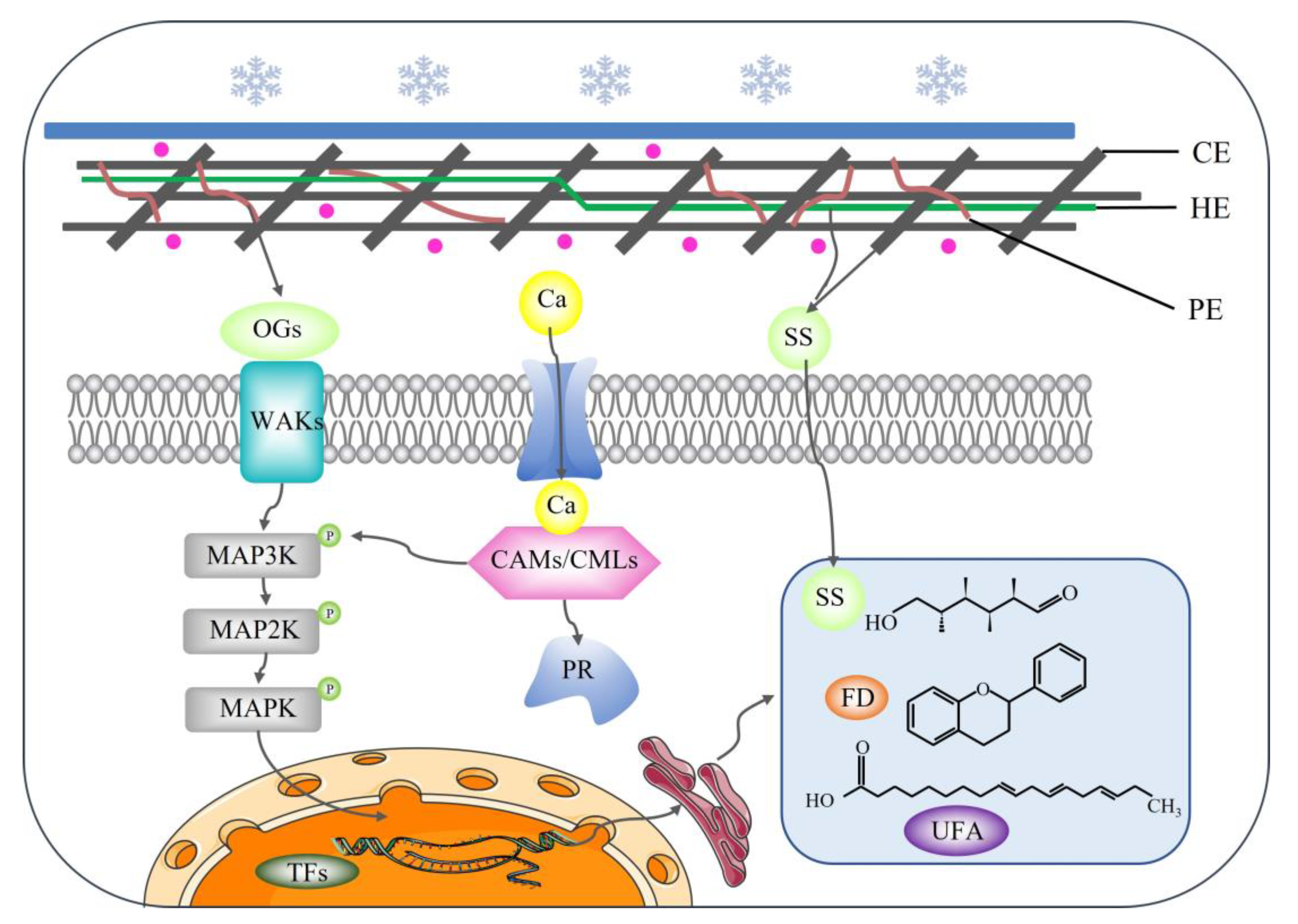
3.5. Hypothetical Model of Grapevine Responding to Low-Temperature Stress
4. Material and Methods
4.1. Plant Materials and Freezing Treatment
4.2. Determination of Electrolyte Leakage
4.3. Determination of the Malondialdehyde Content
4.4. RNA Extraction, Library Construction, and Sequencing
4.5. RNA-Seq Data Analysis
5. Conclusions
- (1)
- More genes were differentially expressed with increasing stress time, but the most common DEGs had peak expression at 6 h. The differential genes under different freezing treatments were mainly enriched in the following GO terms: metabolic processes, cellular processes, binding, catalytic activity, cells, cell parts, and membranes.
- (2)
- Calcium/calmodulin-mediated signaling played a key role in the low-temperature response, most calcium receptors were rapidly activated in the early stages of freezing stress, and genes encoding PR1B1 proteins were up-regulated at low temperatures.
- (3)
- Carbohydrate metabolism, lipid metabolism, and the synthesis of secondary metabolites were the main differential pathways for KEGG enrichment. The hydrolysis of pectin and cellulose in the cell wall may be an important strategy for grapevines to cope with freezing injury.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Lukatkin, A.S.; Brazaitytė, A.; Bobinas, Č.; Duchovskis, P. Chilling injury in chilling-sensitive plants: A review. Zemdirb.-Agric. 2012, 99, 111–124. [Google Scholar]
- Orvar, B.L.; Sangwan, V.; Omann, F.; Dhindsa, R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. Cell Mol. Biol. 2000, 23, 785–794. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.R. Signal transduction leading to low-temperature tolerance in Arabidopsis thaliana. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2002, 357, 871–875. [Google Scholar] [CrossRef]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14 (Suppl. S1), S401–S417. [Google Scholar] [CrossRef]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Costa, A.; Luoni, L.; Marrano, C.A.; Hashimoto, K.; Köster, P.; Giacometti, S.; De Michelis, M.I.; Kudla, J.; Bonza, M.C. Ca2+-dependent phosphoregulation of the plasma membrane Ca2+-ATPase ACA8 modulates stimulus-induced calcium signatures. J. Exp. Bot. 2017, 68, 3215–3230. [Google Scholar] [CrossRef]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J. MAPping Kinase Regulation of ICE1 in Freezing Tolerance. Trends Plant Sci. 2018, 23, 91–93. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S.; Bahk, S.; An, J.; Yoo, Y.; Kim, J.Y.; Chung, W.S. Phosphorylation of the transcriptional repressor MYB15 by mitogen-activated protein kinase 6 is required for freezing tolerance in Arabidopsis. Nucleic Acids Res. 2017, 45, 6613–6627. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. Int. J. Exp. Plant Biol. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Hatier, J.H.; Gould, K.S. Foliar anthocyanins as modulators of stress signals. J. Theor. Biol. 2008, 253, 625–627. [Google Scholar] [CrossRef]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef]
- Pearce, R.S. Extracellular ice and cell shape in frost-stressed cereal leaves: A low-temperature scanning-electron-microscopy study. Planta 1988, 175, 313–324. [Google Scholar] [CrossRef]
- Pearce, R.S.; Ashworth, E.N. Cell shape and localisation of ice in leaves of overwintering wheat during frost stress in the field. Planta 1992, 188, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, C.B.; Lafta, A. Cell-Wall Changes and Cell Tension in Response to Cold Acclimation and Exogenous Abscisic Acid in Leaves and Cell Cultures. Plant Physiol. 1996, 111, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wormit, A.; Usadel, B. The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 878. [Google Scholar] [CrossRef] [PubMed]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Hofte, H.; Peaucelle, A. Probing the mechanical contributions of the pectin matrix: Insights for cell growth. Plant Signal. Behav. 2012, 7, 1037–1041. [Google Scholar] [CrossRef]
- Daher, F.B.; Braybrook, S.A. How to let go: Pectin and plant cell adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef]
- Ashworth, E.N.; Abeles, F.B. Freezing behavior of water in small pores and the possible role in the freezing of plant tissues. Plant Physiol. 1984, 76, 201–204. [Google Scholar] [CrossRef]
- Li, H.; Wang, H. Chinese Wine; NWSUAF Press: Yangling, China, 2010. [Google Scholar]
- Hewer, M.J.; Gough, W.A. Climate change impact assessment on grape growth and wine production in the Okanagan Valley (Canada). Clim. Risk Manag. 2021, 33, 17. [Google Scholar] [CrossRef]
- Schrader, J.A.; Cochran, D.R.; Domoto, P.A.; Nonnecke, G.R. Phenology and winter hardiness of cold-climate grape cultivars and advanced selections in iowa climate. HortTechnology 2019, 29, 906–922. [Google Scholar] [CrossRef]
- Lisek, J.; Lisek, A. Cold Hardiness of Primary Buds of Wine and Table Grape Cultivars in Poland. S. Afr. J. Enol. Vitic. 2020, 41, 189–196. [Google Scholar] [CrossRef]
- Nenko, N.; Ilyina, I.A.; Kiseleva, G.K.; Shalyaho, T. Study of grape resistance to stressors of the winter period in the south of Russia. Agric. Food 2016, 4, 260–266. [Google Scholar]
- Li, H.; Zhang, Z.W. Minor resistant genes’ accumulation by replacement in the grape-powdery mildew pathosystem. Acta Bot. Boreali-Occident. Sin. 1995, 15, 120–124. [Google Scholar]
- Wang, Z.L.; Xue, T.T.; Gao, F.F.; Zhang, L.; Han, X.; Wang, Y.; Hui, M.; Wu, D.; Li, H.; Wang, H. Intraspecific recurrent selection in V. vinifera: An effective method for breeding of high quality, disease-, cold-, and drought-resistant grapes. Euphytica 2021, 217, 111. [Google Scholar] [CrossRef]
- Han, X.; Yao, F.; Xue, T.T.; Wang, Z.L.; Wang, Y.; Cao, X.; Hui, M.; Wu, D.; Li, Y.H.; Wang, H.; et al. Sprayed biodegradable liquid film improved the freezing tolerance of cv. Cabernet Sauvignon by up-regulating soluble protein and carbohydrate levels and alleviating oxidative damage. Front. Plant Sci. 2022, 13, 1021483. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Zhu, W.; Wang, L.; Xiang, Y.; Fang, L.; Li, J.; Sun, X.; Wang, N.; Londo, J.P.; Li, S. Genome wide transcriptional profile analysis of Vitis amurensis and Vitis vinifera in response to cold stress. PLoS ONE 2013, 8, e58740. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, B.; Ding, L.; Li, P.Y.; Shen, L.; Zhang, J.X. Physiological Change and Transcriptome Analysis of Chinese Wild Vitis amurensis and Vitis vinifera in Response to Cold Stress. Plant Mol. Biol. Report. 2020, 38, 478–490. [Google Scholar] [CrossRef]
- Pucker, B.; Schwandner, A.; Becker, S.; Hausmann, L.; Viehöver, P.; Töpfer, R.; Weisshaar, B.; Holtgräwe, D. RNA-Seq Time Series of Vitis vinifera Bud Development Reveals Correlation of Expression Patterns with the Local Temperature Profile. Plants 2020, 9, 1548. [Google Scholar] [CrossRef]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera). Hortic. Res. 2018, 5, 10. [Google Scholar] [CrossRef]
- Sawicki, M.; Rondeau, M.; Courteaux, B.; Rabenoelina, F.; Guerriero, G.; Gomès, E.; Soubigou-Taconnat, L.; Balzergue, S.; Clément, C.; Ait Barka, E.; et al. On a Cold Night: Transcriptomics of Grapevine Flower Unveils Signal Transduction and Impacted Metabolism. Int. J. Mol. Sci. 2019, 20, 1130. [Google Scholar] [CrossRef]
- Jiang, L.B. Physiological Changes and Gene Expression Pattern in Response to Low Temperature Stress in Prunus mume; Beijing Forestry University: Beijing, China, 2020. [Google Scholar]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Zhu, X.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.P. CML8, an Arabidopsis Calmodulin-Like Protein, Plays a Role in Pseudomonas syringae Plant Immunity. Plant Cell Physiol. 2017, 58, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C. Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 1997, 103, 753–765. [Google Scholar] [CrossRef]
- Goyal, R.K.; Fatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-Related Protein 1b1 (PR1b1) Is a Major Tomato Fruit Protein Responsive to Chilling Temperature and Upregulated in High Polyamine Transgenic Genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef] [PubMed]
- Hon, W.C.; Griffith, M.; Mlynarz, A.; Kwok, Y.C.; Yang, D.S. Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol. 1995, 109, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Yin, L.; Qu, J.; Lu, J. Linkage of cold acclimation and disease resistance through plant-pathogen interaction pathway in Vitis amurensis grapevine. Funct. Integr. Genom. 2014, 14, 741–755. [Google Scholar] [CrossRef]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S.; et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Liu, L.J.; Feng, M.F.; Wang, J.H.; Cang, J.; Li, S.; Bao, Y.Z.; Wang, X.T. Research progress of sugar metabolism of plants under cold stress. J. Northeast Agric. Univ. 2015, 46, 95–102. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Bilska-Kos, A.; Solecka, D.; Dziewulska, A.; Ochodzki, P.; Jończyk, M.; Bilski, H.; Sowiński, P. Low temperature caused modifications in the arrangement of cell wall pectins due to changes of osmotic potential of cells of maize leaves (Zea mays L.). Protoplasma 2017, 254, 713–724. [Google Scholar] [CrossRef]
- An, H.J.; Lurie, S.; Greve, L.C.; Rosenquist, D.; Kirmiz, C.; Labavitch, J.M.; Lebrilla, C.B. Determination of pathogen-related enzyme action by mass spectrometry analysis of pectin breakdown products of plant cell walls. Anal. Biochem. 2005, 338, 71–82. [Google Scholar] [CrossRef]
- Kohorn, B.D.; Kohorn, S.L. The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Shahryar, N.; Maali-Amiri, R. Metabolic acclimation of tetraploid and hexaploid wheats by cold stress-induced carbohydrate accumulation. J. Plant Physiol. 2016, 204, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, E.; Van den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Liu, T.; Fang, H.; Liu, J.; Reid, S.; Hou, J.; Zhou, T.; Tian, Z.; Song, B.; Xie, C. Cytosolic glyceraldehyde-3-phosphate dehydrogenases play crucial roles in controlling cold-induced sweetening and apical dominance of potato (Solanum tuberosum L.) tubers. Plant Cell Environ. 2017, 40, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Patyi, G.; Takács, Z.; Szekeres, A.; Bódi, N.; Bagyánszki, M.; Tari, I. Salicylic acid-induced ROS production by mitochondrial electron transport chain depends on the activity of mitochondrial hexokinases in tomato (Solanum lycopersicum L.). J. Plant Res. 2019, 132, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Li, B.; Nakayama, T.; Kawamura, Y.; Uemura, M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Joubès, J.; Raffaele, S.; Bourdenx, B.; Garcia, C.; Laroche-Traineau, J.; Moreau, P.; Domergue, F.; Lessire, R. The VLCFA elongase gene family in Arabidopsis thaliana: Phylogenetic analysis, 3D modelling and expression profiling. Plant Mol. Biol. 2008, 67, 547–566. [Google Scholar] [CrossRef]
- Yi, T.; Zhang, Z.S.; Tang, B.Q.; Xie, L.L.; Zou, X.X. Identification and Expression Analysis of the KCS Gene Family in Pepper. Acta Hortic. Sin. 2020, 47, 370–380. [Google Scholar] [CrossRef]
- Sakamoto, T.; Murata, N. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 2002, 5, 208–210. [Google Scholar] [CrossRef]
- Lindqvist, Y.; Huang, W.; Schneider, G.; Shanklin, J. Crystal structure of delta9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins. EMBO J. 1996, 15, 4081–4092. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, M.; Hu, J.; Li, J. Modification of the fatty acid composition in Arabidopsis and maize seeds using a stearoyl-acyl carrier protein desaturase-1 (ZmSAD1) gene. BMC Plant Biol. 2016, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef]
- Bate, N.J.; Rothstein, S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. Cell Mol. Biol. 1998, 16, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Guo, L.; Xu, Q.; Zhao, S.; Li, F.; Yan, X.; Liu, S.; Wei, C. Characterization and Alternative Splicing Profiles of the Lipoxygenase Gene Family in Tea Plant (Camellia sinensis). Plant Cell Physiol. 2018, 59, 1765–1781. [Google Scholar] [CrossRef]
- López, M.A.; Vicente, J.; Kulasekaran, S.; Vellosillo, T.; Martínez, M.; Irigoyen, M.L.; Cascón, T.; Bannenberg, G.; Hamberg, M.; Castresana, C. Antagonistic role of 9-lipoxygenase-derived oxylipins and ethylene in the control of oxidative stress, lipid peroxidation and plant defence. Plant J. Cell Mol. Biol. 2011, 67, 447–458. [Google Scholar] [CrossRef]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Schulz, E.; Tohge, T.; Zuther, E.; Fernie, A.R.; Hincha, D.K. Natural variation in flavonol and anthocyanin metabolism during cold acclimation in Arabidopsis thaliana accessions. Plant Cell Environ. 2015, 38, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef]
- Kubra, G.; Khan, M.; Munir, F.; Gul, A.; Shah, T.; Hussain, A.; Caparrós-Ruiz, D.; Amir, R. Expression Characterization of Flavonoid Biosynthetic Pathway Genes and Transcription Factors in Peanut Under Water Deficit Conditions. Front. Plant Sci. 2021, 12, 680368. [Google Scholar] [CrossRef]
- Li, X.; Kim, Y.B.; Kim, Y.; Zhao, S.; Kim, H.H.; Chung, E.; Lee, J.H.; Park, S.U. Differential stress-response expression of two flavonol synthase genes and accumulation of flavonols in tartary buckwheat. J. Plant Physiol. 2013, 170, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A. The role of amphiphiles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 527–533. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Pampel, A.; Nissler, L.; Gebhardt, R.; Huster, D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim. Biophys. Acta 2004, 1663, 97–107. [Google Scholar] [CrossRef]
- Hannah, M.A.; Heyer, A.G.; Hincha, D.K. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 2005, 1, e26. [Google Scholar] [CrossRef]
- Hannah, M.A.; Wiese, D.; Freund, S.; Fiehn, O.; Heyer, A.G.; Hincha, D.K. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol. 2006, 142, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.; Peterek, S.; Mock, H.P.; Heyer, A.G.; Hincha, D.K. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant Cell Environ. 2008, 31, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Agbayani, R.; Jackson, M.C.; Tang, C.S.; Moore, P.H. Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta 2004, 220, 241–250. [Google Scholar] [CrossRef]
- Yu, C.K.; Springob, K.; Schmidt, J.; Nicholson, R.L.; Chu, I.K.; Yip, W.K.; Lo, C. A stilbene synthase gene (SbSTS1) is involved in host and nonhost defense responses in sorghum. Plant Physiol. 2005, 138, 393–401. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Ding, J.; Hua, Z.; Wang, Y. Characterization of a novel stilbene synthase promoter involved in pathogen- and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta 2010, 231, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.11–11.10.33. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||
|---|---|---|---|---|
| Classification | Pathway ID | Pathway | Genes | Description |
| Signal Transduction | ko04626 | Plant–pathogen interaction | VIT_07s0141g00060 (KCS2) | KCS; 3-ketoacyl-CoA synthase [EC:2.3.1.199] |
| ko04712 | Circadian rhythm—plant | VIT_16s0100g01140 (CHS-1) | CHS; chalcone synthase [EC:2.3.1.74] | |
| VIT_16s0100g00990 (CHS-2) | CHS; chalcone synthase [EC:2.3.1.74] | |||
| Lipid metabolism | ko00591 | Linoleic acid metabolism | VIT_06s0004g01480 (LOX2.1) | LOX2S; lipoxygenase [EC:1.13.11.12] |
| ko00592 | Alpha-linolenic acid metabolism | VIT_06s0004g01480 (LOX2.1) | LOX2S; lipoxygenase [EC:1.13.11.12] | |
| ko00062 | Fatty acid elongation | VIT_07s0141g00060 (KCS2) | KCS; 3-ketoacyl-CoA synthase [EC:2.3.1.199] | |
| Carbohydrate metabolism | ko00040 | Pentose and glucuronate interconversions | VIT_08s0007g07760 (PG2) | PG; polygalacturonase [EC:3.2.1.15] |
| VIT_08s0007g07750 (PG2) | PG; polygalacturonase [EC:3.2.1.15] | |||
| ko00520 | Amino sugar and nucleotide sugar metabolism | VIT_05s0094g00360 (EP3) | chitinase [EC:3.2.1.14] | |
| Amino acid metabolism | ko01230 | Biosynthesis of amino acids | VIT_08s0007g05000 (METK3) | metK; S-adenosylmethionine synthetase [EC:2.5.1.6] |
| ko00270 | Cysteine and methionine metabolism | VIT_08s0007g05000 (METK3) | metK; S-adenosylmethionine synthetase [EC:2.5.1.6] | |
| Biosynthesis of secondary metabolites | ko00941 | Flavonoid biosynthesis | VIT_16s0100g01140 (CHS-1) | CHS; chalcone synthase [EC:2.3.1.74] |
| VIT_16s0100g00990 (CHS-2) | CHS; chalcone synthase [EC:2.3.1.74] | |||
| ko00945 | Stilbenoid, diarylheptanoid and gingerol biosynthesis | VIT_16s0100g00900 (STS) | ST; stilbene synthase [EC:2.3.1.95] | |
| ko00909 | Sesquiterpenoid and triterpenoid biosynthesis | VIT_09s0054g01220 (OSCBPY-1) | LUP4; beta-amyrin synthase [EC:5.4.99.39] | |
| (B) | ||||
| Classification | K_ID | Pathway | Gene | Description |
| Signal Transduction | ko04626 | Plant–pathogen interaction | VIT_03s0088g00690 (PR1B1) | PR1; pathogenesis-related protein 1 |
| VIT_00s0179g00280 (CML8) | CALM; calmodulin | |||
| ko04075 | Plant hormone signal transduction | VIT_03s0088g00690 (PR1B1) | PR1; pathogenesis-related protein 1 | |
| VIT_19s0014g04690 (GH3.6) | GH3; auxin responsive GH3 gene family | |||
| ko04016 | MAPK signaling pathway-plant | VIT_03s0088g00690 (PR1B1) | PR1; pathogenesis-related protein 1 | |
| VIT_00s0179g00280 (CML8) | CALM; calmodulin | |||
| ko04070 | Phosphatidylinos-itol signaling system | VIT_00s0179g00280 (CML8) | CALM; calmodulin | |
| ko04712 | Circadian rhythm—plant | VIT_16s0100g01150 (CHS-3) | CHS; chalcone synthase [EC:2.3.1.74] | |
| Lipid metabolism | ko01212 | Fatty acid metabolism | VIT_18s0001g15460 (SAD6) | FAB2, SSI2, desA1; acyl-[acyl-carrier-protein] desaturase [EC:1.14.19.2 1.14.19.11 1.14.19.26] |
| ko00061 | Fatty acid biosynthesis | VIT_18s0001g15460 (SAD6) | FAB2, SSI2, desA1; acyl-[acyl-carrier-protein] desaturase [EC:1.14.19.2 1.14.19.11 1.14.19.26] | |
| ko01040 | Biosynthesis of unsaturated fatty acids | VIT_18s0001g15460 (SAD6) | FAB2, SSI2, desA1; acyl-[acyl-carrier-protein] desaturase [EC:1.14.19.2 1.14.19.11 1.14.19.26] | |
| ko00592 | alpha-Linolenic acid metabolism | VIT_18s0041g02060 (OPR2) | OPR; 12-oxophytodienoic acid reductase [EC:1.3.1.42] | |
| VIT_18s0041g02010 (OPR11) | OPR; 12-oxophytodienoic acid reductase [EC:1.3.1.42] | |||
| Carbohydrate metabolism | ko01200 | Carbon metabolism | VIT_01s0010g02460 (GAPC2) | GAPDH, gapA; glyceraldehyde 3-phosphate dehydrogenase [EC:1.2.1.12] |
| VIT_08s0058g01000 (ASP1) | GOT2; aspartate aminotransferase, mitochondrial [EC:2.6.1.1] | |||
| ko00010 | Glycolysis/Gluconeogenesis | VIT_01s0010g02460 (GAPC2) | GAPDH, gapA; glyceraldehyde 3-phosphate dehydrogenase [EC:1.2.1.12] | |
| ko00710 | Carbon fixation in photosynthetic organisms | VIT_01s0010g02460 (GAPC2) | GAPDH, gapA; glyceraldehyde 3-phosphate dehydrogenase [EC:1.2.1.12] | |
| ko00500 | Starch and sucrose metabolism | VIT_06s0004g01430 (BGLU12) | BG; beta-glucosidase [EC:3.2.1.21] | |
| VIT_16s0022g00670 (INV*DC4) | INV, sacA; beta-fructofuranosidase [EC:3.2.1.26] | |||
| ko00040 | Pentose and glucuronate interconversions | VIT_08s0007g07760 (PG2) | PG; polygalacturonase [EC:3.2.1.15] | |
| VIT_15s0048g00510 (PECS-2.1) | PE; pectinesterase [EC:3.1.1.11] | |||
| ko00052 | Galactose metabolism | VIT_16s0022g00670 (INV*DC4) | INV, sacA; beta-fructofuranosidase [EC:3.2.1.26] | |
| Amino acid metabolism | ko01230 | Biosynthesis of amino acids | VIT_01s0010g02460 (GAPC2) | GAPDH, gapA; glyceraldehyde 3-phosphate dehydrogenase [EC:1.2.1.12] |
| VIT_06s0004g01430 (BGLU12) | BG; beta-glucosidase [EC:3.2.1.21] | |||
| VIT_08s0058g01000 (ASP1) | GOT2; aspartate aminotransferase, mitochondrial [EC:2.6.1.1] | |||
| ko00460 | Cyanoamino acid metabolism | VIT_02s0033g00670 (NIT4B) | NIT4; beta-cyano-L-alanine hydratase/nitrilase [EC:3.5.5.4 4.2.1.65] | |
| VIT_02s0033g00770 (NIT4B) | NIT4; beta-cyano-L-alanine hydratase/nitrilase [EC:3.5.5.4 4.2.1.65] | |||
| ko00270 | Cysteine and methionine metabolism | VIT_08s0058g01000 (ASP1) | GOT2; aspartate aminotransferase, mitochondrial [EC:2.6.1.1] | |
| Biosynthesis of secondary metabolites | ko00940 | Phenylpropanoid biosynthesis | VIT_06s0004g01430 (BGLU12) | BG; beta-glucosidase [EC:3.2.1.21] |
| VIT_18s0001g06850 (PNC1) | POD; peroxidase [EC:1.11.1.7] | |||
| VIT_10s0003g05420 (MEE23) | K22395; cinnamyl-alcohol dehydrogenase [EC:1.1.1.195] | |||
| ko01210 | 2-Oxocarboxylic acid metabolism | VIT_08s0058g01000 (ASP1) | GOT2; aspartate aminotransferase, mitochondrial [EC:2.6.1.1] | |
| ko00941 | Flavonoid biosynthesis | VIT_16s0100g01150 (CHS-3) | CHS; chalcone synthase [EC:2.3.1.74] | |
| ko00909 | Sesquiterpenoid and triterpenoid biosynthesis | VIT_10s0003g03650 (OSCBPY-2) | LUP4; beta-amyrin synthase [EC:5.4.99.39] | |
| Stress Temperature/°C | Stress Time/h | Number |
|---|---|---|
| −10 | 3 | CK-1-1 |
| 6 | CK-1-2 | |
| 12 | CK-1-3 | |
| −16 | 3 | CK-2-1 |
| 6 | CK-2-2 | |
| 12 | CK-2-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Li, Y.-H.; Yao, M.-H.; Yao, F.; Wang, Z.-L.; Wang, H.; Li, H. Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine. Int. J. Mol. Sci. 2023, 24, 3884. https://doi.org/10.3390/ijms24043884
Han X, Li Y-H, Yao M-H, Yao F, Wang Z-L, Wang H, Li H. Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine. International Journal of Molecular Sciences. 2023; 24(4):3884. https://doi.org/10.3390/ijms24043884
Chicago/Turabian StyleHan, Xing, Yi-Han Li, Mo-Han Yao, Fei Yao, Zhi-Lei Wang, Hua Wang, and Hua Li. 2023. "Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine" International Journal of Molecular Sciences 24, no. 4: 3884. https://doi.org/10.3390/ijms24043884
APA StyleHan, X., Li, Y.-H., Yao, M.-H., Yao, F., Wang, Z.-L., Wang, H., & Li, H. (2023). Transcriptomics Reveals the Effect of Short-Term Freezing on the Signal Transduction and Metabolism of Grapevine. International Journal of Molecular Sciences, 24(4), 3884. https://doi.org/10.3390/ijms24043884





