Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF
Abstract
1. Introduction
2. Results
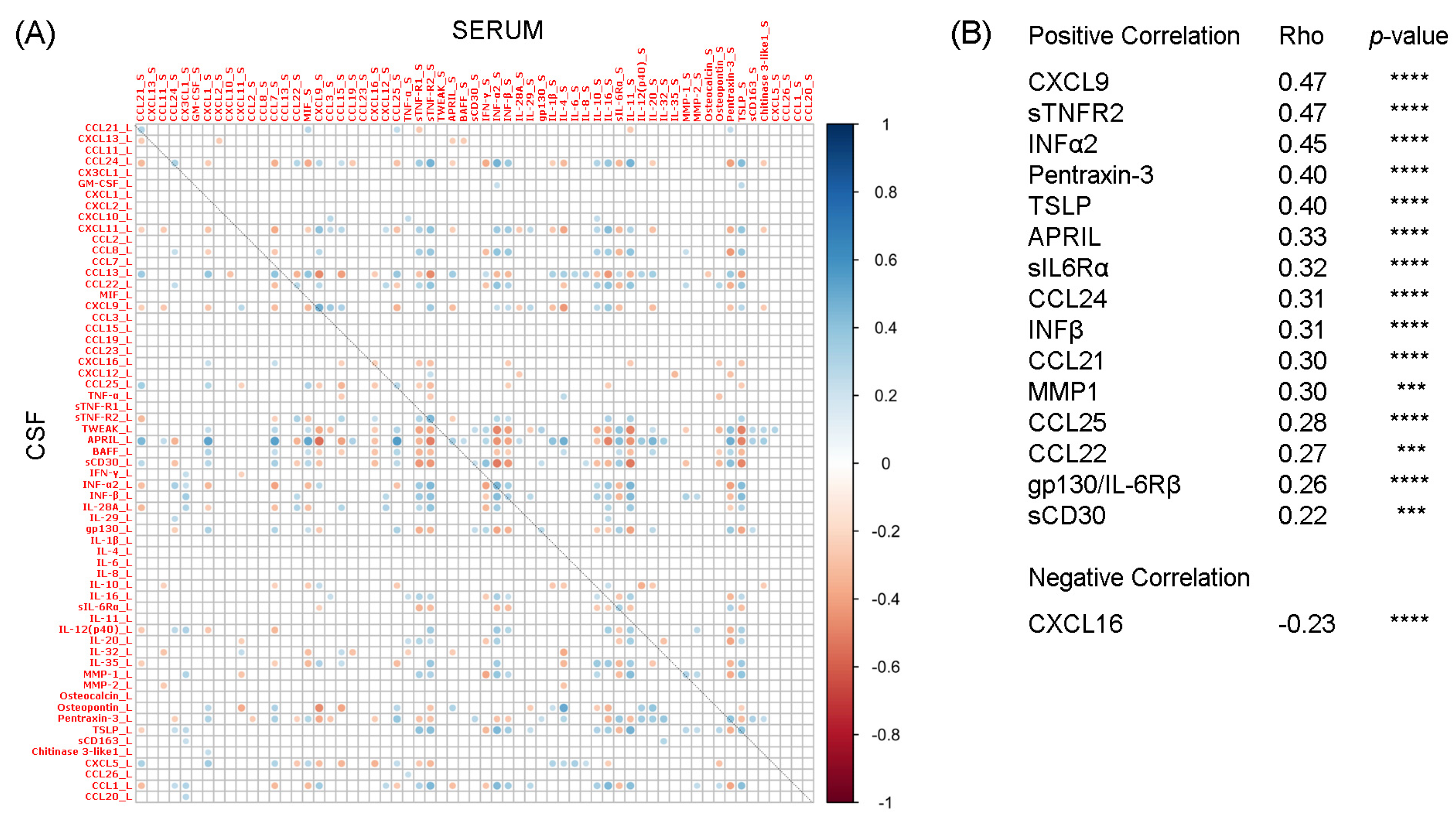
2.1. Correlation Analysis between Liquoral and Serum Expression Levels of 61 Inflammatory Mediators
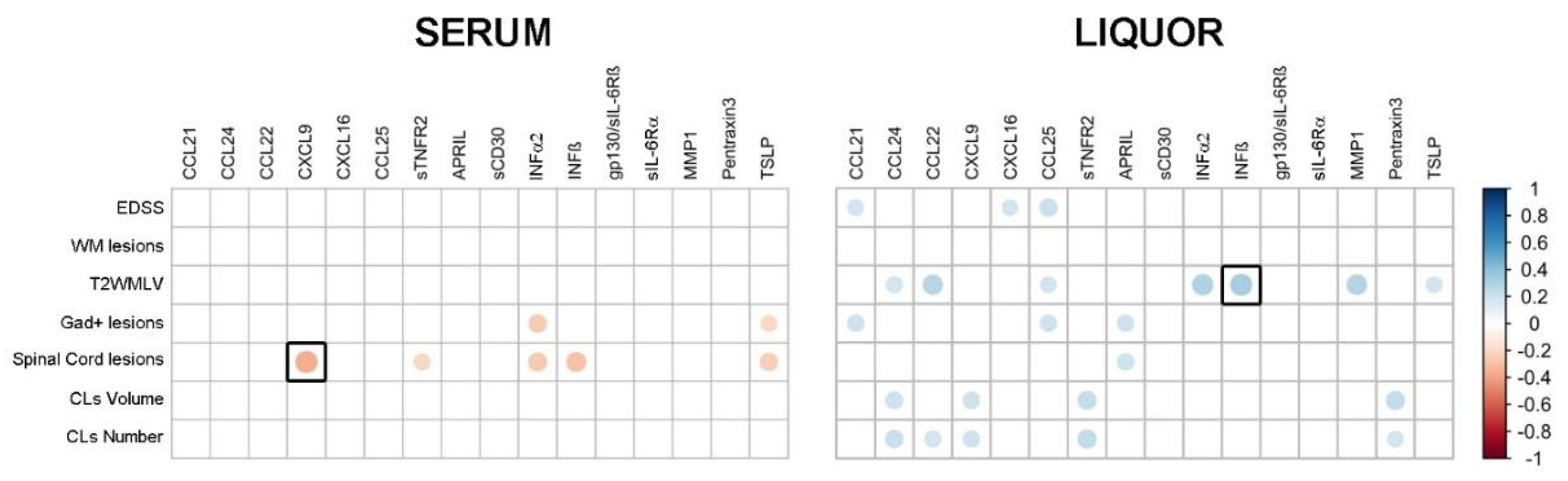
2.2. Correlations between Serum-CSF Profiles and Clinical-MRI Parameters of MS Patients at the Time of Diagnosis
2.3. Serum Expression Level of the 16 Molecules in MS Patients Compared to OND and HS
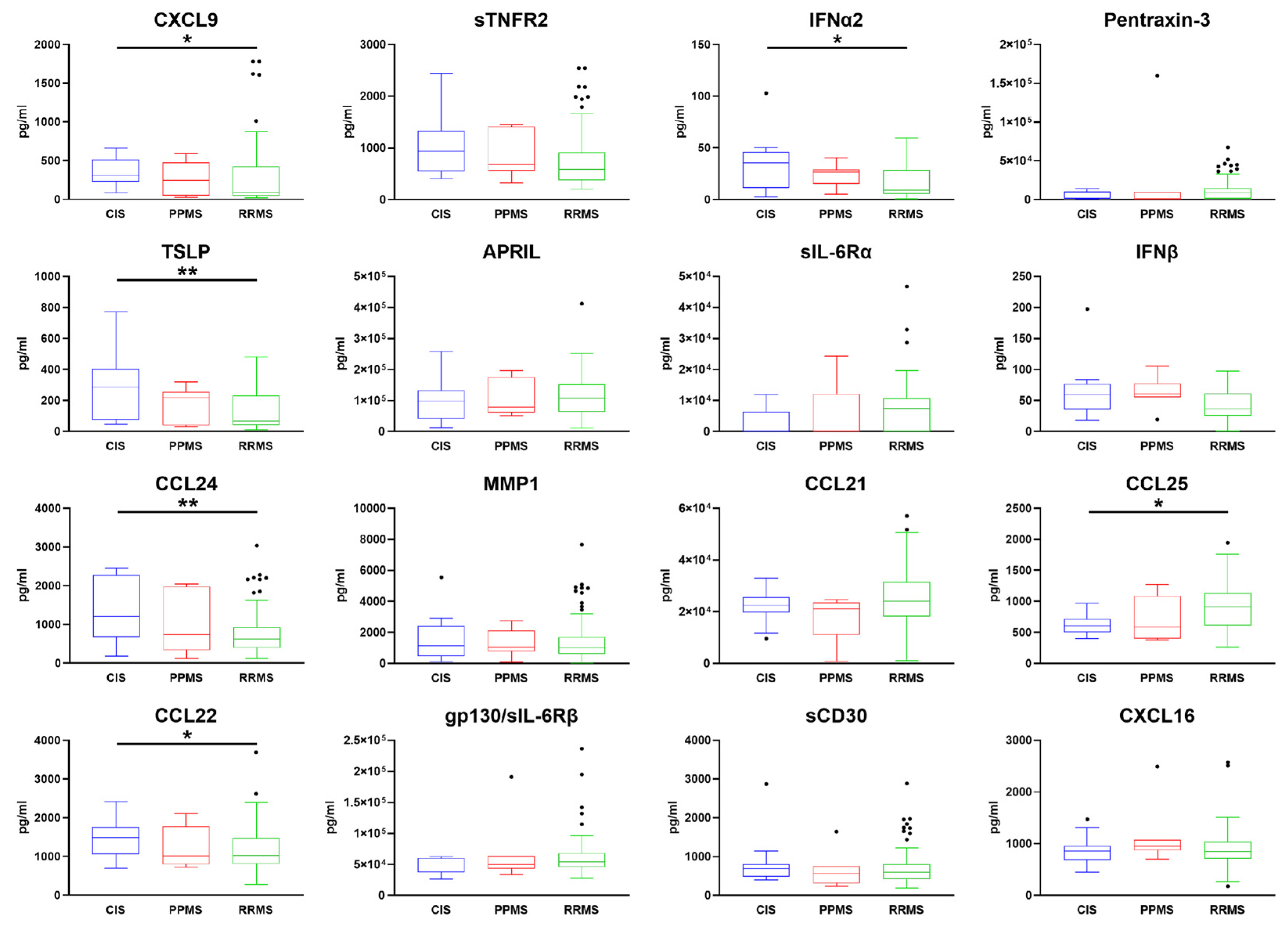
2.4. Comparison of Serum Expression Levels between RR, PP, and CIS MS Subgroups
3. Discussion
4. Materials and Methods
4.1. MS Patient Cohort and Clinical Evaluation
4.2. MRI
4.3. Serum and CSF Immunoassay Protein Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teunissen, C.E.; Malekzadeh, A.; Leurs, C.; Bridel, C.; Killestein, J. Body Fluid Biomarkers for Multiple Sclerosis—The Long Road to Clinical Application. Nat. Rev. Neurol. 2015, 11, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.; Kraus, J. Laboratory Biomarkers of Multiple Sclerosis (MS). Clin. Biochem. 2022, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meinl, E.; Krumbholz, M.; Derfuss, T.; Junker, A.; Hohlfeld, R. Compartmentalization of Inflammation in the CNS: A Major Mechanism Driving Progressive Multiple Sclerosis. J. Neurol. Sci. 2008, 274, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Nicholas, R.; Reynolds, R.; Magliozzi, R. Intrathecal Inflammation in Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 8217. [Google Scholar] [CrossRef]
- Komori, M.; Blake, A.; Greenwood, M.; Lin, Y.C.; Kosa, P.; Ghazali, D.; Winokur, P.; Natrajan, M.; Wuest, S.C.; Romm, E.; et al. Cerebrospinal Fluid Markers Reveal Intrathecal Inflammation in Progressive Multiple Sclerosis. Ann. Neurol. 2015, 78, 3–20. [Google Scholar] [CrossRef]
- Kabat, E.A.; Moore, D.H.; Landow, H. An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J. Clin. Investig. 1942, 21, 571–577. [Google Scholar] [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Grant, D.; Morant, S.; Furby, J.; Hayton, T.; Teunissen, C.E.; Leoni, V.; Marta, M.; Brenner, R.; Palace, J.; et al. Biomarker Report from the Phase II Lamotrigine Trial in Secondary Progressive MS—Neurofilament as a Surrogate of Disease Progression. PLoS ONE 2013, 8, e70019. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory Intrathecal Profiles and Cortical Damage in Multiple Sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [CrossRef]
- Magliozzi, R.; Scalfari, A.; Pisani, A.I.; Ziccardi, S.; Marastoni, D.; Pizzini, F.B.; Bajrami, A.; Tamanti, A.; Guandalini, M.; Bonomi, S.; et al. The CSF Profile Linked to Cortical Damage Predicts Multiple Sclerosis Activity. Ann. Neurol. 2020, 88, 562–573. [Google Scholar] [CrossRef]
- Pitteri, M.; Magliozzi, R.; Nicholas, R.; Ziccardi, S.; Pisani, A.I.; Pezzini, F.; Marastoni, D.; Calabrese, M. Cerebrospinal Fluid Inflammatory Profile of Cognitive Impairment in Newly Diagnosed Multiple Sclerosis Patients. Mult. Scler. J. 2022, 28, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Marastoni, D.; Magliozzi, R.; Bolzan, A.; Pisani, A.I.; Rossi, S.; Crescenzo, F.; Montemezzi, S.; Pizzini, F.B.; Calabrese, M. CSF Levels of CXCL12 and Osteopontin as Early Markers of Primary Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1083. [Google Scholar] [CrossRef] [PubMed]
- Bajrami, A.; Magliozzi, R.; Pisani, A.I.; Pizzini, F.B.; Crescenzo, F.; Marastoni, D.; Calabrese, M. Volume Changes of Thalamus, Hippocampus and Cerebellum Are Associated with Specific CSF Profile in MS. Mult. Scler. 2022, 28, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.L.; Barbour, C.R.; Jackson, K.; Kosa, P.; Bielekova, B. Intrathecal, Not Systemic Inflammation Is Correlated with Multiple Sclerosis Severity, Especially in Progressive Multiple Sclerosis. Front. Neurol. 2019, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Interferons and Their Role In Inflammation. Curr. Pharm. Des. 1999, 5, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, E.N.; Qin, H. Type I Interferons as Anti-Inflammatory Mediators. Sci. STKE 2007, 2007, pe70. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F.; Pujol-Borrell, R. Lymphoid Neogenesis in Chronic Inflammatory Diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef]
- Pilz, G.; Sakic, I.; Wipfler, P.; Kraus, J.; Haschke-Becher, E.; Hitzl, W.; Trinka, E.; Harrer, A. Chemokine CXCL13 in Serum, CSF and Blood–CSF Barrier Function: Evidence of Compartment Restriction. Fluids Barriers CNS 2020, 17, 7. [Google Scholar] [CrossRef]
- DiSano, K.D.; Gilli, F.; Pachner, A.R. Are CSF CXCL13 Concentrations Solely Dependent on Intrathecal Production? A Commentary on “Chemokine CXCL13 in Serum, CSF, and Blood–CSF Barrier Function.”. Fluids Barriers CNS 2021, 18, 9. [Google Scholar] [CrossRef]
- Lycke, J.; Zetterberg, H. The Role of Blood and CSF Biomarkers in the Evaluation of New Treatments against Multiple Sclerosis. Expert Rev. Clin. Immunol. 2017, 13, 1143–1153. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.G.; Roosendaal, S.D.; Calabrese, M.; Ciccarelli, O.; Agosta, F.; Chard, D.T.; Gass, A.; Huerga, E.; Moraal, B.; Pareto, D.; et al. Consensus Recommendations for MS Cortical Lesion Scoring Using Double Inversion Recovery MRI. Neurology 2011, 76, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Banwell, B.L.; Barkhof, F.; Ciccarelli, O.; de Stefano, N.; Geurts, J.J.G.; Paul, F.; Reich, D.S.; Toosy, A.T.; et al. Assessment of Lesions on Magnetic Resonance Imaging in Multiple Sclerosis: Practical Guidelines. Brain 2019, 142, 1858–1875. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A Consensus Protocol for the Standardization of Cerebrospinal Fluid Collection and Biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef]
- Lepennetier, G.; Hracsko, Z.; Unger, M.; Van Griensven, M.; Grummel, V.; Krumbholz, M.; Berthele, A.; Hemmer, B.; Kowarik, M.C. Cytokine and Immune Cell Profiling in the Cerebrospinal Fluid of Patients with Neuro-Inflammatory Diseases. J. Neuroinflamm. 2019, 16, 219. [Google Scholar] [CrossRef]
| Serum Expression Values (pg/mL) | MS | OND | HS | p-Value | ||
|---|---|---|---|---|---|---|
| (n = 143) | (n = 30) | (n = 18) | MS vs. OND | MS vs. HS | OND vs. HS | |
| MIG/CXCL9 | 278.7 ± 335.3 | 166.6 ± 257.7 | 93.9 ± 76.2 | ns | ns | ns |
| sTNF-R2 | 770.9 ± 517.7 | 884.4 ± 467.3 | 440 ± 160.9 | ns | 0.007 | 0.0001 |
| INF-α2 | 19.1 ± 16.8 | 29.7 ± 48 | 108.8 ± 227.3 | ns | 0.03 | 0.02 |
| Pentraxin-3 | 11,681.1 ± 17,389 | 26,546.9 ± 44,145.5 | 12,287.8 ± 7533.9 | 0.0005 | ns | ns |
| TSLP | 148.2 ± 138.6 | 31.8 ± 19.7 | 22.5 ± 18.7 | <0.0001 | <0.0001 | ns |
| APRIL/TNFSF13 | 111,867.1 ± 65,472.8 | 135,471.3 ± 116,679.2 | 174,024 ± 125,614 | ns | ns | ns |
| sIL-6Rα | 6786.6 ± 7412.3 | 19,961.4 ± 12,826.3 | 12,770.6 ± 10,725.5 | <0.0001 | 0.01 | 0.01 |
| EOTAXIN-2/CCL24 | 829 ± 606.5 | 671.6 ± 543.2 | 336.8 ± 310.7 | ns | 0.0001 | 0.0001 |
| INF-β | 46.8 ± 26.4 | 28.7 ± 14.7 | 16.7 ± 15 | 0.0001 | 0.0001 | 0.007 |
| 6Ckine/CCL21 | 24,651.8 ± 10,681.2 | 25,802.4 ± 8115.5 | 20,757.4 ± 13,639.5 | ns | ns | ns |
| MMP-1 | 1419.5 ± 1253.9 | 3471 ± 9088.8 | 628.5 ± 816.3 | ns | 0.005 | 0.005 |
| TECK/CCL25 | 872.3 ± 344.1 | 1648.7 ± 840.8 | 955.3 ± 393 | <0.0001 | ns | 0.003 |
| MDC/CCL22 | 1183.2 ± 528.5 | 1134.3 ± 652.7 | 782.7 ± 438.9 | ns | 0.01 | ns |
| gp130/sIL-6Rβ | 59,752.2 ± 28,655.5 | 107,413 ± 59,655.2 | 69,055.1 ± 48,022.6 | <0.0001 | ns | 0.004 |
| sCD30/TNFRSF8 | 701.7 ± 445.6 | 1422.4 ± 985.7 | 1096.7 ± 1452.5 | <0.001 | ns | 0.03 |
| SCYB16/CXCL16 | 908.2 ± 338.8 | 710.2 ± 261.2 | 559.1 ± 157.9 | 0.004 | <0.001 | 0.01 |
| MS (n = 143) | OND (n = 30) | |
|---|---|---|
| Gender (M/F) | 38/105 | 13/17 |
| Age at PL (years) | 39 ± 12.4 | 46 ± 12.3 |
| MS type (CIS/RRMS/PPMS) | 13/123/7 | - |
| EDSS | 2 (0–5) | - |
| OCB (−/+) | 44/99 | - |
| Qalb (<7/>7/no data) | 111/21/11 | - |
| Inflammatory/Non-inflammatory OtherNeurological Disorders (Y/N) | - | 16/14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzini, F.; Pisani, A.; Mazziotti, V.; Marastoni, D.; Tamanti, A.; Borroni, E.; Magon, S.; Zinnhardt, B.; Magliozzi, R.; Calabrese, M. Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF. Int. J. Mol. Sci. 2023, 24, 3768. https://doi.org/10.3390/ijms24043768
Pezzini F, Pisani A, Mazziotti V, Marastoni D, Tamanti A, Borroni E, Magon S, Zinnhardt B, Magliozzi R, Calabrese M. Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF. International Journal of Molecular Sciences. 2023; 24(4):3768. https://doi.org/10.3390/ijms24043768
Chicago/Turabian StylePezzini, Francesco, Annalisa Pisani, Valentina Mazziotti, Damiano Marastoni, Agnese Tamanti, Edilio Borroni, Stefano Magon, Bastian Zinnhardt, Roberta Magliozzi, and Massimiliano Calabrese. 2023. "Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF" International Journal of Molecular Sciences 24, no. 4: 3768. https://doi.org/10.3390/ijms24043768
APA StylePezzini, F., Pisani, A., Mazziotti, V., Marastoni, D., Tamanti, A., Borroni, E., Magon, S., Zinnhardt, B., Magliozzi, R., & Calabrese, M. (2023). Intrathecal versus Peripheral Inflammatory Protein Profile in MS Patients at Diagnosis: A Comprehensive Investigation on Serum and CSF. International Journal of Molecular Sciences, 24(4), 3768. https://doi.org/10.3390/ijms24043768







