Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo
Abstract
1. Introduction
2. Results
2.1. In Vitro Assay for α-MSH-SM-Liposome and Mg and/or Mg-10Gd Interaction
2.1.1. Fluorometric Analysis
2.1.2. Liposome Effect on Degradation of Mg
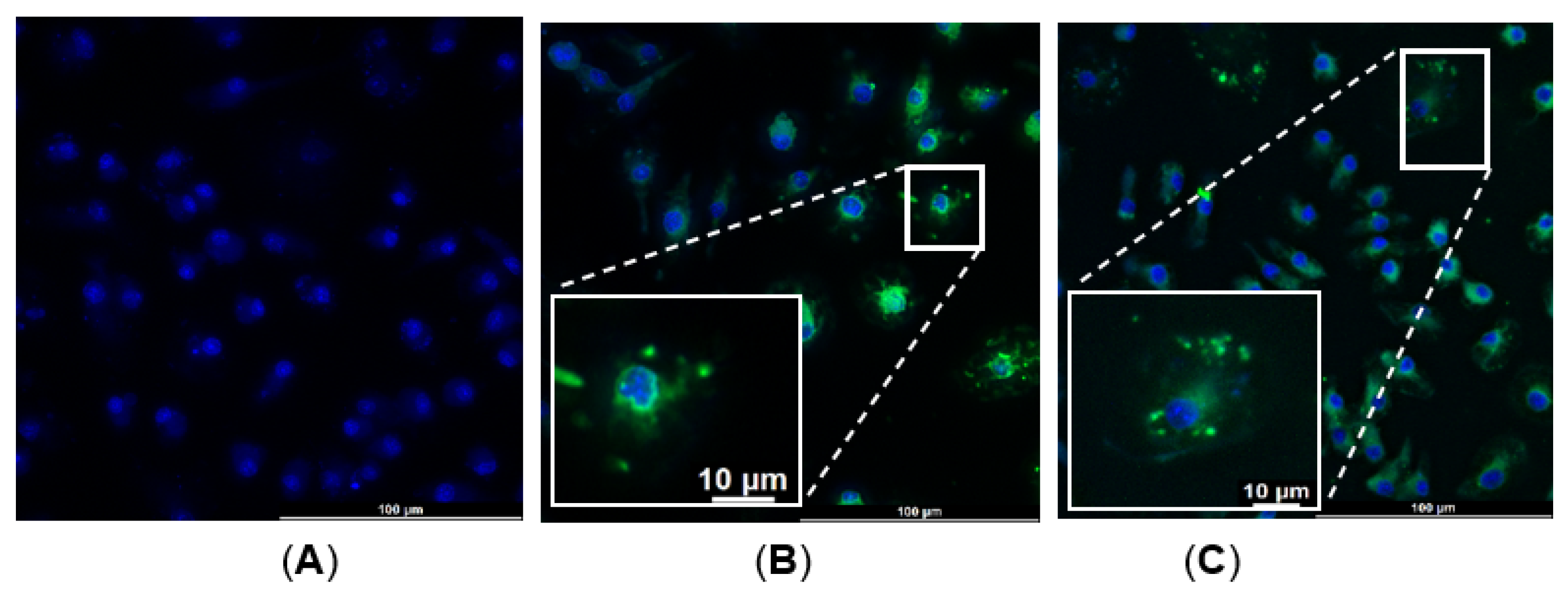
2.1.3. Bone-Marrow-Derived Macrophages (BMDM)—α-MSH-SM-Liposome Uptake Assay
2.2. In Vivo Evaluation of α-MSH-SM-Liposomes
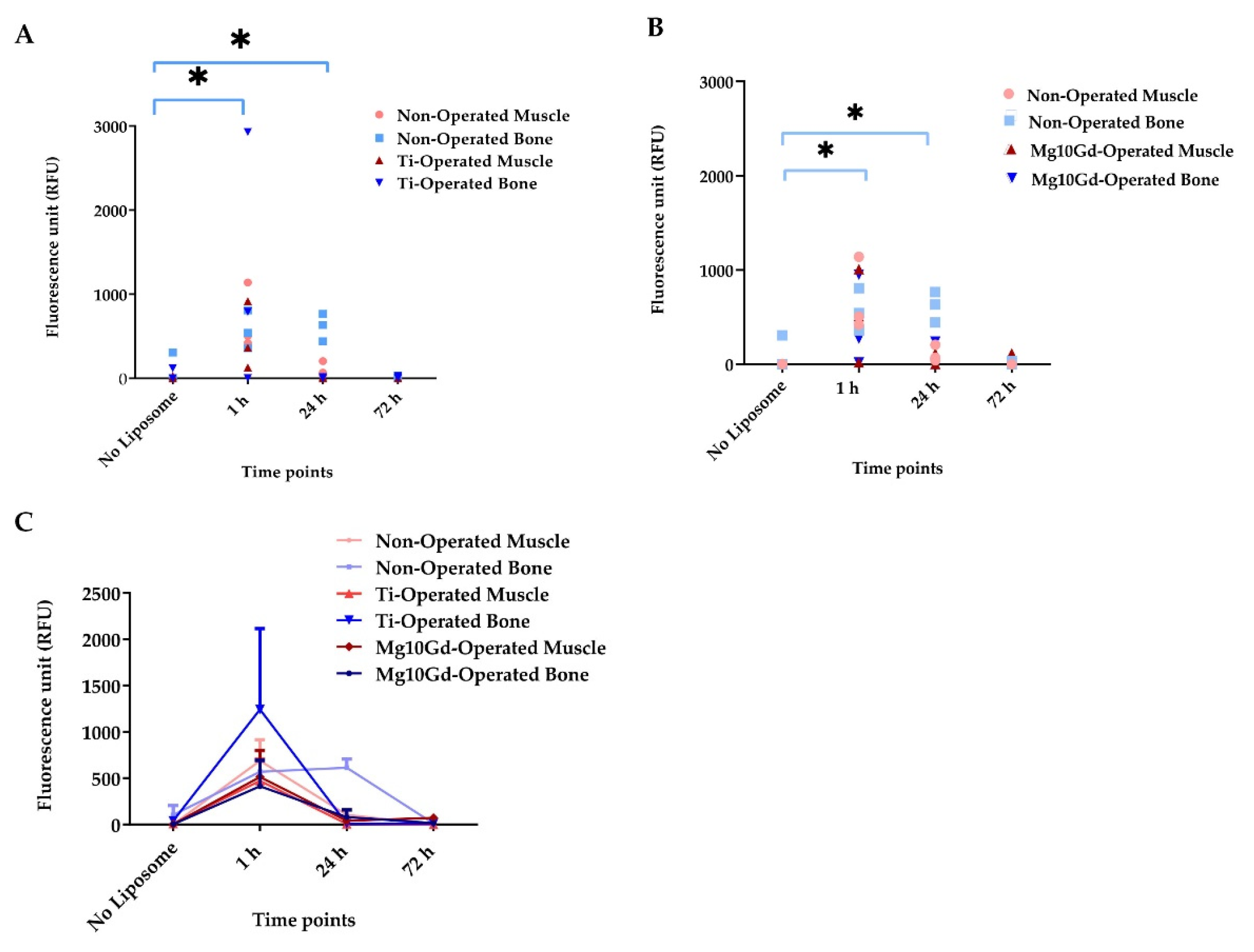
2.3. Ex Vivo Biodistribution Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of the Implants
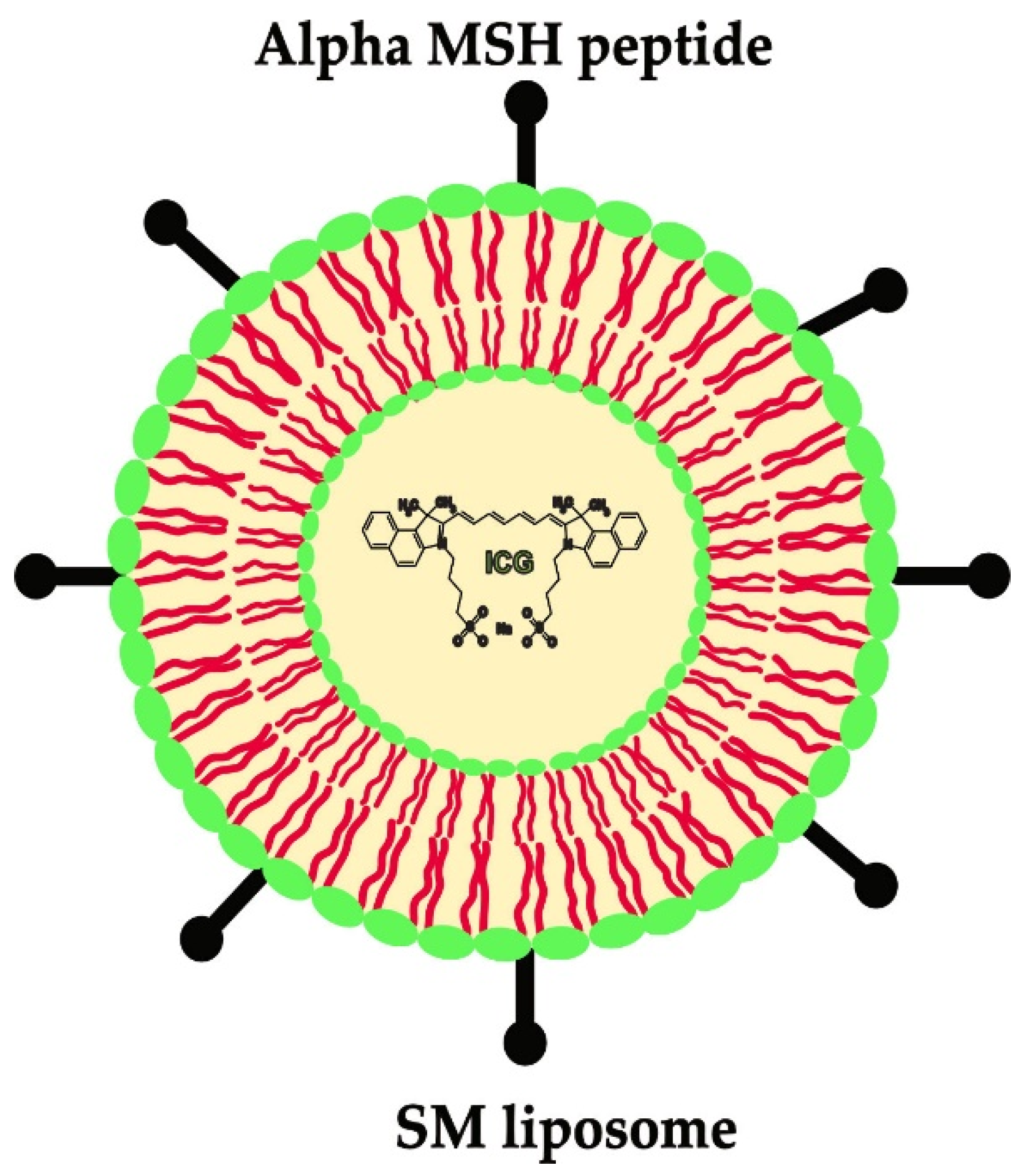
4.2. Preparation of α-MSH-SM-Liposome
4.3. In Vitro Studies: Material and Liposome Effect on Each Other
4.3.1. Fluorescence Measurement to Analyze the Ion Effect on Liposomal ICG Fluorescence
4.3.2. Liposome Effect on Degradation of Mg
4.4. Macrophage and α-MSH-SM-Liposomes Uptake Assay
4.4.1. Preparation of Bone-Marrow-Derived Macrophages In Vitro
4.4.2. BMDM Quality Check with Flow Cytometry
4.4.3. Liposome Assay with BMDM
4.5. Animal Experiments
4.6. Imaging of α-MSH-SM-Liposomes
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MSH | Alpha-melanocyte-stimulating hormone |
| Mg | Magnesium |
| Gd | Gadolinium |
| SM | Sphingomyelin |
| PLGA | Polylactide–co-glycolide |
| RE | Rare earth |
| ICG | Indocyanine green |
| IBD | Inflammatory bowel disease |
| MC | Melanocortin |
| PBS | Phosphate buffer saline |
| DMEM | Dulbecco’s modified eagle medium |
| ROI | Region of interest |
| NPD–αMSH | [Nle4,D-Phe7] α-melanocyte-stimulating hormone |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| RT | Room temperature |
| Ex/Em | Excitation/Emission |
| BMDM | Bone-marrow-derived macrophage |
References
- Jorge, J.R.; Barao, V.A.; Delben, J.A.; Faverani, L.P.; Queiroz, T.P.; Assuncao, W.G. Titanium in dentistry: Historical development, state of the art and future perspectives. J. Indian Prosthodont. Soc. 2013, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Ignatius, A. Development of new, biodegradable implants. Der Chir. 2002, 73, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys with Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Makkar, P.; Sarkar, S.K.; Padalhin, A.R.; Moon, B.G.; Lee, Y.S.; Lee, B.T. In vitro and in vivo assessment of biomedical Mg-Ca alloys for bone implant applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 126–136. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Cecchinato, F.; Agha, N.A.; Martinez-Sanchez, A.H.; Luthringer, B.J.; Feyerabend, F.; Jimbo, R.; Willumeit-Romer, R.; Wennerberg, A. Influence of Magnesium Alloy Degradation on Undifferentiated Human Cells. PLoS ONE 2015, 10, e0142117. [Google Scholar] [CrossRef]
- Bian, D.; Deng, J.; Li, N.; Chu, X.; Liu, Y.; Li, W.; Cai, H.; Xiu, P.; Zhang, Y.; Guan, Z.; et al. In Vitro and in Vivo Studies on Biomedical Magnesium Low-Alloying with Elements Gadolinium and Zinc for Orthopedic Implant Applications. ACS Appl. Mater. Interfaces 2018, 10, 4394–4408. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Fechner, D.; Stormer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drucker, H.; Willumeit, R.; Kainer, K.U.; et al. Magnesium alloys as implant materials--principles of property design for Mg-RE alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef]
- Myrissa, A.; Agha, N.A.; Lu, Y.; Martinelli, E.; Eichler, J.; Szakacs, G.; Kleinhans, C.; Willumeit-Romer, R.; Schafer, U.; Weinberg, A.M. In vitro and in vivo comparison of binary Mg alloys and pure Mg. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Kubasek, J.; Vojtech, D. Structural characteristics and corrosion behavior of biodegradable Mg-Zn, Mg-Zn-Gd alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drucker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Romer, R.; Luthringer-Feyerabend, B.J.C. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Charyeva, O.; Dakischew, O.; Sommer, U.; Heiss, C.; Schnettler, R.; Lips, K.S. Biocompatibility of magnesium implants in primary human reaming debris-derived cells stem cells in vitro. J. Orthop. Traumatol. 2016, 17, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zeller-Plumhoff, B.; Malich, C.; Kruger, D.; Campbell, G.; Wiese, B.; Galli, S.; Wennerberg, A.; Willumeit-Romer, R.; Wieland, D.C.F. Analysis of the bone ultrastructure around biodegradable Mg-xGd implants using small angle X-ray scattering and X-ray diffraction. Acta Biomater. 2020, 101, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Duparc, J.; Massin, P. Results of 203 total hip replacements using a smooth, cementless femoral component. J. Bone Jt. Surg. Br. Vol. 1992, 74, 251–256. [Google Scholar] [CrossRef]
- Goodman, S.B. The effects of micromotion and particulate materials on tissue differentiation. Bone chamber studies in rabbits. Acta Orthop. Scand. Suppl. 1994, 258, 1–43. [Google Scholar] [CrossRef]
- Schaller, B.; Matthias Burkhard, J.P.; Chagnon, M.; Beck, S.; Imwinkelried, T.; Assad, M. Fracture Healing and Bone Remodeling with Human Standard-Sized Magnesium Versus Polylactide-Co-Glycolide Plate and Screw Systems Using a Mini-Swine Craniomaxillofacial Osteotomy Fixation Model. J. Oral Maxillofac. Surg. 2018, 76, 2138–2150. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Patel, H.B.; Montero-Melendez, T.; Greco, K.V.; Perretti, M. Melanocortin receptors as novel effectors of macrophage responses in inflammation. Front. Immunol. 2011, 2, 41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.; Lyytikainen, L.P.; Raitoharju, E.; Kadiri, J.J.; Kholova, I.; Kahonen, M.; Lehtimaki, T.; Oksala, N. Pro-opiomelanocortin and its Processing Enzymes Associate with Plaque Stability in Human Atherosclerosis—Tampere Vascular Study. Sci. Rep. 2018, 8, 15078. [Google Scholar] [CrossRef] [PubMed]
- Humbert, J.; Will, O.; Penate-Medina, T.; Penate-Medina, O.; Jansen, O.; Both, M.; Gluer, C.C. Comparison of photoacoustic and fluorescence tomography for the in vivo imaging of ICG-labelled liposomes in the medullary cavity in mice. Photoacoustics 2020, 20, 100210. [Google Scholar] [CrossRef]
- Penate-Medina, T.; Damoah, C.; Benezra, M.; Will, O.; Kairemo, K.; Humbert, J.; Sebens, S.; Penate-Medina, O. Alpha-MSH Targeted Liposomal Nanoparticle for Imaging in Inflammatory Bowel Disease (IBD). Curr. Pharm. Des. 2020, 26, 3840–3846. [Google Scholar] [CrossRef]
- Ferreira, M.; Aguiar, S.; Bettencourt, A.; Gaspar, M.M. Lipid-based nanosystems for targeting bone implant-associated infections: Current approaches and future endeavors. Drug Deliv. Transl. Res. 2021, 11, 72–85. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Niska, J.A.; Meganck, J.A.; Pribaz, J.R.; Shahbazian, J.H.; Lim, E.; Zhang, N.; Rice, B.W.; Akin, A.; Ramos, R.I.; Bernthal, N.M.; et al. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and muCT imaging in an orthopaedic implant infection in mice. PLoS ONE 2012, 7, e47397. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Taylor, B.N.; Meganck, J.A.; Wang, Y.; Shahbazian, J.H.; Niska, J.A.; Francis, K.P.; Miller, L.S. Combined in vivo optical and microCT imaging to monitor infection, inflammation, and bone anatomy in an orthopaedic implant infection in mice. J. Vis. Exp. 2014, 92, e51612. [Google Scholar] [CrossRef]
- Marelli, G.; Avigni, R.; Allavena, P.; Garlanda, C.; Mantovani, A.; Doni, A.; Erreni, M. Optical in vivo imaging detection of preclinical models of gut tumors through the expression of integrin αVβ3. Oncotarget 2018, 9, 31380–31396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daghighi, S.; Sjollema, J.; Dijkstra, R.J.; Jaspers, V.; Zaat, S.A.; van der Mei, H.C.; Busscher, H.J. Real-time quantification of matrix metalloproteinase and integrin αVβ3 expression during biomaterial-associated infection in a murine model. Eur. Cells Mater. 2014, 27, 26–37; discussion 28–37. [Google Scholar] [CrossRef] [PubMed]
- Scales, H.E.; Ierna, M.; Smith, K.M.; Ross, K.; Meiklejohn, G.R.; Patterson-Kane, J.C.; McInnes, I.B.; Brewer, J.M.; Garside, P.; Maffia, P. Assessment of murine collagen-induced arthritis by longitudinal non-invasive duplexed molecular optical imaging. Rheumatology 2016, 55, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Penate Medina, T.; Kolb, J.P.; Huttmann, G.; Huber, R.; Penate Medina, O.; Ha, L.; Ulloa, P.; Larsen, N.; Ferrari, A.; Rafecas, M.; et al. Imaging Inflammation—From Whole Body Imaging to Cellular Resolution. Front. Immunol. 2021, 12, 692222. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Strijkers, G.J.; Kluza, E.; Van Tilborg, G.A.; van der Schaft, D.W.; Griffioen, A.W.; Mulder, W.J.; Nicolay, K. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 2010, 13, 161–173. [Google Scholar] [CrossRef]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Ferrari, M. Nanovector therapeutics. Curr. Opin. Chem. Biol. 2005, 9, 343–346. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Peñate Medina, T.; Gerle, M.; Humbert, J.; Chu, H.; Köpnick, A.L.; Barkmann, R.; Garamus, V.M.; Sanz, B.; Purcz, N.; Will, O.; et al. Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release. Cancers 2020, 12, 3767. [Google Scholar] [CrossRef]
- Heneweer, C.; Penate Medina, T.; Tower, R.; Kalthoff, H.; Kolesnick, R.; Larson, S.; Penate Medina, O. Acid-Sphingomyelinase Triggered Fluorescently Labeled Sphingomyelin Containing Liposomes in Tumor Diagnosis after Radiation-Induced Stress. Int. J. Mol. Sci. 2021, 22, 3864. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.; Will, O.; Penate-Medina, T.; Humbert, J.; Damm, T.; Luthringer-Feyerabend, B.; Willumeit-Romer, R.; Gluer, C.C.; Penate-Medina, O. Tissue responses after implantation of biodegradable Mg alloys evaluated by multimodality 3D micro-bioimaging in vivo. J. Biomed. Mater. Res. Part A 2021, 109, 1521–1529. [Google Scholar] [CrossRef]
- Dapunt, U.; Giese, T.; Lasitschka, F.; Reinders, J.; Lehner, B.; Kretzer, J.P.; Ewerbeck, V.; Hänsch, G.M. On the inflammatory response in metal-on-metal implants. J. Transl. Med. 2014, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Medina, O.P.; Tower, R.J.; Medina, T.P.; Ashkenani, F.; Appold, L.; Botcher, M.; Huber, L.; Will, O.; Ling, Q.; Hauser, C.; et al. Multimodal Targeted Nanoparticle-Based Delivery System for Pancreatic Tumor Imaging in Cellular and Animal Models. Curr. Pharm. Des. 2022, 28, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bhoil, A.; Caw, H.; Vinjamuri, S. Role of 18F-flurodeoxyglucose in orthopaedic implant-related infection: Review of literature and experience. Nucl. Med. Commun. 2019, 40, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Penate Medina, T.; Pan, J.; Damoah, C.; Humbert, J.; Kopnick, A.L.; Will, O.; Sebens, S.; Penate Medina, O. Utilizing Sphingomyelinase Sensitizing Liposomes in Imaging Intestinal Inflammation in Dextran Sulfate Sodium-Induced Murine Colitis. Biomedicines 2022, 10, 413. [Google Scholar] [CrossRef]
- Xu, L.; Willumeit-Romer, R.; Luthringer-Feyerabend, B. Hypoxia influences the effects of magnesium degradation products on the interactions between endothelial and mesenchymal stem cells. Acta Biomater. 2020, 101, 624–636. [Google Scholar] [CrossRef]
- Bessa-Goncalves, M.; Silva, A.M.; Bras, J.P.; Helmholz, H.; Luthringer-Feyerabend, B.J.C.; Willumeit-Romer, R.; Barbosa, M.A.; Santos, S.G. Fibrinogen and magnesium combination biomaterials modulate macrophage phenotype, NF-kB signaling and crosstalk with mesenchymal stem/stromal cells. Acta Biomater. 2020, 114, 471–484. [Google Scholar] [CrossRef]
- Nidadavolu, E.P.S. On the Determination of Magnesium Degradation Rates under Physiological Conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Willumeit-Romer, R.; Luthringer-Feyerabend, B.J.C. Effect of magnesium-degradation products and hypoxia on the angiogenesis of human umbilical vein endothelial cells. Acta Biomater. 2019, 98, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.Q.; Scharnagl, N.; Willumeit-Romer, R.; Feyerabend, F. Different effects of single protein vs. protein mixtures on magnesium degradation under cell culture conditions. Acta Biomater. 2019, 98, 256–268. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyaz, S.; Helmholz, H.; Penate Medina, T.; Peñate Medina, O.; Will, O.; Sun, Y.; Wiese, B.; Glüer, C.-C.; Willumeit-Römer, R. Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo. Int. J. Mol. Sci. 2023, 24, 1103. https://doi.org/10.3390/ijms24021103
Riyaz S, Helmholz H, Penate Medina T, Peñate Medina O, Will O, Sun Y, Wiese B, Glüer C-C, Willumeit-Römer R. Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo. International Journal of Molecular Sciences. 2023; 24(2):1103. https://doi.org/10.3390/ijms24021103
Chicago/Turabian StyleRiyaz, Sana, Heike Helmholz, Tuula Penate Medina, Oula Peñate Medina, Olga Will, Yu Sun, Björn Wiese, Claus-Christian Glüer, and Regine Willumeit-Römer. 2023. "Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo" International Journal of Molecular Sciences 24, no. 2: 1103. https://doi.org/10.3390/ijms24021103
APA StyleRiyaz, S., Helmholz, H., Penate Medina, T., Peñate Medina, O., Will, O., Sun, Y., Wiese, B., Glüer, C.-C., & Willumeit-Römer, R. (2023). Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo. International Journal of Molecular Sciences, 24(2), 1103. https://doi.org/10.3390/ijms24021103






