Alterations in Antioxidant Micronutrient Concentrations in Placental Tissue, Maternal Blood and Urine and the Fetal Circulation in Pre-eclampsia
Abstract
1. Introduction
2. Results
2.1. Participants
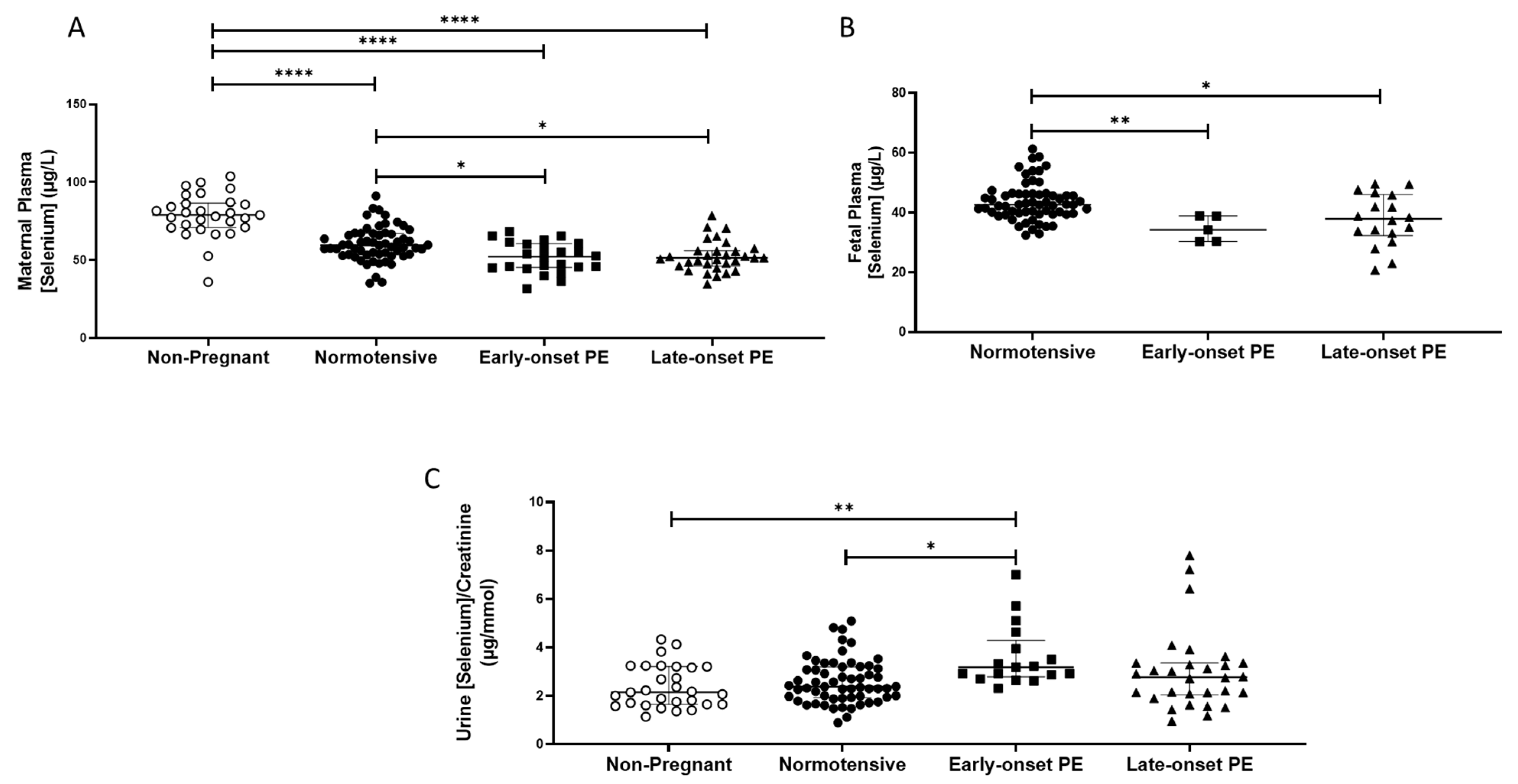
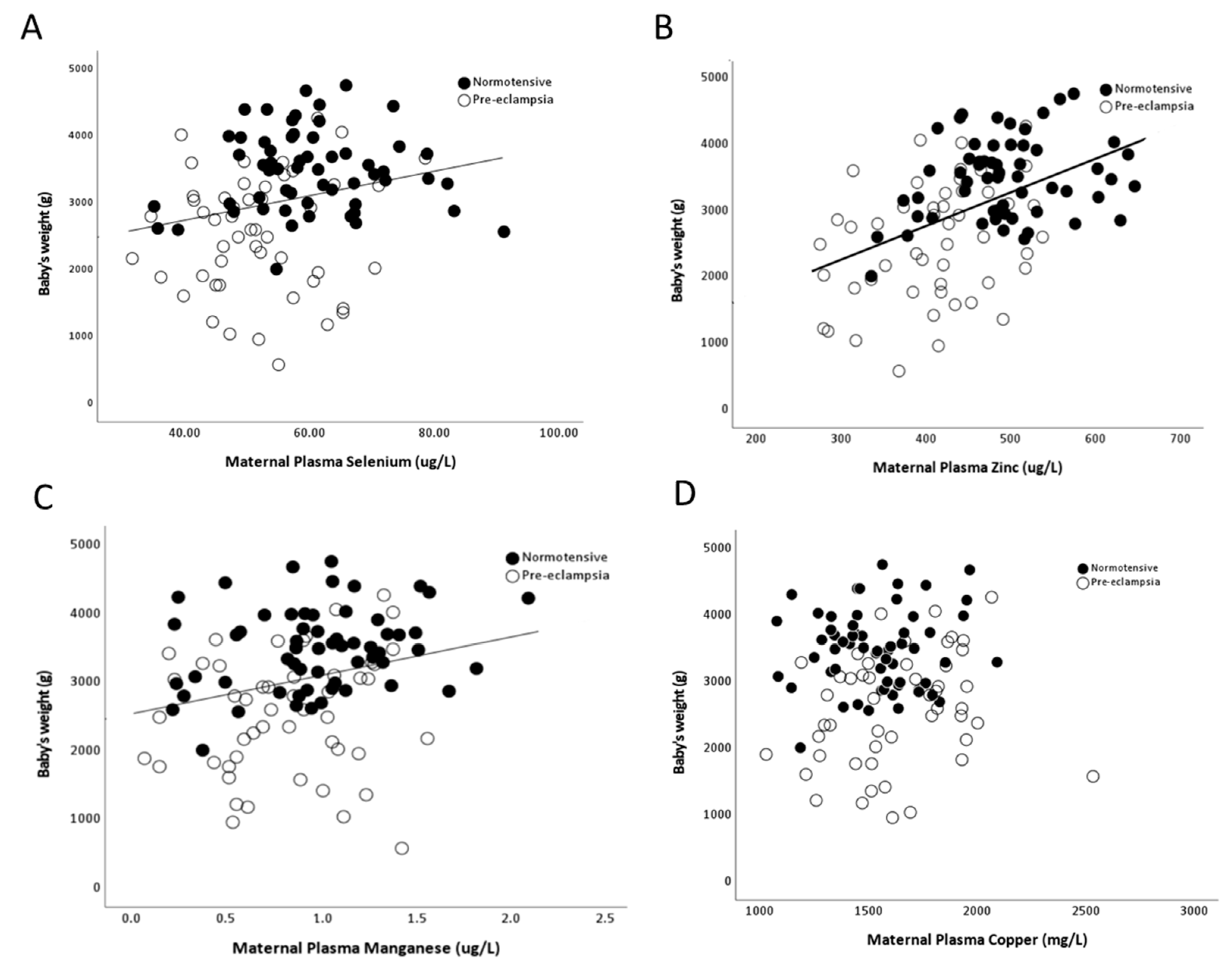
2.2. Selenium
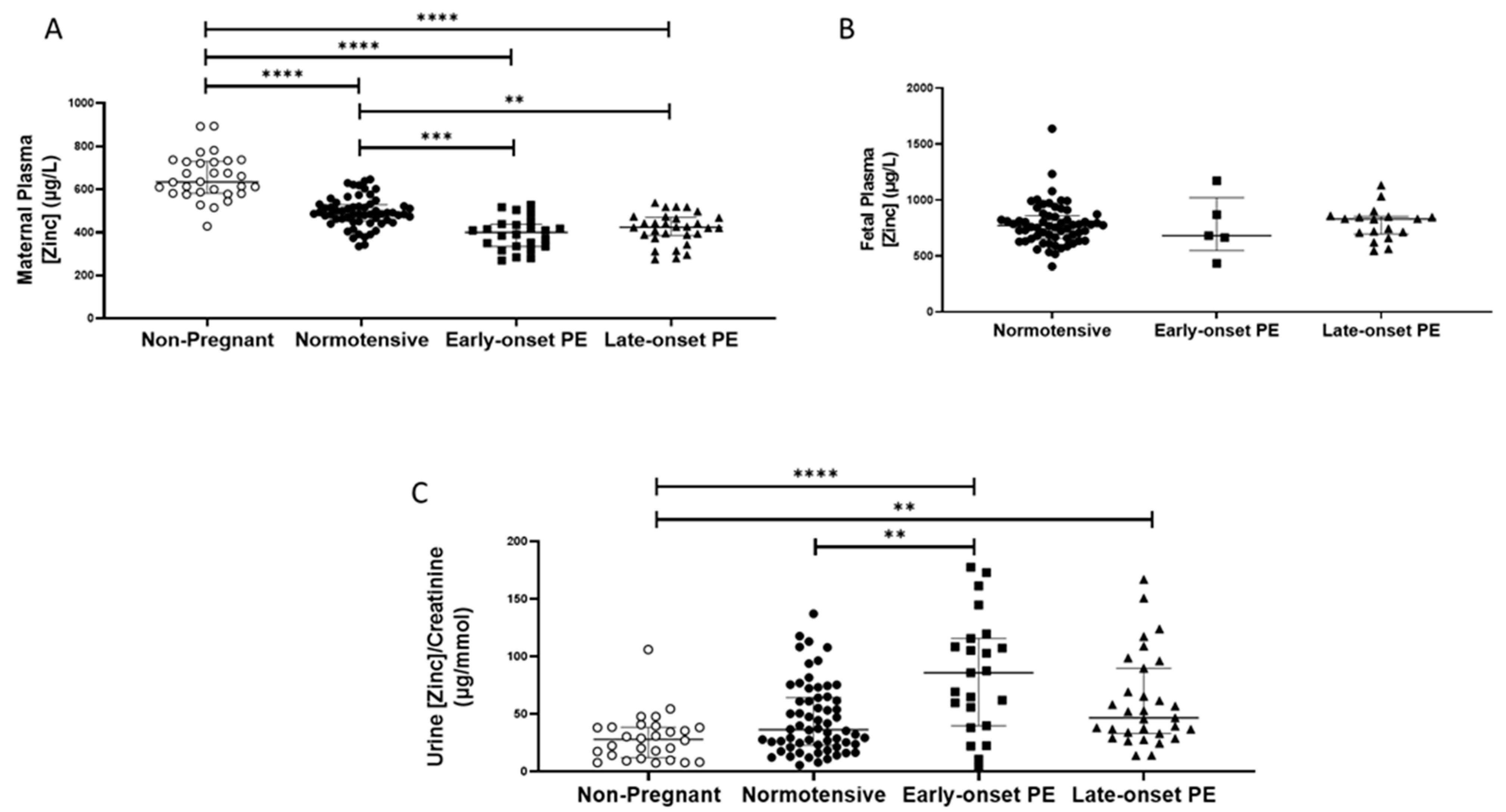
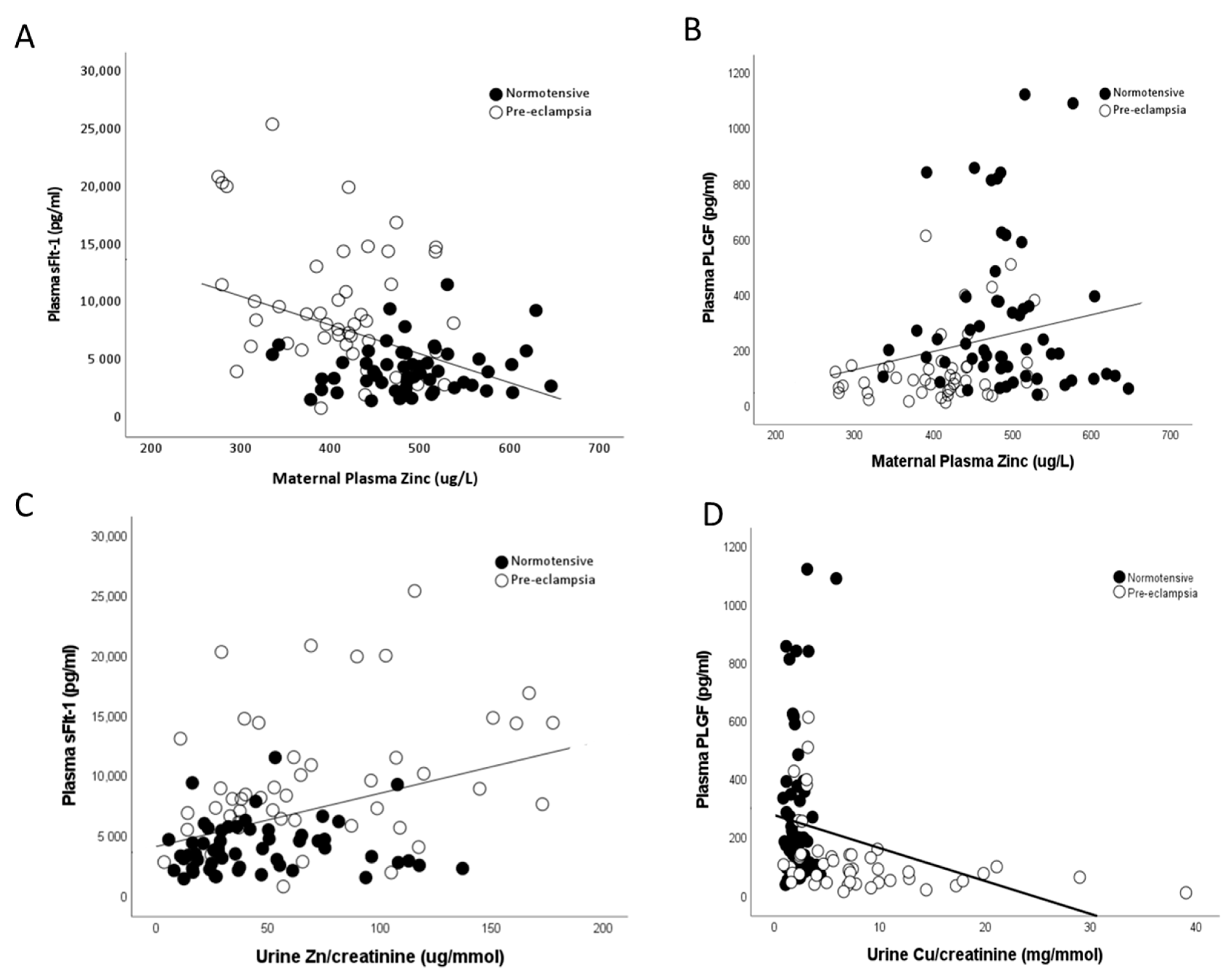
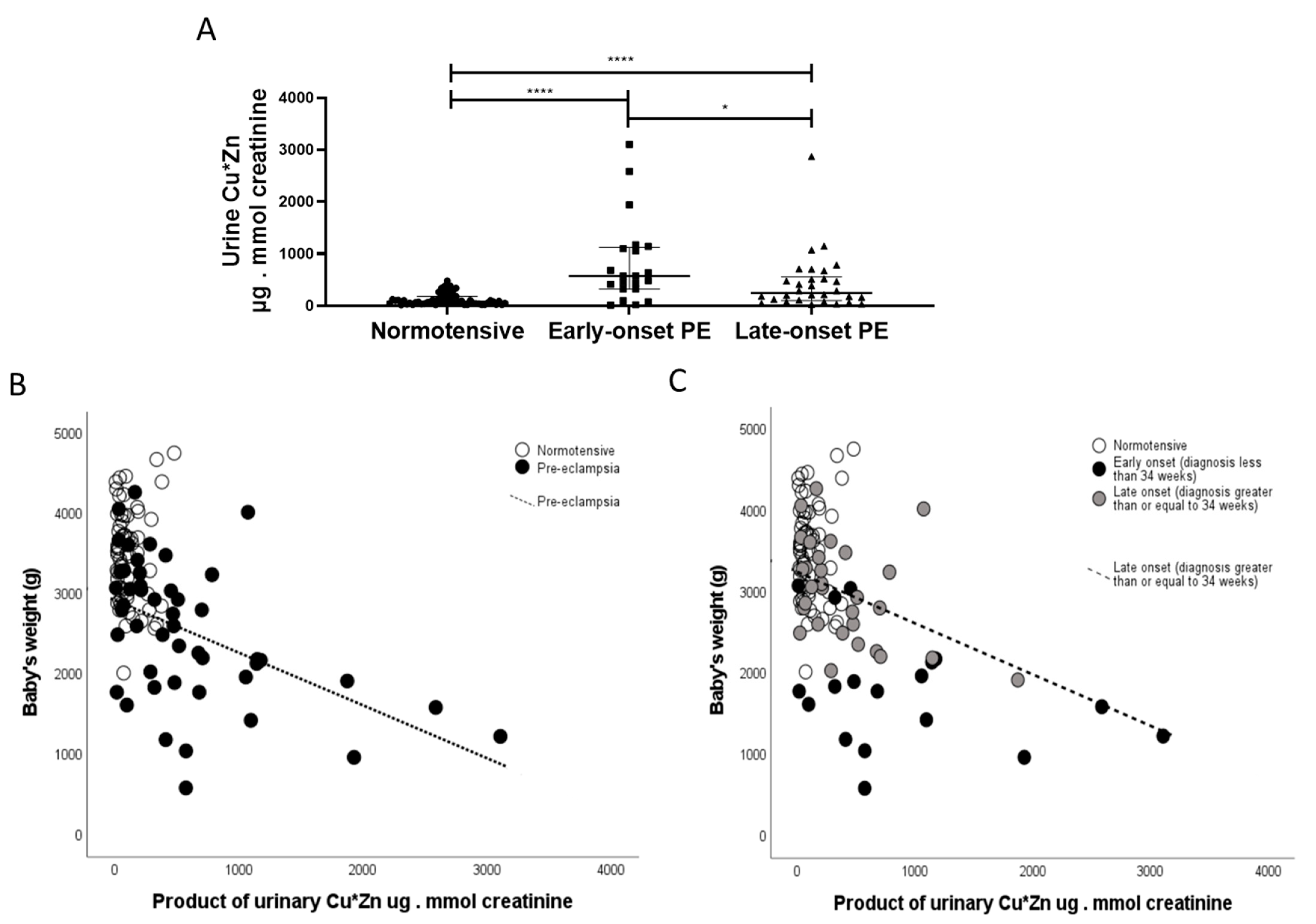
2.3. Zinc
2.4. Manganese
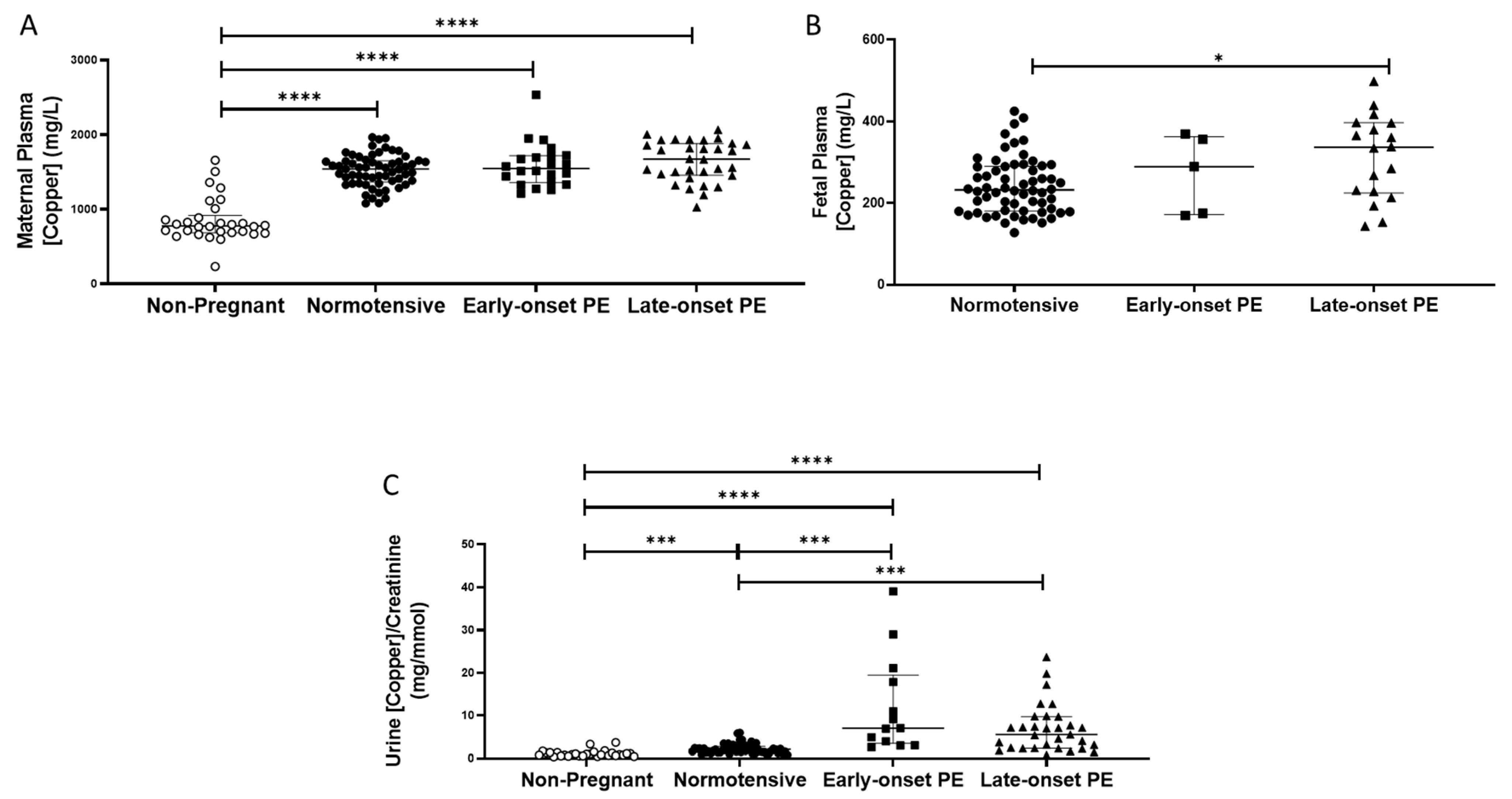
2.5. Copper
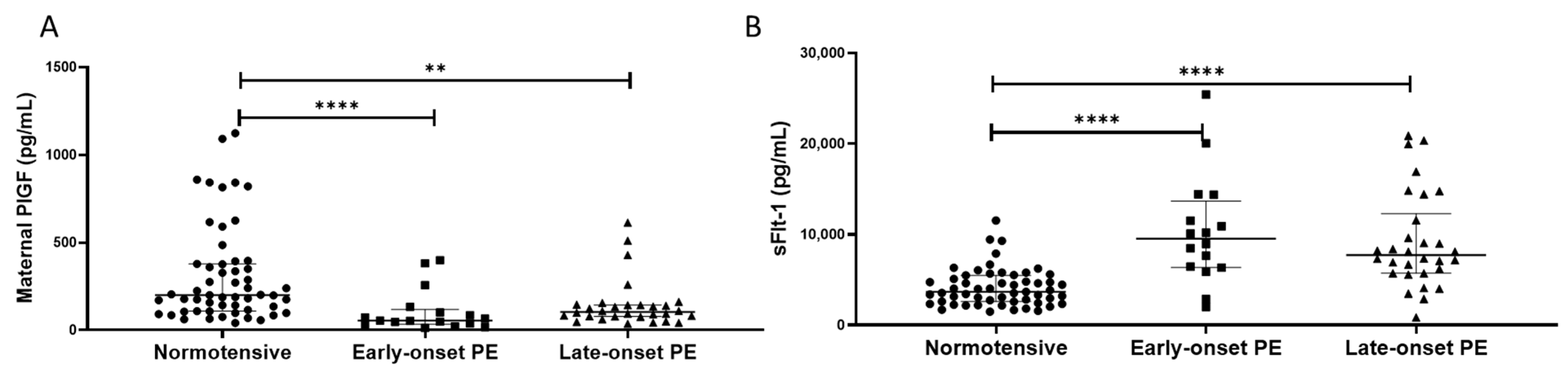
2.6. PlGF and sFlt-1
2.7. Correlations
3. Discussion
4. Materials and Methods
4.1. Cohort and Sample Collection
4.2. Measurement of Micronutrient Content in Maternal Plasma, Urine and Placenta
4.3. Measurement of PlGF and sFlt-1
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.P.; Myers, J.; Poon, L.C.; et al. The hypertensive disorders of pregnancy: The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis and management recommendations for international practice. Pregnancy Hypertens. 2021, 27, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Huang, L.; Umbach, D.M.; Klungsoyr, K.; Engel, S.M.; Magnus, P.; Skjaerven, R.; Zhang, J.; Wilcox, A.J. Risk of fetal death with preeclampsia. Obstet. Gynecol. 2015, 125, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.M.; Cohn, B.A. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 2015, 132, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Williams, P.J. The importance of antioxidant micronutrients in pregnancy. Oxid. Med. Cell. Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef]
- Williams, P.J.; Mistry, H.D. Antioxidant micronutrients in pregnancy and early childhood. In Maternal and Infant Nutrition and Nurture: Controversies and Challenges, 2nd ed.; Moran, V.H., Ed.; Quay Books: London, UK, 2013; pp. 1–44. [Google Scholar]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Reduced selenium concentrations and glutathione peroxidase activity in pre-eclamptic pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef]
- Katz, O.; Paz-Tal, O.; Lazer, T.; Aricha-Tamir, B.; Mazor, M.; Wiznitzer, A.; Sheiner, E. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J. Matern. Fetal Neonatal Med. 2011, 25, 1127–1130. [Google Scholar] [CrossRef]
- Kiilholma, P.; Paul, R.; Pakarinen, P.; Gronroos, M. Copper and zinc in pre-eclampsia. Acta Obstet. Gynecol. Scand. 1984, 63, 629–631. [Google Scholar] [CrossRef]
- Serdar, Z.; Gur, E.; Develioglu, O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem. Funct. 2006, 24, 209–215. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Bode, P.; Redman, C.W. Low selenium status is associated with the occurrence of the pregnancy disease preeclampsia in women from the United Kingdom. Am. J. Obstet. Gynecol. 2003, 189, 1343–1349. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, P.; Kulshreshtha, S.; Mohan, G.; Singh, S. The role of calcium, magnesium, and zinc in pre-eclampsia. Biol. Trace Elem. Res. 2010, 133, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E. Micronutrients in pregnancy. Br. J. Nutr. 2001, 85 (Suppl. S2), S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, O.; Odusoga, O.L.; Adebawo, O.O.; Ugbaja, R.N. Endogenous antioxidant defences in plasma and erythrocytes of pregnant women during different trimesters of pregnancy. Acta Obstet. Gynecol. Scand. 2007, 86, 1175–1180. [Google Scholar] [CrossRef]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Fakhriazad, E.; Seyedaghamiri, Z.; Araki, S. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol. 2008, 25, 219–223. [Google Scholar] [CrossRef]
- Jones, E.A.; Wright, J.M.; Rice, G.; Buckley, B.T.; Magsumbol, M.S.; Barr, D.B.; Williams, B.L. Metal exposures in an inner-city neonatal population. Environ. Int. 2010, 36, 649–654. [Google Scholar] [CrossRef]
- Liu, T.; Hivert, M.F.; Rifas-Shiman, S.L.; Rahman, M.L.; Oken, E.; Cardenas, A.; Mueller, N.T. Prospective Association Between Manganese in Early Pregnancy and the Risk of Preeclampsia. Epidemiology 2020, 31, 677–680. [Google Scholar] [CrossRef]
- Gambling, L.; Andersen, H.S.; McArdle, H.J. Iron and copper, and their interactions during development. Biochem. Soc. Trans. 2008, 36 Pt 6, 1258–1261. [Google Scholar] [CrossRef]
- Kolusari, A.; Kurdoglu, M.; Yildizhan, R.; Adali, E.; Edirne, T.; Cebi, A.; Demir, H.; Yoruk, I.H. Catalase activity, serum trace element and heavy metal concentrations, and vitamin A, D and E levels in pre-eclampsia. J. Int. Med. Res. 2008, 36, 1335–1341. [Google Scholar] [CrossRef]
- Mistry, H.D.; Gill, C.A.; Kurlak, L.O.; Seed, P.T.; Hesketh, J.E.; Meplan, C.; Schomburg, L.; Chappell, L.C.; Morgan, L.; Poston, L.; et al. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 2015, 78, 147–155. [Google Scholar] [CrossRef]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: A randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef]
- Gardner, D.S.; Allen, J.C.; Goodson, D.; Harvey, D.; Sharman, A.; Skinner, H.; Szafranek, A.; Young, J.S.; Bailey, E.H.; Devonald, M.A.J. Urinary Trace Elements Are Biomarkers for Early Detection of Acute Kidney Injury. Kidney Int. Rep. 2022, 7, 1524–1538. [Google Scholar] [CrossRef]
- Rayman, M.P.; Abou-Shakra, F.R.; Ward, N.I.; Redman, C.W. Comparison of selenium levels in pre-eclamptic and normal pregnancies. Biol. Trace Elem. Res. 1996, 55, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, A.C.; Hammond, W.S. Renal iron handling in the nephrotic syndrome. Kidney Int. 1990, 37, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The argument for increasing selenium intake. Proc. Nutr. Soc. 2002, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.; Perkins, A.V. Selenoproteins in the human placenta: How essential is selenium to a healthy start to life? Int. J. Mol. Sci. 2022, 14, 628. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Williams, P.J.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Differential expression and distribution of placental glutathione peroxidases 1, 3 and 4 in normal and preeclamptic pregnancy. Placenta 2010, 31, 401–408. [Google Scholar] [CrossRef]
- Tahan, F.; Karakukcu, C. Zinc status in infantile wheezing. Pediatr. Pulmonol. 2006, 41, 630–634. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J. Nutr. 1995, 125, 823–829. [Google Scholar]
- King, J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000, 71 (Suppl. S5), 1334S–1343S. [Google Scholar] [CrossRef]
- Bassiouni, B.A.; Foda, A.I.; Rafei, A.A. Maternal and fetal plasma zinc in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1979, 9, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Betrie, A.H.; Brock, J.A.; Harraz, O.F.; Bush, A.I.; He, G.W.; Nelson, M.T.; Angus, J.A.; Wright, C.E.; Ayton, S. Zinc drives vasorelaxation by acting in sensory nerves, endothelium and smooth muscle. Nat. Commun. 2021, 12, 3296. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef] [PubMed]
- Atherton, D.J.; Muller, D.P.; Aggett, P.J.; Harries, J.T. A defect in zinc uptake by jejunal biopsies in acrodermatitis enteropathica. Clin. Sci. 1979, 56, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Ford, D. Intestinal and placental zinc transport pathways. Proc. Nutr. Soc. 2004, 63, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fowles, E.R.; Walker, L.O.; Marti, C.N.; Ruiz, R.J.; Wommack, J.; Bryant, M.; Kim, S.; Timmerman, G.M. Relationships among maternal nutrient intake and placental biomarkers during the 1st trimester in low-income women. Arch. Gynecol. Obstet. 2012, 285, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Guallar, E.; Wang, G.; Hong, X.; Wang, X.; Mueller, N.T. Trace Minerals, Heavy Metals, and Preeclampsia: Findings from the Boston Birth Cohort. J. Am. Heart Assoc. 2019, 8, e012436. [Google Scholar] [CrossRef]
- Michaelis, V.; Aengenheister, L.; Tuchtenhagen, M.; Rinklebe, J.; Ebert, F.; Schwerdtle, T.; Buerki-Thurnherr, T.; Bornhorst, J. Differences and Interactions in Placental Manganese and Iron Transfer across an In Vitro Model of Human Villous Trophoblasts. Int. J. Mol. Sci. 2022, 23, 3296. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, R.; Cai, X.; Xiao, R.; Yu, H. Trace elements profiles of maternal blood, umbilical cord blood, and placenta in Beijing, China. J. Matern. Fetal Neonatal Med. 2019, 32, 1755–1761. [Google Scholar] [CrossRef]
- Wu, Q.; Mu, Q.; Xia, Z.; Min, J.; Wang, F. Manganese homeostasis at the host-pathogen interface and in the host immune system. Semin. Cell Dev. Biol. 2021, 115, 45–53. [Google Scholar] [CrossRef]
- de Moraes, M.L.; de Faria Barbosa, R.; Santo, R.E.; da Silva Santos, F.; de Jesus, E.F.; Sardinha, F.L.; Tavares do Carmo, M.D. Maternal-Fetal Distribution of Calcium, Iron, Copper, and Zinc in Pregnant Teenagers and Adults. Biol. Trace Elem. Res. 2010, 139, 126–136. [Google Scholar] [CrossRef]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in fetal growth and development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef]
- Krachler, M.; Rossipal, E.; Micetic-Turk, D. Trace element transfer from the mother to the newborn—Investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 1999, 53, 486–494. [Google Scholar] [CrossRef]
- Rossipal, E.; Krachler, M.; Li, F.; Micetic-Turk, D. Investigation of the transport of trace elements across barriers in humans: Studies of placental and mammary transfer. Acta Paediatr. 2000, 89, 1190–1195. [Google Scholar] [CrossRef]
- Raghunath, R.; Tripathi, R.M.; Sastry, V.N.; Krishnamoorthy, T.M. Heavy metals in maternal and cord blood. Sci. Total Environ. 2000, 250, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bommarito, P.A.; Kim, S.S.; Meeker, J.D.; Fry, R.C.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Urinary trace metals, maternal circulating angiogenic biomarkers, and preeclampsia: A single-contaminant and mixture-based approach. Environ. Health 2019, 18, 63. [Google Scholar] [CrossRef]
- Gardner, D.S.; De Brot, S.; Dunford, L.J.; Grau-Roma, L.; Welham, S.J.; Fallman, R.; O’Sullivan, S.E.; Oh, W.; Devonald, M.A. Remote effects of acute kidney injury in a porcine model. Am. J. Physiol. Renal Physiol. 2016, 310, F259–F271. [Google Scholar] [CrossRef] [PubMed]
- Mariath, A.B.; Bergamaschi, D.P.; Rondo, P.H.; Tanaka, A.C.; Hinnig Pde, F.; Abbade, J.F.; Diniz, S.G. The possible role of selenium status in adverse pregnancy outcomes. Br. J. Nutr. 2011, 105, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Klapec, T.; Cavar, S.; Kasac, Z.; Rucevic, S.; Popinjac, A. Selenium in placenta predicts birth weight in normal but not intrauterine growth restriction pregnancy. J. Trace Elem. Med. Biol. 2008, 22, 54–58. [Google Scholar] [CrossRef]
- Strambi, M.; Longini, M.; Vezzosi, P.; Berni, S.; Buoni, S. Selenium status, birth weight, and breast-feeding: Pattern in the first month. Biol. Trace Elem. Res. 2004, 99, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Kurlak, L.O.; Young, S.D.; Briley, A.L.; Broughton Pipkin, F.; Baker, P.N.; Poston, L. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern. Child Nutr. 2014, 10, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Tamura, T.; Neggers, Y.; Copper, R.L.; Johnston, K.E.; DuBard, M.B.; Hauth, J.C. The effect of zinc supplementation on pregnancy outcome. JAMA 1995, 274, 463–468. [Google Scholar] [CrossRef] [PubMed]
- McKeating, D.R.; Fisher, J.J.; MacDonald, T.; Walker, S.; Tong, S.; Bennett, W.W.; Kaitu’u-Lino, T.J.; Perkins, A.V. Circulating trace elements for the prediction of preeclampsia and small for gestational age babies. Metabolomics 2021, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Lindheimer, M.D.; de Swiet, M.; Van Assche, A.; Moutquin, J.M. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens. Pregnancy 2001, 20, IX–XIV. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013, 3, 44–47. [Google Scholar] [CrossRef]
- Kurlak, L.O.; Broughton Pipkin, F.; Mohaupt, M.G.; Mistry, H.D. Responses of the renin-angiotensin-aldosterone system in pregnant chronicl kidney disease patients with and without superimposed pre-eclampsia. Clin. Kidney J. 2019, 12, 847–854. [Google Scholar] [CrossRef]
- Oh, W.C.; Mafrici, B.; Rigby, M.; Harvey, D.; Sharman, A.; Allen, J.C.; Mahajan, R.; Gardner, D.S.; Devonald, M.A.J. Micronutrient and Amino Acid Losses During Renal Replacement Therapy for Acute Kidney Injury. Kidney Int. Rep. 2019, 4, 1094–1108. [Google Scholar] [CrossRef]
- Gray, C.; Al-Dujaili, E.A.; Sparrow, A.J.; Gardiner, S.M.; Craigon, J.; Welham, S.J.; Gardner, D.S. Excess maternal salt intake produces sex-specific hypertension in offspring: Putative roles for kidney and gastrointestinal sodium handling. PLoS ONE 2013, 8, e72682. [Google Scholar] [CrossRef]

| Parameter | Non-Pregnant (n = 30) | Normotensive (n = 60) | Early-Onset Pre-eclampsia (n = 24) | Late-Onset Pre-eclampsia (n = 31) |
|---|---|---|---|---|
| Maternal age (years) | 27 ± 7.6 | 32 ± 5.4 | 33 ± 5.4 | 29 ± 6.4 |
| Booking body mass index (kg/m2) | 24.7 ± 4.7 | 27.3 ± 6.3 | 32.3 ± 8.0 | 29.1 ± 6.8 |
| Nulliparous (n (%)) | 17 (57) | 31 (52) | 13 (54) | 17 (55) |
| Max. systolic blood pressure outside labour at booking (mmHg) | 124 ± 13.1 | 128 ± 12.7 | 163 ± 10.0 ** | 154 ± 9.0 ** |
| Max. diastolic blood pressure outside labour at booking (mmHg) | 78 ± 9.3 | 80 ± 8.1 | 103 ± 6.8 ** | 99 ± 5.8 ** |
| Protein:creatinine ratio (g/mmol) | - | - | 177 (80, 378) | 127 (58, 269) |
| Gestation age at delivery (weeks) | - | 39.1 ± 1.1 | 32.7 ± 2.8 * | 38.4 ± 1.8 * |
| Birthweight (kg) | - | 3.50 (2.99, 3.88) | 1.77 (1.29, 2.04) * | 2.99 (2.49, 3.48) * |
| Caesarean Sections (n (%) | - | 46 (76) | 16 (66) | 17 (50) |
| Location of Biopsy | Normotensive Control (n = 60) | Early-Onset Pre-eclampsia (n = 5) | Late-Onset Pre-eclampsia (n = 13) |
|---|---|---|---|
| Selenium (µg/Kg) | |||
| Periphery | 1.03 [0.98, 1.09] a | 1.05 [1.01, 1.08] | 0.95 [0.87, 1.02] a |
| Middle | 1.02 [0.94, 1.07] | 0.98 [0.91, 1.03] | 0.91 [0.88, 0.93] |
| Near Cord | 1.01 [0.95, 1.05] a | 1.02 [1.01, 1.20] b | 0.91 [0.88, 0.93] a,b |
| Zinc (µg/Kg) | |||
| Periphery | 58.6 [52.7, 62.4] | 52.2 [52.1, 58.2] | 55.8 [52.6, 62.4] |
| Middle | 56.9 [52.1, 62.1] | 54.6 [44.0, 55.5] | 55.3 [53.7, 58.7] |
| Near Cord | 56.6 [52.5, 61.8] | 51.2 [45.9, 55.1] | 56.7 [54.2, 61.2] |
| Manganese (µg/Kg) | |||
| Periphery | 0.48 [0.37, 0.56] | 0.48 [0.41, 0.58] | 0.47 [0.41, 0.58] |
| Middle | 0.44 [0.34, 0.52] | 0.35 [0.33, 0.39] | 0.39 [0.33, 0.43] |
| Near Cord | 0.46 [0.36, 0.57] | 0.37 [0.31, 0.41] | 0.46 [0.31, 0.63] |
| Copper (mg/Kg) | |||
| Periphery | 5.48 [4.79, 6.17] | 5.19 [4.73, 6.06] | 5.02 [4.93, 5.86] |
| Middle | 5.03 [4.55, 5.53] | 4.70 [4.04, 5.24] | 5.33 [4.86, 6.58] |
| Near Cord | 5.06 [4.59, 5.57] | 5.05 [4.59, 5.79] | 5.16 [4.57, 5.48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurlak, L.O.; Scaife, P.J.; Briggs, L.V.; Broughton Pipkin, F.; Gardner, D.S.; Mistry, H.D. Alterations in Antioxidant Micronutrient Concentrations in Placental Tissue, Maternal Blood and Urine and the Fetal Circulation in Pre-eclampsia. Int. J. Mol. Sci. 2023, 24, 3579. https://doi.org/10.3390/ijms24043579
Kurlak LO, Scaife PJ, Briggs LV, Broughton Pipkin F, Gardner DS, Mistry HD. Alterations in Antioxidant Micronutrient Concentrations in Placental Tissue, Maternal Blood and Urine and the Fetal Circulation in Pre-eclampsia. International Journal of Molecular Sciences. 2023; 24(4):3579. https://doi.org/10.3390/ijms24043579
Chicago/Turabian StyleKurlak, Lesia O., Paula J. Scaife, Louise V. Briggs, Fiona Broughton Pipkin, David S. Gardner, and Hiten D. Mistry. 2023. "Alterations in Antioxidant Micronutrient Concentrations in Placental Tissue, Maternal Blood and Urine and the Fetal Circulation in Pre-eclampsia" International Journal of Molecular Sciences 24, no. 4: 3579. https://doi.org/10.3390/ijms24043579
APA StyleKurlak, L. O., Scaife, P. J., Briggs, L. V., Broughton Pipkin, F., Gardner, D. S., & Mistry, H. D. (2023). Alterations in Antioxidant Micronutrient Concentrations in Placental Tissue, Maternal Blood and Urine and the Fetal Circulation in Pre-eclampsia. International Journal of Molecular Sciences, 24(4), 3579. https://doi.org/10.3390/ijms24043579





