Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review
Abstract
1. Introduction to Urinary Bladder
2. Nanotechnology and Urinary Bladder Imaging
3. Quick Overview of the Literature
- Nanoparticles: NPs or NP-based compounds tested by the authors. Brief description of the particles and abbreviations used throughout the papers by the authors. When reported, the size of the NPs is given. The instrument used to measure NP size, i.e., transmission electron microscopy (TEM), dynamic light scattering (DLS), or atomic force microscopy (AFM), is also reported. The size of NPs is not reported if they are inserted into a more complex system; in this case, the overall size of the system is reported;
- Imaging techniques: The techniques used for imaging purposes are reported, generally FLI (CLSM or OI) or MRI. Bioluminescent imaging was also used to monitor tumor growth. Few details regarding the detector/tomograph are reported, and, for FLI, the excitation and emission wavelengths used;
- Experimental design: Cancer model, in vitro, in vivo, or ex vivo experimentation, route of NPs administration, animal species, NP concentration, and incubation/visualization time;
- Main results: Among the results reported by the authors, particular attention was focused on the imaging results and other observations considered relevant for the readers.
4. Technical Clues for Nanoparticle-Based Imaging of Bladder Cancer Cells
4.1. Nanoparticles
4.2. Imaging Techniques and Detectors
4.3. Cancer Models and Targeting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef] [PubMed]
- Dielubanza, E.J.; Schaeffer, A.J. Urinary tract infections in women. Med. Clin. N. Am. 2011, 95, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory (International Agency for Research on Cancer WHO). Cancer Fact Sheets (Bladder). 2020. Available online: https://gco.iarc.fr/ (accessed on 9 February 2023).
- Hurst, R.E. Structure, function, and pathology of proteoglycans and glycosaminoglycans in the urinary tract. World J. Urol. 1994, 12, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Min, G.; Zhou, G.; Schapira, M.; Sun, T.T.; Kong, X.P. Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J. Cell Sci. 2003, 116, 4087–4094. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef]
- Russo, G.I.; Sholklapper, T.N.; Cocci, A.; Broggi, G.; Caltabiano, R.; Smith, A.B.; Lotan, Y.; Morgia, G.; Kamat, A.M.; Witjes, J.A.; et al. Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers 2021, 13, 4378. [Google Scholar] [CrossRef]
- Zaak, D.; Hungerhuber, E.; Schneede, P.; Stepp, H.; Frimberger, D.; Corvin, S.; Schmeller, N.; Kriegmair, M.; Hofstetter, A.; Knuechel, R. Role of 5-aminolevulinic acid in the detection of urothelial premalignant lesions. Cancer 2002, 95, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; Bosch, J.L. Predictors of false positives in 5-aminolevulinic acid-inducedphotodynamic diagnosis of bladder carcinoma: Identification of patient groups that may benefit most from highly specific optical diagnostics. Urology 2009, 74, 851–856. [Google Scholar] [CrossRef]
- Chernyak, V. Novel imaging modalities for lymph node imaging in urologic oncology. Urol. Clin. N. Am. 2011, 38, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Jayasimha, S. Nanotechnology in Urology. Indian J. Urol. 2017, 33, 13–18. [Google Scholar] [CrossRef]
- Crane, A.; Isharwal, S.; Zhu, H. Current Therapeutic Strategies in Clinical Urology. Mol. Pharm. 2018, 15, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Hosseinikhah, S.M.; Rahdar, A.; Farhoudi, L.; Arshad, R.; Cucchiarini, M.; Pandey, S. Nanotechnology in Bladder Cancer: Diagnosis and Treatment. Cancers 2021, 13, 2214. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Momin, M. Clinical therapies and nano drug delivery systems for urinary bladder cancer. Pharmacol. Ther. 2021, 226, 107871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J. Nanobiotechnol. 2021, 19, 393. [Google Scholar] [CrossRef]
- Zupančič, D.; Veranič, P. Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis. Int. J. Mol. Sci. 2022, 23, 8183. [Google Scholar] [CrossRef]
- António, M.; Vitorino, R.; Daniel-da-Silva, A.L. Gold nanoparticles-based assays for biodetection in urine. Talanta 2021, 230, 122345. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Han, B.; Chen, G.; Song, S.; Bo, H. Mouse Model to Explore the Therapeutic Effect of Nano-Doxorubicin Drug Delivery System on Bladder Cancer. J. Nanosci. Nanotechnol. 2021, 21, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.K.; Luo, Y.; O'Donnell, M.A.; Assouline, J. Nanotechnology and cancer: Improving real-time monitoring and staging of bladder cancer with multimodal mesoporous silica nanoparticles. Cancer Nanotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tao, H.; Li, Z.; Lei, Y.; Bai, B.; Zhang, N.; Yang, P.; Ma, H.; Chen, W. Preparation of Composite Cypate Nanoparticles and Its Application in the Treatment of Pediatric Bladder Tumors. J. Nanosci. Nanotechnol. 2021, 21, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Malatesta, M. In Vitro Models of Biological Barriers for Nanomedical Research. Int. J. Mol. Sci. 2022, 23, 8910. [Google Scholar] [CrossRef]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W.G.; et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, e1906936. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xia, Q.; Zhang, Y.; Li, Y.; Feng, Z.; Zhou, J.; Qi, J.; Tang, B.Z.; Qian, J.; Lin, H. Aggregation-Induced Emission (AIE) Nanoparticles-Assisted NIR-II Fluorescence Imaging-Guided Diagnosis and Surgery for Inflammatory Bowel Disease (IBD). Adv. Healthc. Mater. 2021, 10, e2101043. [Google Scholar] [CrossRef]
- Bruchez Jr, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.L.; et al. Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 2020, 587, 588–593. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids Surf. B Biointerfaces 2013, 102, 534–539. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, K.; Kwon, J.E.; Park, H.; Lee, S.; Kim, S.; Park, S.Y. Dual-color fluorescent nanoparticles showing perfect color-specific photoswitching for bioimaging and super-resolution microscopy. Nat. Commun. 2019, 10, 3089. [Google Scholar] [CrossRef]
- Mannucci, S.; Boschi, F.; Cisterna, B.; Esposito, E.; Cortesi, R.; Nastruzzi, C.; Cappellozza, E.; Bernardi, P.; Sbarbati, A.; Malatesta, M.; et al. A Correlative Imaging Study of in vivo and ex vivo Biodistribution of Solid Lipid Nanoparticles. Int. J. Nanomed. 2020, 15, 1745–1758. [Google Scholar] [CrossRef]
- Pandey, N.; Menon, J.U.; Takahashi, M.; Hsieh, J.T.; Yang, J.; Nguyen, K.T.; Wadajkar, A.S. Thermo-responsive Fluorescent Nanoparticles for Multimodal Imaging and Treatment of Cancers. Nanotheranostics 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boschi, F.; De Sanctis, F. Overview of the optical properties of fluorescent nanoparticles for optical imaging. Eur. J. Histochem. 2017, 61, 2830. [Google Scholar] [CrossRef]
- Lifante, J.; Shen, Y.; Ximendes, E.; Martín Rodríguez, E.; Ortgies, D.H. The role of tissue fluorescence in in vivo optical bioimaging featured. J. Appl. Phys. 2020, 128, 171101. [Google Scholar] [CrossRef]
- Rezende, T.K.L.; Barbosa, H.P.; Dos Santos, L.F.; de O Lima, K.; Alves de Matos, P.; Tsubone, T.M.; Gonçalves, R.R.; Ferrari, J.L. Upconversion rare Earths nanomaterials applied to photodynamic therapy and bioimaging. Front. Chem. 2022, 10, 1035449. [Google Scholar] [CrossRef]
- Lu, C.; Joulin, E.; Tang, H.; Pouri, H.; Zhang, J. Upconversion Nanostructures Applied in Theranostic Systems. Int. J. Mol. Sci. 2022, 23, 9003. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, C.A.; Hirsch, T.; Marín, M.J. Recent advances in near infrared upconverting nanomaterials for targeted photodynamic therapy of cancer. Methods Appl. Fluoresc. 2022, 10, 034003. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, K.; Tamaki, Y.; Taguchi, T.; Tanji, Y.; Miyoshi, Y.; Kim, S.J.; Shimazu, K.; Ueda, S.; Yanagisawa, T.; Okishiro, N.; et al. Potential of reduction in total tumor volume measured with 3D-MRI as a prognostic factor for locally-advanced breast cancer patients treated with primary chemotherapy. Breast J. 2008, 14, 523–531. [Google Scholar] [CrossRef]
- Mannucci, S.; Tambalo, S.; Conti, G.; Ghin, L.; Milanese, A.; Carboncino, A.; Nicolato, E.; Marinozzi, M.R.; Benati, D.; Bassi, R.; et al. Magnetosomes Extracted from Magnetospirillum gryphiswaldense as Theranostic Agents in an Experimental Model of Glioblastoma. Contrast Media Mol. Imaging 2018, 2018, 2198703. [Google Scholar] [CrossRef]
- Azhdeh, S.; Kaviani, A.; Sadighi, N.; Rahmani, M. Accurate Estimation of Breast Tumor Size: A Comparison Between Ultrasonography, Mammography, Magnetic Resonance Imaging, and Associated Contributing Factors. Eur. J. Breast Health 2020, 17, 53–61. [Google Scholar] [CrossRef]
- Lura, N.; Wagner-Larsen, K.S.; Forsse, D.; Trovik, J.; Halle, M.K.; Bertelsen, B.I.; Salvesen, Ø.; Woie, K.; Krakstad, C.; Haldorsen, I.S. What MRI-based tumor size measurement is best for predicting long-term survival in uterine cervical cancer? Insights Imaging 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef]
- Tomlinson, B.; Lin, T.Y.; Dall'Era, M.; Pan, C.X. Nanotechnology in bladder cancer: Current state of development and clinical practice. Nanomedicine 2015, 10, 1189–1201. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; de Haas, R.J.; Pearson, R.A.; Kennish, S.J.; Giannarini, G.; Catto, J.W.F. Improving Staging in Bladder Cancer: The Increasing Role of Multiparametric Magnetic Resonance Imaging. Eur. Urol. Focus 2016, 2, 113–121. [Google Scholar] [CrossRef]
- Pan, Y.; Volkmer, J.-P.; Mach, K.E.; Rouse, R.V.; Liu, J.-J.; Sahoo, D.; Chang, T.C.; Metzner, T.J.; Kang, L.; Van De Rijn, M.; et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med. 2014, 6, 260ra148. [Google Scholar] [CrossRef]
- Isaac, K.M.; Sabaraya, I.V.; Ghousifam, N.; Das, D.; Pekkanen, A.M.; Romanovicz, D.K.; Long, T.E.; Saleh, N.B.; Rylander, M.N. Functionalization of single-walled carbon nanohorns for simultaneous fluorescence imaging and cisplatin delivery in vitro. Carbon 2018, 138, 309–318. [Google Scholar] [CrossRef]
- Alifu, N.; Zebibula, A.; Qi, J.; Zhang, H.; Sun, C.; Yu, X.; Xue, D.; Lam, J.W.Y.; Li, G.; Qian, J.; et al. Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy. ACS Nano 2018, 12, 11282–11293. [Google Scholar] [CrossRef]
- Yuan, R.; Rao, T.; Cheng, F.; Yu, W.-M.; Ruan, Y.; Zhang, X.-B.; Larré, S. Quantum dot-based fluorescent probes for targeted imaging of the EJ human bladder urothelial cancer cell line. Exp. Ther. Med. 2018, 16, 4779–4783. [Google Scholar] [CrossRef]
- Cho, S.K.; Su, L.-J.; Mao, C.; Wolenski, C.D.; Flaig, T.W.; Park, W. Multifunctional nanoclusters of NaYF 4:Yb 3+, Er 3+ upconversion nanoparticle and gold nanorod for simultaneous imaging and targeted chemotherapy of bladder cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Kanehira, K.; Yano, Y.; Hasumi, H.; Fukuhara, H.; Inoue, K.; Hanazaki, K.; Yao, M. Fluorescence enhancement effect of TiO2 nanoparticles and application for photodynamic diagnosis. Int. J. Mol. Sci. 2019, 20, 3698. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zang, Z.; Chen, Z.; Cui, L.; Chang, Z.; Ma, A.; Yin, T.; Liang, R.; Han, Y.; Wu, Z.; et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 2019, 214, 119226. [Google Scholar] [CrossRef] [PubMed]
- An, H.W.; Li, L.L.; Wang, Y.; Wang, Z.; Hou, D.; Lin, Y.X.; Qiao, S.L.; Wang, M.D.; Yang, C.; Cong, Y.; et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nature Commun. 2019, 10, 4861. [Google Scholar] [CrossRef]
- Ding, K.; Wang, L.; Zhu, J.; He, D.; Huang, Y.; Zhang, W.; Wang, Z.; Qin, A.; Hou, J.; Tang, B.Z. Photo-Enhanced Chemotherapy Performance in Bladder Cancer Treatment via Albumin Coated AIE Aggregates. ACS Nano 2021, 16, 7535–7546. [Google Scholar] [CrossRef]
- Li, G.; He, S.; Schätzlein, A.G.; Weiss, R.M.; Martin, D.T.; Uchegbu, I.F. Achieving highly efficient gene transfer to the bladder by increasing the molecular weight of polymer-based nanoparticles. J. Control. Release 2021, 332, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Mullapudi, S.S.; Zhang, Y.; Neoh, K.G. Glycosylated phospholipid-coated upconversion nanoparticles for bioimaging of non-muscle invasive bladder cancers. Mikrochim. Acta 2022, 189, 349. [Google Scholar] [CrossRef]
- Hiranmartsuwan, P.; Ma, X.; Nootem, J.; Daengngern, R.; Kamkaew, A.; Pinyou, P.; Wattanathana, W.; Promarak, V.; Li, Z.; Chansaenpak, K. Synthesis and properties of AIE-active Triazaborolopyridiniums toward fluorescent nanoparticles for cellular imaging and their biodistribution in vivo and ex vivo. Mater. Today Chem. 2022, 26, 101121. [Google Scholar] [CrossRef]
- Richard, S.; Boucher, M.; Saric, A.; Herbet, A.; Lalatonne, Y.; Petit, P.X.; Mériaux, S.; Boquet, D.; Motte, L. Optimization of pegylated iron oxide nanoplatforms for antibody coupling and bio-targeting. J. Mater. Chem. B 2017, 5, 2896–2907. [Google Scholar] [CrossRef]
- Sweeney, S.K.; Luo, Y.; O'Donnell, M.A.; Assouline, J.G. Peptide-mediated targeting mesoporous silica nanoparticles: A novel tool for fighting bladder cancer. J. Biomed. Nanotechnol. 2017, 13, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, H.; Chen, L.; Chen, J.; Zhang, D.; Cheng, Q.; Yang, F.; Zeng, Q.; Chen, T. Pre-clinical MRI-guided intravesical instillation theranosis of bladder cancer by tumor-selective oxygen nanogenerator. Nano Today 2021, 38, 101124. [Google Scholar] [CrossRef]
- Deng, X.; Liu, H.; Xu, Y.; Chan, L.; Xie, J.; Xiong, Z.; Tang, Z.; Yang, F.; Chen, T. Designing highly stable ferrous selenide-black phosphorus nanosheets heteronanostructure via P-Se bond for MRI-guided photothermal therapy. J. Nanobiotechnol. 2021, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.M.; Olivo, M.; Shuter, B.; Yi, J.B.; Bhuvaneswari, R.; Tan, H.R.; Xing, G.-C.; Ng, C.T.; Liu, L.; Lucky, S.S.; et al. Quantum dot capped magnetite nanorings as high performance nanoprobe for multiphoton fluorescence and magnetic resonance imaging. J. Am. Chem. Soc. 2010, 132, 14803–14811. [Google Scholar] [CrossRef]
- Key, J.; Dhawan, D.; Cooper, C.L.; Knapp, D.W.; Kim, K.; Kwon, I.C.; Choi, K.; Park, K.; Decuzzi, P.; Leary, J.F. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging. Int. J. Nanomed. 2016, 11, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, X.; Zhao, S.; Yu, H.; Cao, W.; Chen, W.; Wei, H.; Guo, H. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 2018, 8, 990–1004. [Google Scholar] [CrossRef]
- Bhandari, P.; Wang, X.; Irudayaraj, J. Oxygen Nanobubble Tracking by Light Scattering in Single Cells and Tissues. ACS Nano 2017, 11, 2682–2688. [Google Scholar] [CrossRef]
- Strobbia, P.; Cupil-Garcia, V.; Crawford, B.M.; Fales, A.M.; Pfefer, T.J.; Liu, Y.; Maiwald, M.; Sumpf, B.; Vo-Dinh, T. Accurate in vivo tumor detection using plasmonic-enhanced shifted-excitation Raman difference spectroscopy (SERDS). Theranostics 2021, 11, 4090–4102. [Google Scholar] [CrossRef]
- Hong, F.; Geng, X.; Min, G.; Sun, X.; Zhang, B.; Yao, Y.; Li, R.; Wang, J.; Zhao, H.; Guo, P.; et al. Deep NIR-II optical imaging combined with minimally invasive interventional photothermal therapy for orthotopic bladder cancer. Chem. Eng. J. 2022, 449, 137846. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Kaufman, S.C.; Musch, D.C.; Belin, M.W.; Cohen, E.J.; Meisler, D.M.; Reinhart, W.J.; Udell, I.J.; Van Meter, W.S. Confocal microscopy: A report by the American Academy of Ophthalmology. Ophthalmology 2004, 111, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Pian, Q.; Wang, C.; Chen, X.; Liang, J.; Zhao, L.; Wang, G.; Intes, X. Multimodal Biomedical Optical Imaging Review: Towards Comprehensive Investigation of Biological Tissues. Curr. Mol. Imaging 2014, 3, 72–87. [Google Scholar] [CrossRef]
- Liba, O.; SoRelle, E.D.; Sen, D.; de la Zerda, A. Contrast-enhanced optical coherence tomography with picomolar sensitivity for functional in vivo imaging. Sci. Rep. 2016, 6, 23337. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, T.; Soni, S. Pre-operative Assessment of Ablation Margins for Variable Blood Perfusion Metrics in a Magnetic Resonance Imaging Based Complex Breast Tumour Anatomy: Simulation Paradigms in Thermal Therapies. Comput. Methods Programs Biomed. 2021, 198, 105781. [Google Scholar] [CrossRef] [PubMed]


| Nanoparticles | Imaging Technique | Experimental Design | Main Results | Ref. |
|---|---|---|---|---|
| QD 625 conjugated with antibody anti-CD47 | Clinical confocal endomicroscopy system based on a 2.6 mm fiber optic probe with a microscopic field of view (240 mm) and acquired video sequences at 12 frames/s. | Humans: QD 625 were instilled into fresh, intact bladders obtained from human subjects after radical cystectomy for muscle-invasive or high-risk non-muscle-invasive bladder cancer. | Successful endoscopic imaging of human bladder cancer cells by targeting the protein CD47, highly expressed in a variety of cancer cells but undetectable in normal urothelium. | [49] |
| Single-walled carbon nanohorns QD-cisplatin (SWNH-QD+cis) TEM: 337 ± 11 nm | Fluorescence microscopy. | In vitro: rat AY-27 cancer cells. Incubation time: 1 h. Visualization time: 24, 48, and 72 h. | SWNH-QD+cis were well-trackable for 3 days. SWNH-QD+cis efficiently releases cisplatin in vitro. | [50] |
| BPN-BBTD AIEgen encapsulated into amphiphilic polymer NPs (BPN-BBTD NPs) TEM: 37.1 ± 2.3 nm | FLI: whole-body imaging in NIR II window and NIR I window. In particular: NIR II window using 785 nm laser beam InGaAs camera, a 1000 nm long-pass filter. NIR I window: exc 700, ems < 900 nm by IVIS® Spectrum. | In vivo: nude mice with subcutaneously xenografted bladder tumors or orthotopic bladder tumors (human UMUC3 cancer cells) were injected i.v. with BPN-BBTD NPs. Incubation/visualization time: 1 h. | BPN-BBTD NPs were capable of monitoring subcutaneous and orthotopic tumors for a long time (32 days). | [51] |
| QDs605 conjugated with an antibody against the prostate stem cell antigen (QD-PSCA). | Fluorescence microscopy. | In vitro: human EJ cancer cells. Concentration: 10 nM. Incubation time: 30 min. Visualization time: 6, 24, and 48 h. | QD-PSCA was able to specifically recognize the PSCA protein expressed in bladder cancer cells; fluorescence was stable and long-lasting. | [52] |
| Upconverting NPs coupled with gold nanorods conjugated with an antibody to epidermal growth factor (EGF) receptor (UCNP-AuNR nanocluster) TEM: 48.2 ± 5.17 nm | CLSM (exc 980 nm) | In vitro: human T24T cancer cells. Concentration: 8 × 1010/mL. Incubation/visualization time: 1–2 h. | High contrast imaging and high sensitivity detection of bladder cancer cells. Nanobubbles forming in the vicinity of the AuNRs after irradiation by a femtosecond pulsed laser were able to disrupt the cell membrane (useful for PDT). | [53] |
| Alexa-PEG-modified titanium dioxide NPs (TiO2-PEG NPs) DLS: 123.8 nm | Super-resolution fluorescence microscopy. | In vitro: human UMUC3 TCC. Incubation/visualization time: 1, 2, and 4 h. | The high uptake by bladder cancer cells led to the intracellular accumulation of TiO2-PEG NPs, thus increasing their fluorescence. | [54] |
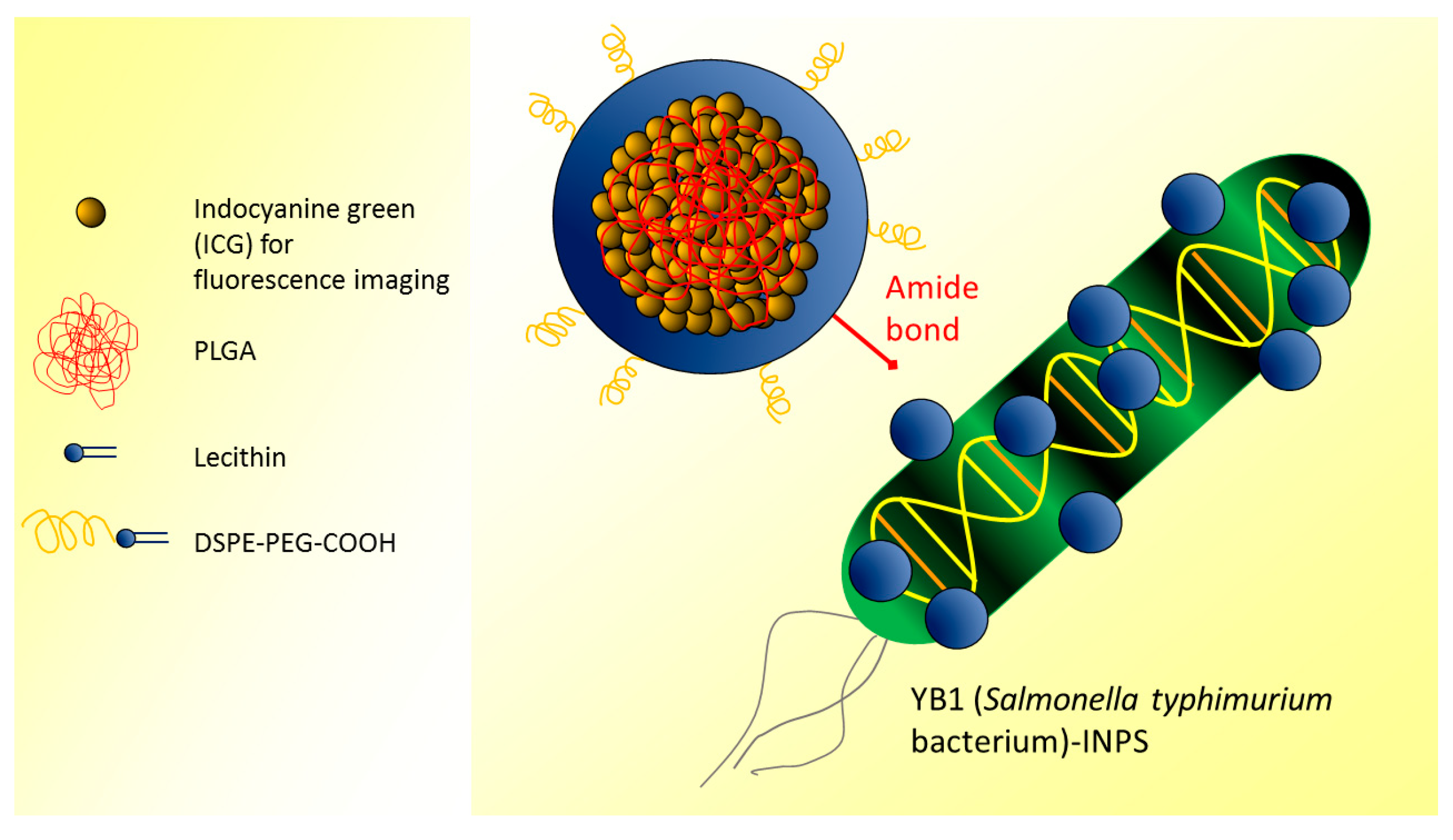
| Indocyanine green-loaded PLGA NPs covalently attached to YB1 (Salmonella typhimurium bacterium) (YB1-INPs) | FLI: OI by CRi Maestro (exc 704 nm, ems 735 nm) | In vivo: mice were subcutaneously injected with mouse MB49 cancer cells and injected i.v. with YB1-INPs. Concentration: 107 µg/mL. Incubation/visualization time: 12 h. Ex vivo: analysis of tumor sections. Incubation/visualization time: 72 h. | YB1-INPs acted as nanophotosensitizers leading to specific hypoxia and perfect photothermal conversion, targeting solid tumors, and showing efficient fluorescence imaging properties. | [55] |
| SiO2 NPs and liposomes labeled by cyanine (named tumor-selective cascade activatable self-detained system-TCASS) | FLI: OI by Maestro II and IVIS® Spectrum CT | In vivo: nude mice with xenografted EJ urothelial cancer cells were injected i.v. with NPs. Concentration: 14 mg/kg. Incubation/visualization time: from 1–120 h (Maestro II); from 2–48 h (IVIS). Humans: NPs instilled in intact excised human bladders. Concentration: 50 µM. Incubation/visualization time: 1 h. | The in vivo self-assembled molecules, combined with the NIR probe, showed high specificity and sensitivity for detecting bladder cancer cells. | [56] |
| BSA-multifunctional BITT@DSP NPs with an albumin-based NP decorated with the cisplatin (IV) prodrug and loaded to produce strong NIR FLI (BSA-BITT@DSP NPs) TEM and DLS: 70.2 ± 22.0 nm | FLI: CLSM In vivo OI by ChemiDoc MP imaging system (exc 647, ems 695 nm) | In vitro: mouse MB49 cancer cells. In vivo: mice bearing subcutaneous MB49 tumors were injected i.v. with BITT@BSA−DSP NPs. Incubation/visualization time: from 2–10 min. Ex vivo: analysis of excised organs. Incubation/visualization time: 6 h. | BITT@BSA−DSP NPs were efficiently taken up by bladder cancer cells both in vitro and in vivo. | [57] |
| Cationic polymer mucoadhesive EAGC-DOPE hybrid lipid NPs (EGCDNPs) complexed with Cy5-GFP-pDNA or FLuc-pDNA TEM: 30 ± 13 nm (EGCDNPs); from 67 ± 15 to 98 ± 28 nm (complexes) | FLI: CLSM Bioluminescent imaging: OI by IVIS® Spectrum. | In vitro: human UMUC3 and TCC-SUP bladder cancer cells; human PC-3prostate cancer cells; human U87-MG glioblastoma cells; human HEK-293 T embryonic kidney cells were treated with EGCDNPs—Cy5-GFP-pDNA complexes. In vivo: EGCDNPs—Cy5-GFP-pDNA complexes were instilled in the bladder of healthy mice. Concentration: 2.4 or 6.4 µg/mouse. Incubation/visualization time: 24 and 48 h. Ex vivo: bladder tissue was analyzed immunohistochemically for Luc detection. | Tuning the molecular weight of the mucoadhesive cationic polymer in NPs increased gene transfer by improving adherence and penetration through the bladder barrier. | [58] |
| Glycosylated PEGylated phospholipid upconverting NPs oleic acid-capped NaYF4: Yb 20%, Er 2%@NaYF4 core–shell structured with phospholipid mixture (X = 0, 25, 50, 75, or 100). (UCNP-GX) DLS: from 42.3 to 60 nm, depending on the phospholipid mixture | Multiphoton fluorescence imaging (exc 908 nm, ems 545 nm) | In vitro: UMUC3 cells. Concentration: 20 or 80 µg/mL. Incubation/visualization time: 2 h. | UCNP-G100 improved the contrast between bladder cancer and normal cells. For PDD, these NPs may be used together with a cystoscope equipped with a NIR light source. | [59] |
| AIE molecules obtained by incorporation of the tetraphenylethylene unit to the triazaborolopyridiniu-m encapsulated within phospholipid- connected PEG (TT-1@DSPE-PEG) DLS: 80.7–83.7 nm | FLI: CLSM (exc 488 nm, ems 550–590 nm and OI by IVIS® Spectrum (exc 500 nm, ems 560 nm) | In vitro: human H1299 lung cancer cells. Concentration: 5 or 10 mM. Incubation/visualization time: 2 h. In vivo: BBN-driven bladder cancer model mice were injected i.v. with TT-1@DSPE-PEG. Concentration: 40 mM. Incubation/visualization time: from 5 min to 4 h. Ex vivo: analysis of excised major organs. | NPs showed bleft red fluorescence within the cells after a short incubation time. The increased fluorescence signal observed ex vivo in the tumor and intestine of treated mice indicated NP accumulation. | [60] |
| Nanoparticles | Imaging Technique | Experimental Design | Main Results | Ref. |
|---|---|---|---|---|
| Nanoplatform SPIO with phosphonate group (PO)-PEG and antibody against ETA receptor labeled with Alexafluor 488 (γFe2O3@PO-PEGx-Ab-AF488) TEM: 9.6 nm | MRI (7 T) | In vivo: mice injected i.v. with NPs. Concentration: 200 µM Fe/kg | Efficiency of the antibody to target specifically ETA receptor overexpressed on different bladder cancer cells. The high r2/r1 ratios confirmed the great potential of these NPs as T2-shortening contrast agents for contrast-enhanced MRI applications. Labeling with Alexafluor 488 made these NPs potentially useful for bimodal imaging (MRI and FLI). | [61] |
| Cyc6-functionalizedMesoporous Silica NPs (Cyc6-FITC-Gd2O3-MSN) DLS: 187.3 nm | MRI (4.7 T) | In vivo: MSN instillation into the bladder of mice bearing Luc+ murine MB49 TCC and human T2442 TCC orthotopic tumor. Incubation/visualization time: 6–8 days. | Enhanced T1- and T2-weighted MRI signals, improving the detection of the tumor boundaries. Cyc6 peptide improved binding efficiency and specificity to bladder cancer cells. | [62] |
| Nanoscale oxygen generator (PLZ4@SeD) encapsulating SPIO NPs and organoselenium with PLZ4 peptide for bladder cancer targeting (PLZ4@SeD) TEM: ~150 nm | MRI (1.5 T) | In vitro: human EJ cancer cells. Concentration: until 4 µM. Incubation/visualization time: from 1 to 8 h. Humans: PLZ4@SeD was instilled into bladders from patients after the radical cystectomy. Concentration: 1 mM. Incubation/visualization time: from 1–8 h. | PLZ4@SeD precisely targeted the tumor inside the bladder and enhanced the T2 MRI contrast | [63] |
| Black phosphorus nanosheets covalently bond with SPIO selenide to construct heteronanostructure NPs modified with methoxy PEG (mPEG-NH2) (BPs-FeSe2-PEG) AFM: ~10 nm | MRI (9.4 T) | In vitro: human EJ cancer cells. Concentration: until 0.02 mM. In vivo: BPs-FeSe2-PEG were injected i.v. in nude mice with a subcutaneous cancer model. Concentration: 10 mg/kg. Incubation/visualization time: from 2 to 24 h. | BPs-FeSe2-PEG acted as a T2 MRI contrast agent. NPs enhanced photothermal conversion efficiency and photostability to realize MRI-guided PTT. | [64] |
| Nanoparticles | Imaging Technique | Experimental Design | Main Results | Ref. |
|---|---|---|---|---|
| QD-capped magnetite Nanorings (QD-FVIO) TEM: 210 and 100 nm DLS: 310 and 155 nm | Bimodal imaging: FLI (two-photon microscopy exc 756 nm, ems long pass 560 nm) and MRI (1.5 T) | In vitro: MGH cancer cells. Concentration: 0.05 mg/mL. Incubation and visualization: 24 h. | QD-FVIO’s r2* relaxivity and r2*/r1 ratio were 4 times and 2 orders of magnitude, respectively, greater than those of commercial SPIOs (ferucarbotran). The uptake and intracellular fate of QD-FVIOs were monitored | [65] |
| Bimodal Mesoporous Silica NPs (PEG-TRITC-Gd2O3-MSN) DLS: 80–180 nm | Bimodal imaging: FLI (OI by IVIS® 200) and MRI (4.7 T). Fluorescence microscopy | In vitro: Luc+ murine MB49 TCC and human T24 TCC. In vivo: (1) Subcutaneous injection of TCC labeled with MSN or instillation into the bladder of mice. Concentration: 1 × 105 cells. (2) Installation of free MSN into the bladder after tumor development. Concentration: 5 × 105 cells Ex vivo: Microscopy analysis of excised bladders. | High cell uptake of MSN. MRI revealed in vivo detailed structural features of the tumor boundaries. MSN further functionalized with a peptide (CF3) bound specifically bladder cancer cells. | [22] |
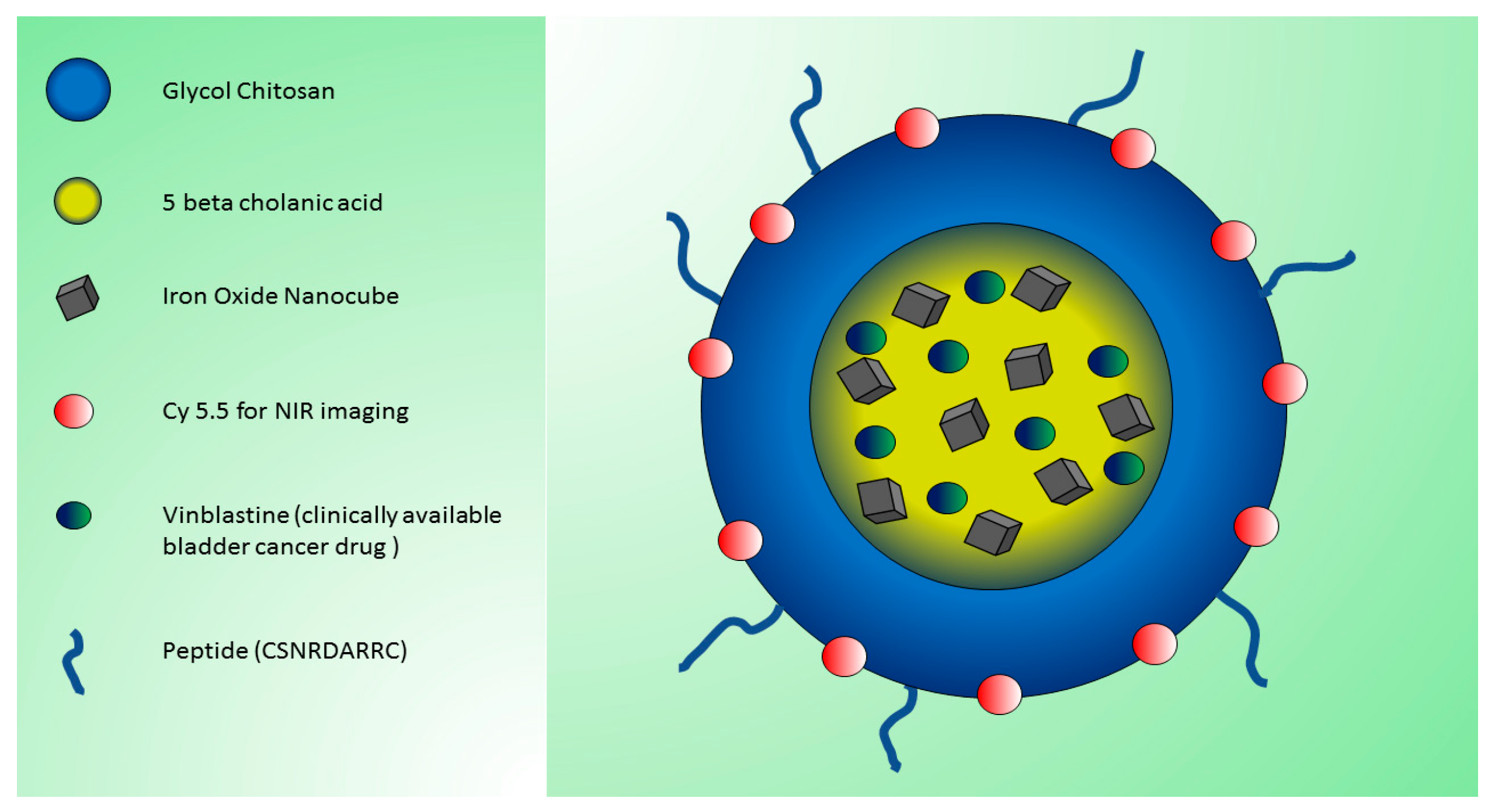
| Bimodal dual-modality peptide (CSNRDARRC)-conjugated NPs with iron oxide nanocubes and glycol chitosan derivatives (pMCNPs) DLS: 481.8 ± 8.7 nm | Bimodal imaging: FLI (OI by IVIS® II Lumina using cyanine 5.5; exc 675 nm, ems 695 nm) and MRI (3.0 T) | In vivo: subcutaneous injection in nude mice of tumor canine K9TCC cancer cells incubated with NPs. Incubation/visualization time: 24 h. Ex vivo: analysis of major organs. | Development of novel MRI and NIRF dual-modality NPs. pMCNPs showed preferential accumulation and longer retention in small tumors (useful as 3T MRI contrast agents). pMCNPs acted as therapeutic agents using vinblastine. | [66] |
| Bimodal human serum albumin-MNO2-chlorin e6-NPs (HSA-MnO2-Ce6 NPs) DLS: 118.6 ± 8.1 nm Other NPs for single imaging technique: HSA-MnO2 (18.5 ± 4.8 nm) HSA-Ce6 (112.8 ± 7.4 nm) | Bimodal imaging: FLI (OI by IVIS® Lumina; exc 675 nm, ems 710–900 nm) and MRI (7.0 T) | In vitro: mouse MB49 cancer cells In vivo: orthotopic bladder cancer model obtained by MB49 cells injection; NPs were administered i.v. Concentration: from 0.05 to 0.4 mM. Incubation/visualization time: 12 h. Ex vivo: analysis of excised major organs. | Excellent bladder tumor-targeting property of HSA-MnO2-Ce6 NPs. PDT with HSA-MnO2-Ce6 NPs showed therapeutic efficacy and significantly prolonged the lifetime of mice. | [67] |
| Nanoparticles | Imaging Technique | Experimental Design | Main Results | Ref. |
|---|---|---|---|---|
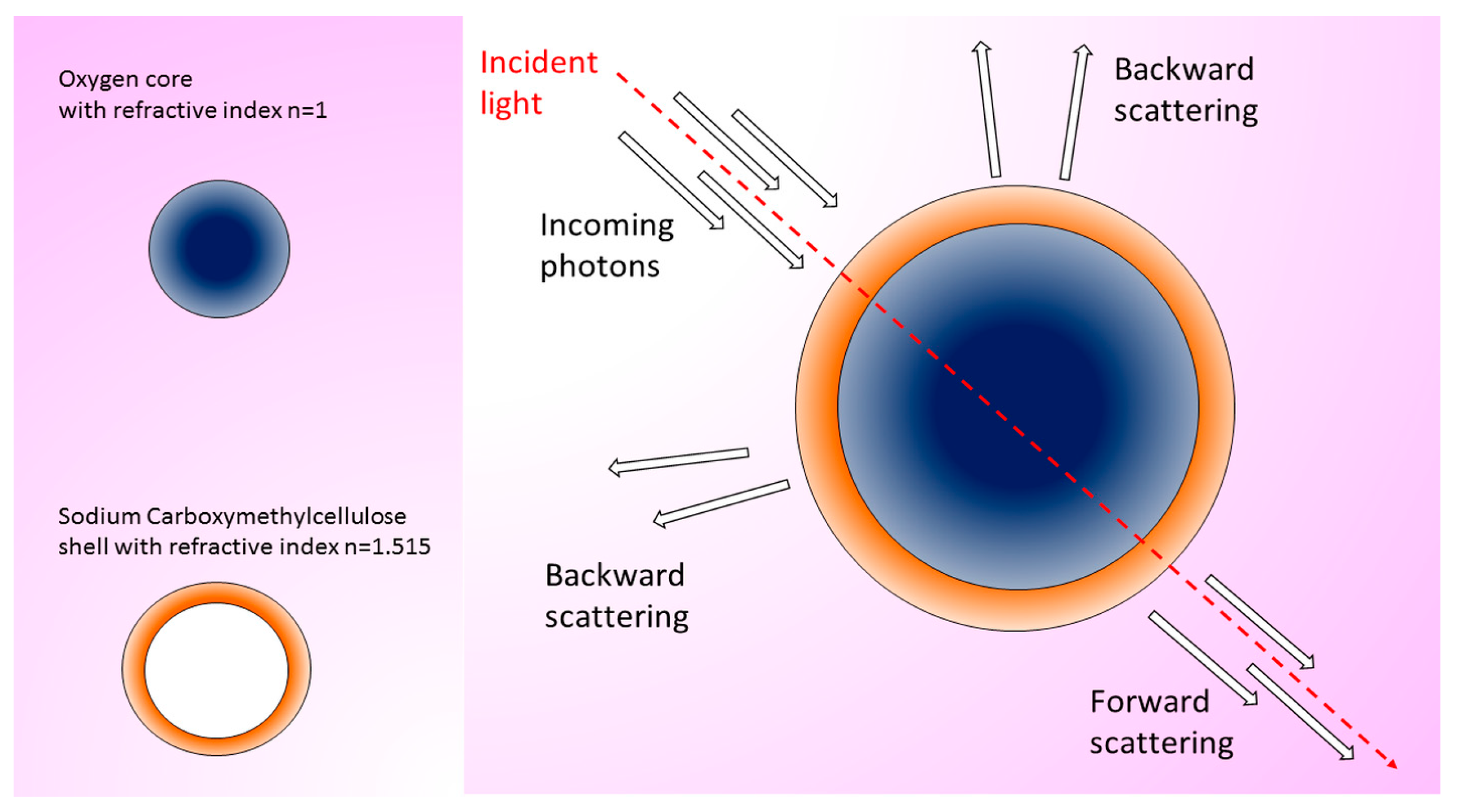
| Two-sized oxygen nanobubbles (ONBs) TEM: 400 and 800 nm | HyperSpectral Dark Field Microscope (HSDFM) | In vitro: mouse MB49 cancer cells. Ex vivo: subcutaneous injection of MB49 cells in mice and treatment with ONBs. Excision of tumor and analysis of wax-embedded tissue slices. Concentration: 100 µg/mL. Incubation/visualization time: 4 days. | ONBs are suitable for in vitro imaging in single cells and ex vivo imaging in bladder cancer tissues thanks to the intense scattering signal. | [68] |
| Gold nanostars coated with silver and silica (AuNS@Ag@SiO2) | Surface-enhanced Raman scattering (SERS) in vivo, but visible ex vivo with multiphoton microscopy (exc 800 nm) | In vivo: mice were injected subcutaneously with MB49 cancer cells; after tumor development, NPs were injected i.v. Concentration: 3.3 mg/mL. Incubation/visualization time: 24 h. Ex vivo: analysis of excised tumors | Nanostars accumulated in the tumor but not in the healthy tissue. | [69] |
| Hyaluronic acid modified and liposome-coated IR1048 NPs (HAPO-1048 NPs) TEM: 135.2 nm | Optical coherence tomography angiography (OCTA) and photoacoustic (PA) imaging NIR-II region two peaks around 930 nm and 1100 nm | In vitro: Luc+ UMUC3 cells. In vivo: HAPO-1048 NPs were injected i.v. in an orthotopic murine model of bladder cancer. Concentration: 200 µg/mL. Incubation/visualization time: from 3–72 h Ex vivo: analysis of excised major organs. | HAPO-1048 proved to be a NIR-II photothermal agent for CD44-overexpressing bladder cancer, showing strong NIR II optical absorption, preferential tumor targeting, excellent biocompatibility, and high PTT efficacy. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschi, F.; Malatesta, M. Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review. Int. J. Mol. Sci. 2023, 24, 3812. https://doi.org/10.3390/ijms24043812
Boschi F, Malatesta M. Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review. International Journal of Molecular Sciences. 2023; 24(4):3812. https://doi.org/10.3390/ijms24043812
Chicago/Turabian StyleBoschi, Federico, and Manuela Malatesta. 2023. "Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review" International Journal of Molecular Sciences 24, no. 4: 3812. https://doi.org/10.3390/ijms24043812
APA StyleBoschi, F., & Malatesta, M. (2023). Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review. International Journal of Molecular Sciences, 24(4), 3812. https://doi.org/10.3390/ijms24043812







