Human Omentin-1 Administration Ameliorates Hypertensive Complications without Affecting Hypertension in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Results
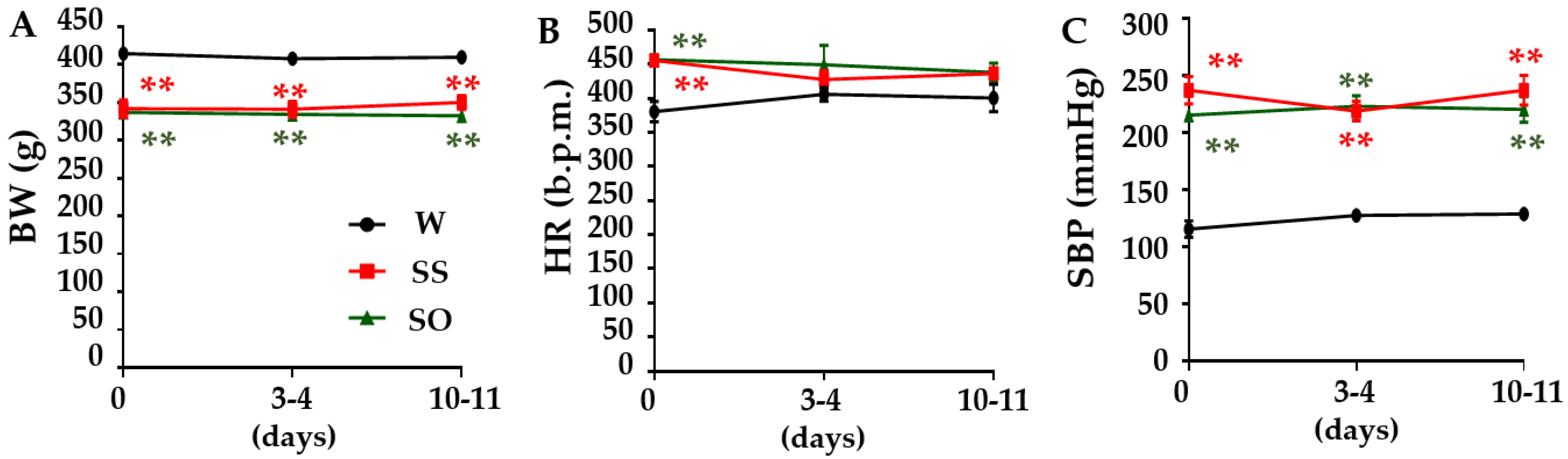
2.1. Human Omentin-1 Had No Effect on Body Weight (BW), Heart Rate (HR), and Systolic Blood Pressure (SBP) in SHR
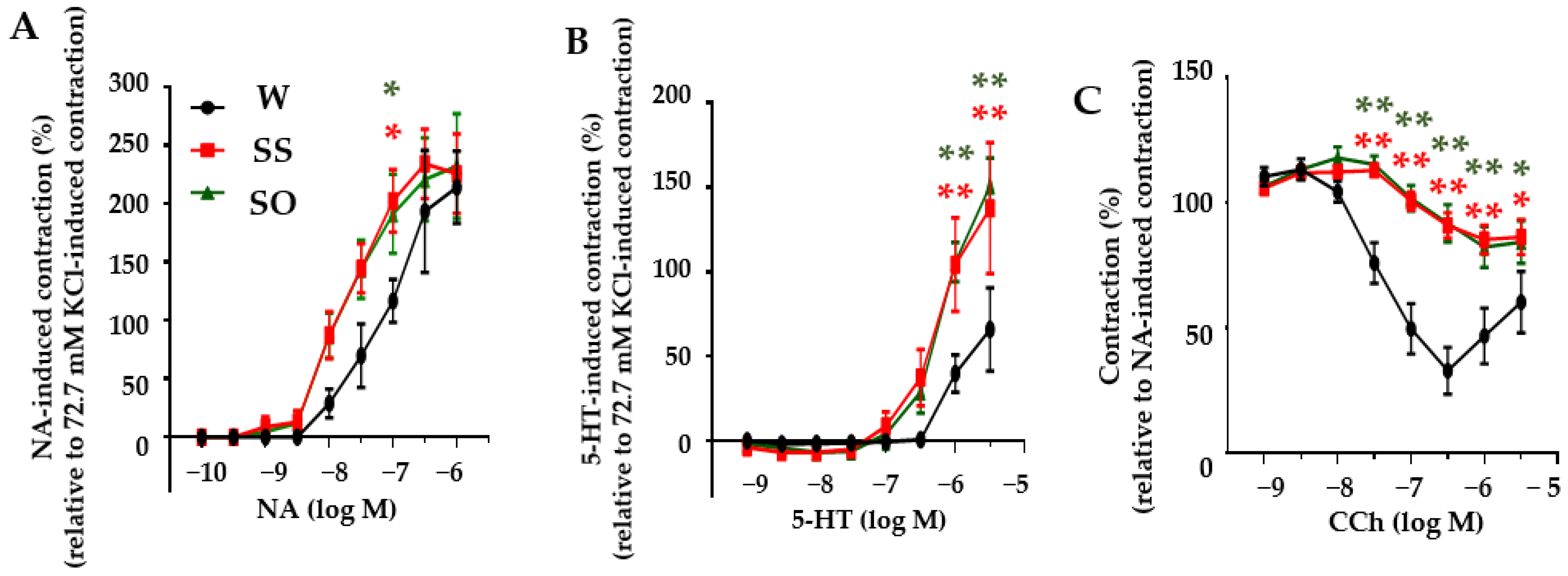
2.2. Human Omentin-1 Had No Effect on Agonist-Induced Contraction and Relaxation in Isolated Thoracic Aorta
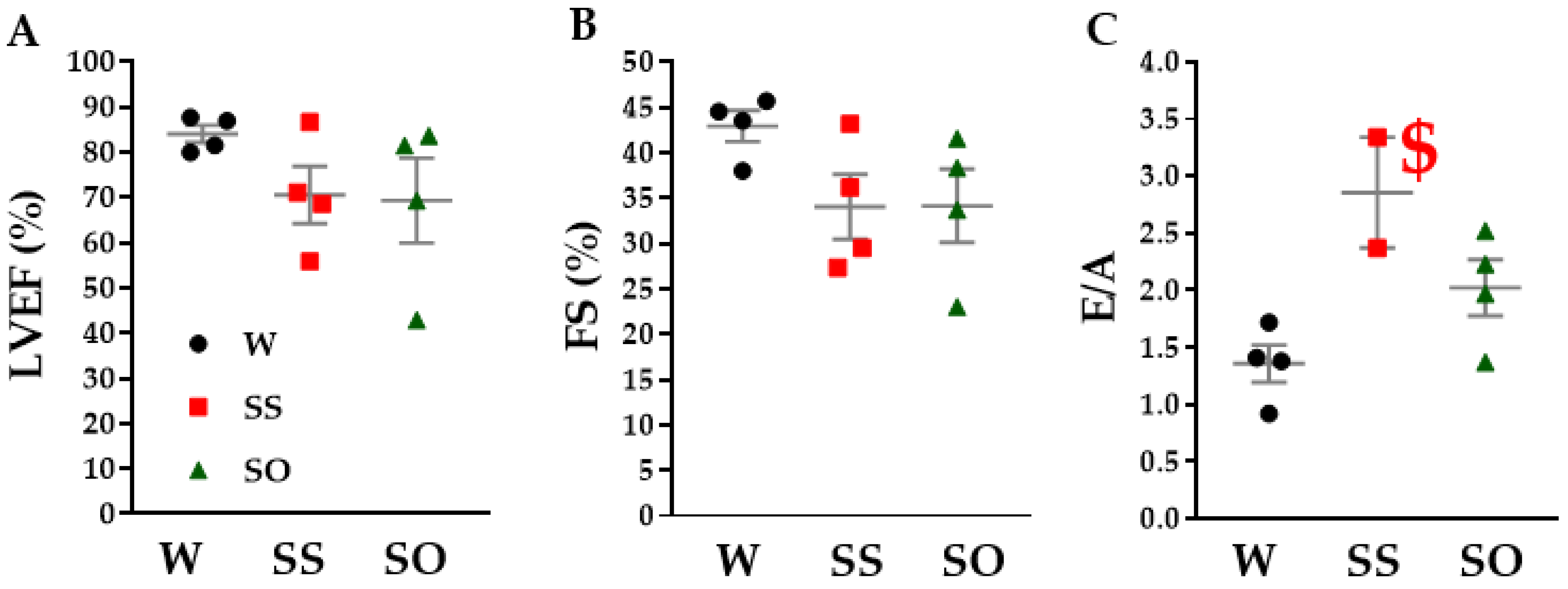
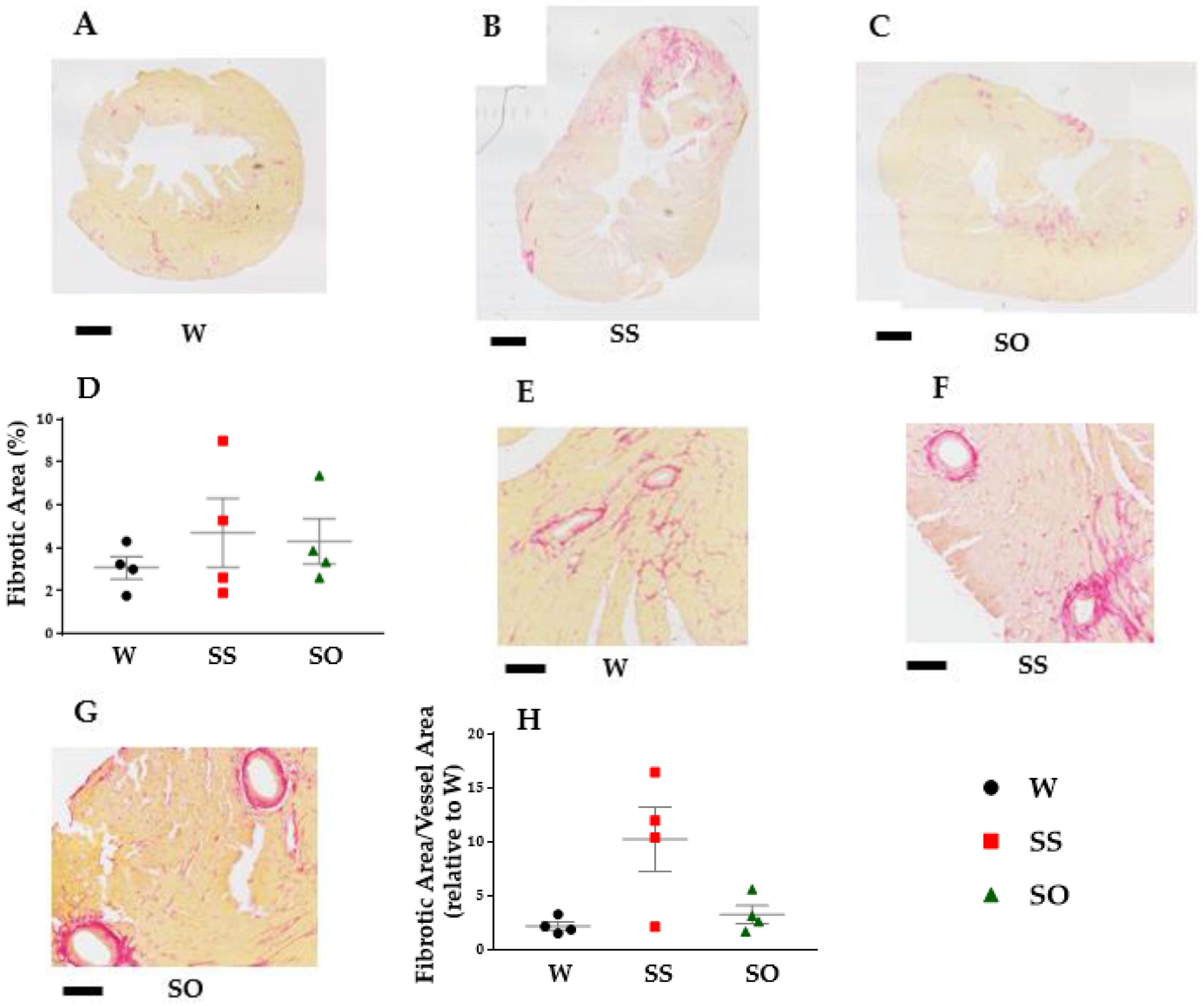
2.3. Human Omentin-1 Tended to Improve Heart Failure in SHR
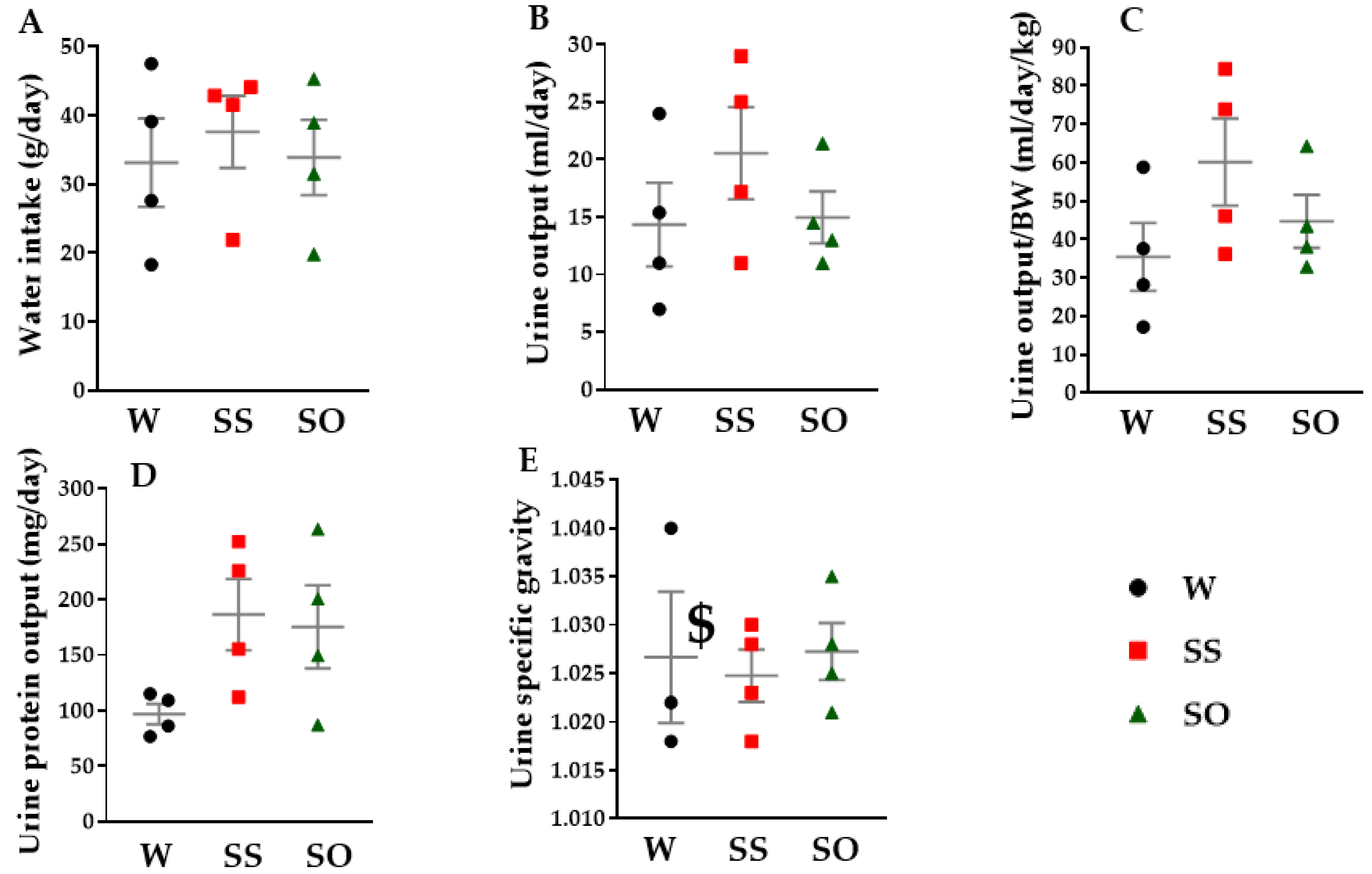
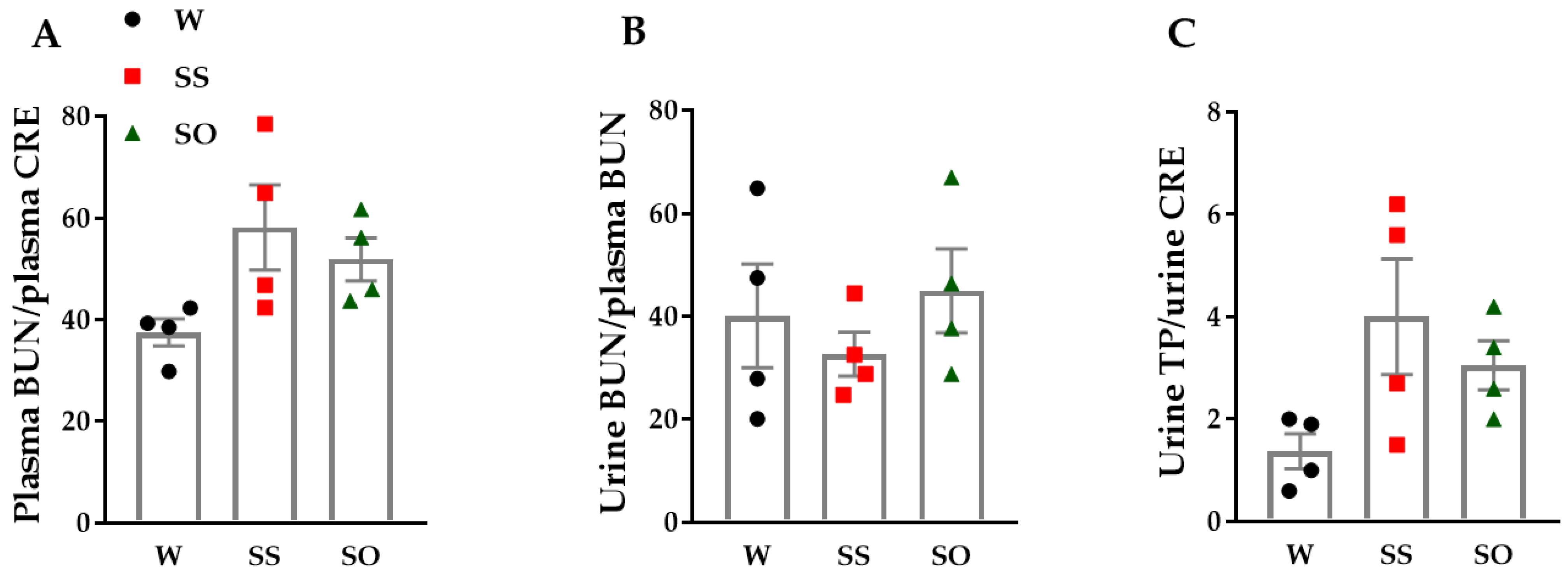
2.4. Human Omentin-1 Tended to Improve Renal Dysfunction in SHR
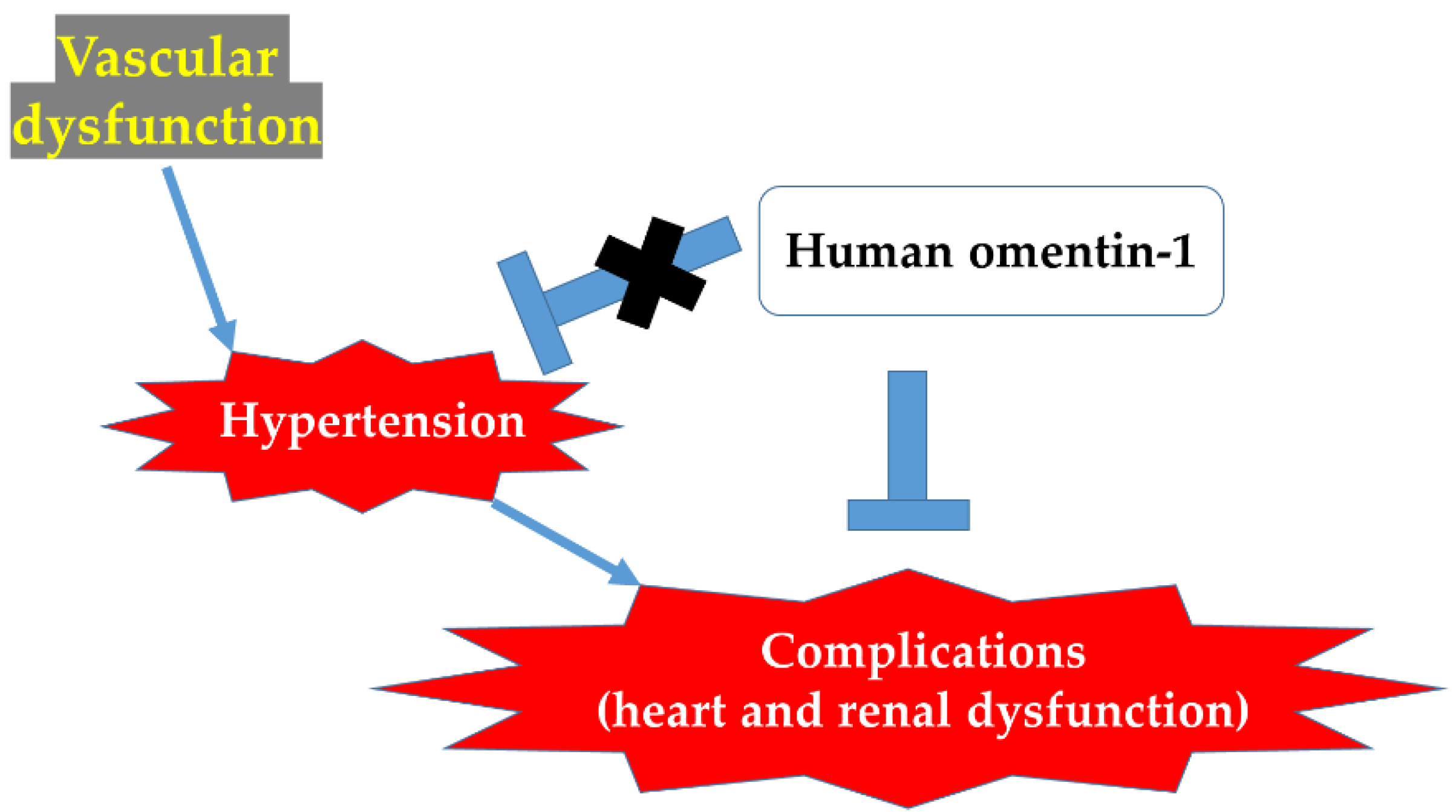
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Measurement of BW, HR, and SBP
4.4. Measurement of Isometric Contraction
4.5. Measurement of Heart Weight
4.6. Echocardiographic Examination
4.7. Picrosirius Red (PSR) Staining
4.8. Measurement of Water Intake, Urine Output, and Urine Specific Gravity
4.9. Blood and Urine Chemical Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytriptamine |
| b.p.m. | Beats per minutes |
| BUN | Blood urea nitrogen |
| BW | Body weight |
| CCh | Carbachol |
| CRE | Creatinine |
| DBP | Diastolic blood pressure |
| E/A | the ratio between velocities of early diastole (E) and late diastole (A) inflow across mitral valves |
| FS | fractional shortening |
| HR | Heart rate |
| i.p. | Intraperitoneal |
| LA | Left atrium |
| LV | Left ventricle |
| LVDd | Left ventricular end-diastolic diameter |
| LVDs | Left ventricular end-systolic diameter |
| LVEDV | Left ventricular end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVESV | Left ventricular end-systolic volume |
| NA | Noradrenaline |
| NO | Nitric oxide |
| PSR | Picrosirius red |
| PSS | Physiological salt solution |
| RA | Right atrium |
| RV | Right ventricle |
| SBP | Systolic blood pressure |
| S.E.M. | Standard error of the mean |
| TGF | Transforming growth factor |
| TL | Tail length |
| TP | Total protein |
References
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Shimizu, S.; Nagao, Y.; Kurabayashi, A.; Shimizu, T.; Higashi, Y.; Karashima, T.; Saito, M. Aging-related severe hypertension induces detrusor underactivity in rats. Life Sci. 2021, 283, 119855. [Google Scholar] [CrossRef]
- Yamawaki, H. Vascular Effects of Novel Adipocytokines: Focus on Vascular Contractility and Inflammatory Responses. Biol. Pharm. Bull. 2011, 34, 307–310. [Google Scholar] [CrossRef]
- Yamawaki, H. Mechanisms of action of novel adipocytokines on the vascular system. Folia Pharmacol. Jpn. 2011, 137, 131–135. [Google Scholar] [CrossRef]
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Çelik, M.; Nar, R.; Nar, G.; Sökmen, E.; Günver, G. Serum omentin-1 levels in hypertensive patients. J. Hum. Hypertens. 2020, 35, 290–295. [Google Scholar] [CrossRef]
- Dong, Q.; Xing, W.; Li, K.; Zhou, X.; Wang, S.; Zhang, H. Tetrahydroxystilbene glycoside improves endothelial dysfunction and hypertension in obese rats: The role of omentin-1. Biochem. Pharmacol. 2021, 186, 114489. [Google Scholar] [CrossRef]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010, 393, 668–672. [Google Scholar] [CrossRef]
- Kazama, K.; Okada, M.; Hara, Y.; Yamawaki, H. A Novel Adipocytokine, Omentin, Inhibits Agonists-Induced Increases of Blood Pressure in Rats. J. Veter-Med. Sci. 2013, 75, 1029–1034. [Google Scholar] [CrossRef]
- Kazama, K.; Okada, M.; Yamawaki, H. A novel adipocytokine, omentin, inhibits monocrotaline-induced pulmonary arterial hypertension in rats. Biochem. Biophys. Res. Commun. 2014, 452, 142–146. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Kataoka, Y.; Shibata, R.; Ohashi, K.; Kambara, T.; Enomoto, T.; Uemura, Y.; Ogura, Y.; Yuasa, D.; Matsuo, K.; Nagata, T.; et al. Omentin Prevents Myocardial Ischemic Injury Through AMP-Activated Protein Kinase- and Akt-Dependent Mechanisms. J. Am. Coll. Cardiol. 2014, 63, 2722–2733. [Google Scholar] [CrossRef]
- Lee, H.B.; Blaufox, M.D. Blood Volume in the Rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar]
- Kazama, K.; Okada, M.; Yamawaki, H. Adipocytokine, omentin inhibits doxorubicin-induced H9c2 cardiomyoblasts apoptosis through the inhibition of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2015, 457, 602–607. [Google Scholar] [CrossRef]
- Kazama, K.; Okada, M.; Yamawaki, H. A novel adipocytokine, omentin, inhibits platelet-derived growth factor-BB-induced vascular smooth muscle cell migration through antioxidative mechanism. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H1714–H1719. [Google Scholar] [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Reed, A.L.; Tanaka, A.; Sorescu, D.; Liu, H.; Jeong, E.-M.; Sturdy, M.; Walp, E.R.; Dudley, S.C.; Sutliff, R.L. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H824–H831. [Google Scholar] [CrossRef]
- Brooks, W.W.; Shen, S.; Conrad, C.H.; Goldstein, R.H.; Deng, L.L.; Bing, O.H. Transcriptional changes associated with recovery from heart failure in the SHR. J. Mol. Cell Cardiol. 2010, 49, 390–401. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Han, F.; Lv, L.; Tang, C.-E.; Xie, Z.; Luo, F. Omentin-1 is associated with atrial fibrillation in patients with cardiac valve disease. BMC Cardiovasc. Disord. 2020, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-H.; Chiou, K.-R. Diastolic Heart Failure Predicted by Left Atrial Expansion Index in Patients with Severe Diastolic Dysfunction. PLoS ONE 2016, 11, e0162599. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, H.; Sun, Y.; Guo, R.; Zhong, D.; Xu, R.; Song, M. Omentin-1 protects renal function of mice with type 2 diabetic nephropathy via regulating miR-27a-Nrf2/Keap1 axis. Biomed. Pharmacother. 2018, 107, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kutlay, Ö.; Kaygısız, Z.; Kaygısız, B. Effect of omentin on cardiovascular functions and gene expressions in isolated rat hearts. Anatol. J. Cardiol. 2019, 21, 91–97. [Google Scholar] [CrossRef]
- Maseda, J.; Hilberman, M.; Derby, G.C.; Spencer, R.J.; Stinson, E.B.; Myers, B.D. The Renal Effects of Sodium Nitroprusside in Postoperative Cardiac Surgical Patients. Anesthesiology 1981, 54, 284–288. [Google Scholar] [CrossRef]
- Okamura, Y.; Otani, K.; Sekiguchi, A.; Kogane, T.; Kakuda, C.; Sakamoto, Y.; Kodama, T.; Okada, M.; Yamawaki, H. Vasculo-protective effect of BMS-309403 is independent of its specific inhibition of fatty acid-binding protein 4. Pflüg. Arch. Eur. J. Physiol. 2017, 469, 1177–1188. [Google Scholar] [CrossRef]
- Otani, K.; Yokoya, M.; Kodama, T.; Hori, K.; Matsumoto, K.; Okada, M.; Yamawaki, H. Plasma exosomes regulate systemic blood pressure in rats. Biochem. Biophys. Res. Commun. 2018, 503, 776–783. [Google Scholar] [CrossRef]
- Yamamoto, A.; Otani, K.; Okada, M.; Yamawaki, H. Chemokine-like Receptor 1 in Brain of Spontaneously Hypertensive Rats Mediates Systemic Hypertension. Int. J. Mol. Sci. 2021, 22, 1812. [Google Scholar] [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef]
- Sugiyama, A.; Okada, M.; Yamawaki, H. Canstatin suppresses isoproterenol-induced cardiac hypertrophy through inhibition of calcineurin/nuclear factor of activated T-cells pathway in rats. Eur. J. Pharmacol. 2020, 871, 172849. [Google Scholar] [CrossRef]
- Sugiyama, A.; Hirano, Y.; Okada, M.; Yamawaki, H. Endostatin Stimulates Proliferation and Migration of Myofibroblasts Isolated from Myocardial Infarction Model Rats. Int. J. Mol. Sci. 2018, 19, 741. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kaisho, M.; Okada, M.; Otani, K.; Yamawaki, H. Decreased Expression of Canstatin in Rat Model of Monocrotaline-Induced Pulmonary Arterial Hypertension: Protective Effect of Canstatin on Right Ventricular Remodeling. Int. J. Mol. Sci. 2020, 21, 6797. [Google Scholar] [CrossRef]
| W (n = 4) | SS (n = 4) | SO (n = 4) | |
|---|---|---|---|
| Whole heart (g) | 1.301 ± 0.009 | 1.575 ± 0.050 * | 1.531 ± 0.082 |
| LV (g) | 0.967 ± 0.005 | 1.286 ± 0.061 ** | 1.238 ± 0.077 * |
| RV (g) | 0.256 ± 0.007 | 0.206 ± 0.011 ** | 0.220 ± 0.006 * |
| LA (g) | 0.034 ± 0.002 | 0.045 ± 0.005 | 0.037 ± 0.005 |
| RA (g) | 0.044 ± 0.002 | 0.039 ± 0.001 | 0.037 ± 0.002 |
| 100 × LV/TL | 4.955 ± 0.066 | 7.359 ± 0.349 ** | 7.333 ± 0.375 ** |
| TL (cm) | 19.5 ± 0.4 | 17.5 ± 0.2 * | 16.9 ± 0.6 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamura, Y.; Niijima, R.; Kameshima, S.; Kodama, T.; Otani, K.; Okada, M.; Yamawaki, H. Human Omentin-1 Administration Ameliorates Hypertensive Complications without Affecting Hypertension in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2023, 24, 3835. https://doi.org/10.3390/ijms24043835
Okamura Y, Niijima R, Kameshima S, Kodama T, Otani K, Okada M, Yamawaki H. Human Omentin-1 Administration Ameliorates Hypertensive Complications without Affecting Hypertension in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences. 2023; 24(4):3835. https://doi.org/10.3390/ijms24043835
Chicago/Turabian StyleOkamura, Yuta, Ryo Niijima, Satoshi Kameshima, Tomoko Kodama, Kosuke Otani, Muneyoshi Okada, and Hideyuki Yamawaki. 2023. "Human Omentin-1 Administration Ameliorates Hypertensive Complications without Affecting Hypertension in Spontaneously Hypertensive Rats" International Journal of Molecular Sciences 24, no. 4: 3835. https://doi.org/10.3390/ijms24043835
APA StyleOkamura, Y., Niijima, R., Kameshima, S., Kodama, T., Otani, K., Okada, M., & Yamawaki, H. (2023). Human Omentin-1 Administration Ameliorates Hypertensive Complications without Affecting Hypertension in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences, 24(4), 3835. https://doi.org/10.3390/ijms24043835






