Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy
Abstract
1. Introduction
2. Results
2.1. MyoMed-205 Acute Toxicological Study
2.2. MyoMed-205 Dose–Response Curve in a DIDD Experimental Model
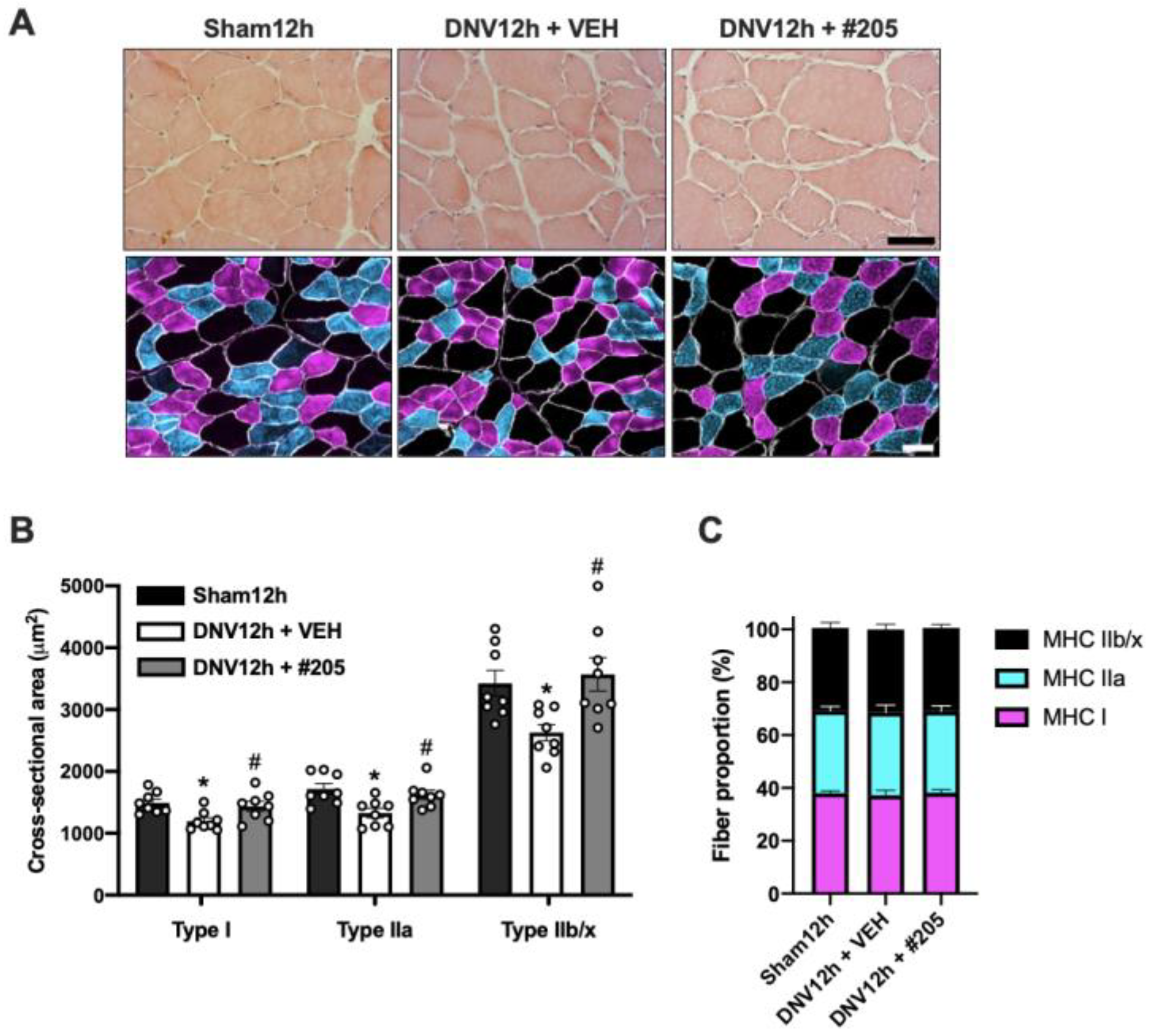
2.3. MuRF1 Inhibition Prevents Early Disuse-Induced Diaphragmatic Contractile Dysfunction and Atrophy after 12 h of Denervation
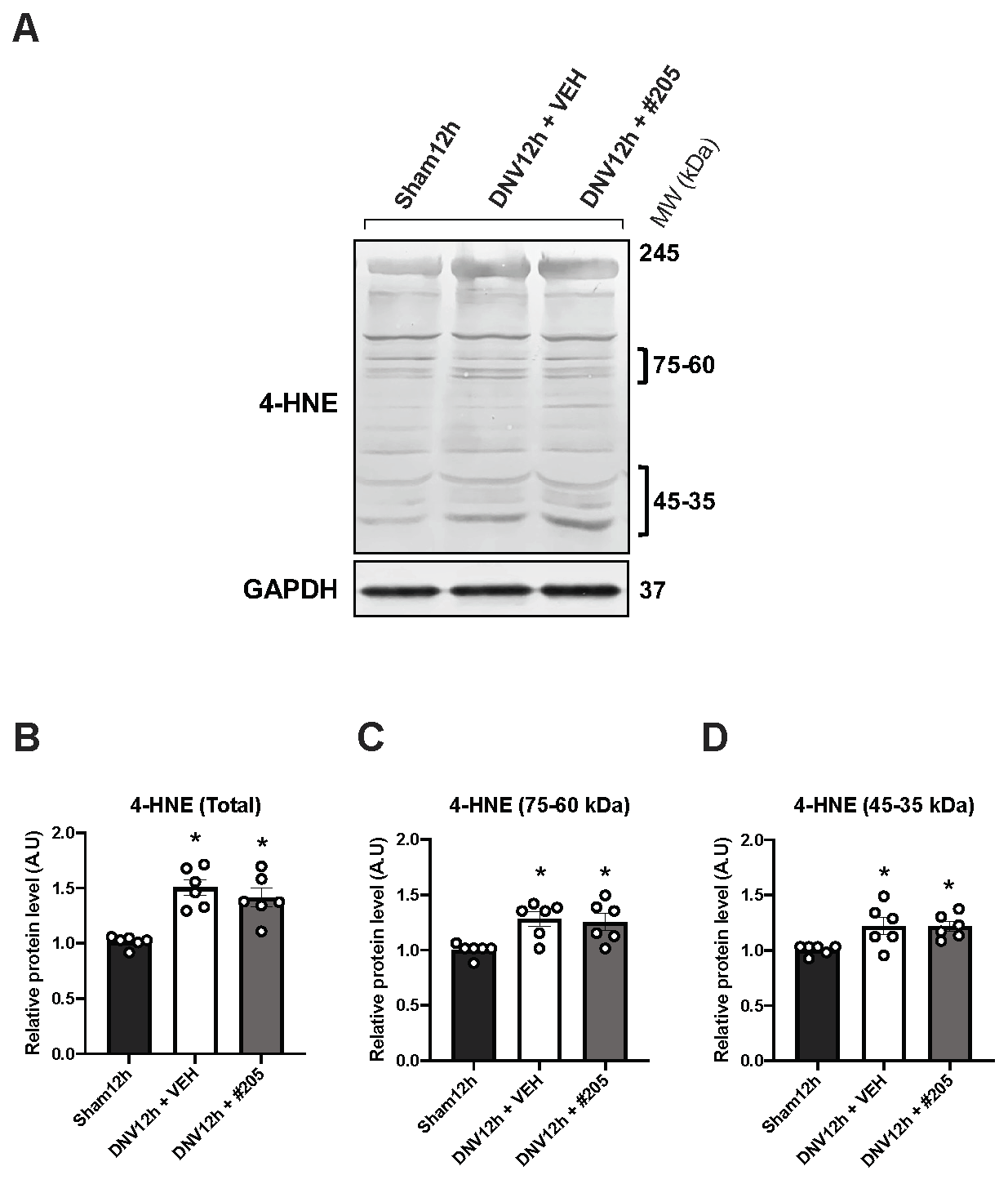
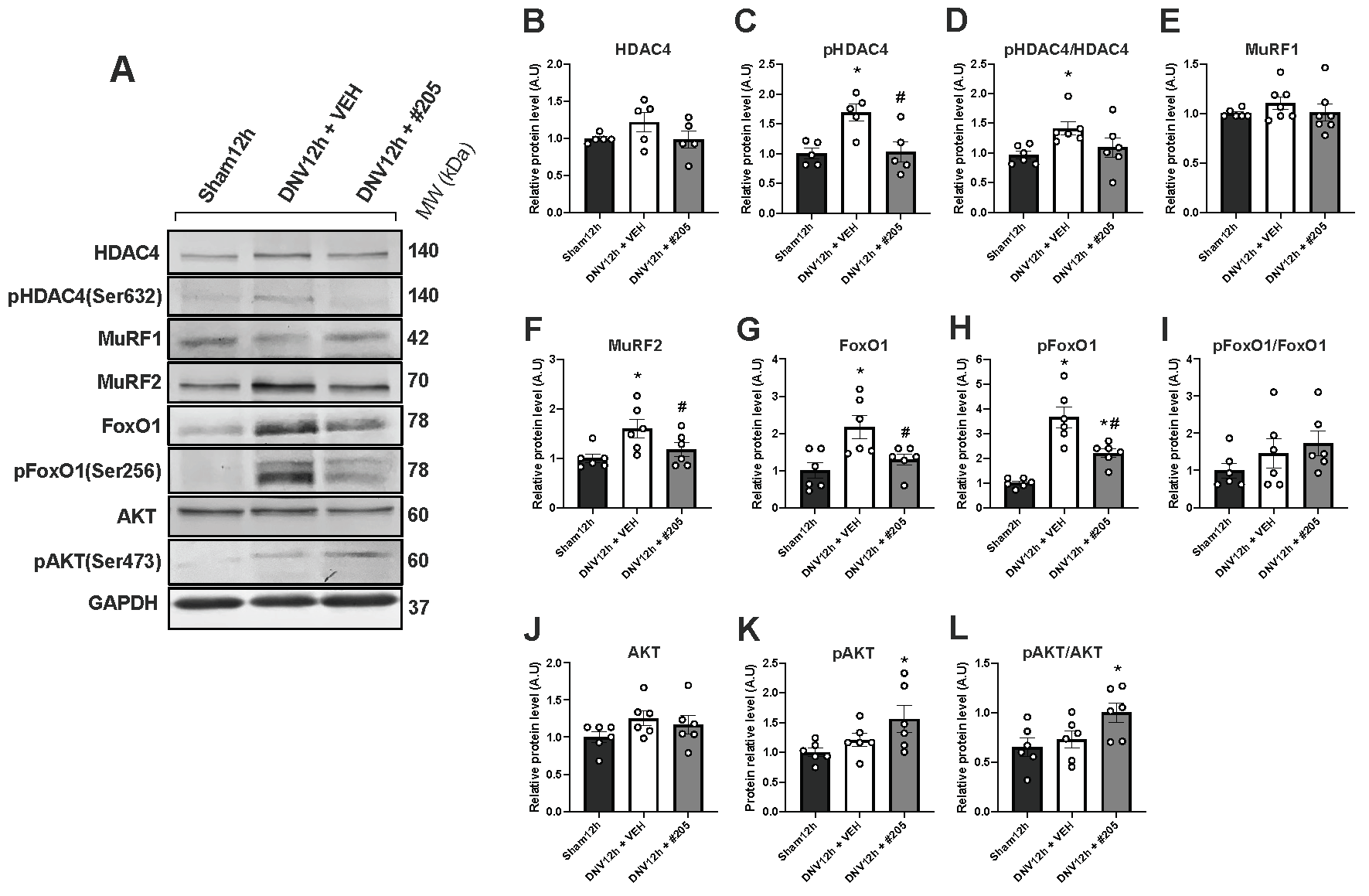
2.4. MuRF1 Inhibition Affects HDAC4, FoxO1, MuRF2 and Akt, but Not Oxidative Stress, in the Early DIDD
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. MyoMed-205 Acute Toxicity
4.4. MyoMed-205 Formulation for Intravenous Administration
4.5. Unilateral Diaphragm Denervation (UDD)
4.6. Contractile Function
4.7. Histomorphometry
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubé, B.-P.; Dres, M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J. Clin. Med. 2016, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Ricoy, J.; Rodríguez-Núñez, N.; Álvarez-Dobaño, J.M.; Toubes, M.E.; Riveiro, V.; Valdés, L. Diaphragmatic Dysfunction. Pulmonology 2019, 25, 223–235. [Google Scholar] [CrossRef]
- Goligher, E.; Ferguson, N.D. Mechanical Ventilation: Epidemiological Insights into Current Practices. Curr. Opin. Crit. Care 2009, 15, 44–51. [Google Scholar] [CrossRef]
- Núñez-Seisdedos, M.N.; Lázaro-Navas, I.; López-González, L.; López-Aguilera, L. Intensive Care Unit- Acquired Weakness and Hospital Functional Mobility Outcomes Following Invasive Mechanical Ventilation in Patients with COVID-19: A Single-Centre Prospective Cohort Study. J. Intensive Care Med. 2022, 37, 1005–1014. [Google Scholar] [CrossRef]
- Rodriguez, B.; Branca, M.; Gutt-Will, M.; Roth, M.; Söll, N.; Nansoz, S.; Cameron, D.R.; Tankisi, H.; Tan, S.V.; Bostock, H.; et al. Development and Early Diagnosis of Critical Illness Myopathy in COVID-19 Associated Acute Respiratory Distress Syndrome. J. Cachexia. Sarcopenia Muscle 2022, 13, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Friedrich, O. Critical Illness Myopathy (CIM) and Ventilator-Induced Diaphragm Muscle Dysfunction (VIDD): Acquired Myopathies Affecting Contractile Proteins. Compr. Physiol. 2017, 7, 105–112. [Google Scholar] [CrossRef]
- Goligher, E.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Vorona, S.; Sklar, M.C.; Rittayamai, N.; Lanys, A.; et al. Mechanical Ventilation–Induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 204–213. [Google Scholar] [CrossRef]
- Sklar, M.C.; Dres, M.; Fan, E.; Rubenfeld, G.D.; Scales, D.C.; Herridge, M.S.; Rittayamai, N.; Harhay, M.O.; Reid, W.D.; Tomlinson, G.; et al. Association of Low Baseline Diaphragm Muscle Mass With Prolonged Mechanical Ventilation and Mortality Among Critically Ill Adults. JAMA Netw. Open 2020, 3, e1921520. [Google Scholar] [CrossRef]
- Le Bourdelles, G.; Viires, N.; Boczkowski, J.; Seta, N.; Pavlovic, D.; Aubier, M. Effects of Mechanical Ventilation on Morphological Properties of the Diaphragm Fibers in Rats. Am. J. Respir. Crit. Care Med. 1994, 149, 1539–1544. [Google Scholar] [CrossRef]
- Powers, S.K.; Andrew Shanely, R.; Coombes, J.S.; Koesterer, T.J.; McKenzie, M.; Van Gammeren, D.; Cicale, M.; Dodd, S.L. Mechanical Ventilation Results in Progressive Contractile Dysfunction in the Diaphragm. J. Appl. Physiol. 2002, 92, 1851–1858. [Google Scholar] [CrossRef]
- Shindoh, C.; Hida, W.; Kurosawa, H.; Ebihara, S.; Kikuchi, Y.; Takishima, T.; Kunio, S. Effects of Unilateral Phrenic Nerve Denervation on Diaphragm Contractility in Rat. Tohoku J. Exp. Med. 1994, 173, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, L.E.; Brice, G.; Carlson, B.; Prakash, Y.S.; Sieck, G.C. Changes in Satellite Cell Mitotic Activity during Acute Period of Unilateral Diaphragm Denervation. J. Appl. Physiol. 1994, 77, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Jaber, S.; Petrof, B.J.; Jung, B.; Chanques, G.; Berthet, J.P.; Rabuel, C.; Bouyabrine, H.; Courouble, P.; Koechlin-Ramonatxo, C.; Sebbane, M.; et al. Rapidly Progressive Diaphragmatic Weakness and Injury during Mechanical Ventilation in Humans. Am. J. Respir. Crit. Care Med. 2011, 183, 364–371. [Google Scholar] [CrossRef]
- Schepens, T.; Verbrugghe, W.; Dams, K.; Corthouts, B.; Parizel, P.M.; Jorens, P.G. The Course of Diaphragm Atrophy in Ventilated Patients Assessed with Ultrasound: A Longitudinal Cohort Study. Crit. Care 2015, 19, 422. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Greco, M.; Bocchino, S.; Cabrini, L.; Beccaria, P.F.; Zangrillo, A. Assessment of Diaphragmatic Dysfunction in the Critically Ill Patient with Ultrasound: A Systematic Review. Intensive Care Med. 2017, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gayan-Ramirez, G.; de Paepe, K.; Cadot, P.; Decramer, M. Detrimental Effects of Short-Term Mechanical Ventilation on Diaphragm Function and IGF-I MRNA in Rats. Intensive Care Med. 2003, 29, 825–833. [Google Scholar] [CrossRef]
- Mcclung, J.M.; Kavazis, A.N.; Whidden, M.A.; Deruisseau, K.C.; Falk, D.J.; Criswell, D.S.; Powers, S.K. Antioxidant Administration Attenuates Mechanical Ventilation-Induced Rat Diaphragm Muscle Atrophy Independent of Protein Kinase B (PKB–Akt) Signalling. J. Phisiology 2007, 585, 203–215. [Google Scholar] [CrossRef]
- Argadine, H.M.; Mantilla, C.B.; Zhan, W.Z.; Sieck, G.C. Intracellular Signaling Pathways Regulating Net Protein Balance Following Diaphragm Muscle Denervation. Am. J. Physiol.—Cell Physiol. 2011, 300, 318–327. [Google Scholar] [CrossRef]
- Bernard, N.; Matecki, S.; Py, G.; Lopez, S.; Mercier, J.; Capdevila, X. Effects of Prolonged Mechanical Ventilation on Respiratory Muscle Ultrastructure and Mitochondrial Respiration in Rabbits. Intensive Care Med. 2003, 29, 111–118. [Google Scholar] [CrossRef]
- Picard, M.; Azuelos, I.; Jung, B.; Giordano, C.; Matecki, S.; Hussain, S.; White, K.; Li, T.; Liang, F.; Benedetti, A.; et al. Mechanical Ventilation Triggers Abnormal Mitochondrial Dynamics and Morphology in the Diaphragm. J. Appl. Physiol. 2015, 118, 1161–1171. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zergeroglu, M.A.; Lennon, S.L.; Sugiura, T.; Yimlamai, T.; Enns, D.; Belcastro, A.; Powers, S.K. Mechanical Ventilation–Induced Diaphragmatic Atrophy Is Associated with Oxidative Injury and Increased Proteolytic Activity. Am. J. Respir. Crit. Care Med. 2002, 166, 1369–1374. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Sollanek, K.J.; Smuder, A.J. Ventilator-Induced Diaphragm Dysfunction: Cause and Effect. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R464–R477. [Google Scholar] [CrossRef] [PubMed]
- DeRuisseau, K.C.; Shanely, R.A.; Akunuri, N.; Hamilton, M.T.; Van Gammeren, D.; Zergeroglu, A.M.; McKenzie, M.; Powers, S.K. Diaphragm Unloading via Controlled Mechanical Ventilation Alters the Gene Expression Profile. Am. J. Respir. Crit. Care Med. 2005, 172, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, C.S.H.; Zhu, E.; Caiozzo, V.J. Assist-Control Mechanical Ventilation Attenuates Ventilator-Induced Diaphragmatic Dysfunction. Am. J. Respir. Crit. Care Med. 2004, 170, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Smuder, A.J.; Nelson, W.B.; Hudson, M.B.; Kavazis, A.N.; Powers, S.K. Inhibition of the Ubiquitin–Proteasome Pathway Does Not Protect against Ventilator-Induced Accelerated Proteolysis or Atrophy in the Diaphragm. Anesthesiology 2014, 121, 115–126. [Google Scholar] [CrossRef]
- Agten, A.; Maes, K.; Thomas, D.; Cielen, N.; Van Hees, H.W.H.; Dekhuijzen, R.P.N.; Decramer, M.; Gayan-Ramirez, G. Bortezomib Partially Protects the Rat Diaphragm from Ventilator-Induced Diaphragm Dysfunction. Crit. Care Med. 2012, 40, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Ozdemir, M.; Yoshihara, T.; Nguyen, B.L.; Deminice, R.; Powers, S.K. Calpains Play an Essential Role in Mechanical Ventilation-Induced Diaphragmatic Weakness and Mitochondrial Dysfunction. Redox Biol. 2021, 38, 101802. [Google Scholar] [CrossRef]
- Smuder, A.J.; Morton, A.B.; Hall, S.E.; Wiggs, M.P.; Ahn, B.; Wawrzyniak, N.R.; Sollanek, K.J.; Min, K.; Kwon, O.S.; Nelson, W.B.; et al. Effects of Exercise Preconditioning and HSP72 on Diaphragm Muscle Function during Mechanical Ventilation. J. Cachexia. Sarcopenia Muscle 2019, 10, 767–781. [Google Scholar] [CrossRef]
- Hooijman, P.E.; Beishuizen, A.; Witt, C.C.; de Waard, M.C.; Girbes, A.R.J.; Spoelstra-de Man, A.M.E.; Niessen, H.W.M.; Manders, E.; van Hees, H.W.H.; van den Brom, C.E.; et al. Diaphragm Muscle Fiber Weakness and Ubiquitin–Proteasome Activation in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2015, 191, 1126–1138. [Google Scholar] [CrossRef]
- Witt, C.C.; Witt, S.H.; Lerche, S.; Labeit, D.; Back, W.; Labeit, S. Cooperative Control of Striated Muscle Mass and Metabolism by MuRF1 and MuRF2. EMBO J. 2008, 27, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Khurram, O.U.; Sieck, G.C.; Mantilla, C.B. Compensatory Effects Following Unilateral Diaphragm Paralysis. Respir. Physiol. Neurobiol. 2017, 246, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Peris-Moreno, D.; Taillandier, D.; Polge, C. MuRF1/TRIM63, Master Regulator of Muscle Mass. Int. J. Mol. Sci. 2020, 21, 6663. [Google Scholar] [CrossRef]
- Adams, V.; Gußen, V.; Zozulya, S.; Cruz, A.; Moriscot, A.; Linke, A.; Labeit, S. Small-Molecule Chemical Knockdown of MuRF1 in Melanoma Bearing Mice Attenuates Tumor Cachexia Associated Myopathy. Cells 2020, 9, 2272. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; Hughes, D.C.; Lynch, S.A.; Van Haver, D.; Maia, T.M.; Marshall, A.G.; Radoshevich, L.; Impens, F.; Waddell, D.S.; Bodine, S.C. Identification of the MuRF1 Skeletal Muscle Ubiquitylome Through Quantitative Proteomics. Function 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Labeit, S.; Hirner, S.; Bogomolovas, J.; Cruz, A.; Myrzabekova, M.; Moriscot, A.; Bowen, T.S.; Adams, V. Regulation of Glucose Metabolism by MuRF1 and Treatment of Myopathy in Diabetic Mice with Small Molecules Targeting MuRF1. Int. J. Mol. Sci. 2021, 22, 2225. [Google Scholar] [CrossRef]
- Moroz, N.; Maes, K.; Leduc-Gaudet, J.-P.; Goldberg, P.; Petrof, B.J.; Mayaki, D.; Vassilakopoulos, T.; Rassier, D.; Gayan-Ramirez, G.; Hussain, S.N. Oxidants Regulated Diaphragm Proteolysis during Mechanical Ventilation in Rats. Anesthesiology 2019, 131, 605–618. [Google Scholar] [CrossRef]
- Corpeno, R.; Dworkin, B.; Cacciani, N.; Salah, H.; Bergman, H.-M.; Ravara, B.; Vitadello, M.; Gorza, L.; Gustafson, A.-M.; Hedström, Y.; et al. Time Course Analysis of Mechanical Ventilation-Induced Diaphragm Contractile Muscle Dysfunction in the Rat. J. Physiol. 2014, 592, 3859–3880. [Google Scholar] [CrossRef]
- Lyu, Q.; Wen, Y.; Zhang, X.; Addinsall, A.B.; Cacciani, N.; Larsson, L. Multi-Omics Reveals Age-Related Differences in the Diaphragm Response to Mechanical Ventilation: A Pilot Study. Skelet. Muscle 2021, 11, 11. [Google Scholar] [CrossRef]
- McClung, J.M.; Whidden, M.A.; Kavazis, A.N.; Falk, D.J.; DeRuisseau, K.C.; Powers, S.K. Redox Regulation of Diaphragm Proteolysis during Mechanical Ventilation. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2008, 294, 1608–1617. [Google Scholar] [CrossRef]
- Tang, H.; Kennedy, C.L.; Lee, M.; Gao, Y.; Xia, H.; Olguin, F.; Fraga, D.A.; Ayers, K.; Choi, S.; Kim, M.; et al. Smad3 Initiates Oxidative Stress and Proteolysis That Underlies Diaphragm Dysfunction during Mechanical Ventilation. Sci. Rep. 2017, 7, 14530. [Google Scholar] [CrossRef]
- DeRuisseau, K.C.; Kavazis, A.N.; Deering, M.A.; Falk, D.J.; Van Gammeren, D.; Yimlamai, T.; Ordway, G.A.; Powers, S.K. Mechanical Ventilation Induces Alterations of the Ubiquitin-Proteasome Pathway in the Diaphragm. J. Appl. Physiol. 2005, 98, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Smuder, A.J.; Sollanek, K.J.; Min, K.; Nelson, W.B.; Powers, S.K. Inhibition of Forkhead BoxO–Specific Transcription Prevents Mechanical Ventilation–Induced Diaphragm Dysfunction. Crit. Care Med. 2015, 43, e133–e142. [Google Scholar] [CrossRef]
- Ochala, J.; Gustafson, A.M.; Diez, M.L.; Renaud, G.; Li, M.; Aare, S.; Qaisar, R.; Banduseela, V.C.; Hedström, Y.; Tang, X.; et al. Preferential Skeletal Muscle Myosin Loss in Response to Mechanical Silencing in a Novel Rat Intensive Care Unit Model: Underlying Mechanisms. J. Physiol. 2011, 589, 2007–2026. [Google Scholar] [CrossRef] [PubMed]
- Centner, T.; Yano, J.; Kimura, E.; McElhinny, A.S.; Pelin, K.; Witt, C.C.; Bang, M.-L.; Trombitas, K.; Granzier, H.; Gregorio, C.C.; et al. Identification of Muscle Specific Ring Finger Proteins as Potential Regulators of the Titin Kinase Domain. J. Mol. Biol. 2001, 306, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.H.; Granzier, H.; Witt, C.C.; Labeit, S. MURF-1 and MURF-2 Target a Specific Subset of Myofibrillar Proteins Redundantly: Towards Understanding MURF-Dependent Muscle Ubiquitination. J. Mol. Biol. 2005, 350, 713–722. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal Muscle Atrophy and the E3 Ubiquitin Ligases MuRF1 and MAFbx/Atrogin-1. Am. J. Physiol.—Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Nguyen, T.; Bowen, T.S.; Augstein, A.; Schauer, A.; Gasch, A.; Linke, A.; Labeit, S.; Adams, V. Expression of MuRF1 or MuRF2 Is Essential for the Induction of Skeletal Muscle Atrophy and Dysfunction in a Murine Pulmonary Hypertension Model. Skelet. Muscle 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Bowen, T.S.; Werner, S.; Barthel, P.; Amberger, C.; Konzer, A.; Graumann, J.; Sehr, P.; Lewis, J.; Provaznik, J.; et al. Small-molecule-mediated Chemical Knock-down of MuRF1/MuRF2 and Attenuation of Diaphragm Dysfunction in Chronic Heart Failure. J. Cachexia. Sarcopenia Muscle 2019, 10, 1102–1115. [Google Scholar] [CrossRef]
- Luo, L.; Martin, S.C.; Parkington, J.; Cadena, S.M.; Zhu, J.; Ibebunjo, C.; Summermatter, S.; Londraville, N.; Patora-Komisarska, K.; Widler, L.; et al. HDAC4 Controls Muscle Homeostasis through Deacetylation of Myosin Heavy Chain, PGC-1α, and Hsc70. Cell Rep. 2019, 29, 749–763.e12. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Levine, S.; Biswas, C.; Dierov, J.; Barsotti, R.; Shrager, J.B.; Nguyen, T.; Sonnad, S.; Kucharchzuk, J.C.; Kaiser, L.R.; Singhal, S.; et al. Increased Proteolysis, Myosin Depletion, and Atrophic AKT-FOXO Signaling in Human Diaphragm Disuse. Am. J. Respir. Crit. Care Med. 2011, 183, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Adams, V.; Werner, S.; Fischer, T.; Vinke, P.; Brogger, M.N.; Mangner, N.; Linke, A.; Sehr, P.; Lewis, J.; et al. Small-Molecule Inhibition of MuRF1 Attenuates Skeletal Muscle Atrophy and Dysfunction in Cardiac Cachexia. J. Cachexia. Sarcopenia Muscle 2017, 8, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.J.; Graça, F.A.; Cruz, A.; Silvestre, J.G.; Labeit, S.; Miyabara, E.H.; Yan, C.Y.I.; Wang, D.Z.; Moriscot, A.S. MiR-29c Improves Skeletal Muscle Mass and Function throughout Myocyte Proliferation and Differentiation and by Repressing Atrophy-Related Genes. Acta Physiol. 2019, 226, e13320. [Google Scholar] [CrossRef]


| Dose (mg.Kg−1 bw) | Step | Animal # | observation day | |||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||||
| 0:30 h * |
10:20 am |
12:00 pm | 02:00 pm | |||||||||||||||||
| 300 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Dose (mg.Kg−1 bw) | Step | Animal # | observation day | |||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||||
| 0:30 h * | 09:30 am | 11:55 am | 12:50 pm | |||||||||||||||||
| 300 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Dose (mg.Kg−1 bw) | Step | Animal # | observation day | |||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||||
| 0:30 h * | 09:30 am | 11:00 am | 2:00 pm | |||||||||||||||||
| 2000 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Dose (mg.Kg−1 bw) | Step | Animal # | observation day | |||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||||
| 0:30 h * | 10:00 am | 11:03 am | 12:50 pm | |||||||||||||||||
| 2000 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Treatment | Dose (mg.Kg−1) | Animal # | Macroscopic Alterations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Brain | Eyes | Lungs | Heart | Liver | Spleen | Urinary System | G.I.T | R.T | Carcass | |||
| 1st | 300 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2nd | 300 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3rd | 2000 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 4th | 2000 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, F.; Alves, P.K.N.; Bechara, L.R.G.; Ferreira, J.C.B.; Labeit, S.; Moriscot, A.S. Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy. Int. J. Mol. Sci. 2023, 24, 3637. https://doi.org/10.3390/ijms24043637
Ribeiro F, Alves PKN, Bechara LRG, Ferreira JCB, Labeit S, Moriscot AS. Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy. International Journal of Molecular Sciences. 2023; 24(4):3637. https://doi.org/10.3390/ijms24043637
Chicago/Turabian StyleRibeiro, Fernando, Paula K. N. Alves, Luiz R. G. Bechara, Julio C. B. Ferreira, Siegfried Labeit, and Anselmo S. Moriscot. 2023. "Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy" International Journal of Molecular Sciences 24, no. 4: 3637. https://doi.org/10.3390/ijms24043637
APA StyleRibeiro, F., Alves, P. K. N., Bechara, L. R. G., Ferreira, J. C. B., Labeit, S., & Moriscot, A. S. (2023). Small-Molecule Inhibition of MuRF1 Prevents Early Disuse-Induced Diaphragmatic Dysfunction and Atrophy. International Journal of Molecular Sciences, 24(4), 3637. https://doi.org/10.3390/ijms24043637








