Synthesis and Characterization of Nanoparticle-Based Dexamethasone-Polypeptide Conjugates as Potential Intravitreal Delivery Systems
Abstract
1. Introduction
2. Results and Discussion
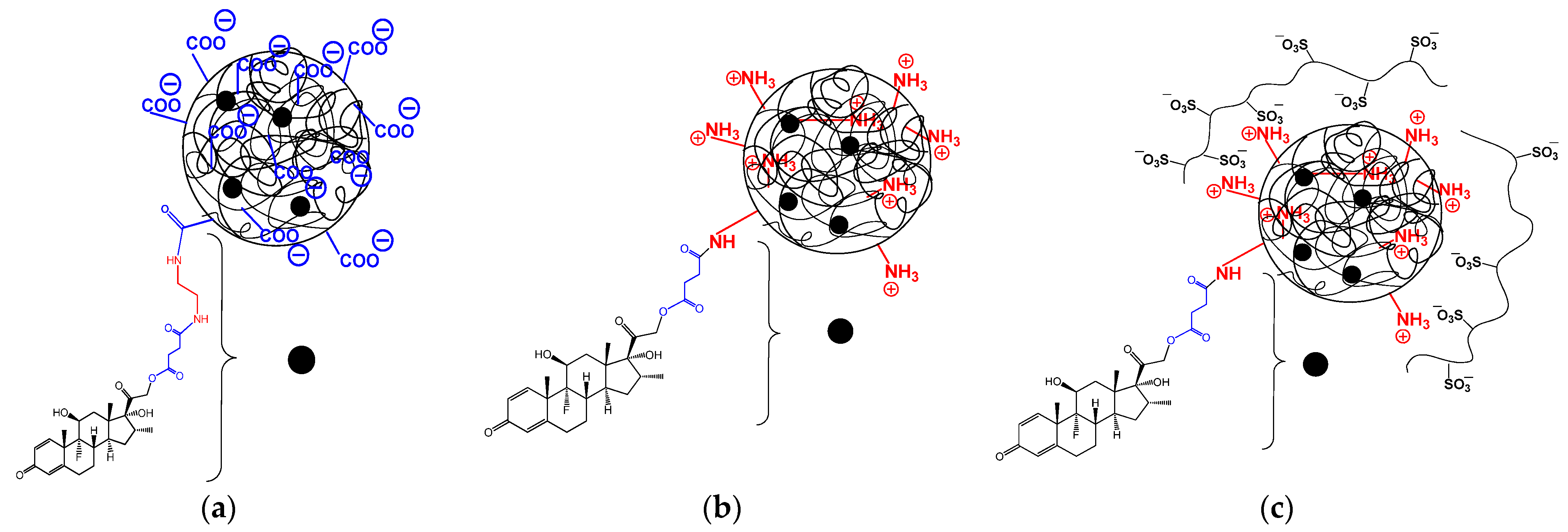
2.1. Polymer Carriers and Their Characteristics
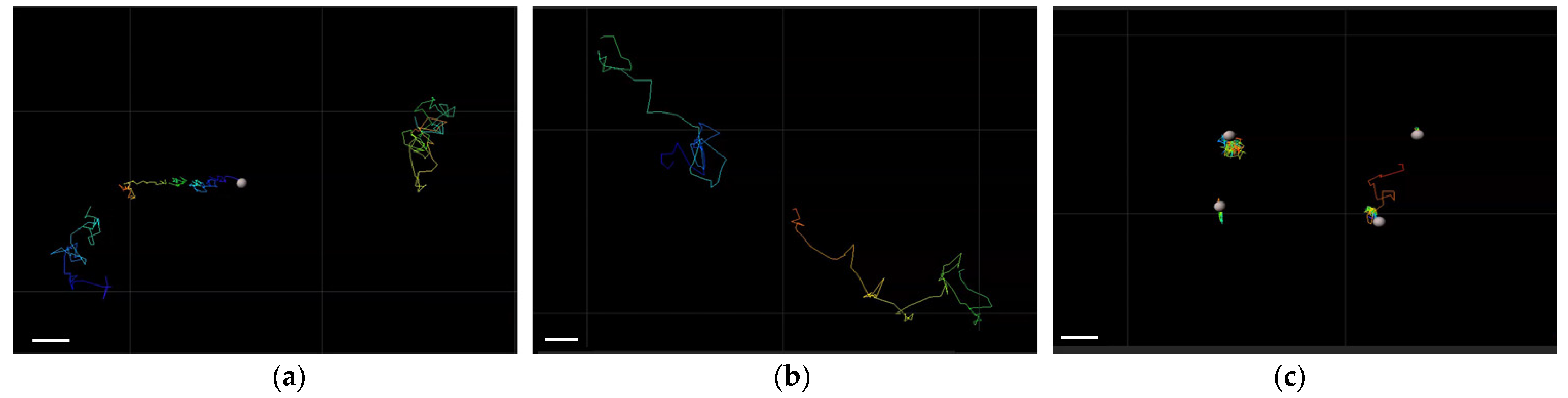
2.2. Mobility of Different Nanoparticles in Vitreous Humor
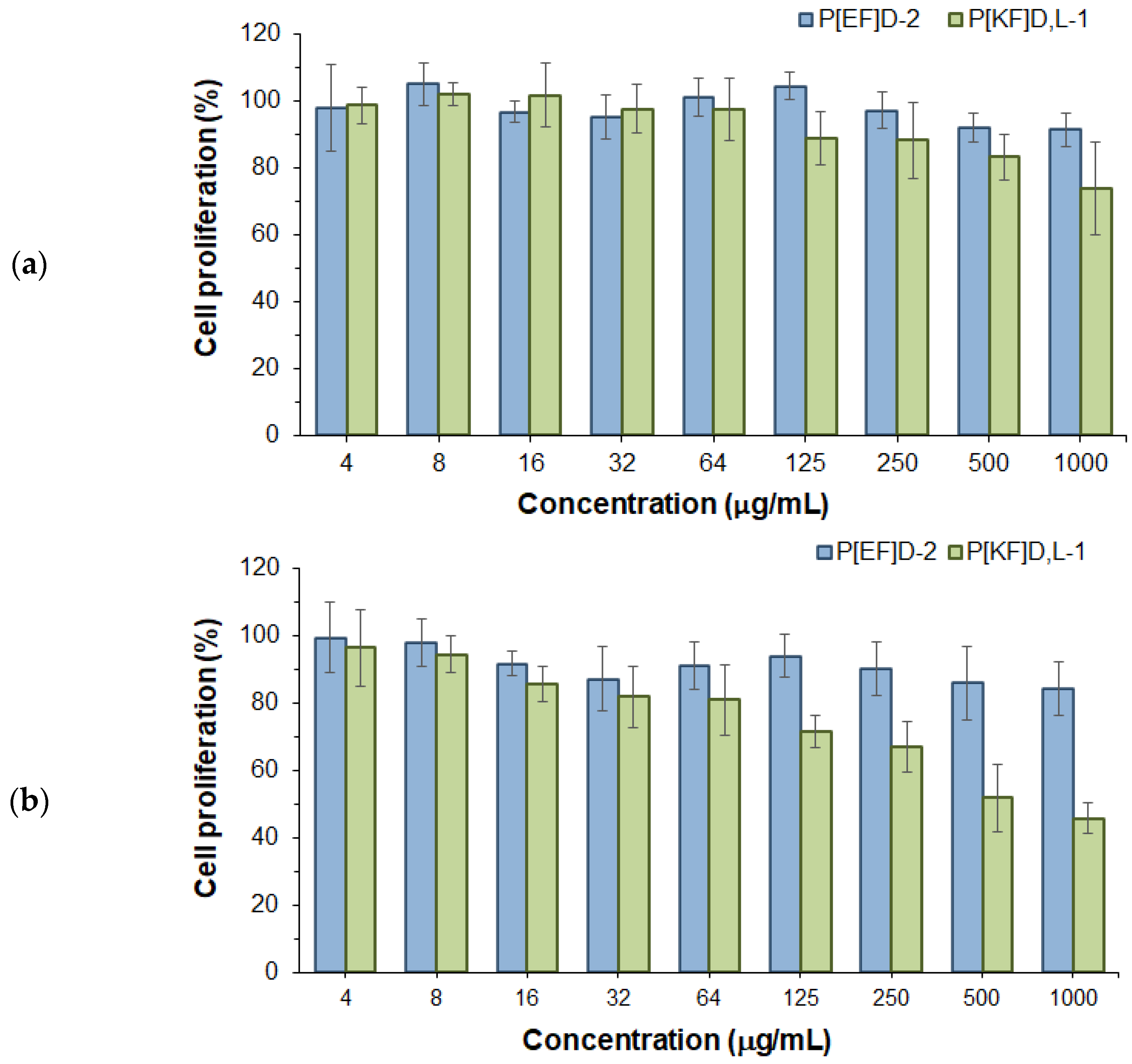
2.3. Effect of Polypeptide Nanoparticles on ARPE-19 Cell Proliferation
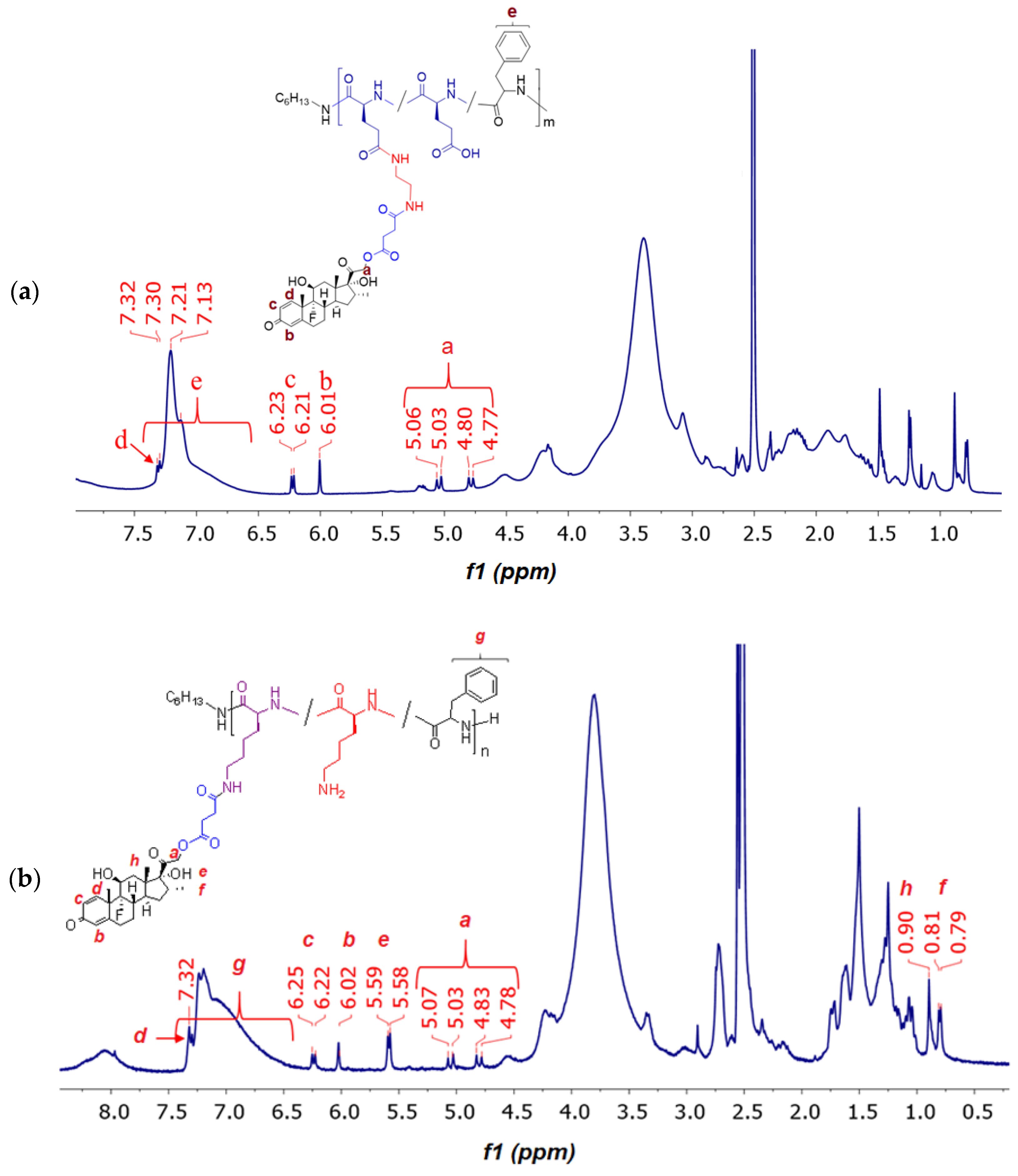
2.4. Synthesis and Characterization of Polypeptide-DEX Conjugates
2.4.1. DEX Modification and General Strategy for Conjugation with Polypeptides
2.4.2. Conjugation of DEX with Glutamic Acid- and Lysine-Containing Polypeptides
2.4.3. Storage Stability
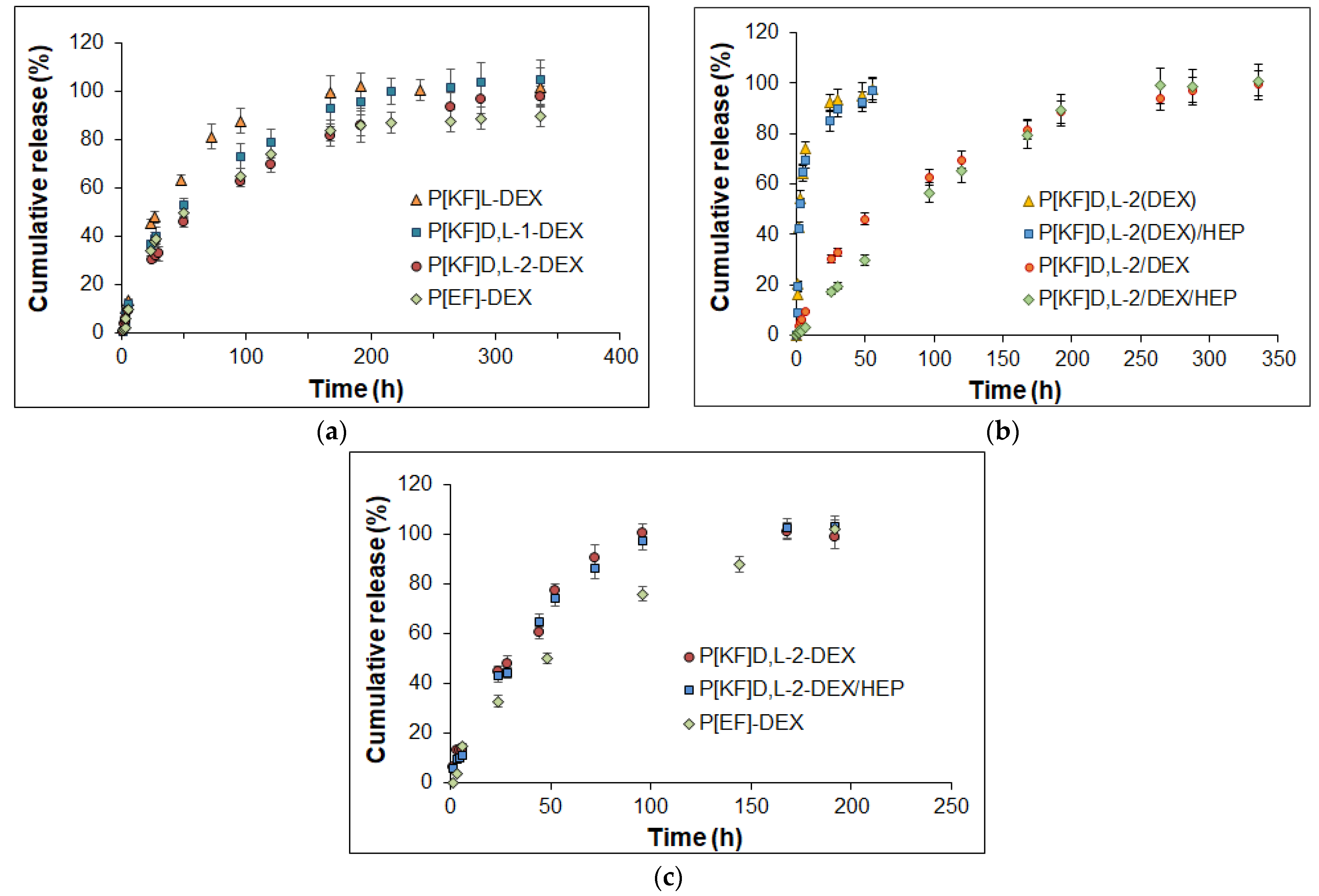
2.5. Release of DEX from Conjugates in Different Media
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis and Characterization of Delivery Systems
3.2.2. Stability of Nanoparticles and Movement in Vitreous Humor
3.2.3. Proliferation Assay
3.2.4. Modification of DEX
3.2.5. Modification of poly(Glutamic Acid-co-Phenylalanine) with Ethylenediamine
3.2.6. Synthesis and Characterization of Conjugates
3.2.7. DEX Release Study
3.2.8. DEX Quantitative HPLC Analysis
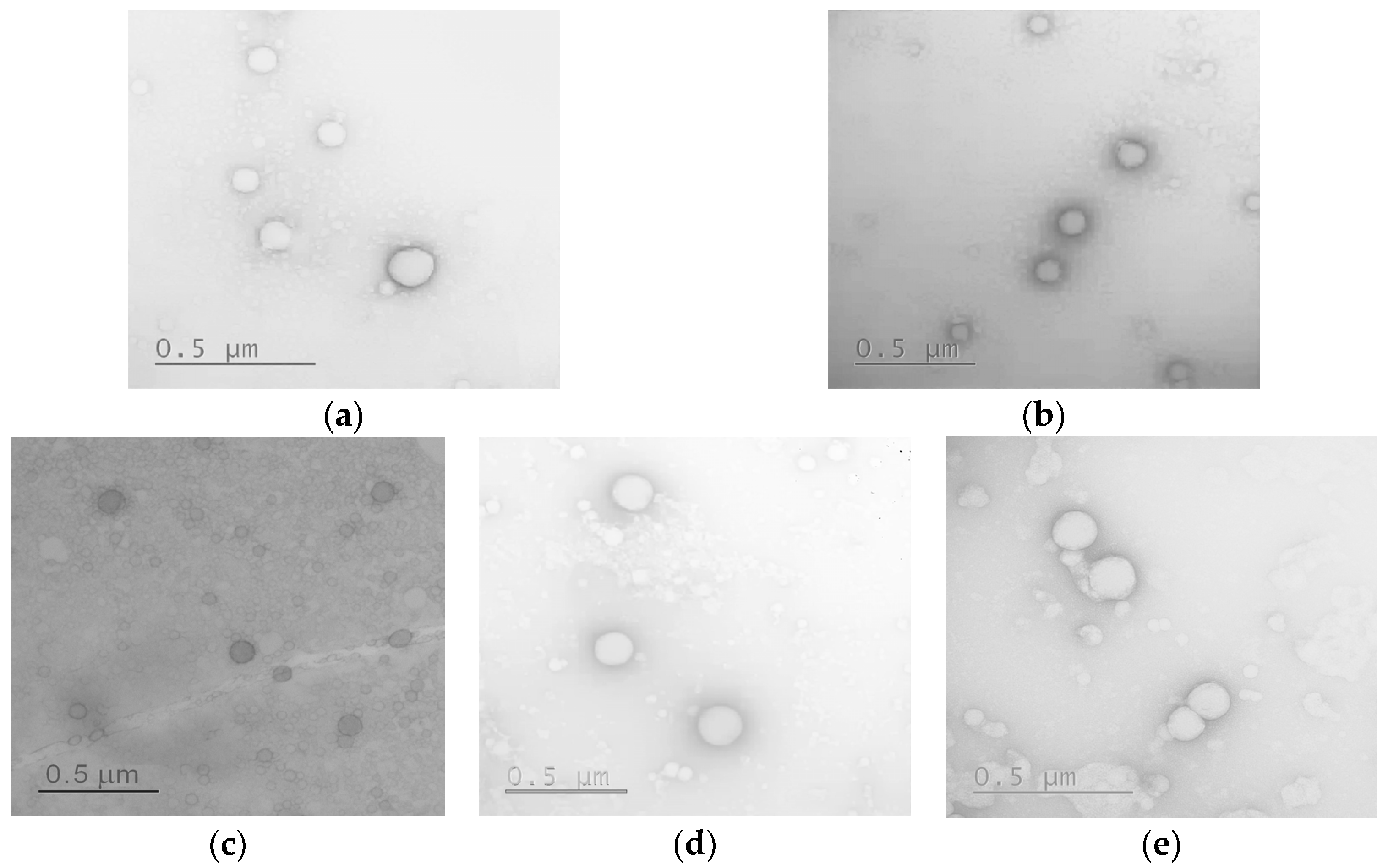
3.2.9. Morphology and Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madamsetty, V.S.; Mohammadinejad, R.; Uzieliene, I.; Nabavi, N.; Dehshahri, A.; García-Couce, J.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Makvandi, P.; et al. Dexamethasone: Insights into Pharmacological Aspects, Therapeutic Mechanisms, and Delivery Systems. ACS Biomater. Sci. Eng. 2022, 8, 1763–1790. [Google Scholar]
- Rodríguez Villanueva, J.; Rodríguez Villanueva, L.; Guzmán Navarro, M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 2017, 516, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Barcia, E.; Herrero-Vanrell, R.; Díez, A.; Alvarez-Santiago, C.; López, I.; Calonge, M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp. Eye Res. 2009, 89, 238–245. [Google Scholar] [CrossRef]
- Ryu, W.M.; Kim, S.-N.; Min, C.H.; Choy, Y. Bin Dry Tablet Formulation of PLGA Nanoparticles with a Preocular Applicator for Topical Drug Delivery to the Eye. Pharmaceutics 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.S.Y.; To, K.K.W. Biodegradable Thermosensitive PLGA-PEG-PLGA Polymer for Non-irritating and Sustained Ophthalmic Drug Delivery. AAPS J. 2019, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.R.; Lima, T.H.; Fernandes-Cunha, G.M.; Oréfice, R.L.; Da Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. Ocular biocompatibility of dexamethasone acetate loaded poly(ɛ-caprolactone) nanofibers. Eur. J. Pharm. Biopharm. 2019, 142, 20–30. [Google Scholar] [CrossRef]
- Ozurdex. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ozurdex (accessed on 18 November 2022).
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomedicine 2017, 12, 1329–1339. [Google Scholar] [CrossRef]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Wang, C.; Nan, K.; Freeman, W.R.; Sailor, M.J.; Cheng, L. Controlled Release of Dexamethasone From an Intravitreal Delivery System Using Porous Silicon Dioxide. Investig. Opthalmology Vis. Sci. 2016, 57, 557. [Google Scholar] [CrossRef]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A Nanoparticle-Based Ophthalmic Formulation of Dexamethasone Enhances Corneal Permeability of the Drug and Prolongs Its Corneal Residence Time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef]
- Al-Amin, M.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone Loaded Liposomes by Thin-Film Hydration and Microfluidic Procedures: Formulation Challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar]
- Budai-Szűcs, M.; Kiss, E.; Szilágyi, B.; Szilágyi, A.; Gyarmati, B.; Berkó, S.; Kovács, A.; Horvát, G.; Aigner, Z.; Soós, J.; et al. Mucoadhesive Cyclodextrin-Modified Thiolated Poly(aspartic acid) as a Potential Ophthalmic Drug Delivery System. Polymers 2018, 10, 199. [Google Scholar] [CrossRef]
- Bongiovì, F.; Di Prima, G.; Palumbo, F.S.; Licciardi, M.; Pitarresi, G.; Giammona, G. Hyaluronic Acid-Based Micelles as Ocular Platform to Modulate the Loading, Release, and Corneal Permeation of Corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef] [PubMed]
- Choksi, A.; Sarojini, K.V.L.; Vadnal, P.; Dias, C.; Suresh, P.K.; Khandare, J. Comparative anti-inflammatory activity of poly(amidoamine) (PAMAM) dendrimer–dexamethasone conjugates with dexamethasone-liposomes. Int. J. Pharm. 2013, 449, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Everts, M.; Kok, R.J.; Ásgeirsdóttir, S.A.; Melgert, B.N.; Moolenaar, T.J.M.; Koning, G.A.; van Luyn, M.J.A.; Meijer, D.K.F.; Molema, G. Selective Intracellular Delivery of Dexamethasone into Activated Endothelial Cells Using an E-Selectin-Directed Immunoconjugate. J. Immunol. 2002, 168, 883–889. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Golovkin, A.S.; Kudryavtsev, I.V.; Serebryakova, M.K.; Trulioff, A.S.; Dubrovskii, Y.A.; Skorik, Y.A. Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. Int. J. Mol. Sci. 2021, 22, 10960. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Song, W.; Xu, Y.; Si, X.; Zhang, D.; Lv, S.; Yang, C.; Ma, L.; Tang, Z.; Chen, X. Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials 2020, 232, 119676. [Google Scholar] [CrossRef]
- Cho, S.; Choi, J.; Kim, A.; Lee, Y.; Kwon, Y.-U. Efficient Solid-Phase Synthesis of a Series of Cyclic and Linear Peptoid−Dexamethasone Conjugates for the Cell Permeability Studies. J. Comb. Chem. 2010, 12, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Rezaee, M.; Gholami, L.; Behzad, M.; Mohajeri, M.; Kazemi Oskuee, R. Dexamethasone conjugated polyallylamine: Synthesis, characterization, and in vitro transfection and cytotoxicity. J. Drug Deliv. Sci. Technol. 2017, 40, 172–179. [Google Scholar] [CrossRef]
- Quan, L.; Yuan, F.; Liu, X.; Huang, J.; Alnouti, Y.; Wang, D. Pharmacokinetic and Biodistribution Studies of N-(2-Hydroxypropyl)methacrylamide Copolymer-Dexamethasone Conjugates in Adjuvant-Induced Arthritis Rat Model. Mol. Pharm. 2010, 7, 1041–1049. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef]
- Tarasenko, I.; Zashikhina, N.; Guryanov, I.; Volokitina, M.; Biondi, B.; Fiorucci, S.; Formaggio, F.; Tennikova, T.; Korzhikova-Vlakh, E. Amphiphilic polypeptides with prolonged enzymatic stability for the preparation of self-assembled nanobiomaterials. RSC Adv. 2018, 8, 34603–34613. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for Improving Peptide Stability and Delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef]
- Zagorodko, O.; Arroyo-Crespo, J.J.; Nebot, V.J.; Vicent, M.J. Polypeptide-Based Conjugates as Therapeutics: Opportunities and Challenges. Macromol. Biosci. 2017, 17, 1600316. [Google Scholar] [CrossRef]
- Dvoretckaia, A.; Egorova, T.; Dzhuzha, A.; Levit, M.; Sivtsov, E.; Demyanova, E.; Korzhikova-Vlakh, E. Polymyxin B Conjugates with Bio-Inspired Synthetic Polymers of Different Nature. Int. J. Mol. Sci. 2023, 24, 1832. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.A.; MacKay, J.A. Protein and polypeptide mediated delivery to the eye. Adv. Drug Deliv. Rev. 2022, 188, 114441. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Sorenson, C.M.; Sheibani, N.; Albert, D.M.; Senanayake, T.; Vinogradov, S.; Henkin, J.; Zhang, H.F. Sustaining Intravitreal Residence With L-Arginine Peptide-Conjugated Nanocarriers. Investig. Opthalmology Vis. Sci. 2017, 58, 5142. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kari, O.K.; Turunen, T.; Lajunen, T.; Schmitt, M.; Lehtinen, J.; Tasaka, F.; Parkkila, P.; Ndika, J.; Viitala, T.; et al. Diffusion and Protein Corona Formation of Lipid-Based Nanoparticles in the Vitreous Humor: Profiling and Pharmacokinetic Considerations. Mol. Pharm. 2021, 18, 699–713. [Google Scholar] [CrossRef]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.-Y.; Nance, E.A.; Yang, J.-C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release 2013, 167, 76–84. [Google Scholar] [CrossRef]
- Martens, T.F.; Vercauteren, D.; Forier, K.; Deschout, H.; Remaut, K.; Paesen, R.; Ameloot, M.; Engbersen, J.F.; Demeester, J.; De Smedt, S.C.; et al. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine 2013, 8, 1955–1968. [Google Scholar] [CrossRef]
- Zashikhina, N.; Levit, M.; Dobrodumov, A.; Gladnev, S.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Biocompatible Nanoparticles Based on Amphiphilic Random Polypeptides and Glycopolymers as Drug Delivery Systems. Polymers 2022, 14, 1677. [Google Scholar] [CrossRef] [PubMed]
- Zashikhina, N.N.; Yudin, D.V.; Tarasenko, I.I.; Osipova, O.M.; Korzhikova-Vlakh, E.G. Multilayered Particles Based on Biopolyelectrolytes as Potential Peptide Delivery Systems. Polym. Sci. Ser. A 2020, 62, 43–53. [Google Scholar] [CrossRef]
- Iudin, D.; Zashikhina, N.; Demyanova, E.; Korzhikov-Vlakh, V.; Shcherbakova, E.; Boroznjak, R.; Tarasenko, I.; Zakharova, N.; Lavrentieva, A.; Skorik, Y.; et al. Polypeptide self-assembled nanoparticles as delivery systems for polymyxins B and E. Pharmaceutics 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Raup, A.; Wang, H.; Synatschke, C.V.; Jérôme, V.; Agarwal, S.; Pergushov, D.V.; Müller, A.H.E.; Freitag, R. Compaction and Transmembrane Delivery of pDNA: Differences between l-PEI and Two Types of Amphiphilic Block Copolymers. Biomacromolecules 2017, 18, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Osipova, O.; Sharoyko, V.; Zashikhina, N.; Zakharova, N.; Tennikova, T.; Urtti, A.; Korzhikova-Vlakh, E. Amphiphilic polypeptides for VEGF siRNA delivery into retinal epithelial cells. Pharmaceutics 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yue, Y.; Maidana, D.E.; Bouzika, P.; Atik, A.; Matsumoto, H.; Miller, J.W.; Vavvas, D.G. Drug Delivery Nanoparticles: Toxicity Comparison in Retinal Pigment Epithelium and Retinal Vascular Endothelial Cells. Semin. Ophthalmol. 2016, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Posritong, P.; Ariyawong, P. Stability, Cytotoxicity, and Retinal Pigment Epithelial Cell Binding of Hyaluronic Acid-Coated PLGA Nanoparticles Encapsulating Lutein. AAPS PharmSciTech 2019, 20, 4. [Google Scholar] [CrossRef]
- Zashikhina, N.; Sharoyko, V.; Antipchik, M.; Tarasenko, I.; Anufrikov, Y.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Novel Formulations of C-Peptide with Long-Acting Therapeutic Potential for Treatment of Diabetic Complications. Pharmaceutics 2019, 11, 27. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Elsevier. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar]





| Copolymer a | Sample Abbreviation | Composition b (mol%) | [Lys]/[Phe] or [Glu]/[Phe] | Mn (for Protected Copolymers) | Ðc | |
|---|---|---|---|---|---|---|
| Lys or Glu | Phe | |||||
| P(Glu-co-DPhe) | P[EF]-1 | 74 | 26 | 2.8 | 6700 c | 1.19 |
| P[EF]-2 | 81 | 19 | 4.3 | 11,220 d | − | |
| P(Lys-co-LPhe) | P[KF]L | 77 | 23 | 3.4 | 12,250 c | 1.31 |
| P(Lys-co-D,LPhe) | P[KF]DL-1 | 81 | 19 | 4.3 | 11,000 c | 1.33 |
| P[KF]DL-2 | 88 | 12 | 7.3 | 12,150 c | 1.37 | |
| Sample | DH (nm) | PDI | Zeta-Potential a (mV) | CAC b (μg/mL) |
|---|---|---|---|---|
| P[EF]-1 | 90 | 0.08 | −37 ± 2 | n/d |
| P[EF]-2 | 165 | 0.11 | −40 ± 2 | 4.2 ± 0.2 |
| P[KF]L | 210 | 0.27 | 36 ± 1 | 9.4 ± 0.2 |
| P[KF]DL-1 | 110 | 0.16 | 41 ± 1 | 6.7 ± 0.3 |
| P[KF]DL-2 | 135 | 0.19 | 45 ± 2 | 7.4 ± 0.1 |
| P[KF]DL-2/HEP | 110 | 0.22 | −20 ± 1 | − c |
| Nanoparticles | Surface Charge | Dw (µm2/s) | Dv (µm2/s) | Dw/Dv |
|---|---|---|---|---|
| P[KF]DL-2 | positive | 4.9 | 0.015 ± 0.06 | 329 |
| P[KF]DL-2/HEP | negative | 6.9 | 0.61 ± 0.10 | 11.3 |
| P[EF]-2 | negative | 7.7 | 0.67 ± 0.15 | 11.5 |
| Nanoparticle-Based Polypeptide-DEX Conjugates | Glu/Phe or Lys/Phe Molar Ratio | DEX Fraction in Polymer (wt%) | DH (nm) | Zeta-Potential (mV) |
|---|---|---|---|---|
| P[EF]-1-DEX | 2.8 | 3.0 | 200 ± 20 | −30 ± 3 |
| P[EF]-2-DEX | 4.3 | 3.8 | 225 ± 35 | −35 ± 4 |
| P[KF]L-DEX | 3.4 | 4.2 | 370 ± 30 | 11 ± 1 |
| P[KF]DL-1-DEX | 4.3 | 5.2 | 320 ± 10 | 12 ± 1 |
| P[KF]DL-2-DEX | 7.2 | 8.6 | 290 ± 20 | 15 ± 3 |
| P[KF]DL-2-DEX/HEP | 7.2 | 8.6 | 250 ± 10 | −19 ± 1 |
| Sample | Average Diameter Determined by TEM (nm) |
|---|---|
| P[EF]-2 | 119 ± 39 |
| P[EF]-2-DEX | 181 ± 36 |
| P[KF]DL-2 | 71 ± 20 |
| P[KF]DL-2-DEX | 178 ± 90 |
| P[KF]DL-2-DEX/HEP | 158 ± 37 |
| Model | Encapsulated Systems | Conjugated Systems | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.01 M PBS, pH 7.4 | 0.01 M PBS, pH 7.4 | Vitreous/0.01 M PBS, pH 7.4 (50/50, v/v) | ||||||
| P[KF]D,L-2(DEX) | P[KF]D,L-2(DEX)/HEP | P[KF]D,L-2/DEX | P[KF]D,L-2/DEX/HEP | P[EF]-2/DEX | P[KF]D,L-2/DEX | P[KF]D,L-2/DEX/HEP | P[EF]-2/DEX | |
| Zero-order * | R2 = 0.9644 | R2 = 0.9565 | R2 = 0.9653 | R2 = 0.9985 | R2 = 0.9517 | R2 = 0.9811 | R2 = 0.9937 | R2 = 0.9616 |
| Kzo = 14.32 | Kzo = 13.79 | Kzo = 0.764 | Kzo = 0.598 | Kzo = 0.820 | Kzo = 1.564 | Kzo = 1.568 | Kzo = 0.782 | |
| First-order * | R2 = 0.9950 | R2 = 0.9924 | R2 = 0.9945 | R2 = 0.9962 | R2 = 0.9907 | R2 = 0.9943 | R2 = 0.9956 | R2 = 0.9888 |
| kfo = 0.246 | kfo = 0.231 | kfo = 1.2 × 10−2 | kfo = 7.9 × 10−3 | kfo = 1.4 × 10−2 | kfo = 2.3 × 10−2 | kfo = 2.3 × 10−2 | kfo = 1.3 × 10−2 | |
| Higuchi * | R2 = 0.9896 | R2 = 0.9805 | R2 = 0.9941 | R2 = 0.9675 | R2 = 0.9896 | R2 = 0.9892 | R2 = 0.9801 | R2 = 0.9932 |
| KH = 29.629 | KH = 28.418 | KH = 6.085 | KH = 4.486 | KH = 6.558 | KH = 8.714 | KH = 8.562 | KH = 6.600 | |
| Korsmeyer-Peppas * | R2 = 0.9926 | R2 = 0.9845 | R2 = 0.9948 | R2 = 0.9990 | R2 = 0.9885 | R2 = 0.9945 | R2 = 0.9964 | R2 = 0.9932 |
| KKP = 25.842 | KKP = 23.627 | KKP = 3.480 | KKP = 0.752 | KKP = 3.936 | KKP = 5.422 | KKP = 3.393 | KKP = 4.375 | |
| n = 0.605 | n = 0.641 | n = 0.643 | n = 0.947 | n = 0.631 | n = 0.645 | n = 0.780 | n = 0.602 | |
| Hixon-Crowell * | R2 = 0.9900 | R2 = 0.9858 | R2 = 0.9884 | R2 = 0.9981 | R2 = 0.9826 | R2 = 0.9920 | R2 = 0.9965 | R2 = 0.9827 |
| KHC = 7.0 × 10−2 | KHC = 6.6 × 10−2 | KHC = 3.5 × 10−3 | KHC = 2.4 × 10−3 | KHC = 3.9 × 10−3 | KHC = 6.8 × 10−3 | KHC = 6.7 × 10−3 | KHC = 3.7 × 10−3 | |
| Hopfenberg * | R2 = 0.9950 | R2 = 0.9924 | R2 = 0.9945 | R2 = 0.9990 | R2 = 0.9907 | R2 = 0.9943 | R2 = 0.9960 | R2 = 0.9888 |
| KHb = 1.8 × 10−4 | KHb = 6.2 × 10−5 | KHb = 3.4 × 10−6 | KHb = 4.8 × 10−3 | KHb = 5.9 × 10−6 | KHb = 5.2 × 10−5 | KHb = 2.3 × 10−3 | KHb = 4.3 × 10−6 | |
| Baker-Lonsdale * | R2 = 0.9822 | R2 = 0.9736 | R2 = 0.9913 | R2 = 0.9590 | R2 = 0.9882 | R2 = 0.9840 | R2 = 0.9713 | R2 = 0.9909 |
| KBL = 2.0 × 10−2 | KBL = 1.8 × 10−2 | KBL = 7.6 × 10−4 | KBL = 3.8 × 10−4 | KBL = 9.1 × 10−4 | KBL = 1.6 × 10−3 | KBL = 1.5 × 10−3 | KBL = 9.5 × 10−4 | |
| Weibull ** | R2 = 0.9963 | R2 = 0.9940 | R2 = 0.9987 | R2 = 0.9992 | R2 = 0.9990 | R2 = 0.9963 | R2 = 0.9987 | R2 = 0.9912 |
| α = 2.51 | α = 2.26 | α = 53.228 | α = 587.182 | α = 23.440 | α = 325.204 | α = 191.949 | α = 58.592 | |
| β = 0.64 | β = 0.51 | β = 0.887 | β = 1.345 | β = 0.716 | β = 1.504 | β = 1.379 | β = 0.943 | |
| Gompertz ** | R2 = 0.9960 | R2 = 0.9984 | R2 = 0.9911 | R2 = 0.9901 | R2 = 0.9967 | R2 = 0.9875 | R2 = 0.9923 | R2 = 0.9763 |
| α = 1.442 | α = 1.529 | α = 39.371 | α = 168.455 | α = 16.931 | α = 213.930 | α = 223.724 | α = 30.684 | |
| β = 1.903 | β = 1.822 | β = 2.390 | β = 3.007 | β = 1.954 | β = 3.942 | β = 3.935 | β = 2.320 | |
| Peppas-Sahlin ** | R2 = 0.9866 | R2 = 0.9782 | R2 = 0.9992 | R2 = 0.9934 | R2 = 0.9980 | R2 = 0.9971 | R2 = 0.9984 | R2 = 0.9970 |
| K1 = 34.304 | K1 = 32.556 | K1 = 3.146 | K1 = 18.141 | K1 = 3.665 | K1 = 3.697 | K1 = 3.329 | K1 = 4.107 | |
| K2 = 2.965 | K2 = 2.778 | K2 = 0.025 | K2 = 14.802 | K2 = 0.037 | K2 = 0.032 | K2 = 0.026 | K2 = 0.018 | |
| m = 0.492 | m = 0.484 | m = 0.707 | m = 0.210 | m = 0.696 | m = 0.821 | m = 0.835 | m = 0.631 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zashikhina, N.; Gladnev, S.; Sharoyko, V.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E.; Tennikova, T. Synthesis and Characterization of Nanoparticle-Based Dexamethasone-Polypeptide Conjugates as Potential Intravitreal Delivery Systems. Int. J. Mol. Sci. 2023, 24, 3702. https://doi.org/10.3390/ijms24043702
Zashikhina N, Gladnev S, Sharoyko V, Korzhikov-Vlakh V, Korzhikova-Vlakh E, Tennikova T. Synthesis and Characterization of Nanoparticle-Based Dexamethasone-Polypeptide Conjugates as Potential Intravitreal Delivery Systems. International Journal of Molecular Sciences. 2023; 24(4):3702. https://doi.org/10.3390/ijms24043702
Chicago/Turabian StyleZashikhina, Natalia, Sergei Gladnev, Vladimir Sharoyko, Viktor Korzhikov-Vlakh, Evgenia Korzhikova-Vlakh, and Tatiana Tennikova. 2023. "Synthesis and Characterization of Nanoparticle-Based Dexamethasone-Polypeptide Conjugates as Potential Intravitreal Delivery Systems" International Journal of Molecular Sciences 24, no. 4: 3702. https://doi.org/10.3390/ijms24043702
APA StyleZashikhina, N., Gladnev, S., Sharoyko, V., Korzhikov-Vlakh, V., Korzhikova-Vlakh, E., & Tennikova, T. (2023). Synthesis and Characterization of Nanoparticle-Based Dexamethasone-Polypeptide Conjugates as Potential Intravitreal Delivery Systems. International Journal of Molecular Sciences, 24(4), 3702. https://doi.org/10.3390/ijms24043702










