Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats
Abstract
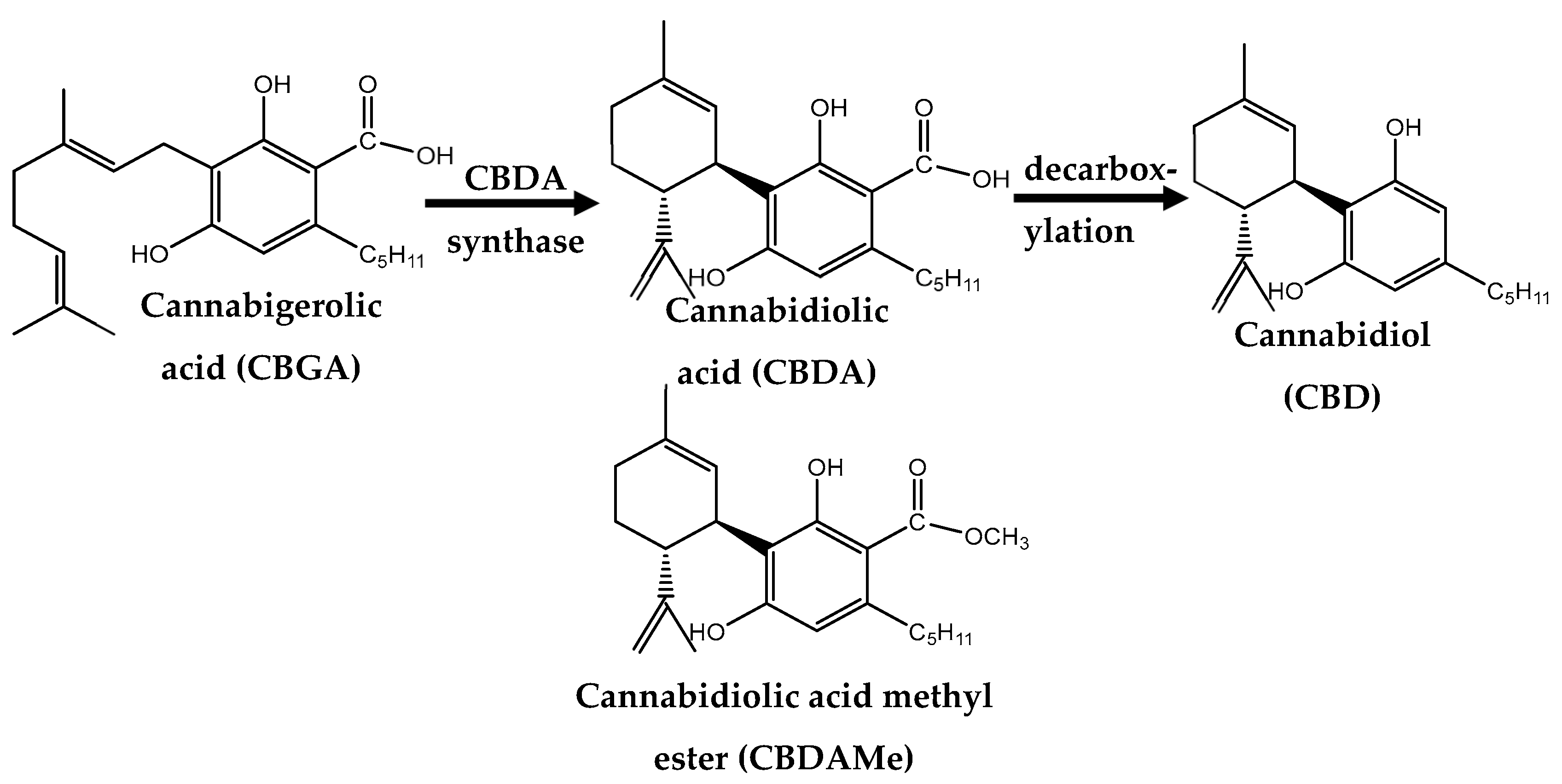
1. Introduction
2. Results
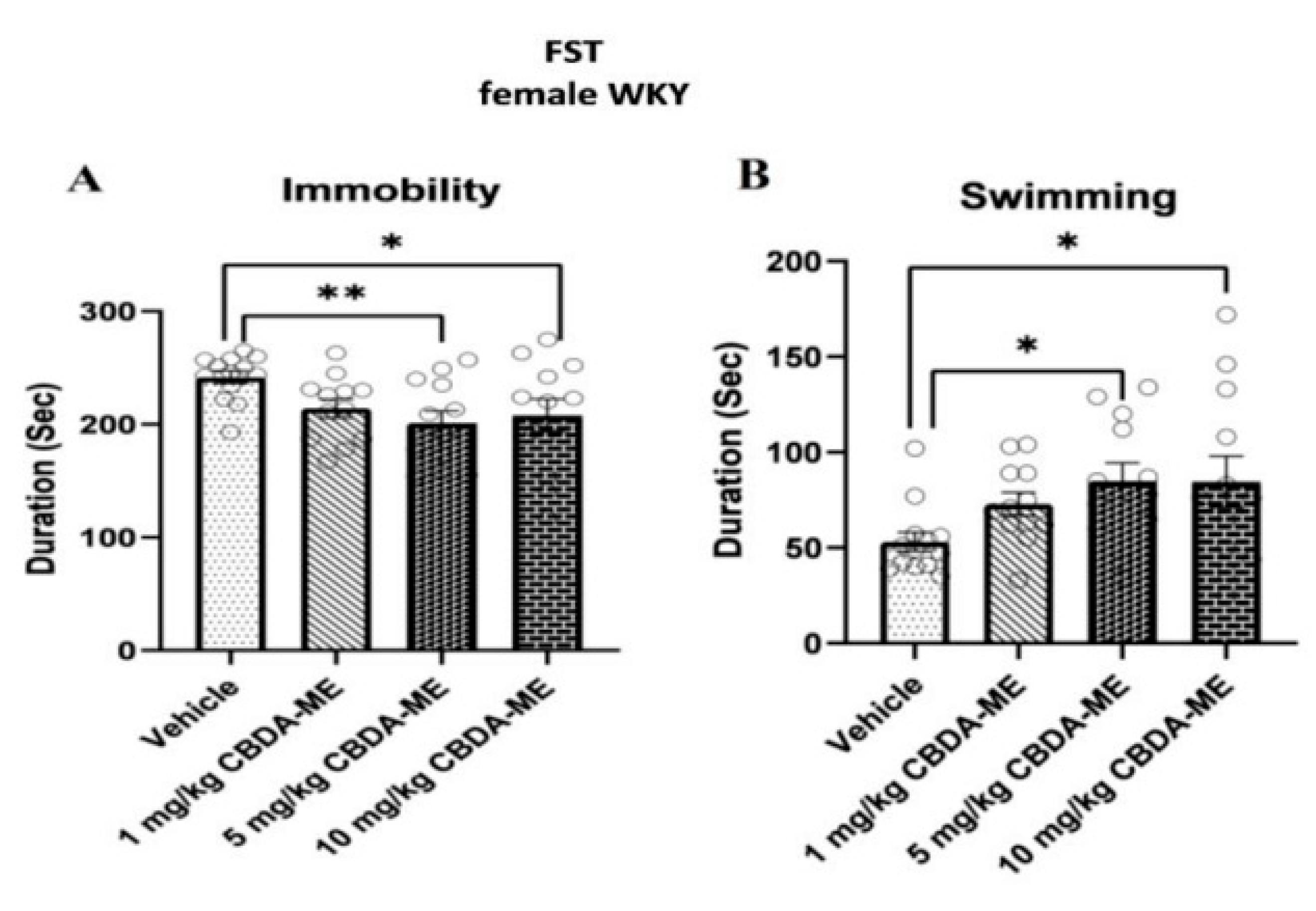
2.1. Experiment 1
- (a)
- FST in female WKY rats
- (b)
- Corticosterone levels in blood
- (c)
- Cytokine levels in blood
2.2. Experiment 2
- (a)
- FST in male WKY rats
- (b)
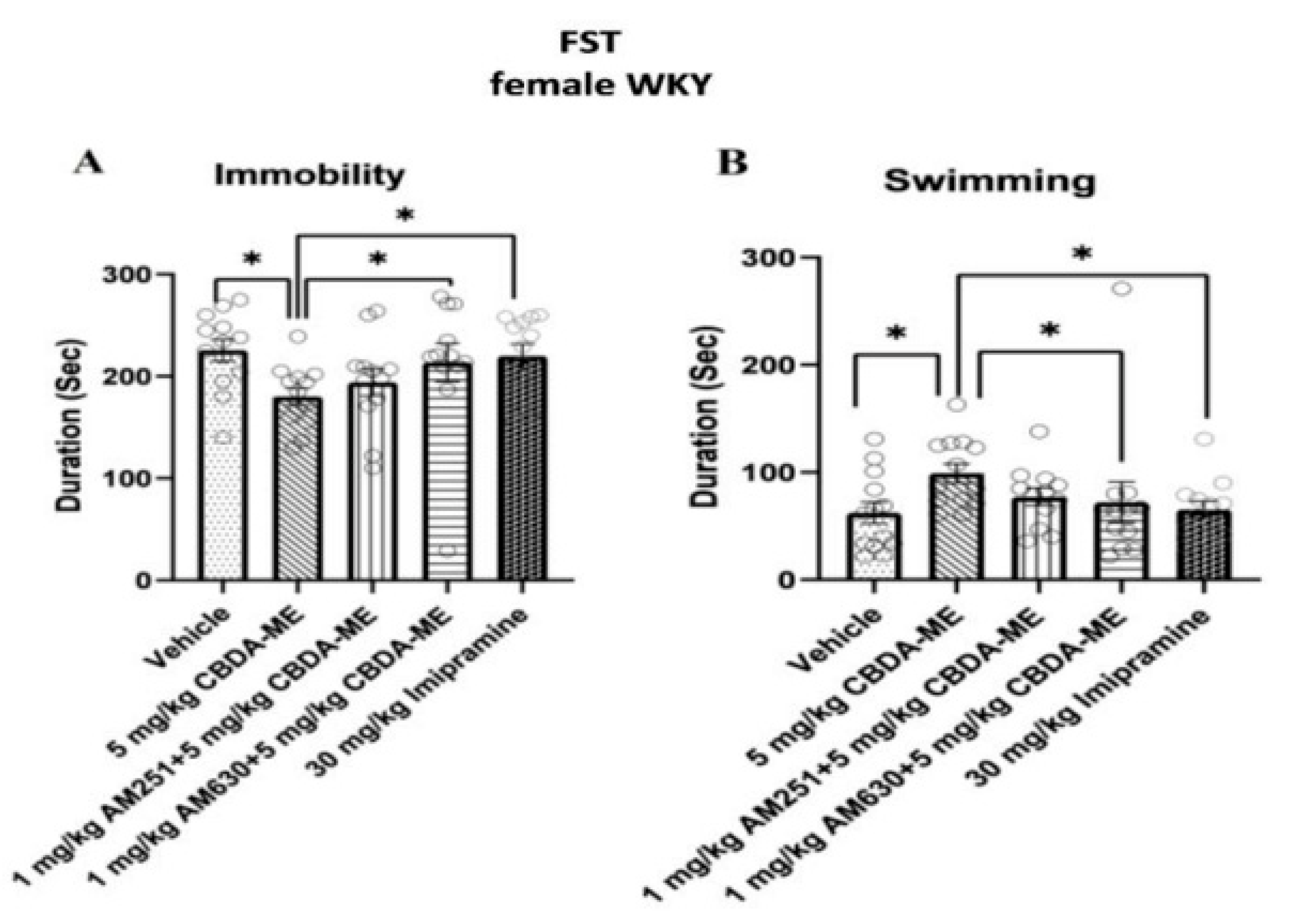
- FST-female WKY rats
- (c)
- Sex differences
- (d)
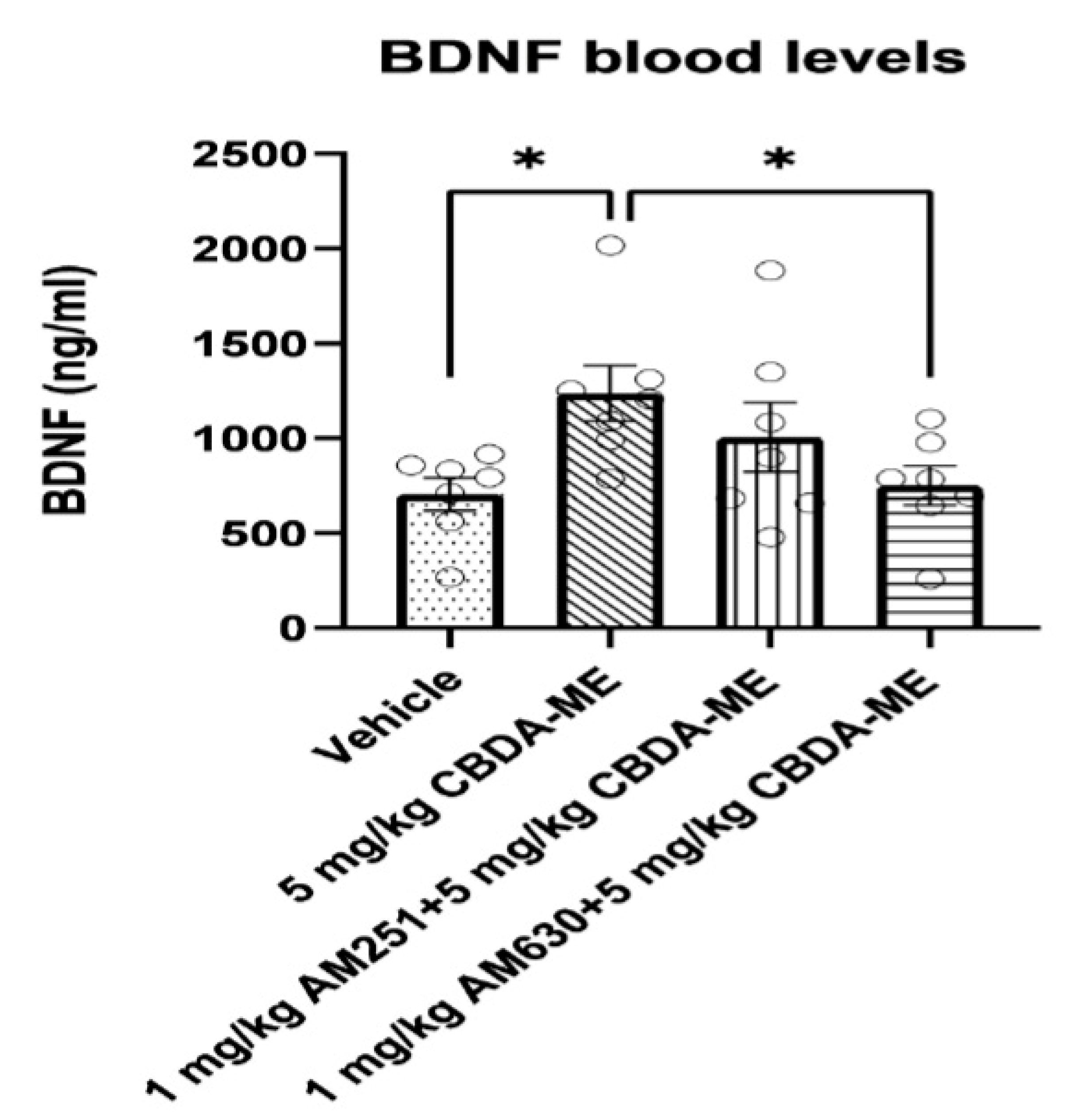
- BDNF measurement in blood
- (e)
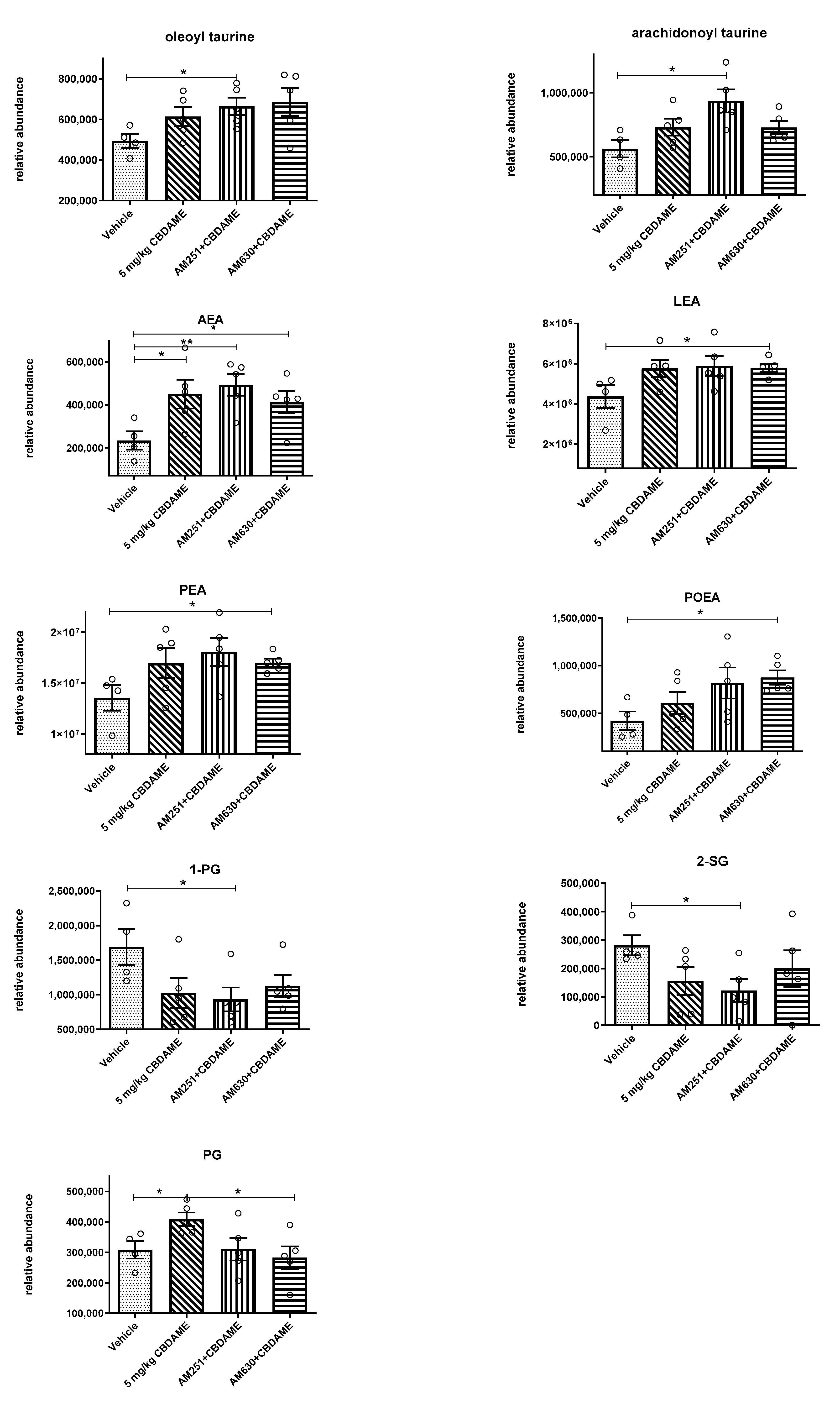
- Endocannabinoid levels in blood
- (f)
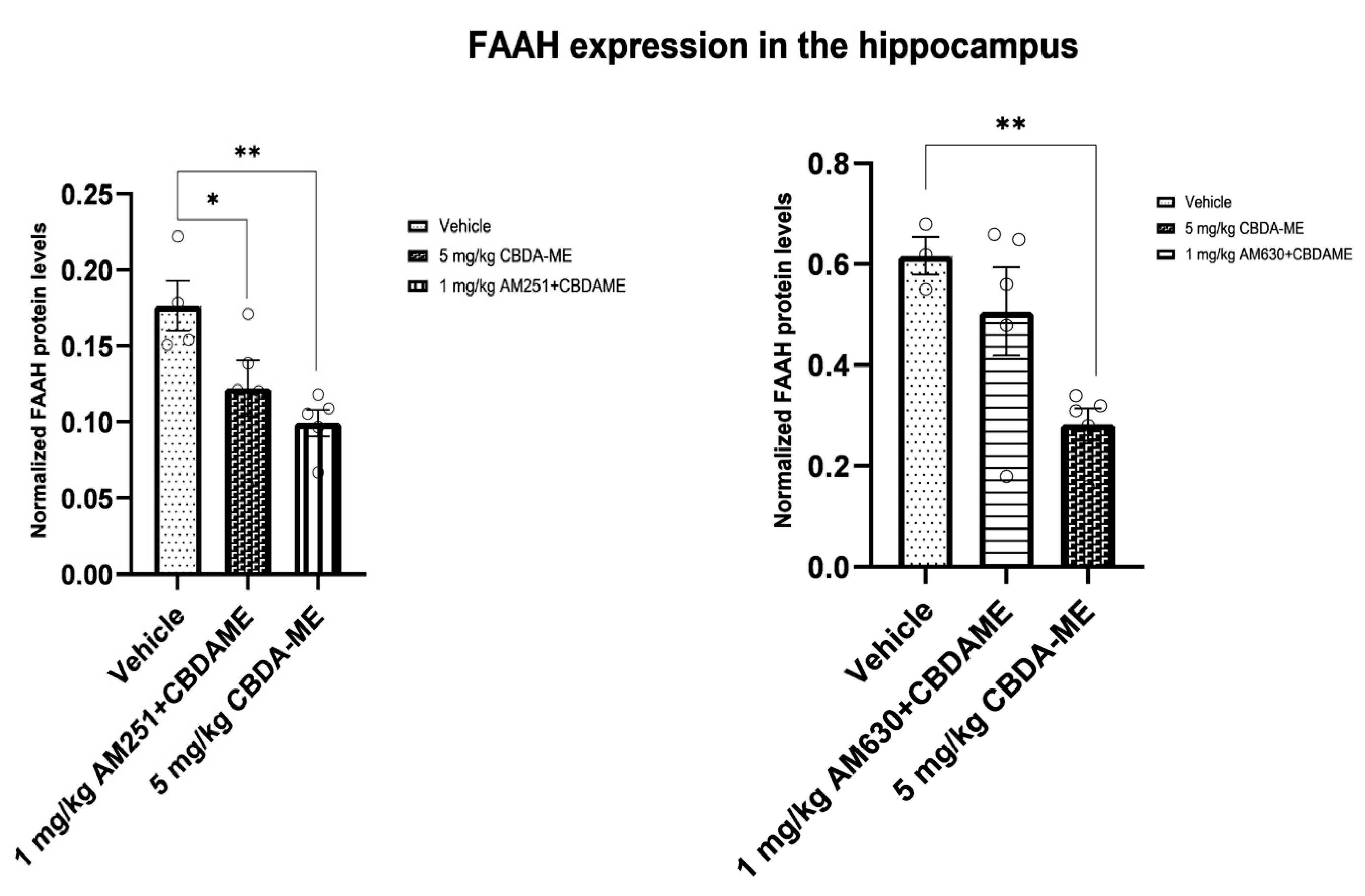
- FAAH expression in the hippocampus
3. Discussion
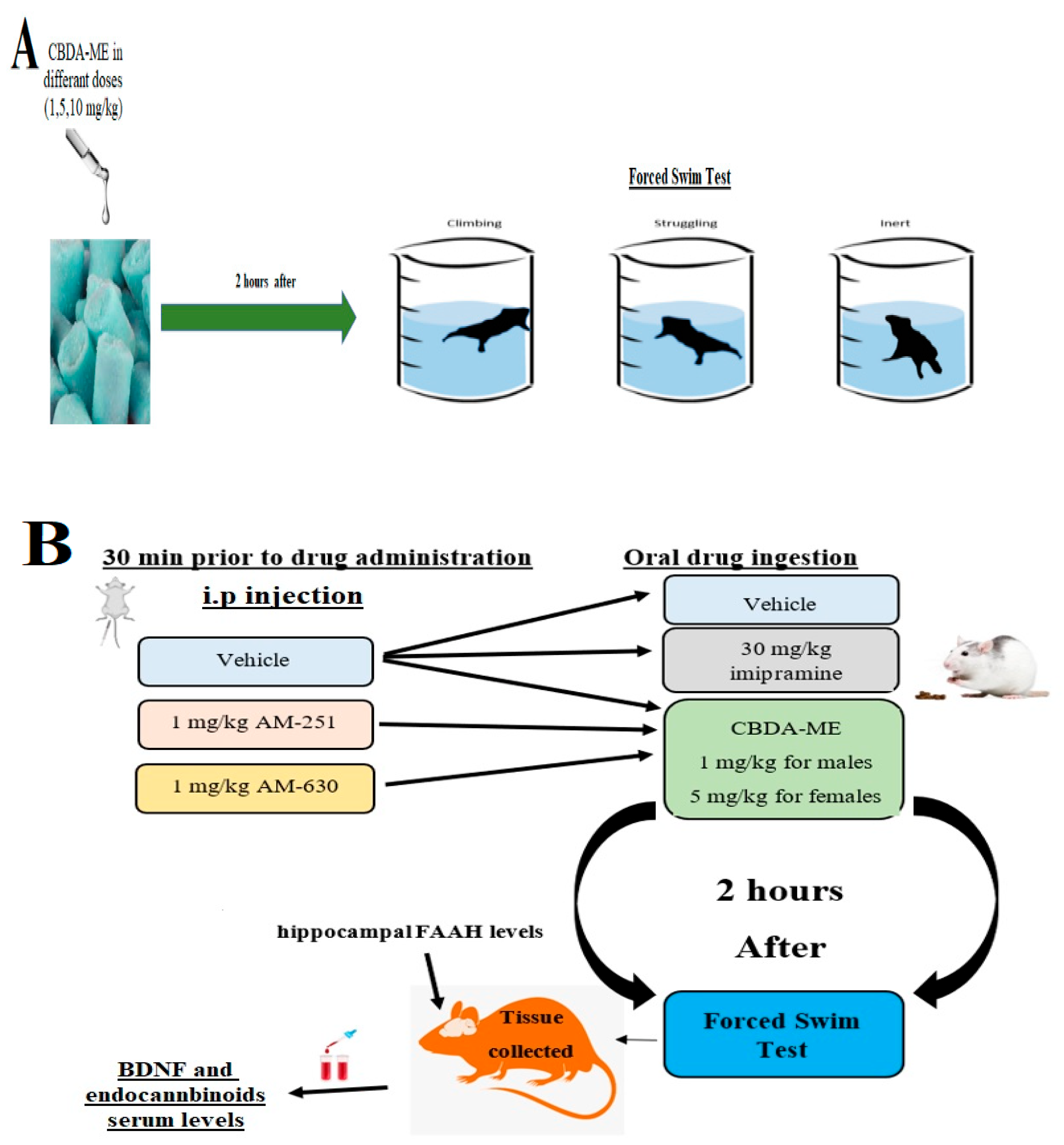
4. Materials and Methods
4.1. Animals
4.2. Forced Swimming Test (FST)
4.3. Drugs
4.4. Cytokines Assessment
4.5. Corticosterone Measurement
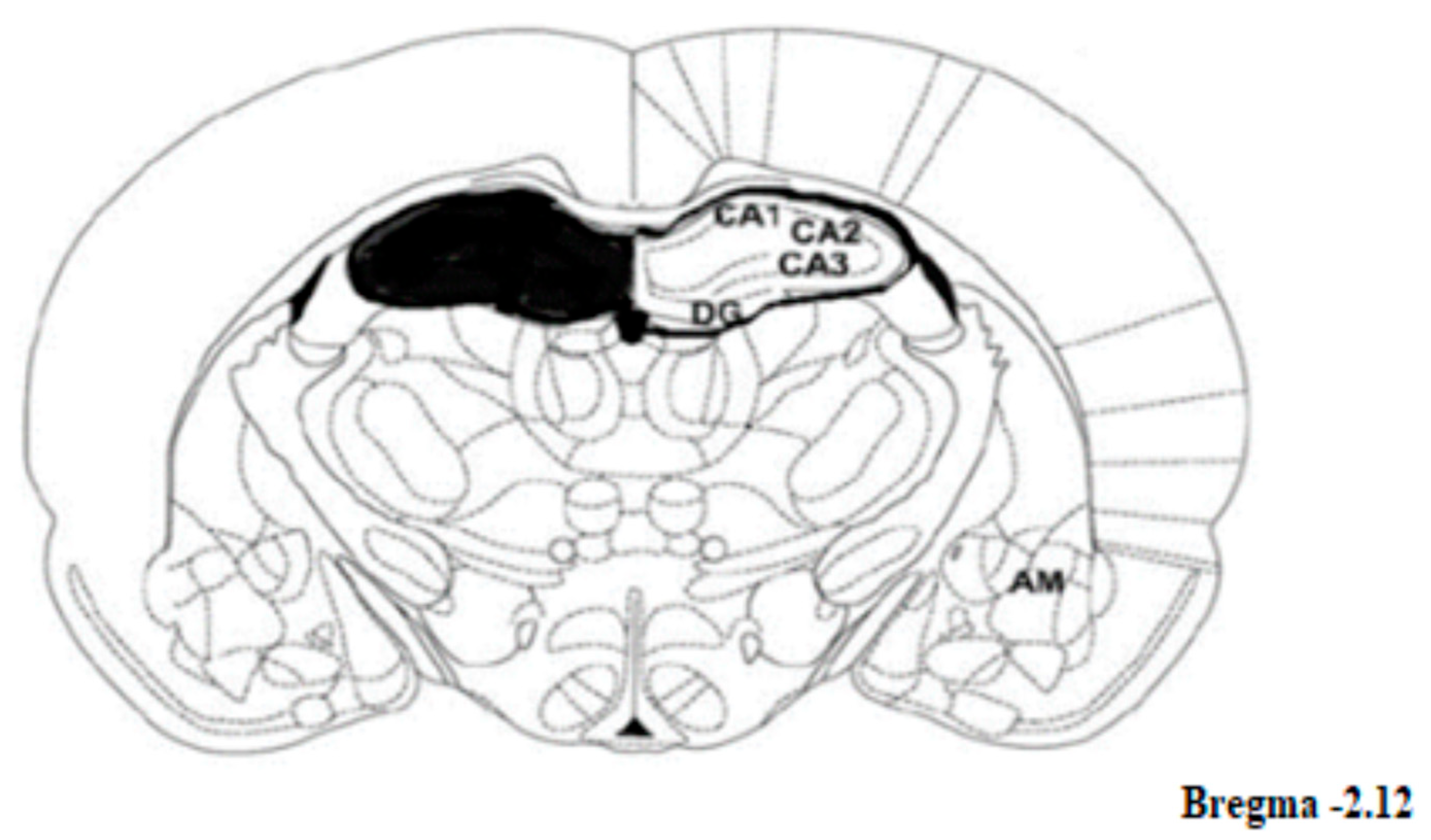
4.6. Brain Regions
4.7. BDNF Levels in Blood
4.8. Endocannabinoid Levels in Blood
4.9. Western Blotting
Protein Extraction
4.10. Analysis of Proteins
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanti, A.; Belzung, C. Open Questions in Current Models of Antidepressant Action. Br. J. Pharmacol. 2010, 159, 1187–1200. [Google Scholar] [CrossRef]
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Kvarta, M.D.; Thompson, S.M. Sex Differences in Antidepressant Efficacy. Neuropsychopharmacology 2019, 44, 140–154. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus (Madison) 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-Resistant Depression: Therapeutic Trends, Challenges, and Future Directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.Y.; Hungund, B.L. Role of the Endocannabinoid System in Depression and Suicide. Trends Pharmacol. Sci. 2006, 27, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.A.; Marsicano, G.; Nestler, E.J.; Holsboer, F.; Lutz, B.; Wotjak, C.T. Antidepressant-like Behavioral Effects of Impaired Cannabinoid Receptor Type 1 Signaling Coincide with Exaggerated Corticosterone Secretion in Mice. Psychoneuroendocrinology 2008, 33, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H.; Moore, P.B. Endocannabinoid System Dysfunction in Mood and Related Disorders Endocannabinoid System Dysfunction in Mood and Related Disorders. Acta Psychiatr. Scand. 2011, 124, 250–261. [Google Scholar] [CrossRef]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of Cannabidiol in Males and Females in Two Different Rat Models of Depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of Chronic Cannabidiol Treatment in the Rat Chronic Unpredictable Mild Stress Model of Depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- Potter, D.J.; Clark, P.; Brown, M.B. Potency of Δ9-THC and Other Cannabinoids in Cannabis in England in 2005: Implications for Psychoactivity and Pharmacology. J. Forensic Sci. 2008, 53, 90–94. [Google Scholar] [CrossRef]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of Nausea and Vomiting by Cannabinoids. Br. J. Pharmacol. 2011, 163, 1327–1562. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Rock, E.M.; Guenther, K.; Limebeer, C.L.; Stevenson, L.A.; Haj, C.; Smoum, R.; Parker, L.A.; Mechoulam, R. Cannabidiolic Acid Methyl Ester, a Stable Synthetic Analogue of Cannabidiolic Acid, Can Produce 5-HT1A Receptor-Mediated Suppression of Nausea and Anxiety in Rats. Br. J. Pharmacol. 2018, 175, 100–112. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Linher-Melville, K.; Niazmand, M.J.; Sharma, M.; Shahid, A.; Zhu, K.L.; Parzei, N.; Sidhu, J.; Haj, C.; Mechoulam, R.; et al. An Evaluation of the Anti-Hyperalgesic Effects of Cannabidiolic Acid-Methyl Ester in a Preclinical Model of Peripheral Neuropathic Pain. Br. J. Pharmacol. 2020, 177, 2712–2725. [Google Scholar] [CrossRef]
- Hen-Shoval, D.; Amar, S.; Shbiro, L.; Smoum, R.; Haj, C.G.; Mechoulam, R.; Zalsman, G.; Weller, A.; Shoval, G. Acute Oral Cannabidiolic Acid Methyl Ester Reduces Depression-like Behavior in Two Genetic Animal Models of Depression. Behav. Brain Res. 2018, 351, 1–3. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Gomes, F.V.; Resstel, L.B.M. Cannabidiol Inhibitory Effect on Marble-Burying Behaviour: Involvement of CB1 Receptors. Behav. Pharmacol. 2010, 4, 353–358. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.C.; Zuardi, A.W.; Crippa, J.A.; Dal-Pizzol, F. Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediators Inflamm. 2015, 2015, 538670. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. ImmunoTargets Ther. 2020, 9, 131–140. [Google Scholar] [CrossRef]
- De Filippis, D.; Iuvone, T.; D’Amico, A.; Esposito, G.; Steardo, L.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y.; De Man, J.G. Effect of Cannabidiol on Sepsis-Induced Motility Disturbances in Mice: Involvement of CB1 Receptors and Fatty Acid Amide Hydrolase. Neurogastroenterol. Motil. 2008, 20, 919–927. [Google Scholar] [CrossRef]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Nolan, M.; Roman, E.; Nasa, A.; Levins, K.J.; O’Hanlon, E.; O’Keane, V.; Willian Roddy, D. Hippocampal and Amygdalar Volume Changes in Major Depressive Disorder: A Targeted Review and Focus on Stress. Chronic Stress 2020, 4. [Google Scholar] [CrossRef]
- Roddy, D.W.; Farrell, C.; Doolin, K.; Roman, E.; Tozzi, L.; Frodl, T.; O’Keane, V.; O’Hanlon, E. Tozzi The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol. Psychiatry 2018, 85, 487–497. [Google Scholar] [CrossRef]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Emerging Evidence for the Antidepressant Effect of Cannabidiol and the Underlying Molecular Mechanisms. J. Chem. Neuroanat. 2019, 98, 104–116. [Google Scholar] [CrossRef]
- Sales, A.J.; Guimarães, F.S.; Joca, S.R.L. CBD Modulates DNA Methylation in the Prefrontal Cortex and Hippocampus of Mice Exposed to Forced Swim. Behav. Brain Res. 2020, 388, 112627. [Google Scholar] [CrossRef]
- Shetty, R.A.; Sadananda, M. Immediate and Delayed Anxiety- and Depression-like Profiles in the Adolescent Wistar-Kyoto Rat Model of Endogenous Depression Following Postweaning Social Isolation. Behav. Brain Res. 2017, 320, 323–332. [Google Scholar] [CrossRef]
- Nam, H.; Clinton, S.M.; Jackson, N.L.; Kerman, I.A. Learned Helplessness and Social Avoidance in the Wistar-Kyoto Rat. Front. Behav. Neurosci. 2014, 8, 109. [Google Scholar] [CrossRef]
- Malkesman, O.; Braw, Y.; Maayan, R.; Weizman, A.; Overstreet, D.H.; Shabat-Simon, M.; Kesner, Y.; Touati-Werner, D.; Yadid, G.; Weller, A. Two Different Putative Genetic Animal Models of Childhood Depression. Biol. Psychiatry 2006, 59, 17–23. [Google Scholar] [CrossRef]
- Seedat, S.; Scott, K.M.; Angermeyer, M.C.; Berglund, P.; Bromet, E.J.; Brugha, T.S.; Demyttenaere, K.; De Girolamo, G.; Haro, J.M.; Jin, R.; et al. Cross-National Associations between Gender and Mental Disorders in the World Health Organization World Mental Health Surveys. Arch. Gen. Psychiatry 2009, 66, 785–795. [Google Scholar] [CrossRef]
- Kuehner, C. Why Is Depression More Common among Women than among Men? The Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Yonkers, K.A.; Brawman-Mintzer, O. The Pharmacologic Treatment of Depression: Is Gender a Critical Factor? J. Clin. Psychiatry 2002, 63, 610–615. [Google Scholar] [CrossRef]
- Millard, S.J.; Weston-Green, K.; Newell, K.A. The Wistar-Kyoto Rat Model of Endogenous Depression: A Tool for Exploring Treatment Resistance with an Urgent Need to Focus on Sex Differences. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Arankowsky-Sandoval, G.; Pertwee, R.G.; Parker, L.; Mechoulam, R. Sleep and Neurochemical Modulation by Cannabidiolic Acid Methyl Ester in Rats. Brain Res. Bull. 2020, 155, 166–173. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Pertwee, R.G.; Mechoulam, R.; Parker, L.A. Therapeutic Potential of Cannabidiol, Cannabidiolic Acid, and Cannabidiolic Acid Methyl Ester as Treatments for Nausea and Vomiting. Cannabis Cannabinoid Res. 2021, 6, 266–274. [Google Scholar] [CrossRef]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Prohedonic Effect of Cannabidiol in a Rat Model of Depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol Induces Rapid-Acting Antidepressant-like Effects and Enhances Cortical 5-HT/Glutamate Neurotransmission: Role of 5-HT1A Receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT 1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex Differences in the Rapid and the Sustained Antidepressant-like Effects of Ketamine in Stress-Naïve and “Depressed” Mice Exposed to Chronic Mild Stress. Neuroscience 2015, 290, 49–60. [Google Scholar] [CrossRef]
- Kokras, N.; Antoniou, K.; Mikail, H.G.; Kafetzopoulos, V.; Papadopoulou-Daifoti, Z.; Dalla, C. Forced Swim Test: What about Females? Neuropharmacology 2015, 99, 408–421. [Google Scholar] [CrossRef]
- Lifschytz, T.; Shalom, G.; Lerer, B.; Newman, M.E. Sex-Dependent Effects of Fluoxetine and Triiodothyronine in the Forced Swim Test in Rats. Eur. Neuropsychopharmacol. 2006, 16, 115–121. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Limphaibool, N.; Puszczewicz, M. Cytokine Secretion and the Risk of Depression Development in Patients with Connective Tissue Diseases. Psychiatry Clin. Neurosci. 2019, 73, 302–316. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Psiquiatr. Biol. 2010, 17, 71–80. [Google Scholar] [CrossRef]
- Wilson, D.R.; Warise, L. Cytokines and Their Role in Depression. Perspect. Psychiatr. Care 2008, 44, 285–289. [Google Scholar] [CrossRef]
- Clark, S.M.; Notarangelo, F.M.; Li, X.; Chen, S.; Schwarcz, R.; Tonelli, L.H. Maternal Immune Activation in Rats Blunts Brain Cytokine and Kynurenine Pathway Responses to a Second Immune Challenge in Early Adulthood. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2019, 89, 286–294. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Gerrard, D.; Dalloul, R. Dynamics of Cytokines and Heat Shock Proteins MRNA Expression in Thermally Manipulated Chicken Challenged with Intraperitoneally Injection of Lipopolysaccharide (LPS). FASEB J. 2020, 34. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Keshavlal, G.P.; Bezbaruah, B.B.; Dwivedi, S.; Gurjar, S.S.; Munde, N.; Jangra, A.; Lahkar, M.; Gogoi, R. Lipopolysaccharide Induced Anxiety- and Depressive-like Behaviour in Mice Are Prevented by Chronic Pre-Treatment of Esculetin. Neurosci. Lett. 2016, 611, 106–111. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Martinez-Mota, L.; Salinas, C.; Marquez-Velasco, R.; Hernandez-Chan, N.G.; Morales-Montor, J.; Pérez-Tapia, M.; Streber, M.L.; Granados-Camacho, I.; Becerril, E.; et al. Chronic Stress Induces Structural Alterations in Splenic Lymphoid Tissue That Are Associated with Changes in Corticosterone Levels in Wistar-Kyoto Rats. Biomed Res. Int. 2013, 2013, 868742. [Google Scholar] [CrossRef]
- Mileva, G.R.; Rooke, J.; Ismail, N.; Bielajew, C. Corticosterone and Immune Cytokine Characterization Following Environmental Manipulation in Female WKY Rats. Behav. Brain Res. 2017, 316, 197–204. [Google Scholar] [CrossRef]
- Rittenhouse, P.A.; López-Rubalcava, C.; Stanwood, G.D.; Lucki, I. Amplified Behavioral and Endocrine Responses to Forced Swim Stress in the Wistar-Kyoto Rat. Psychoneuroendocrinology 2002, 27, 303–318. [Google Scholar] [CrossRef]
- Malkesman, O.; Maayan, R.; Weizman, A.; Weller, A. Aggressive Behavior and HPA Axis Hormones after Social Isolation in Adult Rats of Two Different Genetic Animal Models for Depression. Behav. Brain Res. 2006, 175, 408–414. [Google Scholar] [CrossRef]
- Shetty, R.A.; Sadananda, M. Brief Social Isolation in the Adolescent Wistar-Kyoto Rat Model of Endogenous Depression Alters Corticosterone and Regional Monoamine Concentrations. Neurochem. Res. 2017, 42, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Dow-Edwards, D. Sex Differences in the Interactive Effects of Early Life Stress and the Endocannabinoid System. Neurotoxicol. Teratol. 2020, 80, 106893. [Google Scholar] [CrossRef]
- Danan, D.; Todder, D.; Zohar, J.; Cohen, H. Is Ptsd-phenotype Associated with Hpa-axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats. Int. J. Mol. Sci. 2021, 22, 6416. [Google Scholar] [CrossRef]
- Lahmame, A.; Del Arco, C.; Pazos, A.; Yritia, M.; Armario, A. Are Wistar-Kyoto Rats a Genetic Animal Model of Depression Resistant to Antidepressants? Eur. J. Pharmacol. 1997, 337, 115–123. [Google Scholar] [CrossRef]
- Nagasawa, M.; Otsuka, T.; Yasuo, S.; Furuse, M. Chronic Imipramine Treatment Differentially Alters the Brain and Plasma Amino Acid Metabolism in Wistar and Wistar Kyoto Rats. Eur. J. Pharmacol. 2015, 762, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Holl, K.; He, H.; Wedemeyer, M.; Clopton, L.; Wert, S.; Meckes, J.K.; Cheng, R.; Kastner, A.; Palmer, A.A.; Redei, E.E.; et al. Heterogeneous Stock Rats: A Model to Study the Genetics of Despair-like Behavior in Adolescence. Genes, Brain Behav. 2018, 17, 139–148. [Google Scholar] [CrossRef]
- Preskorn, S.H. Selection of an Antidepressant: Mirtazapine. J. Clin. Psychiatry 1997, 58, 3–8. [Google Scholar]
- Pies, R. Are Antidepressants Effective in the Acute and Long-Term Treatment of Depression? Sic et Non. Innov. Clin. Neurosci. 2012, 9, 31–40. [Google Scholar]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the Pathophysiology and Treatment of Depression: Activity-Dependent Effects Distinguish Rapid-Acting Antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef]
- Gelle, T.; Samey, R.A.; Plansont, B.; Bessette, B.; Jauberteau-Marchan, M.O.; Lalloué, F.; Girard, M. BDNF and Pro-BDNF in Serum and Exosomes in Major Depression: Evolution after Antidepressant Treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110229. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.Y.; Xie, S.; Psychoyos, D.; Hungund, B.L.; Cooper, T.B.; Tejani-Butt, S.M. Dysfunction in Fatty Acid Amide Hydrolase Is Associated with Depressive-like Behavior in Wistar Kyoto Rats. PLoS ONE 2012, 7, e36743. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of Molecular Targets and Signaling Pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of Cannabidiol (CBD) and Its Analogues: Structures, Biological Activities, and Neuroprotective Mechanisms in Epilepsy and Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 192. [Google Scholar] [CrossRef]
- Likar, R.; Koestenberger, M.; Stultschnig, M.; Nahler, G. Concomitant Treatment of Malignant Brain Tumours with CBD-A Case Series and Review of the Literature. Anticancer Res. 2019, 39, 5797–5801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, P.; Xie, Y.; Chen, X.; Solowij, N.; Green, K.; Chew, Y.L.; Huang, X.F. Cannabidiol Regulates CB1-PSTAT3 Signaling for Neurite Outgrowth, Prolongs Lifespan, and Improves Health Span in Caenorhabditis Elegans of Aβ Pathology Models. FASEB J. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Locci, A.; Pinna, G. Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biol. Psychiatry 2019, 85, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Paterniti, I.; Ahmad, A.; Campolo, M.; Esposito, E.; Cuzzocrea, S. Effects of Palmitoylethanolamide and Luteolin in an Animal Model of Anxiety/Depression. CNS Neurol. Disord.-Drug Targets 2013, 12, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Thieme, D.; Schelling, G.; Kolassa, I.T. Endocannabinoid Concentrations in Hair Are Associated with PTSD Symptom Severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.; Liu, Q.Z.; Wang, L.L.; Shang, J. Simvastatin and Bezafibrate Ameliorate Emotional Disorder Induced by High Fat Diet in C57BL/6 Mice. Sci. Rep. 2017, 7, 2335. [Google Scholar] [CrossRef]
- Botsford, C.; Brellenthin, A.G.; Cisler, J.M.; Hillard, C.J.; Koltyn, K.F.; Crombie, K.M. Circulating Endocannabinoids and Psychological Outcomes in Women with PTSD. J. Anxiety Disord. 2023, 93, 102656. [Google Scholar] [CrossRef]
- Walther, A.; Kirschbaum, C.; Wehrli, S.; Rothe, N.; Penz, M.; Wekenborg, M.; Gao, W. Depressive Symptoms Are Negatively Associated with Hair N-Arachidonoylethanolamine (Anandamide) Levels: A Cross-Lagged Panel Analysis of Four Annual Assessment Waves Examining Hair Endocannabinoids and Cortisol. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2023, 121, 110658. [Google Scholar] [CrossRef]
- Behnke, A.; Karabatsiakis, A.; Krumbholz, A.; Karrasch, S.; Schelling, G.; Kolassa, I.T.; Rojas, R. Associating Emergency Medical Services Personnel’s Workload, Trauma Exposure, and Health with the Cortisol, Endocannabinoid, and N-Acylethanolamine Concentrations in Their Hair. Sci. Rep. 2020, 10, 22403. [Google Scholar] [CrossRef]
- Vamvakidès, A. Pharmacologic Profile of N-Palmitoylglycine. Its Effect on Reserpine and Haloperidol Catalepsy. Boll. Chim. Farm. 1995, 134, 258–262. [Google Scholar]
- Rimmerman, N.; Bradshaw, H.B.; Hughes, H.V.; Chen, J.S.C.; Hu, S.S.J.; McHugh, D.; Vefring, E.; Jahnsen, J.A.; Thompson, E.L.; Masuda, K.; et al. N-Palmitoyl Glycine, a Novel Endogenous Lipid That Acts as a Modulator of Calcium Influx and Nitric Oxide Production in Sensory Neurons. Mol. Pharmacol. 2008, 74, 213–224. [Google Scholar] [CrossRef]
- Vollmayr, B.; Sulger, J.; Gabriel, P.; Aldenhoff, J.B. Mitogen Stimulated Rise of Intracellular Calcium Concentration in Single t Lymphocytes from Patients with Major Depression Is Reduced. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 1263–1273. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, J.; Xu, T.; Cai, X. Synaptic Potentiation Mediated by L-Type Voltage-Dependent Calcium Channels Mediates the Antidepressive Effects of Lateral Habenula Stimulation. Neuroscience 2017, 362, 25–32. [Google Scholar] [CrossRef]
- Nghia, N.A.; Hirasawa, T.; Kasai, H.; Obata, C.; Moriishi, K.; Mochizuki, K.; Koizumi, S.; Kubota, T. Long-Term Imipramine Treatment Increases N-Methyl-d-Aspartate Receptor Activity and Expression via Epigenetic Mechanisms. Eur. J. Pharmacol. 2015, 752, 69–77. [Google Scholar] [CrossRef]
- Kabir, Z.D.; Martínez-Rivera, A.; Rajadhyaksha, A.M. From Gene to Behavior: L-Type Calcium Channel Mechanisms Underlying Neuropsychiatric Symptoms. Neurotherapeutics 2017, 14, 588–613. [Google Scholar] [CrossRef]
- Della Pietra, A.; Savinainen, J.; Giniatullin, R. Inhibiting Endocannabinoid Hydrolysis as Emerging Analgesic Strategy Targeting a Spectrum of Ion Channels Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 4407. [Google Scholar] [CrossRef]
- Della Pietra, A.; Giniatullin, R.; Savinainen, J.R. Distinct Activity of Endocannabinoid-Hydrolyzing Enzymes MAGL and FAAH in Key Regions of Peripheral and Central Nervous System Implicated in Migraine. Int. J. Mol. Sci. 2021, 22, 1204. [Google Scholar] [CrossRef] [PubMed]
- Portugalov, A.; Zaidan, H.; Gaisler-Salomon, I.; Hillard, C.J.; Akirav, I. FAAH Inhibition Restores Early Life Stress-Induced Alterations in PFC MicroRNAs Associated with Depressive-Like Behavior in Male and Female Rats. Int. J. Mol. Sci. 2022, 23, 6101. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi Zer-Aviv, T.; Islami, L.; Hamilton, P.J.; Parise, E.M.; Nestler, E.J.; Sbarski, B.; Akirav, I. Enhancing Endocannabinoid Signaling via β-Catenin in the Nucleus Accumbens Attenuates PTSD- and Depression-like Behavior of Male Rats. Biomedicines 2022, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.M.; Asratian, A.; Lindé, J.; Holm, L.; Nätt, D.; Augier, G.; Stensson, N.; Vecchiarelli, H.A.; Balsevich, G.; Aukema, R.J.; et al. Protective Effects of Elevated Anandamide on Stress and Fear-Related Behaviors: Translational Evidence from Humans and Mice. Mol. Psychiatry 2020, 25, 993–1005. [Google Scholar] [CrossRef]
- Difede, J.A.; Rothbaum, B.O.; Rizzo, A.A.; Wyka, K.; Spielman, L.; Reist, C.; Roy, M.J.; Jovanovic, T.; Norrholm, S.D.; Cukor, J.; et al. Enhancing Exposure Therapy for Posttraumatic Stress Disorder (PTSD): A Randomized Clinical Trial of Virtual Reality and Imaginal Exposure with a Cognitive Enhancer. Transl. Psychiatry 2022, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Liebowitz, M.R.; Stein, M.B.; Grunfeld, J.; Van Hove, I.; Simmons, W.K.; Van Der Ark, P.; Palmer, J.A.; Saad, Z.S.; Pemberton, D.J.; et al. The Effects of Inhibition of Fatty Acid Amide Hydrolase (FAAH) by JNJ-42165279 in Social Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Proof-of-Concept Study. Neuropsychopharmacology 2021, 46, 1004–1010. [Google Scholar] [CrossRef]
- Ben-Cnaan, E.; Tam, J.; Permyakova, A.; Azar, S.; Hirsch, S.; Baraghithy, S.; Hinden, L. The Metabolic Efficacy of a Cannabidiolic Acid (CBDA) Derivative in Treating Diet-and Genetic-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 5610. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M.J. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 2, 327–336. [Google Scholar]
- Mechoulam, R.; Ben-Zvi, Z.; Yagnitinsky, B.; Shani, A. A New Tetrahydrocannabinolic Acid; Pergamon Press: Oxford, UK, 1969; Volume 10. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Kang, J.; Wang, D.; Duan, Y.; Zhai, L.; Shi, L.; Guo, F. Aerobic Exercise Prevents Depression via Alleviating Hippocampus Injury in Chronic Stressed Depression Rats. Brain Sci. 2021, 11, 9. [Google Scholar] [CrossRef]
- Malitsky, S.; Ziv, C.; Rosenwasser, S.; Zheng, S.; Schatz, D.; Porat, Z.; Ben-Dor, S.; Aharoni, A.; Vardi, A. Viral Infection of the Marine Alga Emiliania Huxleyi Triggers Lipidome Remodeling and Induces the Production of Highly Saturated Triacylglycerol. New Phytol. 2016, 210, 88–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hen-Shoval, D.; Moshe, L.; Indig-Naimer, T.; Mechoulam, R.; Shoval, G.; Zalsman, G.; Kogan, N.M.; Weller, A. Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats. Int. J. Mol. Sci. 2023, 24, 3828. https://doi.org/10.3390/ijms24043828
Hen-Shoval D, Moshe L, Indig-Naimer T, Mechoulam R, Shoval G, Zalsman G, Kogan NM, Weller A. Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats. International Journal of Molecular Sciences. 2023; 24(4):3828. https://doi.org/10.3390/ijms24043828
Chicago/Turabian StyleHen-Shoval, Danielle, Lital Moshe, Talia Indig-Naimer, Raphael Mechoulam, Gal Shoval, Gil Zalsman, Natalya M. Kogan, and Aron Weller. 2023. "Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats" International Journal of Molecular Sciences 24, no. 4: 3828. https://doi.org/10.3390/ijms24043828
APA StyleHen-Shoval, D., Moshe, L., Indig-Naimer, T., Mechoulam, R., Shoval, G., Zalsman, G., Kogan, N. M., & Weller, A. (2023). Cannabinoid Receptor 2 Blockade Prevents Anti-Depressive-like Effect of Cannabidiol Acid Methyl Ester in Female WKY Rats. International Journal of Molecular Sciences, 24(4), 3828. https://doi.org/10.3390/ijms24043828





