Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation
Abstract
1. Introduction
1.1. Variability in Auditory Development after a Cochlear Implantation
1.2. Molecular Basis of Neuroplasticity after Sensory Deprivation
1.3. Aim of the Study
2. Results
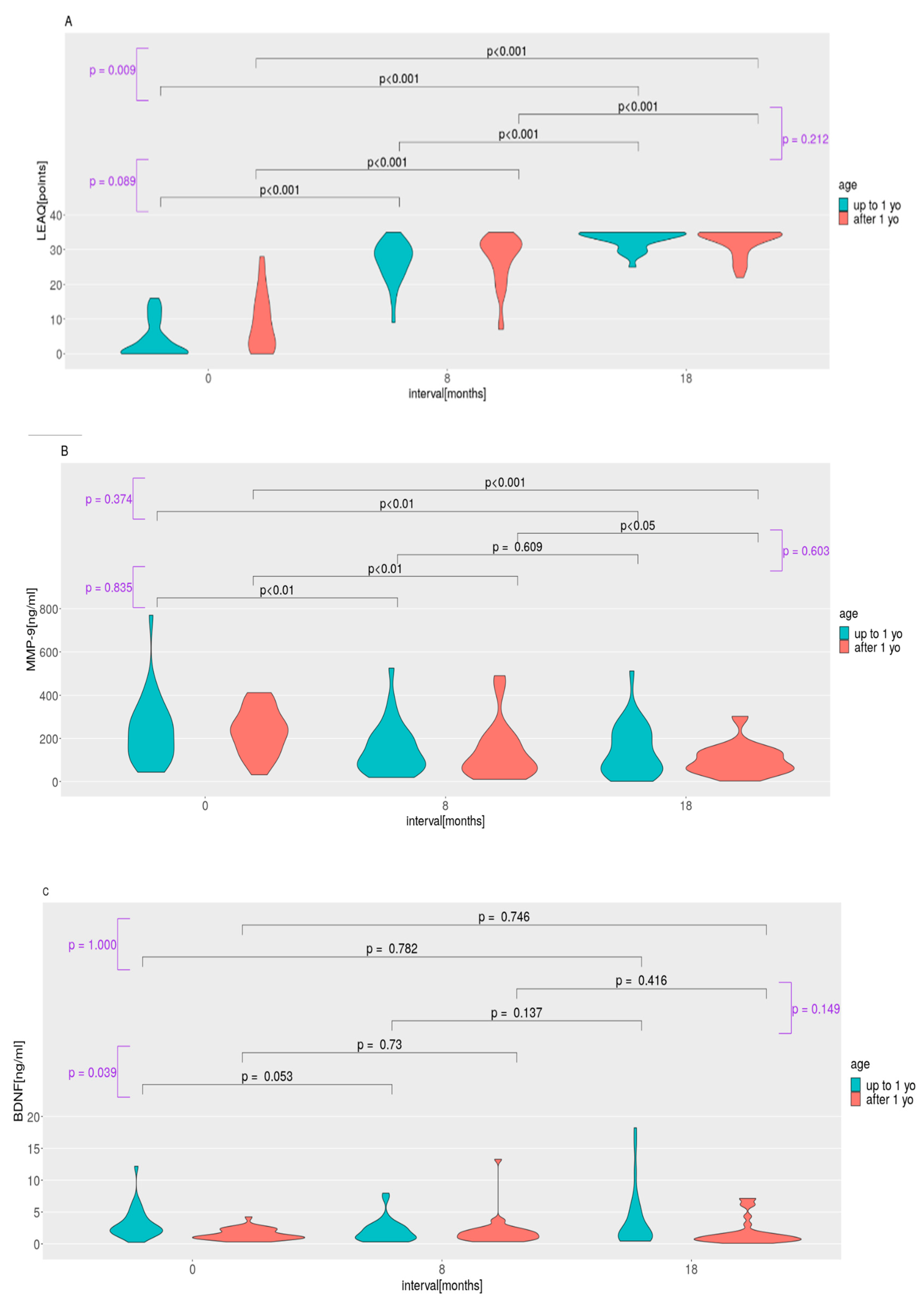
2.1. LEAQ Scores; MMP-9, BDNF, and Pro-BDNF Plasma Levels; and Pro-BDNF/BDNF Ratio in the Study Group
2.2. LEAQ Scores; MMP-9, BDNF, and Pro-BDNF Plasma Levels; and Pro-BDNF/BDNF Ratio: Effect of Age at Implantation
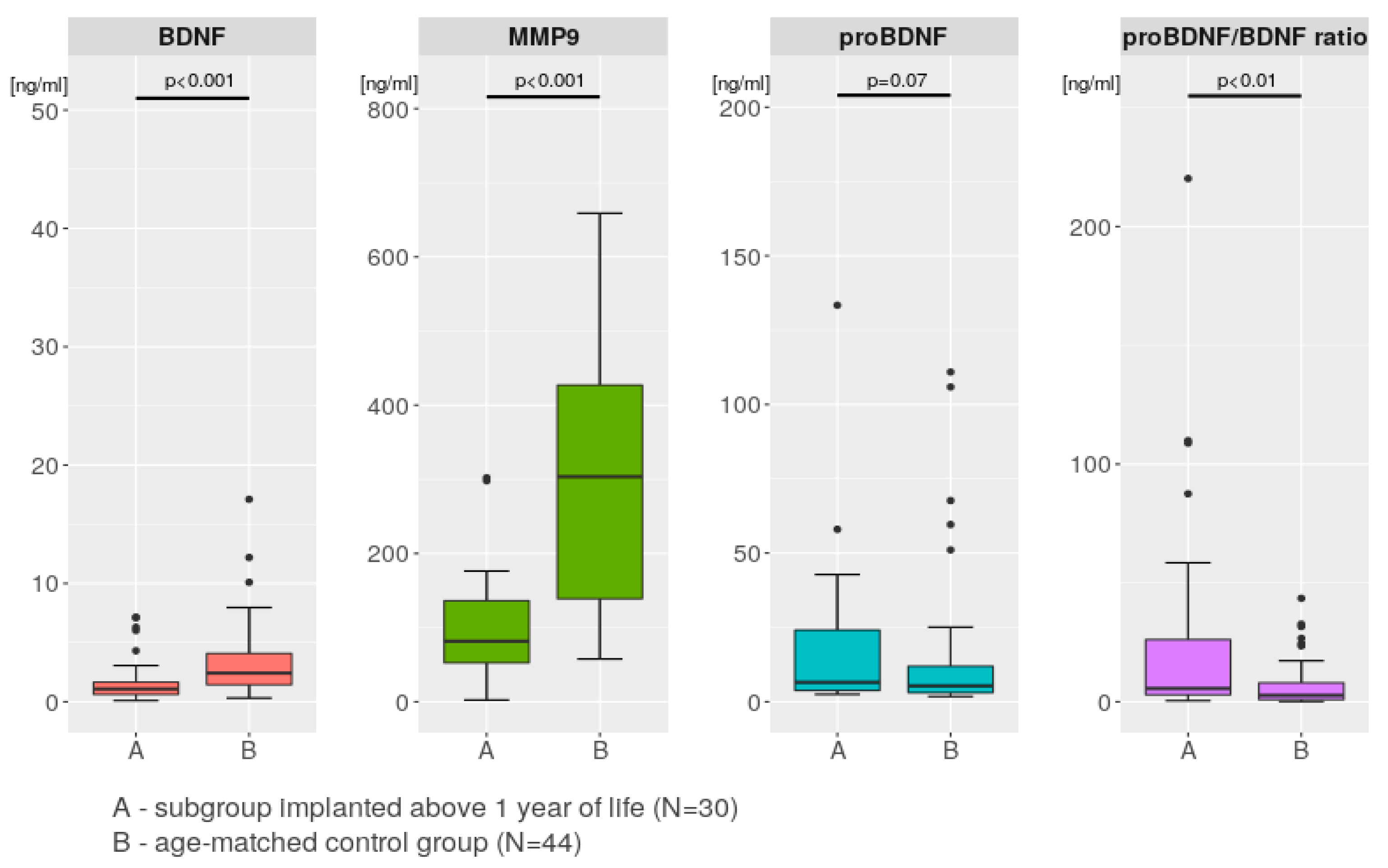
2.3. MMP-9, BDNF, and Pro-BDNF Levels and Pro-BDNF/BDNF Ratio in the Control Group
2.4. Comparisons between the Study Group and the Control Group
2.5. Correlations between Age and Protein Levels in Both the Study and Control Groups
3. Discussion
3.1. Auditory Development and Plasma Protein Levels in Subgroups Implanted before and after 1 Year of Life
3.2. BDNF Plasma Level Variation
3.3. Blood–Brain Barrier for BDNF and MMP-9 after Auditory Deprivation and after CI
3.4. Comparison with Control Group
3.5. Perspective
3.6. Limitations
4. Materials and Methods
4.1. Study Design, Participants, and Ethical Approval
4.2. Auditory Development Assessment
4.3. Plasma Sample Collection
4.4. Plasma MMP-9, BDNF, and Pro-BDNF Levels
4.5. Statistical Analyses
4.5.1. Paired Comparisons
4.5.2. Tests for Significance of Differences between Changes in LEAQ Scores; Plasma Levels of MMP-9, BDNF, and Pro-BDNF; and Pro-BDNF/BDNF Ratio
4.5.3. Assessment of the Significance of Changes in Values in Subsequent Measurements for LEAQ, MMP-9, BDNF, Pro-BDNF, and Pro-BDNF/BDNF Ratio in Subgroups
4.5.4. Correlation Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kral, A.; O’Donoghue, G.M. Profound deafness in childhood. N. Engl. J. Med. 2010, 363, 1438–1478. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.; Kronenberger, W.G.; Pisoni, D.B.; O’Donoghue, G.M. Neurocognitive factors in sensory restoration of early deafness: A connectome model. Lancet Neurol. 2016, 15, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; Strother-Garcia, K.; Michnick-Glinkoff, R.; Hirsh-Pasek, K. Language development in the first year of life: What deaf children might be missing before cochlear implantation. Otol. Neurotol. 2016, 37, 56–62. [Google Scholar] [CrossRef]
- Houston, D.M.; Stewart, J.; Moberly, A.; Hollich, G.; Miyamoto, R.T. Word learning in deaf children with cochlear implants: Effects of early auditory experience. Dev. Sci. 2012, 15, 448–461. [Google Scholar] [CrossRef]
- Leigh, J.; Dettman, S.; Dowell, R.; Briggs, R. Communication development in children who receive a cochlear implant by 12 months of age. Otol. Neurotol. 2013, 34, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kral, A. Auditory critical periods: A review from system’s perspective. Neuroscience 2013, 247, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Niparko, J.; Tobey, E.A.; Thal, D.J.; Eisenbrg, L.S.; Wang, N.Y.; Quittner, A.L.; Fink, N.E.; CDaCI Investigative Team. Spoken language development in children following cochlear implantation. JAMA 2010, 303, 1498–1506. [Google Scholar] [CrossRef]
- May-Mederake, B.; Kuehn, H.; Vogel, A.; Keilmann, A.; Bohnert, A.; Muller, S.; Witt, G.; Neumann, K.; Hey, C.; Stroele, A.; et al. Evaluation of auditory development in infants and toddlers who received cochlear implants under the age of 24 months with the LittlEARS Auditory Questionnaire. Int. J. Pediatr. Otorhniolaryngol. 2010, 74, 1149–1155. [Google Scholar] [CrossRef]
- Oziębło, D.; Obrycka, A.; Lorens, A.; Skarżyński, H.; Ołdak, M. Cochlear implantation outcome in children with DFNB1 locus pathogenic variants. J. Clin. Med. 2020, 9, 228. [Google Scholar] [CrossRef]
- Miyamoto, R.T.; Hay-McCutcheon, M.J.; Kirk, K.I.; Houston, D.M.; Bergeson-Dana, T. Language skills of profoundly deaf children who received cochlear implants under 12-months of age: A preliminary study. Acta Otolaryngol. 2008, 128, 373–377. [Google Scholar] [CrossRef]
- Holtmaat, A.; Caroni, P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat. Neurosci. 2016, 19, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Oziębło, D.; Ołdak, M.; Rejmak, E.; Kaczmarek, L.; Skarżyński, P.H.; Skarżyński, H. Prospective cohort study reveals MMP-9, a neuroplasticity regulator, as a prediction marker of cochlear implantation outcome in congenital deafness. Mol. Neurobiol. 2022, 59, 2190–2203. [Google Scholar] [CrossRef]
- Reinhard, S.M.; Razak, K.; Ethell, I. A delicate balance: Role of MMP9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Beroun, A.; Mitra, S.; Michaluk, P.; Pijet, B.; Stefaniuk, M.; Kaczmarek, L. MMPs in learning and memory and neuropsychiatric disorders. Cell. Mol. Life Sci. 2019, 76, 3207–3228. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagy, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, A.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagy, O.; Huntley, W.H. The extracellular protease matrix metalloproteinase-9 is required by inhibitory avoidance learning and required for long term memory. Learn. Mem. 2007, 14, 655–664. [Google Scholar] [CrossRef]
- Bozdagi, O.; Nagy, V.; Kwei, K.T.; Huntley, G.W. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007, 98, 334–344. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. 2), 91–114. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Kolodziej, L.; Mioduszewska, B.; Wilczynski, G.M.; Dzwonek, A.; Jawoski, J.; Gorecki, D.C.; Ottersen, O.P.; Kaczmarek, L. Beta-dystrogylcan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 2007, 282, 16036–16041. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76 Pt C, 677–683. [Google Scholar] [CrossRef]
- Zagrebelsky, M.; Korte, M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014, 76, 628–638. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Nakade, J.; Tachibana, M.; Ibi, D.; Someya, E.; Koike, H.; Kamei, H.; Nabeshima, T.; Itohara, S.; Takuma, K.; et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J. Neurosci. 2011, 31, 12963–12971. [Google Scholar] [CrossRef]
- Singer, W.; Panford-Walsh, R.; Knipper, M. The function of BDNF in the adult auditory system. Neuropharmacology 2014, 76 Pt C, 719–728. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zheng, Y.B.; Yuan, K.; Han, Y.; Lu, L. Extracellular zinc regulates contextual fear memory formation in male rats through MMP-BDNF-TrkB pathway in dorsal hippocampus and basolateral amygdala. Behav. Brain Res. 2023, 439, 114230. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, H.; Hashimoto, R.; Ishima, T.; Kishi, F.; Yasuda, Y.; Ohi, K.; Hujimoto, M.; Umeda-Yano, S.; Ito, A.; Hasshimoto, K.; et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment –resistant schizophrenia treated with clozapine. Neurosci. Lett. 2013, 556, 37–41. [Google Scholar] [CrossRef]
- LaFrance, W.C.; Leaver, K.; Stopa, E.; Papandonatos, G.D.; Blum, A.S. Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 2010, 75, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Li, W.; Qu, B.; Zou, X.; Chen, J.; Sander, J.W.; Zhou, D. Serum brain-derived neurotrophic factor levels in epilepsy. Eur. J. Neurol. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-C.; Chuang, Y.-C.; Huang, C.-W.; Lui, C.-C.; Lee, C.-C.; Hsu, S.-W.; Lin, P.-H.; Lu, Y.-T.; Chang, Y.-T.; Hsu, C.-W. Interictal serum brain-derived neurotrophic factor level reflects white matter integrity, epilepsy severity and cognitive dysfunction in chronic temporal lobe epilepsy. Epilepsy Behav. 2016, 59, 149–154. [Google Scholar] [CrossRef]
- Vandooren, J.; Van den Steen, P.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Yoshimura, R.; Kishi, T.; Hori, H.; Atake, K.; Katsuki, A.; Nakano-Umene, W.; Ikenouchi-Sugita, A.; Uwata, N.; Nakamura, J. Serum proBDNF/BDNF and response to fluvoxamine in drug naïve first-episode major depressive disorder patients. Ann. Gen. Psychiatry 2014, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ishikawa, M.; Niitsu, T.; Nakazato, M.; Watanabe, H.; Shiraishi, T.; Shiina, A.; Hashimoto, T.; Kanahara, N.; Hasegawa, T.; et al. Decreased serum level of mature Brain-Derived Neurotrophic Factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS ONE 2012, 7, e42676. [Google Scholar] [CrossRef] [PubMed]
- Cudna, A.; Jopowicz, A.; Mierzejewski, P.; Kurkowska-Jastrzębska, I. Serum metalloproteinase 9 levels increase after generalized tonic-clonic seizures. Epilepsy Res. 2017, 129, 33–36. [Google Scholar] [CrossRef]
- Alemi, R.; Zarandy, M.M.; Joghataeri, M.T.; Eftekharian, A.; Zarrindast, M.R.; Vousooghi, N. Plasticity after pediatric cochlear implantation: Implications from changes in peripheral plasma level of BDNF and auditory nerve responses. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Oziębło, D.; Obrycka, A.; Ołdak, M.; Kaczmarek, L.; Skarżyński, P.; Skarżyński, H. Functional polymorphism of MMP9 and BDNF as a potential biomarker of auditory plasticity in prelingual deafness treatment with cochlear implantation: A retrospective cohort analysis. Trends Hear. 2021, 25, 23312165211002140. [Google Scholar]
- Tan, J.; Widjaja, S.; Xu, J.; Shepherd, R.K. Cochlear Implants stimulate activity dependent CREB pathway in the deaf auditory cortex: Implications for molecular plasticity induced by neural prosthetic devices. Cereb. Cortex 2008, 18, 1799–1813. [Google Scholar] [CrossRef]
- Karege, F.; Schwald, M.; Cisse, M. Postnatal development profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002, 328, 261–264. [Google Scholar] [CrossRef]
- Bjelosevic, S.; Pascovici, D.; Ping, H.; Karlaftis, V.; Zaw, T.; Song, X.; Molloy, M.P.; Monagle, P.; Ignjatovic, V. Quantitative age-specific variability of plasma proteins in healthy neonates, children and adults. Mol. Cell. Proteomics 2017, 16, 924–935. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Vrichow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef]
- Iughetti, L.; Casarosa, E.; Predieri, B.; Patianna, V.; Luisi, S. Plasma brain-derived neurotrophic factor concentrations in children and adolescents. Neuropeptides 2011, 26, 115–123. [Google Scholar] [CrossRef]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Kambayashi, J.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metabol. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Yasui, K.; Ichiyawa, T.; Saito, Y.; Nagaoka, Y.; Yashiro, M.; Yamashita, N.; Morishima, T. Increase of tumαor necrosis factor-α in the blood induces early activation of matrix metalloproteinase-9 in the brain. Microbiol. Immunol. 2010, 54, 417–424. [Google Scholar] [PubMed]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Kotak, V.C.; Sanes, D.H. Deafferentiation weakens excitatory synapses in the developing central auditory system. Eur. J. Neurosci. 1997, 9, 2340–2347. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, H.S.; Park, M.-H. Transcriptome analysis of deafness: Intracellular signal transduction signalling pathways regulate neuroplastic changes in the auditory cortex. Otol. Neurotol. 2010, 41, 986–996. [Google Scholar] [CrossRef]
- Tan, J.; Ruttiger, L.; Panford-Walsh, R.; Singer, W.; Schulze, H.; Kilian, S.B.; Hadjab, S.; Zimmermann, U.; Kopschall, I.; Rohbock, K.; et al. Tinnitus behaviour and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience 2007, 145, 715–726. [Google Scholar] [CrossRef]
- Kral, A.; Hartmann, R.; Tillein, J.; Heid, S.; Klinke, R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb. Cortex 2000, 10, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.; Yusuf, P.A.; Land, R. Higher-order auditory areas in congenital deafness: Top-down interactions and corticocortical decoupling. Hear. Res. 2017, 343, 50–63. [Google Scholar] [CrossRef]
- Weichbold, V.; Tsiakpini, L.; Coninx, F.; D’Haese, P. Development of a parent questionnaire for assessment of auditory behaviour of infants up to two years of age. Laryngorhinootologie 2005, 84, 328–334. [Google Scholar] [CrossRef]
- Obrycka, A.; Padilla Garcia, J.P.; Pankowska, A.; Lorens, A.; Skarżynski, H. Production and evaluation of a Polish version of the LittlEars questionnaire for the assessment of auditory development in infants. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Coninx, F.; Weichbold, V.; Tsiakpini, L.; Autrique, E.; Bescond, G.; Tamas, L.; Compernol, A.; Georgescu, M.; Koroleva, I.; Le Maner-Idrissi, G.; et al. Validation of the LittlEARS Auditory Questionnaire in children with normal hearing. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Geal-Dor, M.; Jbarah, R.; Meilijson, S.; Adelman, C.; Levi, H. The Hebrew and the Arabic version of the LittlEARS Auditory Questionnaire for the assessment of auditory development: Results in normal hearing children and children with cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1327–1332. [Google Scholar] [CrossRef]
- Obrycka, A.; Lorens, A.; Padilla, J.L.; Piotrowska, A.; Skarżyński, H. Validation of the LittlEARS Auditory Questionnaire in cochlear implanted infants and toddlers. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 107–116. [Google Scholar] [CrossRef] [PubMed]

| CI Activation | LEAQ_0 | LEAQ_8 | LEAQ_18 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SD | Mean | Min | Max | SD | Mean | Min | Max | SD | |
| <1 y. o. | 3.93 | 0 | 16 | 5.13 | 26.55 | 9 | 35 | 5.87 | 32.90 | 25 | 35 | 2.64 |
| >1 y. o. | 8.73 | 0 | 28 | 8.10 | 27.30 | 7 | 35 | 7.24 | 32.10 | 22 | 35 | 3.75 |
| p-value | <0.01 | 0.277 | 0.619 | |||||||||
| MMP-9_0 (ng/mL) | MMP-9_8 (ng/mL) | MMP-9_18 (ng/mL) | ||||||||||
| mean | min | max | SD | mean | min | max | SD | mean | min | max | SD | |
| <1 y. o. | 235.03 | 43.3 | 769.6 | 151.7 | 150.7 | 19.0 | 525.7 | 116.69 | 142.6 | 0.67 | 512.3 | 118.2 |
| >1 y. o. | 231.1 | 31.1 | 412.0 | 105.1 | 137.7 | 10.3 | 490.4 | 128.4 | 100.78 | 2.39 | 302.09 | 71.38 |
| p-value | 0.712 | 0.435 | 0.292 | |||||||||
| BDNF_0 (ng/mL) | BDNF_8 (ng/mL) | BDNF_18 (ng/mL) | ||||||||||
| mean | min | max | SD | mean | min | max | SD | mean | min | max | SD | |
| <1 y. o. | 3.13 | 0.25 | 2.18 | 2.38 | 2.08 | 0.32 | 7.95 | 1.84 | 3.76 | 0.45 | 18.21 | 4.50 |
| >1 y. o. | 1.51 | 0.32 | 4.24 | 0.86 | 1.96 | 0.36 | 13.27 | 2.31 | 1.85 | 0.09 | 7.14 | 2.10 |
| p-value | <0.001 | 0.682 | <0.05 | |||||||||
| pro-BDNF_0 (ng/mL) | pro-BDNF_8 (ng/mL) | pro-BDNF_18 (ng/mL) | ||||||||||
| mean | min | max | SD | mean | min | max | SD | mean | min | max | SD | |
| <1 y. o. | 16.98 | 1.41 | 158.6 | 32.66 | 20.74 | 2.52 | 99.78 | 24.47 | 31.27 | 1.82 | 220.13 | 50.57 |
| >1 y. o. | 22.18 | 0.00 | 62.16 | 40.50 | 13.59 | 1.72 | 75.29 | 18.42 | 18.09 | 2.47 | 133.38 | 25.92 |
| p-value | 1.000 | 0.126 | 0.481 | |||||||||
| pro-BDNF_0/BDNF_0 ratio | pro-BDNF_8/BDNF_8 ratio | pro-BDNF_18/BDNF_18 ratio | ||||||||||
| mean | min | max | SD | mean | min | max | SD | mean | min | max | SD | |
| <1 y. o. | 10.54 | 0.20 | 102.8 | 22.48 | 21.84 | 0.35 | 114.0 | 30.76 | 20.43 | 0.17 | 127.19 | 31.29 |
| >1 y. o. | 20.03 | 0.00 | 161.5 | 39.12 | 13.35 | 0.19 | 108.5 | 22.40 | 27.56 | 0.39 | 220.2 | 48.02 |
| p-value | 0.097 | 0.386 | 0.484 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusiak, M.; Oziębło, D.; Ołdak, M.; Rejmak, E.; Kaczmarek, L.; Skarżyński, H. Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation. Int. J. Mol. Sci. 2023, 24, 3714. https://doi.org/10.3390/ijms24043714
Matusiak M, Oziębło D, Ołdak M, Rejmak E, Kaczmarek L, Skarżyński H. Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation. International Journal of Molecular Sciences. 2023; 24(4):3714. https://doi.org/10.3390/ijms24043714
Chicago/Turabian StyleMatusiak, Monika, Dominika Oziębło, Monika Ołdak, Emilia Rejmak, Leszek Kaczmarek, and Henryk Skarżyński. 2023. "Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation" International Journal of Molecular Sciences 24, no. 4: 3714. https://doi.org/10.3390/ijms24043714
APA StyleMatusiak, M., Oziębło, D., Ołdak, M., Rejmak, E., Kaczmarek, L., & Skarżyński, H. (2023). Longitudinal Changes in BDNF and MMP-9 Protein Plasma Levels in Children after Cochlear Implantation. International Journal of Molecular Sciences, 24(4), 3714. https://doi.org/10.3390/ijms24043714







