Abstract
Hypomethylating agents (HMAs) have been used for decades in the treatment of hematologic neoplasms, and now, have gathered attention again in terms of their combination with potent molecular-targeted agents such as a BCL-6 inhibitor venetoclax and an IDH1 inhibitor ivosidenib, as well as a novel immune-checkpoint inhibitor (anit-CD47 antibody) megrolimab. Several studies have shown that leukemic cells have a distinct immunological microenvironment, which is at least partially due to genetic alterations such as the TP53 mutation and epigenetic dysregulation. HMAs possibly improve intrinsic anti-leukemic immunity and sensitivity to immune therapies such as PD-1/PD-L1 inhibitors and anti-CD47 agents. This review describes the immuno-oncological backgrounds of the leukemic microenvironment and the therapeutic mechanisms of HMAs, as well as current clinical trials of HMAs and/or venetoclax-based combination therapies.
1. Introduction
Acute myeloid leukemia (AML) is an aggressive, rapidly progressing disease with a poor prognosis. Normal hematopoiesis is often disrupted in patients with AML due to the expansion of leukemic cells within the bone marrow. Although the morbidity rate of AML is higher in the elderly, its treatment can be challenging due to moderate tolerability and comorbidity. Intensive chemotherapy is generally provided to young adults without significant complications. Elderly patients aged 60 years and over have had historically poor performances when they were treated with intensive chemotherapy, with high reported early mortality rates of up to 40% [1]. Even in cases of a response, the duration of the response is limited, with a median overall survival (OS) of from 6 to 8 months. A variety of host- and disease-related risk factors for poor prognosis, including a history of myelodysplastic syndromes (MDS), unfavorable karyotypes, a poor performance status, and complications, limit the treatment options. As a result, older patients are likely to receive only palliative care. The National Comprehensive Cancer Network (NCCN) guidelines recommend standard induction chemotherapy (IC) for AML patients aged 60 years and older with a more favorable prognosis. Older patients eligible for IC may have relatively high complete remission (CR) rates. However, it has not been established that IC provides a clearer survival benefit compared with those of less-intensive treatment options. In a phase III trial, azacitidine (AZA) monotherapy showed better trends toward a favorable prognosis compared with those of conventional care regimens in older patients with newly diagnosed AML, with a median survival of 10 months [2]. In addition, a BCL-2 inhibitor venetoclax (VEN) showed superior survival benefits when it was in combination with AZA or LDAC, with a median OS of up to 14 months [3,4]. NCCN treatment recommendations for older patients with newly diagnosed AML also include decitabine, a hypomethylating agent (HMA). This review focused on immune-biological mechanisms of the AZA/VEN treatment, which have brought the paradigm shift of AML therapy. We also summarize the current status of the preclinical and clinical development of epigenetic immunotherapies.
2. Current Clinical Application of HMAs for AML
2.1. Combination of VEN and AZA in the Elderly
According to a phase III trial (VIALE-A) led by the University of Texas MD Anderson Cancer Centre, the combination of VEN and AZA was safe and improved the OS more than AZA alone did in selected patients with AML [3]. Patients treated with AZA plus VEN had a median OS of 14.7 months. In contrast, this was 9.6 months for patients in the AZA monotherapy arm. Furthermore, 66.4% of the patients in the AZA plus VEN combination reached CR, which was compared with 28.3% of the patients in the AZA monotherapy group. The responses to the treatments in both groups were rapid and sustained. Of the patients in the combination therapy group, 43% had a treatment responses in the first cycle, with a median duration of remission of 17.5 months. This result shows that the combination of VEN and AZA has a safety profile that is comparable to that of the two drugs administered separately. The most common adverse events in both the combination therapy and AZA plus placebo groups were hematological and gastrointestinal symptoms. Overall, the incidence of adverse events was consistent between the two treatment groups, although neutropenia (42% vs. 29%) and febrile neutropenia (42% vs. 19%) were more frequent in the combination therapy group with VEN and AZA than they were the AZA and the placebo group. That is to say, the toxicity profile of the VEN and AZA therapy was acceptable, with early mortality rates of less than 10%, despite an increased risk of myelosuppression and febrile neutropenia of up to 40%. More interestingly, the combination therapy with VEN and AZA was able to induce faster and more sustained remissions, with a median time to response of one month. This new regimen was approved by the FDA for newly diagnosed AML patients older than 75 years or those unfit for intensive chemotherapy.
Another type of AML that is more difficult to treat is treated secondary AML (ts-AML). This is a subtype of AML that develops following treatment for a precursor stage hematological disease such as myelodysplastic syndrome (MDS). Ts-AML is a poor prognosis disease as it does not tend to respond to treatment and has a high risk of early relapse. Despite the availability of approved therapies, patients previously treated with HMAs do not have improved outcomes, which was confirmed by a retrospective analysis of 562 patients with ts-AML with a history of HMA exposure who were treated with different types of treatments (IC, low-intensity chemotherapy (LIC), HAM, and VEN) [5]. The remission rates were investigated separately. This analysis showed that the combination of HAM and VEN resulted in higher remission rates and longer survival rates compared to those of IC and LIC. IC and LIC had similar CR (24% and 26% for IC and LIC, respectively) and OS rates (1 year survival: 14% and 22% for IC and LIC, respectively), while HAM and VEN therapies resulted in similar CR (39%) and OS rates (1 year survival: 35%). These results suggest that the combination of HAM and VEN may be the best option for treating patients with ts-AML with a history of HMA exposure.
2.2. Mechanism of Action of HMAs
In normal cells, DNA methyltransferase (DNMT)s function properly and the on/off balance of genes is maintained. However, in leukemic cells, the DNMTs do not work properly, resulting in the abnormal hypermethylation of DNA sequences that regulate the function of cancer-suppressor genes. As a result, many of the cancer-suppressor genes are switched off, making it easier for cancer to grow. AZA and decitabine are DNMT inhibitors.
They are analogues of the nucleoside cytidine and have two main anti-tumor activities: (i) cytotoxicity by incorporating into DNA or RNA and inducing a DNA damage response; (ii) DNA hypomethylation by inhibiting DNA methyltransferases, restoring normal growth and differentiation mechanisms. In addition, the molecular mechanisms of action of HMA are cellular uptake, intracellular activation, uptake into nucleic acids, and the induction of DNA hypomethylation through the inhibition of DNMTs (Figure 1). Cellular uptake is carried out by various nucleoside transporters; three types of phosphorylation produce the active metabolites 5-azacytidine-triphosphate for azacytidine and 5-aza-2’-deoxycytidine-triphosphate (5-aza-DCTP) for decitabine. HMA is replicated during incorporated into DNA and is, therefore, considered to be an S-phase-specific agent. During replication, 5-aza-dCTP from decitabine is incorporated into newly synthesized DNA. In contrast, 80–90% of AZA is incorporated into RNA as 5-aza-CTP, while 10–20% is multistep converted to 5-aza-dCTP by ribonucleotide reductase and incorporated into DNA.
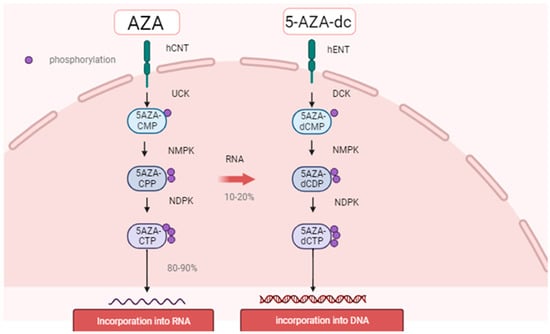
Figure 1.
The figure shows 5-aza-U 5-aza-uridine, 5-aza-dU 5-aza-2’-deoxyuridine, CDP cytidine diphosphate, CMP cytidine monophosphate, hCNT human concentrative nucleoside transporter, hENT human equilibrative nucleoside transporter, NDPK nucleoside diphosphate kinase, NMPK nucleoside monophosphate kinase, and RNR ribonucleotide reductase, and azanucleoside (AZA) uptake and intracellular metabolism. Once inside the cell, the drugs are activated through consecutive ATP-dependent phosphorylation steps.
2.3. Pathophysiologic Features of Leukemic Stem Cells
2.3.1. Metabolic Changes in Leukemic Stem Cells
One study demonstrated that amino acid uptake, steady-state levels, and catabolism were all elevated in a leukemia stem cell (LSC) population. Furthermore, LSCs isolated from neonatal leukemia patients were found to be specifically dependent on amino acid metabolism for oxidative phosphorylation and survival. The pharmacological inhibition of amino acid metabolism reduces oxidative phosphorylation and induces cell death. In contrast, LSCs from patients with relapsed AML are independent of the amino acid metabolism, as they can compensate for this by increasing fatty acid metabolism. Amino acids are essential for oxidative phosphorylation in LSCs [6,7,8,9,10].
2.3.2. Amplification of Anti-Apoptotic Factors
Cell death escape represents a cancer hallmark, and B-cell/CLL lymphoma 2 (BCL-2) family proteins play a pivotal role in tumorigenesis and survival [11]. Among these proteins, an anti-apoptotic factor myeloid leukemia-1 (MCL-1) is often highly amplified in a variety of human cancers [12]. MCL-1 strongly binds to pro-apoptotic effectors such as Bcl-2 homologous antagonist/killer (BAK) and the Bcl-2-interacting mediator of cell death (BIM) on the mitochondrial outer membrane, which results in the prevention of apoptosis [13]. Several studies have demonstrated that MCL-1 is essential for tumorigenesis and survival in solid tumors, lymphomas [14], and myeloid leukemia [15]. A recent multi-omics analysis of patient-derived LSC and a consequent xenograft mouse model experiment suggested that resistance to VEN-based therapy in monocytic AML relies on MCL-1 functions [16]. Potent MCL-1 inhibitors are currently under evaluation in clinical trials [17]. The combination therapy of AZA and MCL-1 inhibition may regulate fatty acid metabolism [18].
2.4. VEN and Gilteritinib
Relapsed/refractory FLT3 mutant (FLT3+) AML is generally resistant to VEN, and a treatment with an FLT3 inhibitor results in a shorter duration of the effects. In an open-label phase Ib study, 54 patients with R/R FLT3+ AML who were treated with VEN and the FLT3 tyrosine kinase inhibitor, gilteritinib, participated [19]. The combination was safe and well tolerated, with 74.5% of the FLT3+ patients achieving a promising composite CR rate, except for frequent but manageable hemophilia, which occurred in 80% of the study participants. Furthermore, the combination therapy may contribute to prolonged survival, as more than half (56.7%) of the patients who responded to the treatment eliminated the FLT mutation. This trial presents promising evidence that VEN and gilteritinib may be effective and safe treatment options for patients with relapsed or refractory FLT3+AML. Further studies are warranted, including the development of a frontline combination of AZA and VEN plus gilteritinib in older AML patients and ineligible AML patients [20].
2.5. Magrolimab Combination Therapy
Patients with AML receiving a combination of VEN and AZA have high frontline remission rates, but they tend to eventually relapse, with very low survival rates in relapsed/refractory AML. A study focused on a treatment that adds magrolimab, an anti-CD47 antibody, to combination therapy [20]. Thirty-eight patients with various types of AML, comprising newly diagnosed AML patients, patients with relapsed/refractory AML who had not previously been treated with VEN, and patients with relapsed/refractory AML after a VEN treatment, participated in the ongoing, phase 2 study. The CR and CR with incomplete hematologic recovery (CRi) rates for newly diagnosed patients were 94%, which was 81% CR in the frontline patients. The CR/CRi rates in relapsed/refractory patients that were not treated with VEN were 63% and 27% in the patients with relapsed/refractory AML after the VEN treatment. Anemia occurring after the first and second doses is a common, but manageable side effect in the study participants, and it is a common side effect in VEN. The combination of AZA and magrolimab was found to be particularly effective in older patients with newly diagnosed AML and those unsuitable for IC. Furthermore, a randomized multinational phase III trial is now underway, and the results are promising (NCT04778397).
2.6. AZA and Ivosidenib, Newly Therapy
Approximately between 6% and 10% of AML patients have a disease induced by mutations in a gene called isocitrate dehydrogenase 1 (IDH1). Ivosidenib (IDB) inhibits the activity of a protein produced by IDH1. In a large international trial of patients with this type of AML who were unable to receive potent chemotherapy, IDB in combination with AZA was significantly more effective at inducing remission than AZA was alone [21]. Patients treated with both drugs in this clinical trial had a median OS of two years, an improvement compared with that of approximately 8 months due to AZA and a placebo. In general, the patients’ side effects were not worsened by receiving combination therapy. Indeed, the patients receiving combination therapy reported a better quality of life during the study compared with those of the patients receiving AZA monotherapy. The CR after 6 months was 11% in the AZA and placebo group compared with 38% in the combination group; beyond 6 months, further remissions were observed in those in the combination group. Overall, about half of the patients who received AZA and IDB achieved a CR compared with only 15% of those who received AZA and a placebo. Out of those who had a CR, more than half had no evidence of the IDH1 mutation in their bone marrow. During the study period, 28 people (39%) died in the combination treatment group compared with 46 (62%) in the AZA and placebo group. Those receiving the combination reported that their health-related quality of life was the same or improved from that at the start of the trial. No improvement was reported in those receiving AZA and a placebo. Almost all of the study participants had one or more serious side effects due to the treatment, such as a decrease in red or white blood cells. Infections were most common in those receiving AZA and a placebo. Hemorrhage and differentiation syndromes (a series of dangerous complications attributable to the immune system) were more common in those who received AZA and IDB. These results suggest that the drug was more effective than the single agent was.
Thus, cancer patients with IDH1 mutations can be treated with AZA and VEN or AZA and IDB. Both combinations, however, showed comparable life-prolonging effects to those of AZA alone in the randomized trials, but direct comparisons between the trials are not possible. In addition, clinical trials have already been initiated to test a combination therapy with AZA, VEN, and IDB. It would be desirable to further reduce the gap period required for the DNA sequencing of these leukemia cells. Trials comparing different targeted treatment options with the current standard of care based on the genetic profile of the patient’s tumor would also be necessary to determine which treatment is the best one.
3. Effect of HMAs on Tumor Immunity
3.1. HMA May Improve the Efficacy of CAR-T Cells
In an in vivo mouse models study, AZA induces the increased expression of CD123 in leukemia cells [22]. The administration of AZA to leukemic mice increases the number of CTLA-4-negative anti-CD123 CAR-T cells after the injection. These CAR-T cells show excellent cytotoxicity against AML cells, which is accompanied by increased production of TNFα and downstream phosphorylation of key T-cell-activating molecules. That is to say, AZA enhances the immunogenicity of AML cells and promotes the recognition and elimination of malignant cells by highly efficient CTLA-4-negative CD123 CART cells [23]. In other words, AZA enhances the immunogenicity of AML cells and promotes the recognition and elimination of malignant cells by high-efficiency CTLA-4-negative CD123CAR-T cells. The study also showed that anti-tumor activities, cytokine production, and proliferation are enhanced in decitabine-treated chimeric antigen receptor T (dCAR-T) cells both in vitro and in vivo. It suggests that HMA may improve the efficacy of CAR-T cells.
CD70 is a member of the tumor necrosis factor (TNF) superfamily and has recently been considered as a potential target for AML; the normal expression of CD70 is restricted to activated immune cells, but its expression is increased in many cancer types. Additionally, unlike other targets, CD70 is not expressed on normal hematopoietic stem cells, and an antibody targeting CD70, ARGX-110 (casatuzumab), showed excellent response rates in phase I trials. These results suggest that CAR-T therapies targeting CD70 may be effective. AZA is thought to increase CD70 surface expression in individual tumor cell lines and primary AML blast and leukemia stem cells. The mouse model also suggested that an increase in CD70 expression density would lead to a significant increase in CAR efficacy [24].
Chimeric antigen receptor T cell (CAR-T) immunotherapy targeting the CD19 antigen has shown clinical efficacy in patients with leukemia and lymphoma. However, it is less effective in lymphomas than it is in ALL. This is because CAR-T cells undergo a state of exhaustion that is characterized by the up-regulation of inhibitory receptors and loss of effector function [25,26,27,28,29]. It has recently been shown that de novo DNA methylation promotes T cell exhaustion and limits anti-PD1 immunotherapy and that methylation inhibition promotes PD1 blockade-mediated T cell rejuvenation [30,31,32,33]. Similarly, chromatin accessibility with functional DNA demethylation was found to be reduced in the transcription start regions in exhausted T cells.
The relapse of ALL is associated with persistence of CAR-T cells, suggesting that the active surveillance of leukemia via the CAR-T cells is lost. Previous studies suggested that decitabine (5-aza-deoxycytidine; DAC) may modify the DNA methylation program. In the present study, CAR-T cells after a low-dose DAC treatment (dCAR-T cells) underwent DNA initialization, induced a higher expression of genes for cell memory, proliferation, cytotoxicity and cytokine production, and reduced attrition following antigen exposure. Very low dCAR-T cell doses were able to efficiently control tumors with a very large tumor burden. Then, dCAR-T cells had a higher proportion of cells with a memory phenotype than CAR-T cells under long-term tumor stimulation. This means that the expression of memory-related genes was very high, and the expression of wasting-related genes was maintained at a low level. This suggests that the addition of DMAT may improve the attrition of CAR-T cells and increase their anti-tumor effect and reduce the tumor recurrence [34].
3.2. HMA-Induced Changes to the Immune System
HMAs are thought to induce an immunostimulatory effect in cancer cells through a process known as viral mimicry [35,36]. This effect is characterized by the up-regulation of repetitive transcription and formation of immunogenic double-stranded RNA (dsRNA) structures, leading to activity in the endogenous viral defense pathways and type I/III interferon responses. The high expression of viral defense gene signatures is also said to significantly correlate with durable responses to immune checkpoint inhibition (ICB) in melanoma patients, and synergistic effects have been reported where the combination of HMA and ICB results in enhanced immune infiltration and anti-tumor responses. The synergistic effect of combining HMA and ICB has been reported.
The intraperitoneal administration of DAC in immunocompromised mice was found to significantly inhibit tumor growth. These anti-tumor effects were quenched by the depletion of CD8+ T cells. A marked increase in CD8+ T cell infiltration into the tumor microenvironment was also observed during the DAC treatment; considering that a high density of CD8+ cytotoxic T cells is associated with prolonged disease-free survival, low-dose DAC was found to inhibit tumor growth by increasing CD8+ T cell infiltration and T cell effector function in vivo. DAC-activated CD8+ T cells were found to increase the protein expression of the T cell activation markers, CD69, CD25, and HLA-DR, which increased T cell activation and enhanced their ability to kill the target cells [37,38].
3.3. The Effect of TP53 Mutation on Anti-Leukemic Immunity
Patients with myelodysplastic syndrome (MDS) and secondary myeloid leukemia (sAML) with TP53 mutations have a poor prognosis, with a median overall survival of 6–12 months regardless of the treatment. The aberrant expression of several immune checkpoints (e.g., PD-1, PDL-1, and CTLA4) in CD34-positive cells has been reported in 34% of MDS patients after an HMA treatment, which may be a poor prognostic factor. One study examined the relationship between checkpoint molecules and TP53 gene mutations in MDS and sAML [39,40,41,42]. The up-regulation of the immune checkpoints PD-L1, PD-L2, and CTLA4 was found in 20–36% of the patients with TP53 mutations compared to only 5–7% of wild-type TP53 patients. Additionally, patients with the up-regulation of immune checkpoints such as PD-L1, PD-L2, and CTLA4 had an inferior OS rate (median OS 6 months vs. 19 months; p = 0.007). TP53 mutant MDS/sAML also had increased HSCs and overexpressed PD-L1 (16.3% vs. 10.9%; p < 0.01).
The inducible T cell co-stimulator (ICOS) is a co-stimulator with the ability to induce tumor immunity by stimulating T cells, including cytotoxic T lymphocytes (CTLs) and Th cells, while promoting immune evasion by stimulating Treg cells. ICOS expression was markedly increased in the CTL and Th populations of TP53 mutant patients relative to that of the wild type. Additionally, CTLs in the TP53 mutant group were ICOS+/4-1BB/PD-1+, whereas Th cells were ICOS+ and Treg cells were low in ICOS+/PD-1. Increased numbers of ICOS-high/PD-1-negative Treg cells infiltrating tumors are associated with an inferior OS in cancer cells. Patients with increased ICOS-high/PD-1-negative Tregs in the bone marrow had a significantly reduced OS (median OS 8.6 months vs. 34.3 months; hazard ratio 2.34; p = 0.0006). This means that the number of OX40+ cytotoxic T cells (Treg) and helper T cells infiltrating the bone marrow was significantly reduced, especially in patients with TP53 mutations, as well as ICOS+ and 4-1BB+ natural killer cells. Furthermore, the number of immunosuppressive regulatory T cells (high levels of ICOS/PD-1-negative ones) and bone marrow-derived suppressor cells (PD1-low) is increased in TP53 mutation cases [43]. The higher proportion of ICOS-high/PD-1-negative Treg cells infiltrating the bone marrow was highly advantageous as an independent prognostic predictor of overall survival. MYC was also significantly elevated in the TP53 mutant and wild-type groups, and the PDL1-positive rate of MDS HSCs was positively correlated with the MYC expression (p = 0.004). MYC expression is negatively regulated by miR-34a, which degrades MYC mRNA and is induced by the wild-type p53 transcription target induced by wild-type p53. When we are comparing the differential expressions of miRNAs in bone marrow MPCs, miR-34a was significantly down-regulated in the TP53 mutant group (p = 0.00003). In summary, PD-L1 expression was markedly increased in HSCs from the TP53 mutant patients and was associated with up-regulation of MYC and the significant down-regulation of miR-34a, a negative regulator of MYC and the p53 transcriptional target.
In conclusion, the microenvironment of TP53 mutant MDS and sAML has an immune-privileged, aversive phenotype that is a major contributor to a poor prognosis, suggesting that immunomodulatory treatment strategies may be effective in the future.
4. Impact of Genetic Backgrounds on DNA-Hypomethylating Therapeutics
4.1. Aberrant Epigenetic Modification in Hematologic Malignancies
4.1.1. Physiologic Epigenetic Regulation of Hematopoiesis (Figure 2)
Hematopoietic stem cells (HSCs) continue self-renewal and generate several specialized progenitor cells to maintain normal hematopoiesis, which is mainly regulated by epigenetic modifications. In general, epigenetic modifications are classified into two major forms: the direct methylation of DNA and histone modification, such as methylation, phosphorylation, and acetylation. DNA methylation mainly occurs at the cytosine base that is linked to the adjacent guanine base through the phosphodiester bond (CpG). Although from two thirds to four fifths of CpGs are methylated through a whole genome, clusters of CpGs (so-called CpG islands) are often located on near promoter regions, and they are usually hypomethylated [44]. Unmethylated CpG island promoters show elevated levels of dimethylation of Lys4 of histone H3 (H3K4me2), suggesting that histone modifications contribute to the regulation of DNA methylation [45]. The genome-wide DNA methylation pattern is also associated with a variety of medical, psychological, and behavioral conditions [46,47,48,49], and these can be altered by extrinsic factors such as smoking [50] and coffee/tea consumption [51].
DNA methylation is carried out by DNA methyltransferase enzymes (DNMT) such as DNMT1, DNMT3A, DNMT3B, and DNMT3L. DNMT1 is involved in the methylation of existing GpC islands. A mouse model experiment demonstrated that the biallelic knockdown of Dnmt1 led to a loss of the important function of embryonic stem cells [52]. DNMT3L does not have a catalytic function, and rather, it works as a co-factor of DNMT3A. DNMT3A proteins form a homo-tetramer or a hetero-tetramer with DNMT3L, which is important for its catalytic function [53]. DNMT3A and DNMT3B are involved in de novo DNA methylation and play a key role in the establishment of DNA methylation patterns during embryonic development [54,55].
High levels of DNA methylation of the promoter region are apparently associated with decreased transcriptional activity and gene silencing [44,56]. However, many key promoters of the regulator genes for embryonal development, which are constantly inactivated in mature cells, frequently have a large hypomethylated region named the DNA methylation valley (DMV) or canyon [57,58]. A large portion of DMVs are marked by the tri-methylation of the 27th lysine residue of histone H3 (H3K27me3), which is mediated by Polycomb repressive complex 2 (PRC2) [59]. PRC2 and PRC1 are polycomb group (PcG) proteins, which are evolutionarily conserved chromatin modifying factors. PcG proteins are known to play an important role in maintaining the stemness of murine embryonic stem cells by silencing key developmental regulators [60,61]. In addition to a pivotal role in embryonic development, PcG proteins also regulate the key function of adult hematopoietic stem cells (HSPs) [60,62]. A mouse model study demonstrated that a defective PRC2 assembly resulted in a decreased number of bone marrow HSPs and suppressed differentiation to mature cells [62]. The deposition of H3K27me3 is directly mediated by the enhancer of zeste homologs 1 (EZH1) and 2 (EZH2), a catalytic core subunit of PRC2, which are stimulated by other PRC2 subunits such as Jumonji and the AT-rich interaction domain containing 2 (JARID2) [63] and inhibited by EZH inhibitory protein (EZHIP) [64]. EZH2 also regulates the DNA methylation of EZH2-target promoters by directly binding with DNMT proteins [65].
PcG proteins keep DMVs hypomethylated in concert with ten–eleven translocation proteins (TET) [59]. TET proteins (TET1, TET2, and TET3) have a compact catalytic domain containing zinc fingers, and they specifically recognize CpGs. TET proteins are an alpha-ketoglutarate (aKG)-dependent dioxygenase that catalyze the conversion of 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC), which is an initiation of DNA demethylation [66]. A genome-wide analysis of mice showed that the loss of TET genes led to increased methylation in DMVs, suggesting that TET proteins are essential in regulating the methylation of DMVs [59]. Recent studies have suggested that vitamin C, a co-factor of TET proteins, negatively regulates HSC function and prevent leukemogenesis, at least partially due to enhanced TET activity [67,68].
Additional sex comb-like 1 (ASXL1) protein was originally recognized as a co-factor of retinoic acid receptor [69]. ASXL1 is a regulatory component of the Polycomb repressive deubiquitinase (PR-DUB) complex, which removes a mono-ubiquitin conjugated to the 119th lysine residue of histone H2A (H2A-K119Ub) [70,71]. The mono-ubiquitination of histone H2A is catalyzed by PRC1 [72], and it is essential for Polycomb-mediated transcriptional repression [73]. ASXL1 also recognize N6-methyladenosine methylation (6mA) on DNA, which leads to its ubiquitination and the degradation by thyroid hormone receptor interactor 12 (TRIP12), and it is detracted by the deposition of 6mA [74].
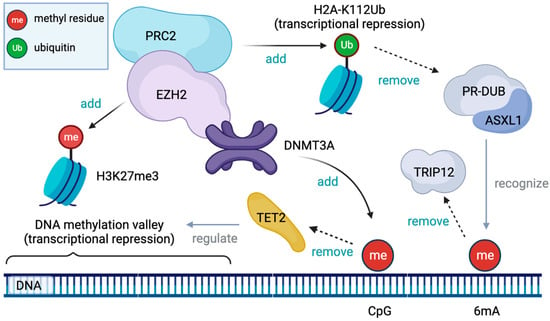
Figure 2.
DNA methyltransferase 3A (DNMT3A) proteins methylate cytidine residue linked to the adjacent guanine residue (CpG). Ten-eleven translocation 2 (TET2) protein initiates the demethylation of CpG and contributes to maintaining the DNA methylation valley (DMV). Enhancer of zeste homolog 2 (EZH2) is a catalytic subunit of Polycomb repressive complex 2 (PRC2) and mediates the tri-methylation of the 27th lysine residue of histone H3 (H3K27me3), an epigenetic mark associated with DMVs. PRC2 also mediates the mono-ubiquitination of the 112th lysine residue of histone H2A, a mark of transcriptional repression, which is reversed by the Polycomb repressive deubiquitinase (PR-DUB) complex involving additional sex comb-like 1 (ASXL1) as its regulatory subunit. ASXL1 also recognizes methylated adenine (6mA) on DNA and helps its degradation by thyroid hormone receptor interactor 12 (TRIP12).
4.1.2. DNMT3A Mutation
DNMT3A mutations are relatively common in patients with cytogenetically normal acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [75], accounting for a quarter of AML cases. The majority of the DNMT3A mutations are heterozygous missense mutations located in the 882nd arginine, though a broad range of pathogenic mutation sites have been reported. Because DNMT proteins function as an oligomer, most heterozygous loss-of-function mutations can disrupt part of its methyltransferase activity [76]. A DNMT3A mutation in AML is linked to decreased DNA methylation and a poor prognosis [77]. An analysis of the DNA methylation profiles in patients with cytogenetically normal AML revealed that DNMT3A mutations are associated with the hypomethylation of genomic regions far from the CpG islands (open see area) and specific promoter regions with a high CpG content such as homeobox (HOX) genes [78]. A mouse model experiment showed that concomitant homozygous Dnmt3a loss and the isocitrate dehydrogenase 2 (IDH2) gene mutation induced murine leukemia, accompanying epigenomic dysregulation such as the substantially decreased trimethylation (H3K9me3) and acetylation (H3K9ac) of the 9th lysine residue of histone H3, which was reversed by a histone deacetylase (HDAC) inhibitor [79]. A clinical retrospective analysis of patients with MDS who were treated with azacytidine and/or decitabine showed that the DNMT3A mutation was associated with a significantly better progression-free survival rate [80]. A similar retrospective analysis of patients with MDS or AML who received azacytidine therapy suggested that the DNMT3A mutation was associated with better response rates only if TET2 and IDH1/2 mutations co-existed [81].
4.1.3. TET2 Mutation
The TET2 mutation is also commonly found in adult AML and MDS, accounting for 10–20% of patients [82,83]. A mouse model in vivo study suggested that the haploinsufficiency of TET2 gene contributes to increased self-renewal of hematopoietic stem cells [84]. Decreased levels of 5hmC, which is catalyzed by TET enzymes, in genomic DNA in patients with myeloid malignancies have been reported, and low 5hmC levels are linked to hypomethylation at differentially methylated CpG sites [85]. The catalytic function of the TET2 enzyme is also impaired by IDH1/2 mutations [86]. Mutant IDH enzymes convert aKG into an onco-metabolite 2-hydroxyglutarate (2HG), and 2HG competitively inhibits aKG-dependent dioxygenases including TET2 [87]. In clinical observations, TET2 mutation in patients with AML or MDS may predict a better response to hypomethylating agents (HMAs) such as azacytidine [88,89]. An in vivo study using transplanted mice with leukemic cells carrying the homozygous TET2 loss with the Flt3ITD mutation demonstrated that an azacytidine treatment resulted in the normalized hypermethylated state of leukemic genome, as well as nearly the complete elimination of leukemic blasts, suggesting that TET2-mutated AML may be specifically responsive to HMAs [90]. A similar, positive predictive value for TET2 mutation was reported in a retrospective analysis of patients with azacytidine-sensitive MDS [91].
4.1.4. ASXL1 Mutation
ASXL1 mutations are prevalent in myeloid malignancies and associated with a poor prognosis especially when they have high variant allele fraction [92,93]. A transgenic mouse model study demonstrated that a gain-of-function mutation of the ASXL1 gene resulted in an MDS-like condition in the mice, suggesting a molecular interaction with bromodomain containing 4 (BRD4) [94]. In a xenograft mouse model study in which human bone marrow cells from patients with chronic myelomonocytic leukemia (CMML) were transplanted into mice, the co-expression of mutant ASXL1 and RUNX family transcription factor 1 (RUNX1) genes showed strong leukemic transformation, accompanying an increased expression of inhibitor of DNA binding 1 (ID1) gene [95]. Similarly, a knock-in mouse model study showed that co-existing ASXL1-G643W and CCAAT enhancer binding protein alpha (CEBPA) mutations synergistically accelerated leukemogenesis [96]. The clinical impacts of the ASXL1 mutation on HMA therapy for patients with myeloid malignancies are still conflicting [80,97]. However, a retrospective analysis of patients with high-risk MDS who were treated with azacytidine showed a longer survival period for patients with the ASXL1 mutation [97].
4.1.5. EZH2 Mutation
EZH2 mutations are found in up to 10% of myeloid malignancies, including some AML cases [98]. Although EZH2 gene is often overexpressed in many solid tumors, as well as malignant lymphoma, a defective EZH2 function should be important in myeloid malignancies [99]. A xenograft model study of MDS demonstrated that EZH2 knockdown resulted in reduced levels of H3K27me3 and the consequent up-regulation of HOX genes, which can contribute to the transformation to AML [100]. Similarly, in a mouse model study of MDS, the loss of EZH2 and concurrent TET2 insufficiency induced the marked hypermethylation of CpG islands of the PcG target, which is characteristic of MDS [101]. Although the loss-of-function EZH2 mutation is associated with a shorter survival in patients with MDS [91], its prognostic impacts on AML patients are somewhat conflicting. A genomic analysis of 200 patients with AML revealed a poor prognosis after the first complete remission in patients with an EZH2-mutated intermediate-risk disease [102]. However, a larger cohort study (n = 1604) reported a comparable overall survival between patients with or without the EZH2 mutation [98]. Although a reduction of EZH2 levels and H3K27me3 can be achieved by DNA demethylation agents in combination with histone deacetylation inhibitors [99,103], it remains to be proved if such a therapeutic approach can be effective for EZH2-defective myeloid malignancies. Given that loss-of-function EZH2 mutation leads to hypermethylation of specific CpG regions [101], the therapeutic role of HMAs may not be ignorable.
4.2. Association between Anti-Leukemic Immunity and Epigenetic Dysregulation
Dufva and colleagues comprehensively analyzed the immunologic landscape of hematologic malignancies using 7092 samples from patients with hematologic malignancies, including 4281 cases of leukemia [104]. They demonstrated that 8.2% of the samples of AML showed high cytolytic activity, as well as the correlated infiltration of cytotoxic T lymphocytes into the bone marrow. Not surprisingly, the TP53 mutation and deletion of chromosome 5p were positively correlated with high cytolytic activity, which may be due to a high mutational burden and more chance to generate neoantigens. In contrast, FLT3 and nucleophosmin 1 (NPM1) mutations were associated with low cytolytic activity. AML samples with MDS-like transcriptome signature were related to high cytolytic activity and were also associated with MDS-related mutations such as RUNX1, TP53, U2AF1, and SRSF2. On the other hand, the expression of HLA class II and its transactivator (CIITA) was particularly low in samples of acute promyelocytic leukemia and NPM1-mutated AML. The methylation of promoter regions of HLA class II genes and CIITA was negatively correlated with cytolytic activity, and, of note, abundant CIITA hypermethylation was observed in subclusters with IHD1, IHD2, and TET2 mutations. Interestingly, a subsequent cell line experiment showed that a co-culture with decitabine resulted in the decreased methylation of CIITA and increased expression of HLA class II. Given that HLA class II is essential for antigen presentation to CD4-positive helper T cells and the subsequent initiation of tumor-specific immunity, these findings indicate that a treatment with HMAs may contribute to enhance intrinsic anti-leukemic immunity and improve the response to immune therapies.
5. Conclusions
This review described the current development of AZA and/or VEN-based combination therapy including potent FLT3 inhibitor gilteritib and novel immune-checkpoint inhibitor magrolimab, which have shown promising results in clinical trials. These approaches should bring benefits especially for patients who are ineligible for highly intensive chemotherapy and/or allogeneic hematopoietic stem cell transplantation. On an immune-oncological note, HMAs affect the leukemic microenvironment and possibly sensitize LSCs to immune-oncologic therapy such as immune checkpoint inhibitors and CAR-T cells. In addition, HMAs possibly reverse the immune-escaping mechanisms mediated by epigenetic dysregulation. HMAs are expected to play a key role in immuno-oncologic strategies for AML.
Author Contributions
A.U., S.C. and Y.M. were responsible for the preparation and writing of the manuscript, and all authors contributed to and approved the final manuscript. A.U. and S.C. equally contributed to this article. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by a National Cancer Research and Development expenses grant (2021-A-11).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
Y.M. received research funding from Ono and received honoraria from Bristol-Myers Squibb, Novartis, and Pfizer. The other authors declare no conflict of interest.
References
- Giles, F.J.; Borthakur, G.; Ravandi, F.; Faderl, S.; Verstovsek, S.; Thomas, D.; Wierda, W.; Ferrajoli, A.; Kornblau, S.; Pierce, S.; et al. The Haematopoietic Cell Transplantation Comorbidity Index Score Is Predictive of Early Death and Survival in Patients over 60 Years of Age Receiving Induction Therapy for Acute Myeloid Leukaemia. Br. J. Haematol. 2007, 136, 624–627. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs. Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Strickland, S.A.; Hou, J.-Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients with Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Kadia, T.M.; Ravandi, F.; DiNardo, C.D.; Daver, N.; Borthakur, G.; Konopleva, M.; Garcia-Manero, G.; Montalban-Bravo, G.; Maiti, A.; et al. Impact of Frontline Treatment Approach in Patients with Secondary AML and Prior Hypomethylating Agent Exposure: A Retrospective Analysis of 562 Patients with Treated Secondary AML. Blood 2021, 138, 794. [Google Scholar] [CrossRef]
- Guan, Y.; Hogge, D.E. Proliferative Status of Primitive Hematopoietic Progenitors from Patients with Acute Myelogenous Leukemia (AML). Leukemia 2000, 14, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Hope, K.J.; Jin, L.; Dick, J.E. Acute Myeloid Leukemia Originates from a Hierarchy of Leukemic Stem Cell Classes That Differ in Self-Renewal Capacity. Nat. Immunol. 2004, 5, 738–743. [Google Scholar] [CrossRef]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-Resistant Human AML Stem Cells Home to and Engraft within the Bone-Marrow Endosteal Region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem Cells, Cancer, and Cancer Stem Cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Terpstra, W.; Ploemacher, R.E.; Prins, A.; van Lom, K.; Pouwels, K.; Wognum, A.W.; Wagemaker, G.; Löwenberg, B.; Wielenga, J.J. Fluorouracil Selectively Spares Acute Myeloid Leukemia Cells with Long-Term Growth Abilities in Immunodeficient Mice and in Culture. Blood 1996, 88, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The Landscape of Somatic Copy-Number Alteration across Human Cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Denis, C.; Sopková-de Oliveira Santos, J.; Bureau, R.; Voisin-Chiret, A.S. Hot-Spots of Mcl-1 Protein. J. Med. Chem. 2020, 63, 928–943. [Google Scholar] [CrossRef] [PubMed]
- Grabow, S.; Delbridge, A.R.D.; Aubrey, B.J.; Vandenberg, C.J.; Strasser, A. Loss of a Single Mcl-1 Allele Inhibits MYC-Driven Lymphomagenesis by Sensitizing Pro-B Cells to Apoptosis. Cell Rep. 2016, 14, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.P.; Lee, E.F.; Trounson, E.; Bouillet, P.; Wei, A.; Fairlie, W.D.; Izon, D.J.; Zuber, J.; Rappaport, A.R.; Herold, M.J.; et al. Anti-Apoptotic Mcl-1 Is Essential for the Development and Sustained Growth of Acute Myeloid Leukemia. Genes Dev. 2012, 26, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Pollyea, D.A.; Gustafson, A.; Stevens, B.M.; Minhajuddin, M.; Fu, R.; Riemondy, K.A.; Gillen, A.E.; Sheridan, R.M.; Kim, J.; et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 536–551. [Google Scholar] [CrossRef]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 Inhibitors, Fast-Lane Development of a New Class of Anti-Cancer Agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2018, 34, 724–740.e4. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.R.; McCloskey, J.; Smith, C.C.; Schiller, G.J.; Bradley, T.; et al. Venetoclax in Combination with Gilteritinib Demonstrates Molecular Clearance of FLT3 Mutation in Relapsed/Refractory FLT3-Mutated Acute Myeloid Leukemia. Blood 2021, 138, 691. [Google Scholar] [CrossRef]
- Daver, N.; Konopleva, M.; Maiti, A.; Kadia, T.M.; DiNardo, C.D.; Loghavi, S.; Pemmaraju, N.; Jabbour, E.J.; Montalban-Bravo, G.; Tang, G.; et al. Phase I/II Study of Azacitidine (AZA) with Venetoclax (VEN) and Magrolimab (Magro) in Patients (Pts) with Newly Diagnosed Older/Unfit or High-Risk Acute Myeloid Leukemia (AML) and Relapsed/Refractory (R/R) AML. Blood 2021, 138, 371. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.; Nomdedéu, J.F.; López, O.; Carnicer, M.J.; Bellido, M.; Aventín, A.; Brunet, S.; Sierra, J. Interleukin-3 Receptor Alpha Chain (CD123) Is Widely Expressed in Hematologic Malignancies. Haematologica 2001, 86, 1261–1269. [Google Scholar]
- El Khawanky, N.; Hughes, A.; Yu, W.; Myburgh, R.; Matschulla, T.; Taromi, S.; Aumann, K.; Clarson, J.; Vinnakota, J.M.; Shoumariyeh, K.; et al. Demethylating Therapy Increases Anti-CD123 CAR T Cell Cytotoxicity against Acute Myeloid Leukemia. Nat. Commun. 2021, 12, 6436. [Google Scholar] [CrossRef]
- Leick, M.B.; Silva, H.; Scarfò, I.; Larson, R.; Choi, B.D.; Bouffard, A.A.; Gallagher, K.; Schmidts, A.; Bailey, S.R.; Kann, M.C.; et al. Non-Cleavable Hinge Enhances Avidity and Expansion of CAR-T Cells for Acute Myeloid Leukemia. Cancer Cell 2022, 40, 494–508.e5. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Georgakilas, G.; Petrovic, J.; Kurachi, M.; Cai, S.; Harly, C.; Pear, W.S.; Bhandoola, A.; Wherry, E.J.; Vahedi, G. Lineage-Determining Transcription Factor TCF-1 Initiates the Epigenetic Identity of T Cell Development. Immunity 2018, 48, 243–257.e10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Greenbaum, U.; Mahadeo, K.M.; Kebriaei, P.; Shpall, E.J.; Saini, N.Y. Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1594. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Wherry, E.J.; Ha, S.-J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hämmerling, G.J.; et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 2016, 45, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Schietinger, A.; Greenberg, P.D. Tolerance and Exhaustion: Defining Mechanisms of T Cell Dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, C.; Dai, H.; Wu, Z.; Han, X.; Guo, Y.; Chen, D.; Wei, J.; Ti, D.; Liu, Z.; et al. Low-Dose Decitabine Priming Endows CAR T Cells with Enhanced and Persistent Antitumour Potential via Epigenetic Reprogramming. Nat. Commun. 2021, 12, 409. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via DsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef]
- Legat, A.; Speiser, D.E.; Pircher, H.; Zehn, D.; Fuertes Marraco, S.A. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than “Exhaustion” of Human CD8 T Cells. Front. Immunol. 2013, 4, 455. [Google Scholar] [CrossRef]
- Loo Yau, H.; Bell, E.; Ettayebi, I.; de Almeida, F.C.; Boukhaled, G.M.; Shen, S.Y.; Allard, D.; Morancho, B.; Marhon, S.A.; Ishak, C.A.; et al. DNA Hypomethylating Agents Increase Activation and Cytolytic Activity of CD8+ T Cells. Mol. Cell 2021, 81, 1469–1483.e8. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.-H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Yoshizato, T.; Nannya, Y.; Atsuta, Y.; Shiozawa, Y.; Iijima-Yamashita, Y.; Yoshida, K.; Shiraishi, Y.; Suzuki, H.; Nagata, Y.; Sato, Y.; et al. Genetic Abnormalities in Myelodysplasia and Secondary Acute Myeloid Leukemia: Impact on Outcome of Stem Cell Transplantation. Blood 2017, 129, 2347–2358. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Gallì, A.; Bacigalupo, A.; Zibellini, S.; Bernardi, M.; Rizzo, E.; Allione, B.; van Lint, M.T.; Pioltelli, P.; Marenco, P.; et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients with Myelodysplastic Syndromes Treated with Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Bravo, G.; Takahashi, K.; Garcia-Manero, G. Decitabine in TP53-Mutated AML. N. Engl. J. Med. 2017, 376, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; McLemore, A.F.; Aldrich, A.L.; Komrokji, R.S.; McGraw, K.L.; Dhawan, A.; Geyer, S.; Hou, H.-A.; Eksioglu, E.A.; Sullivan, A.; et al. TP53 Mutations in Myelodysplastic Syndromes and Secondary AML Confer an Immunosuppressive Phenotype. Blood 2020, 136, 2812–2823. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA Methylation Landscapes: Provocative Insights from Epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef]
- Weber, M.; Hellmann, I.; Stadler, M.B.; Ramos, L.; Pääbo, S.; Rebhan, M.; Schübeler, D. Distribution, Silencing Potential and Evolutionary Impact of Promoter DNA Methylation in the Human Genome. Nat. Genet. 2007, 39, 457–466. [Google Scholar] [CrossRef]
- Katrinli, S.; Maihofer, A.X.; Wani, A.H.; Pfeiffer, J.R.; Ketema, E.; Ratanatharathorn, A.; Baker, D.G.; Boks, M.P.; Geuze, E.; Kessler, R.C.; et al. Epigenome-Wide Meta-Analysis of PTSD Symptom Severity in Three Military Cohorts Implicates DNA Methylation Changes in Genes Involved in Immune System and Oxidative Stress. Mol. Psychiatry 2022, 27, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Sammallahti, S.; Hidalgo, A.P.C.; Tuominen, S.; Malmberg, A.; Mulder, R.H.; Brunst, K.J.; Alemany, S.; McBride, N.S.; Yousefi, P.; Heiss, J.A.; et al. Maternal Anxiety during Pregnancy and Newborn Epigenome-Wide DNA Methylation. Mol. Psychiatry 2021, 26, 1832–1845. [Google Scholar] [CrossRef]
- Wang, X. Blood DNA Methylation and Type 2 Diabetes Mellitus: A Protocol for Systematic Review and Meta-Analysis; INPLASY—International Platform of Registered Systematic Review Protocols: New Castle, DE, USA, 2020. [Google Scholar]
- Jiang, Y.; Forno, E.; Han, Y.-Y.; Xu, Z.; Hu, D.; Boutaoui, N.; Eng, C.; Acosta-Pérez, E.; Huntsman, S.; Colón-Semidey, A.; et al. A Genome-Wide Study of DNA Methylation in White Blood Cells and Asthma in Latino Children and Youth. Epigenetics 2021, 16, 577–585. [Google Scholar] [CrossRef]
- Christiansen, C.; Castillo-Fernandez, J.E.; Domingo-Relloso, A.; Zhao, W.; El-Sayed Moustafa, J.S.; Tsai, P.-C.; Maddock, J.; Haack, K.; Cole, S.A.; Kardia, S.L.R.; et al. Novel DNA Methylation Signatures of Tobacco Smoking with Trans-Ethnic Effects. Clin. Epigenet. 2021, 13, 36. [Google Scholar] [CrossRef]
- Karabegovic, I. Epigenome-Wide Association Meta-Analysis of DNA Methylation with Coffee and Tea Consumption. Nat. Commun. 2021, 12, 2830. [Google Scholar] [CrossRef]
- Brown, K.D.; Robertson, K.D. DNMT1 Knockout Delivers a Strong Blow to Genome Stability and Cell Viability. Nat. Genet. 2007, 39, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Holz-Schietinger, C.; Matje, D.M.; Harrison, M.F.; Reich, N.O. Oligomerization of DNMT3A Controls the Mechanism of de Novo DNA Methylation. J. Biol. Chem. 2011, 286, 41479–41488. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for de Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Heyn, P.; Logan, C.V.; Fluteau, A.; Challis, R.C.; Auchynnikava, T.; Martin, C.-A.; Marsh, J.A.; Taglini, F.; Kilanowski, F.; Parry, D.A.; et al. Gain-of-Function DNMT3A Mutations Cause Microcephalic Dwarfism and Hypermethylation of Polycomb-Regulated Regions. Nat. Genet. 2019, 51, 96–105. [Google Scholar] [CrossRef]
- Putiri, E.L.; Robertson, K.D. Epigenetic Mechanisms and Genome Stability. Clin. Epigenet. 2011, 2, 299–314. [Google Scholar] [CrossRef]
- Xie, W.; Schultz, M.D.; Lister, R.; Hou, Z.; Rajagopal, N.; Ray, P.; Whitaker, J.W.; Tian, S.; Hawkins, R.D.; Leung, D.; et al. Epigenomic Analysis of Multilineage Differentiation of Human Embryonic Stem Cells. Cell 2013, 153, 1134–1148. [Google Scholar] [CrossRef]
- Jeong, M.; Sun, D.; Luo, M.; Huang, Y.; Challen, G.A.; Rodriguez, B.; Zhang, X.; Chavez, L.; Wang, H.; Hannah, R.; et al. Large Conserved Domains of Low DNA Methylation Maintained by Dnmt3a. Nat. Genet. 2014, 46, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Wang, Q.; Zhou, C.; Wei, L.; Liu, X.; Zhang, W.; Zhang, Y.; Du, Z.; Wang, X.; et al. Genome-Wide Analyses Reveal a Role of Polycomb in Promoting Hypomethylation of DNA Methylation Valleys. Genome Biol. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.A.; Plath, K.; Zeitlinger, J.; Brambrink, T.; Medeiros, L.A.; Lee, T.I.; Levine, S.S.; Wernig, M.; Tajonar, A.; Ray, M.K.; et al. Polycomb Complexes Repress Developmental Regulators in Murine Embryonic Stem Cells. Nature 2006, 441, 349–353. [Google Scholar] [CrossRef]
- Leeb, M.; Pasini, D.; Novatchkova, M.; Jaritz, M.; Helin, K.; Wutz, A. Polycomb Complexes Act Redundantly to Repress Genomic Repeats and Genes. Genes Dev. 2010, 24, 265–276. [Google Scholar] [CrossRef]
- Xie, H.; Xu, J.; Hsu, J.H.; Nguyen, M.; Fujiwara, Y.; Peng, C.; Orkin, S.H. Polycomb Repressive Complex 2 Regulates Normal Hematopoietic Stem Cell Function in a Developmental-Stage-Specific Manner. Cell Stem Cell 2014, 14, 68–80. [Google Scholar] [CrossRef]
- Kasinath, V.; Faini, M.; Poepsel, S.; Reif, D.; Feng, X.A.; Stjepanovic, G.; Aebersold, R.; Nogales, E. Structures of Human PRC2 with Its Cofactors AEBP2 and JARID2. Science 2018, 359, 940–944. [Google Scholar] [CrossRef]
- Jain, S.U.; Do, T.J.; Lund, P.J.; Rashoff, A.Q.; Diehl, K.L.; Cieslik, M.; Bajic, A.; Juretic, N.; Deshmukh, S.; Venneti, S.; et al. PFA Ependymoma-Associated Protein EZHIP Inhibits PRC2 Activity through a H3 K27M-like Mechanism. Nat. Commun. 2019, 10, 2146. [Google Scholar] [CrossRef]
- Viré, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.-M.; et al. The Polycomb Group Protein EZH2 Directly Controls DNA Methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef]
- Hu, L.; Li, Z.; Cheng, J.; Rao, Q.; Gong, W.; Liu, M.; Shi, Y.G.; Zhu, J.; Wang, P.; Xu, Y. Crystal Structure of TET2-DNA Complex: Insight into TET-Mediated 5mC Oxidation. Cell 2013, 155, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate Regulates Haematopoietic Stem Cell Function and Leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hong, J.; Han, H.; Park, J.; Kim, D.; Park, H.; Ko, M.; Koh, Y.; Shin, D.-Y.; Yoon, S.-S. Decreased Vitamin C Uptake Mediated by SLC2A3 Promotes Leukaemia Progression and Impedes TET2 Restoration. Br. J. Cancer 2020, 122, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Kim, E.-J.; Park, U.-H.; Sin, H.-S.; Um, S.-J. Additional Sex Comb-like 1 (ASXL1), in Cooperation with SRC-1, Acts as a Ligand-Dependent Coactivator for Retinoic Acid Receptor. J. Biol. Chem. 2006, 281, 17588–17598. [Google Scholar] [CrossRef]
- Kolovos, P.; Nishimura, K.; Sankar, A.; Sidoli, S.; Cloos, P.A.; Helin, K.; Christensen, J. PR-DUB Maintains the Expression of Critical Genes through FOXK1/2- and ASXL1/2/3-Dependent Recruitment to Chromatin and H2AK119ub1 Deubiquitination. Genome Res. 2020, 30, 1119–1130. [Google Scholar] [CrossRef]
- Scheuermann, J.C.; de Ayala Alonso, A.G.; Oktaba, K.; Ly-Hartig, N.; McGinty, R.K.; Fraterman, S.; Wilm, M.; Muir, T.W.; Müller, J. Histone H2A Deubiquitinase Activity of the Polycomb Repressive Complex PR-DUB. Nature 2010, 465, 243–247. [Google Scholar] [CrossRef]
- de Napoles, M.; Mermoud, J.E.; Wakao, R.; Tang, Y.A.; Endoh, M.; Appanah, R.; Nesterova, T.B.; Silva, J.; Otte, A.P.; Vidal, M.; et al. Polycomb Group Proteins Ring1A/B Link Ubiquitylation of Histone H2A to Heritable Gene Silencing and X Inactivation. Dev. Cell 2004, 7, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Tamburri, S.; Lavarone, E.; Fernández-Pérez, D.; Conway, E.; Zanotti, M.; Manganaro, D.; Pasini, D. Histone H2AK119 Mono-Ubiquitination Is Essential for Polycomb-Mediated Transcriptional Repression. Mol. Cell 2020, 77, 840–856.e5. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.-M.; Chen, Y.; Moon, E.; Kvederaviciutė, K.; Klimasauskas, S.; Feldman, D.E. An Adversarial DNA N6-Methyladenine-Sensor Network Preserves Polycomb Silencing. Mol. Cell 2019, 74, 1138–1147.e6. [Google Scholar] [CrossRef] [PubMed]
- El Ghannam, D.; Taalab, M.M.; Ghazy, H.F.; Eneen, A.F. DNMT3A R882 Mutations in Patients with Cytogenetically Normal Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood Cells Mol. Dis. 2014, 53, 61–66. [Google Scholar] [CrossRef]
- Russler-Germain, D.A.; Spencer, D.H.; Young, M.A.; Lamprecht, T.L.; Miller, C.A.; Fulton, R.; Meyer, M.R.; Erdmann-Gilmore, P.; Townsend, R.R.; Wilson, R.K.; et al. The R882H DNMT3A Mutation Associated with AML Dominantly Inhibits Wild-Type DNMT3A by Blocking Its Ability to Form Active Tetramers. Cancer Cell 2014, 25, 442–454. [Google Scholar] [CrossRef]
- Hájková, H.; Marková, J.; Haškovec, C.; Sárová, I.; Fuchs, O.; Kostečka, A.; Cetkovský, P.; Michalová, K.; Schwarz, J. Decreased DNA Methylation in Acute Myeloid Leukemia Patients with DNMT3A Mutations and Prognostic Implications of DNA Methylation. Leuk. Res. 2012, 36, 1128–1133. [Google Scholar] [CrossRef]
- Qu, Y.; Lennartsson, A.; Gaidzik, V.I.; Deneberg, S.; Karimi, M.; Bengtzén, S.; Höglund, M.; Bullinger, L.; Döhner, K.; Lehmann, S. Differential Methylation in CN-AML Preferentially Targets Non-CGI Regions and Is Dictated by DNMT3A Mutational Status and Associated with Predominant Hypomethylation of HOX Genes. Epigenetics 2014, 9, 1108–1119. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, X.Q.D.; Su, J.; Putluri, N.; Zhou, T.; Qu, Y.; Jeong, M.; Guzman, A.; Rosas, C.; et al. Dnmt3a Loss and Idh2 Neomorphic Mutations Mutually Potentiate Malignant Hematopoiesis. Blood 2020, 135, 845–856. [Google Scholar] [CrossRef]
- Traina, F.; Visconte, V.; Elson, P.; Tabarroki, A.; Jankowska, A.M.; Hasrouni, E.; Sugimoto, Y.; Szpurka, H.; Makishima, H.; O’Keefe, C.L.; et al. Impact of Molecular Mutations on Treatment Response to DNMT Inhibitors in Myelodysplasia and Related Neoplasms. Leukemia 2014, 28, 78–87. [Google Scholar] [CrossRef]
- Kuendgen, A.; Müller-Thomas, C.; Lauseker, M.; Haferlach, T.; Urbaniak, P.; Schroeder, T.; Brings, C.; Wulfert, M.; Meggendorfer, M.; Hildebrandt, B.; et al. Efficacy of Azacitidine Is Independent of Molecular and Clinical Characteristics—An Analysis of 128 Patients with Myelodysplastic Syndromes or Acute Myeloid Leukemia and a Review of the Literature. Oncotarget 2018, 9, 27882–27894. [Google Scholar] [CrossRef]
- Weissmann, S.; Alpermann, T.; Grossmann, V.; Kowarsch, A.; Nadarajah, N.; Eder, C.; Dicker, F.; Fasan, A.; Haferlach, C.; Haferlach, T.; et al. Landscape of TET2 Mutations in Acute Myeloid Leukemia. Leukemia 2012, 26, 934–942. [Google Scholar] [CrossRef]
- Langemeijer, S.M.C.; Kuiper, R.P.; Berends, M.; Knops, R.; Aslanyan, M.G.; Massop, M.; Stevens-Linders, E.; van Hoogen, P.; van Kessel, A.G.; Raymakers, R.A.P.; et al. Acquired Mutations in TET2 Are Common in Myelodysplastic Syndromes. Nat. Genet. 2009, 41, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Moran-Crusio, K.; Reavie, L.; Shih, A.; Abdel-Wahab, O.; Ndiaye-Lobry, D.; Lobry, C.; Figueroa, M.E.; Vasanthakumar, A.; Patel, J.; Zhao, X.; et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell 2011, 20, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Huang, Y.; Jankowska, A.M.; Pape, U.J.; Tahiliani, M.; Bandukwala, H.S.; An, J.; Lamperti, E.D.; Koh, K.P.; Ganetzky, R.; et al. Impaired Hydroxylation of 5-Methylcytosine in Myeloid Cancers with Mutant TET2. Nature 2010, 468, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Scourzic, L.; Mouly, E.; Bernard, O.A. TET Proteins and the Control of Cytosine Demethylation in Cancer. Genome Med. 2015, 7, 9. [Google Scholar] [CrossRef]
- Bejar, R.; Lord, A.; Stevenson, K.; Bar-Natan, M.; Pérez-Ladaga, A.; Zaneveld, J.; Wang, H.; Caughey, B.; Stojanov, P.; Getz, G.; et al. TET2 Mutations Predict Response to Hypomethylating Agents in Myelodysplastic Syndrome Patients. Blood 2014, 124, 2705–2712. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Cluzeau, T.; Mansat-De Mas, V.; Dreyfus, F.; Beyne-Rauzy, O.; Quesnel, B.; Vey, N.; Gelsi-Boyer, V.; Raynaud, S.; et al. Impact of TET2 Mutations on Response Rate to Azacitidine in Myelodysplastic Syndromes and Low Blast Count Acute Myeloid Leukemias. Leukemia 2011, 25, 1147–1152. [Google Scholar] [CrossRef]
- Shih, A.H.; Meydan, C.; Shank, K.; Garrett-Bakelman, F.E.; Ward, P.S.; Intlekofer, A.; Nazir, A.; Stein, E.; Knapp, K.; Glass, J.; et al. Combination Targeted Therapy to Disrupt Aberrant Oncogenic Signaling and Reverse Epigenetic Dysfunction in IDH2- and TET2-Mutant Acute Myeloid Leukemia. Cancer Discov. 2017, 7, 494–505. [Google Scholar] [CrossRef]
- Cedena, M.T.; Rapado, I.; Santos-Lozano, A.; Ayala, R.; Onecha, E.; Abaigar, M.; Such, E.; Ramos, F.; Cervera, J.; Díez-Campelo, M.; et al. Mutations in the DNA Methylation Pathway and Number of Driver Mutations Predict Response to Azacitidine in Myelodysplastic Syndromes. Oncotarget 2017, 8, 106948–106961. [Google Scholar] [CrossRef]
- Sasaki, K.; Kanagal-Shamanna, R.; Montalban-Bravo, G.; Assi, R.; Jabbour, E.; Ravandi, F.; Kadia, T.; Pierce, S.; Takahashi, K.; Nogueras Gonzalez, G.; et al. Impact of the Variant Allele Frequency of ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 on the Outcomes of Patients with Newly Diagnosed Acute Myeloid Leukemia. Cancer 2020, 126, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, W.; Wang, M.; Li, Y.; Wang, X.; Yang, E.; Ming, J.; Quan, R.; Hu, X. Prognostic Value of ASXL1 Mutations in Patients with Primary Myelofibrosis and Its Relationship with Clinical Features: A Meta-Analysis. Ann. Hematol. 2021, 100, 465–479. [Google Scholar] [CrossRef]
- Yang, H.; Kurtenbach, S.; Guo, Y.; Lohse, I.; Durante, M.A.; Li, J.; Li, Z.; Al-Ali, H.; Li, L.; Chen, Z.; et al. Gain of Function of ASXL1 Truncating Protein in the Pathogenesis of Myeloid Malignancies. Blood 2018, 131, 328–341. [Google Scholar] [CrossRef]
- Bera, R.; Chiu, M.-C.; Huang, Y.-J.; Lin, T.-H.; Kuo, M.-C.; Shih, L.-Y. RUNX1 Mutations Promote Leukemogenesis of Myeloid Malignancies in ASXL1-Mutated Leukemia. J. Hematol. Oncol. 2019, 12, 104. [Google Scholar] [CrossRef]
- D’Altri, T.; Wilhelmson, A.S.; Schuster, M.B.; Wenzel, A.; Kalvisa, A.; Pundhir, S.; Hansen, A.M.; Porse, B.T. The ASXL1-G643W Variant Accelerates the Development of CEBPA Mutant Acute Myeloid Leukemia. Haematologica 2021, 106, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Tobiasson, M.; McLornan, D.P.; Karimi, M.; Dimitriou, M.; Jansson, M.; Ben Azenkoud, A.; Jädersten, M.; Lindberg, G.; Abdulkadir, H.; Kulasekararaj, A.; et al. Mutations in Histone Modulators Are Associated with Prolonged Survival during Azacitidine Therapy. Oncotarget 2016, 7, 22103–22115. [Google Scholar] [CrossRef]
- Stasik, S.; Middeke, J.M.; Kramer, M.; Röllig, C.; Krämer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. EZH2 Mutations and Impact on Clinical Outcome: An Analysis in 1604 Patients with Newly Diagnosed Acute Myeloid Leukemia. Haematologica 2020, 105, e228–e231. [Google Scholar] [CrossRef] [PubMed]
- Chase, A.; Cross, N.C.P. Aberrations of EZH2 in Cancer. Clin. Cancer Res. 2011, 17, 2613–2618. [Google Scholar] [CrossRef]
- Xu, F.; Liu, L.; Chang, C.-K.; He, Q.; Wu, L.-Y.; Zhang, Z.; Shi, W.-H.; Guo, J.; Zhu, Y.; Zhao, Y.-S.; et al. Genomic Loss of EZH2 Leads to Epigenetic Modifications and Overexpression of the HOX Gene Clusters in Myelodysplastic Syndrome. Oncotarget 2016, 7, 8119–8130. [Google Scholar] [CrossRef]
- Hasegawa, N.; Oshima, M.; Sashida, G.; Matsui, H.; Koide, S.; Saraya, A.; Wang, C.; Muto, T.; Takane, K.; Kaneda, A.; et al. Impact of Combinatorial Dysfunctions of Tet2 and Ezh2 on the Epigenome in the Pathogenesis of Myelodysplastic Syndrome. Leukemia 2017, 31, 861–871. [Google Scholar] [CrossRef]
- Saygin, C.; Hirsch, C.; Przychodzen, B.; Sekeres, M.A.; Hamilton, B.K.; Kalaycio, M.; Carraway, H.E.; Gerds, A.T.; Mukherjee, S.; Nazha, A.; et al. Mutations in DNMT3A, U2AF1, and EZH2 Identify Intermediate-Risk Acute Myeloid Leukemia Patients with Poor Outcome after CR1. Blood Cancer J 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, X. The Role of Histone Methyltransferase EZH2 in Myelodysplastic Syndromes. Expert Rev. Hematol. 2012, 5, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dufva, O.; Pölönen, P.; Brück, O.; Keränen, M.A.I.; Klievink, J.; Mehtonen, J.; Huuhtanen, J.; Kumar, A.; Malani, D.; Siitonen, S.; et al. Immunogenomic Landscape of Hematological Malignancies. Cancer Cell 2020, 38, 380–399.e13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).