The Prognostic Value and the Oncogenic and Immunological Roles of Vacuolar Protein Sorting Associated Protein 26 A in Pancreatic Adenocarcinoma
Abstract
1. Introduction
2. Results
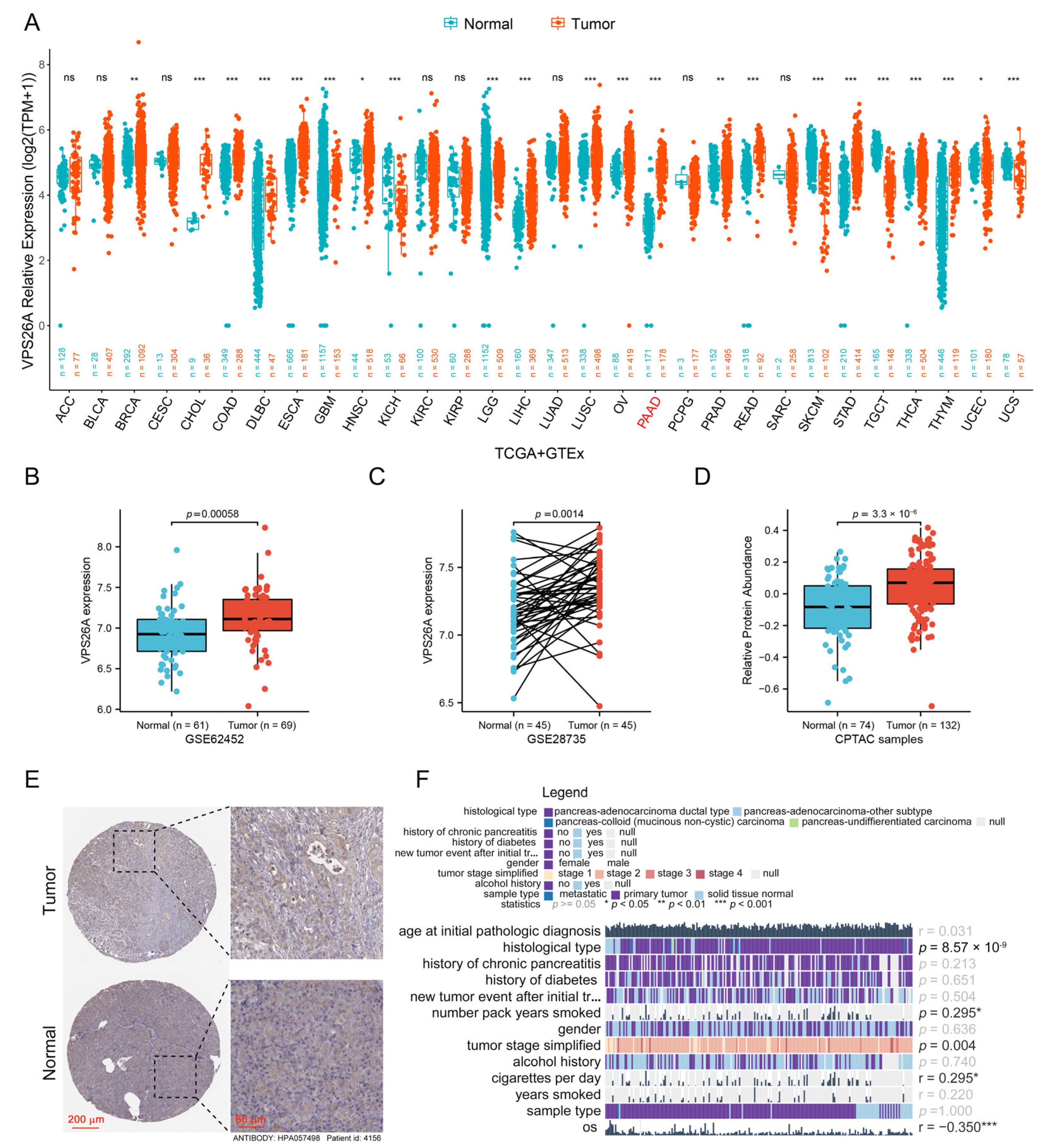
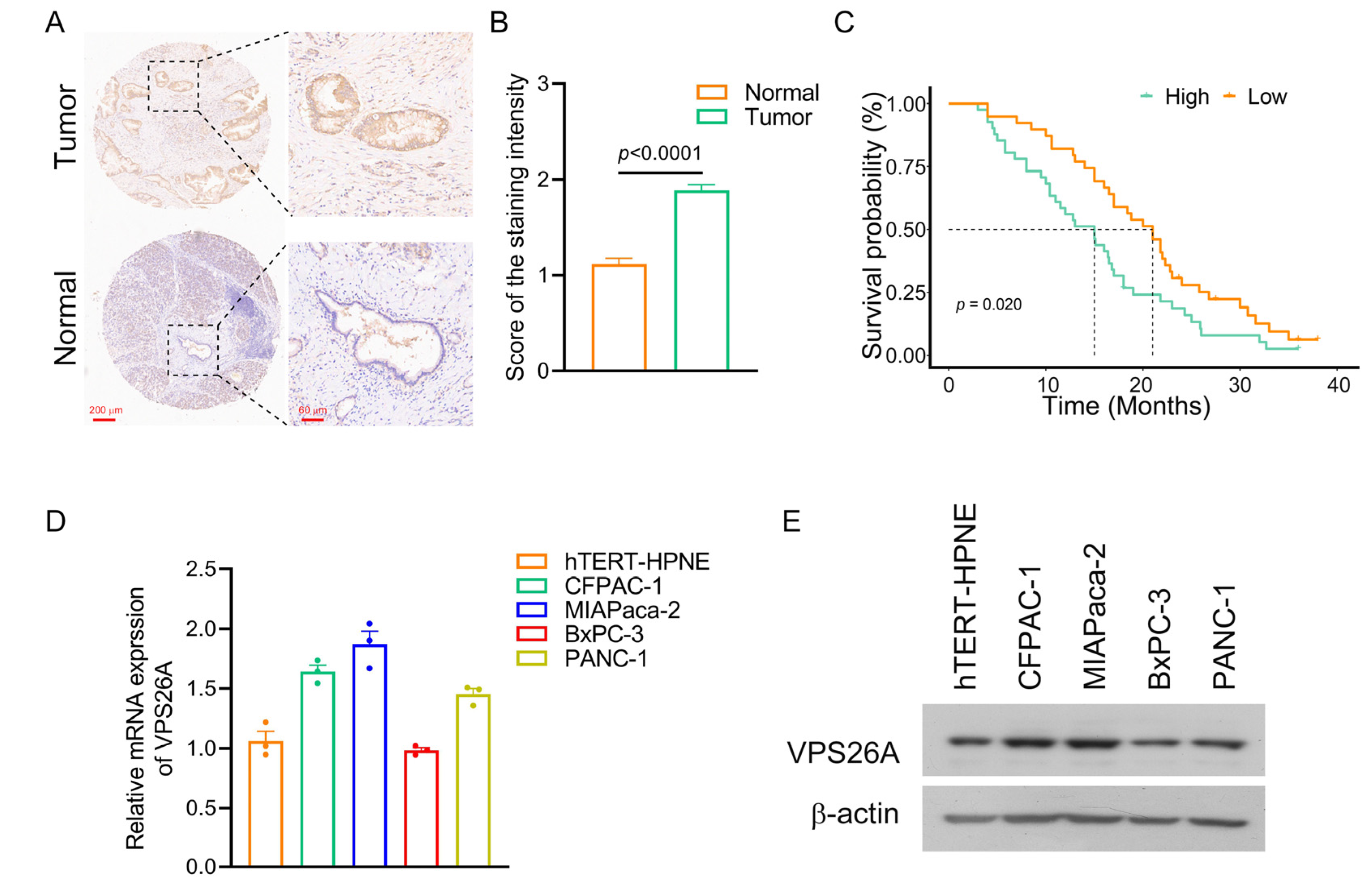
2.1. Increased Expression of VPS26A in PAAD
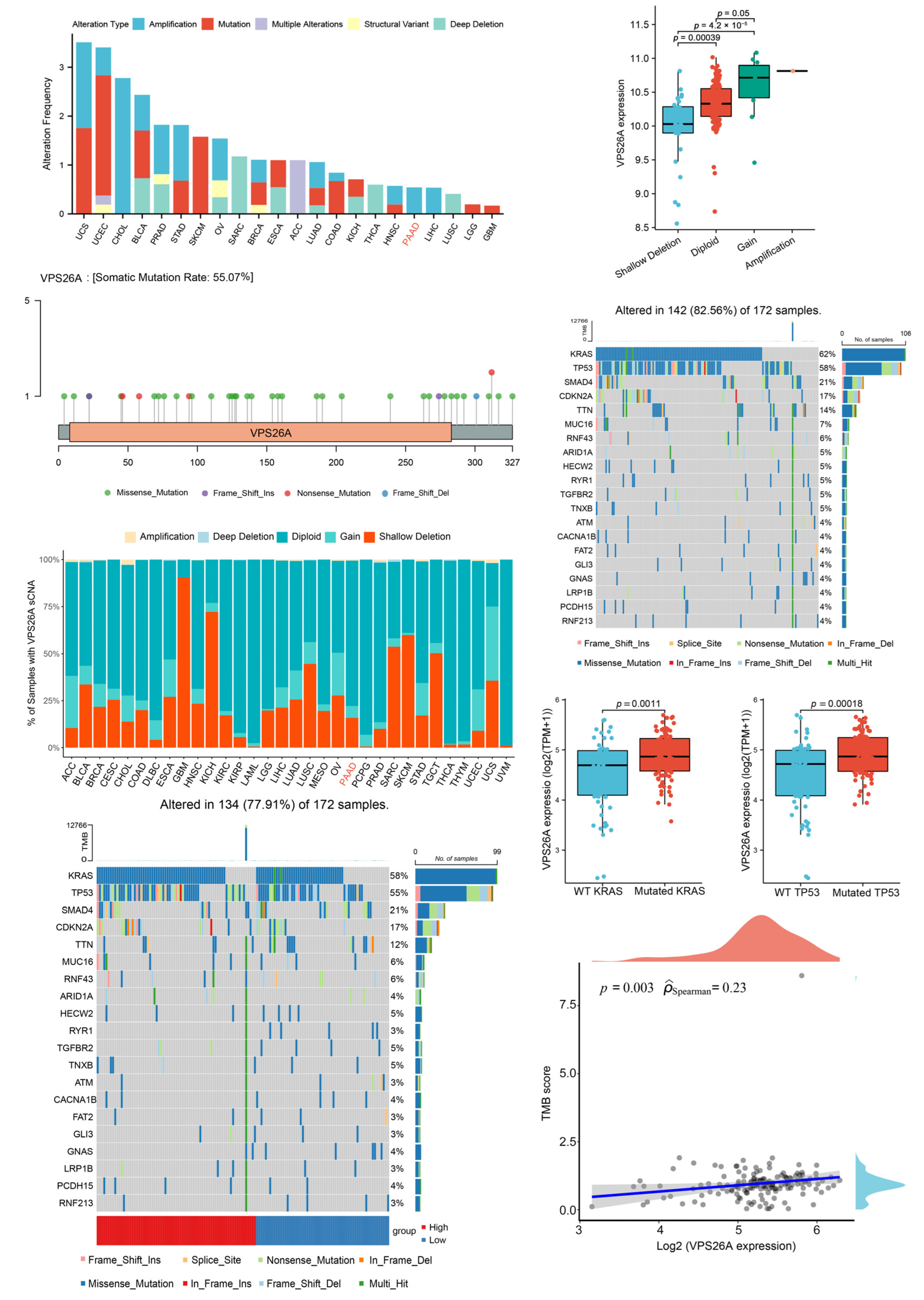
2.2. Analysis of VPS26A Mutation and SCNAs
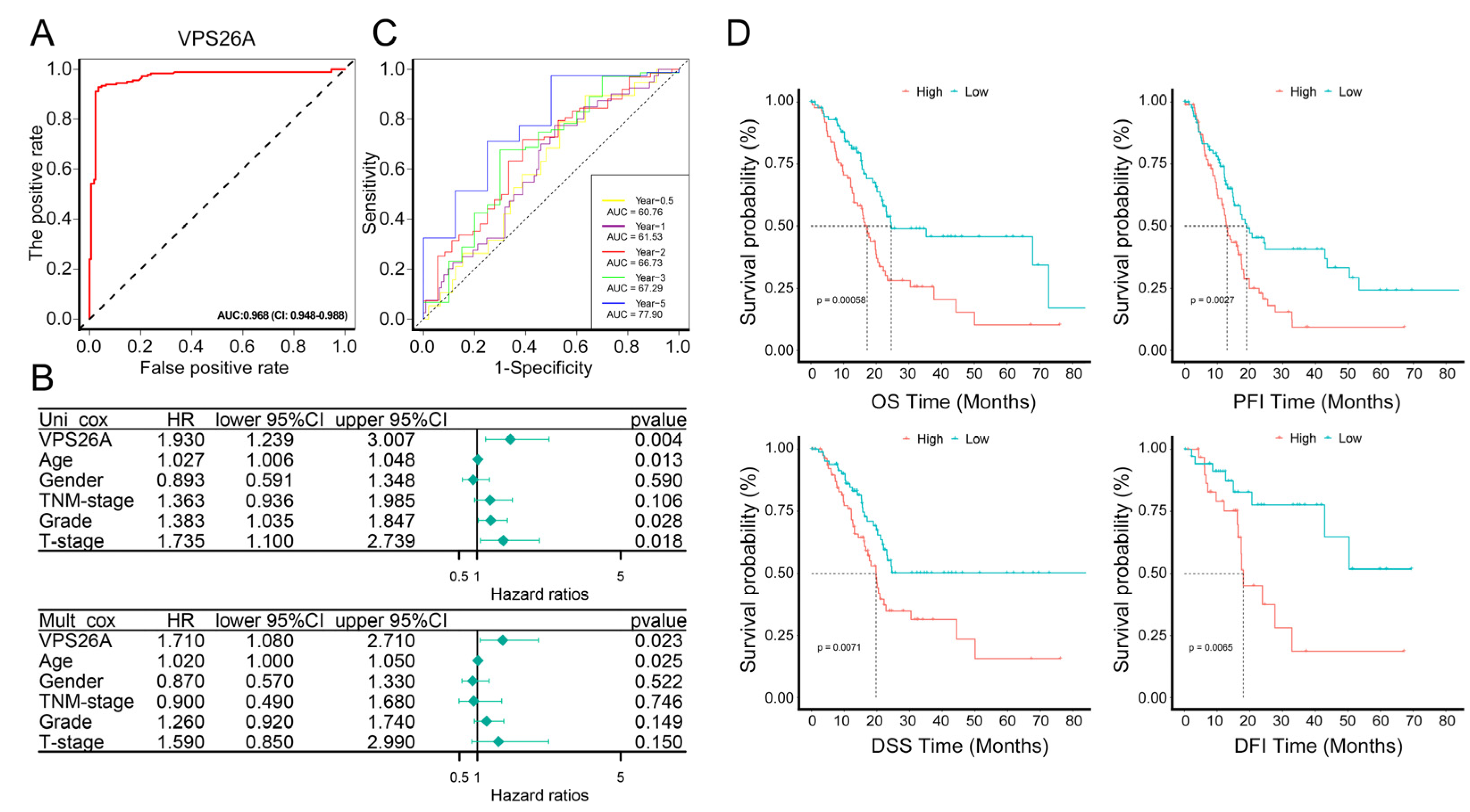
2.3. Diagnostic and Prognostic Analysis of VPS26A in PAAD
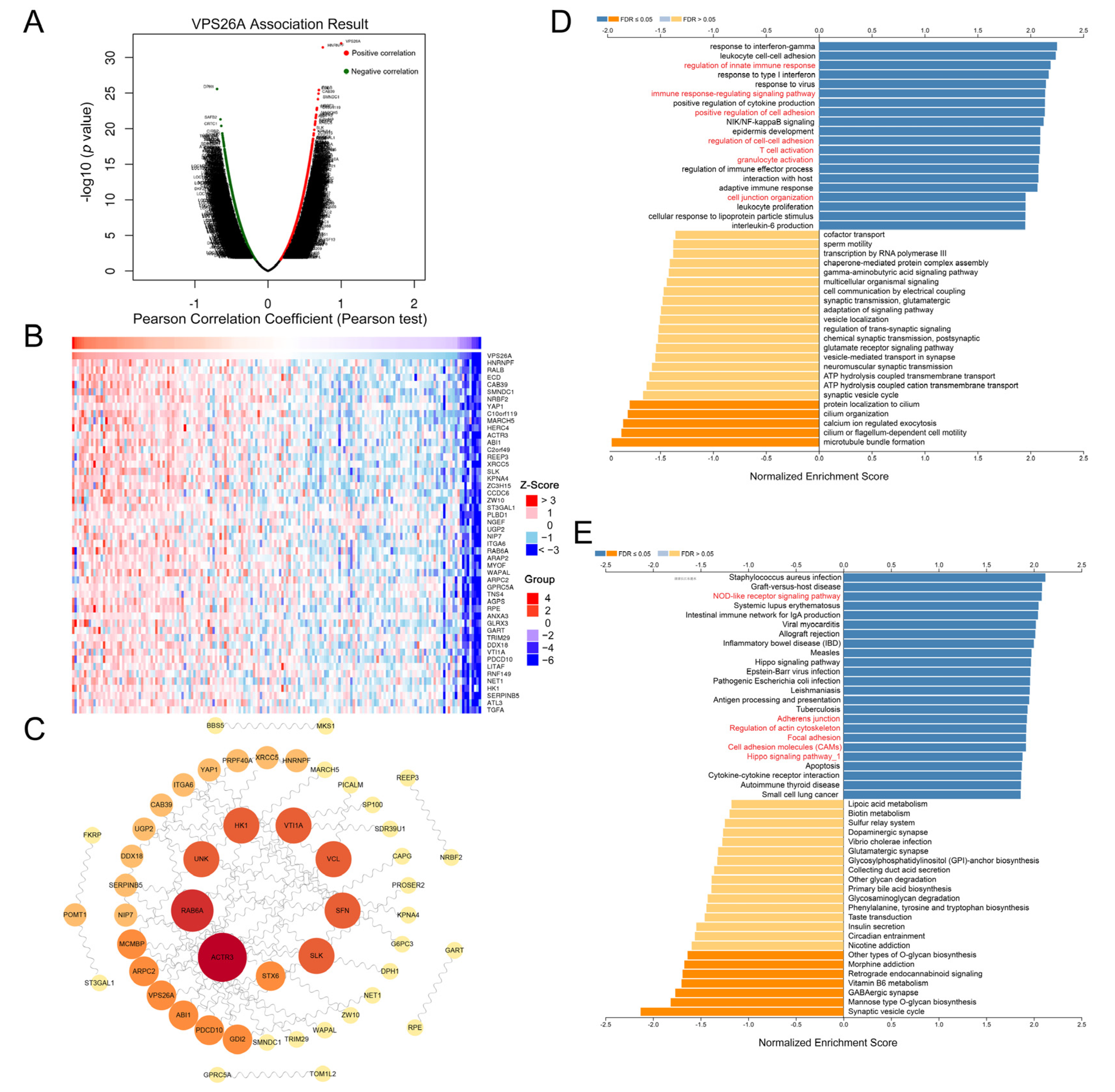
2.4. Immune-Related Analysis of VPS26A
2.5. Protein–Protein Interaction (PPI) Network and Pathway Enrichment Analysis of VPS26A
2.6. Validation of VPS26A Expression in PAAD Tissues and PAAD Cell Lines
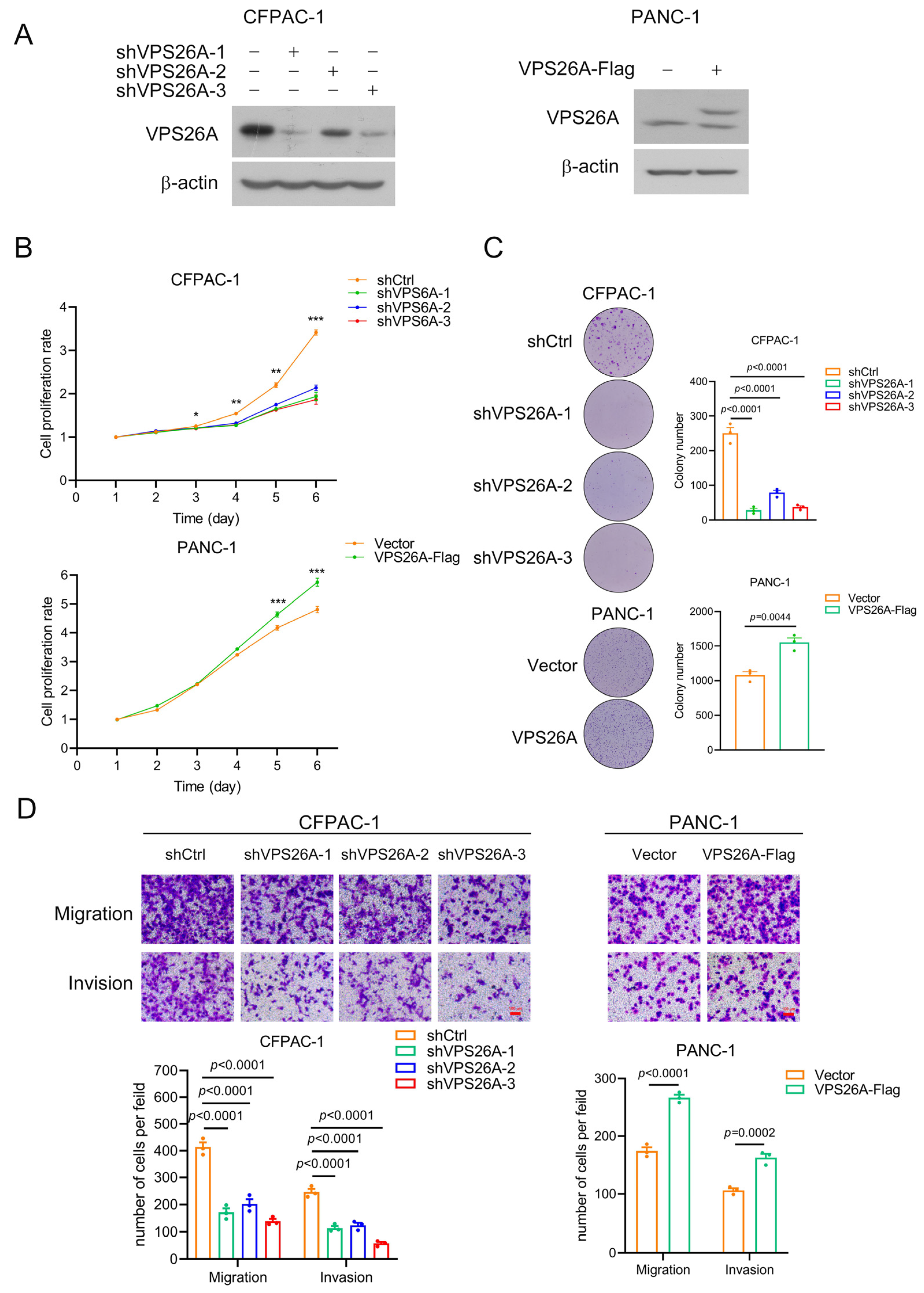
2.7. VPS26A Promoted the Proliferation, Migration and Invasion of PAAD Cells
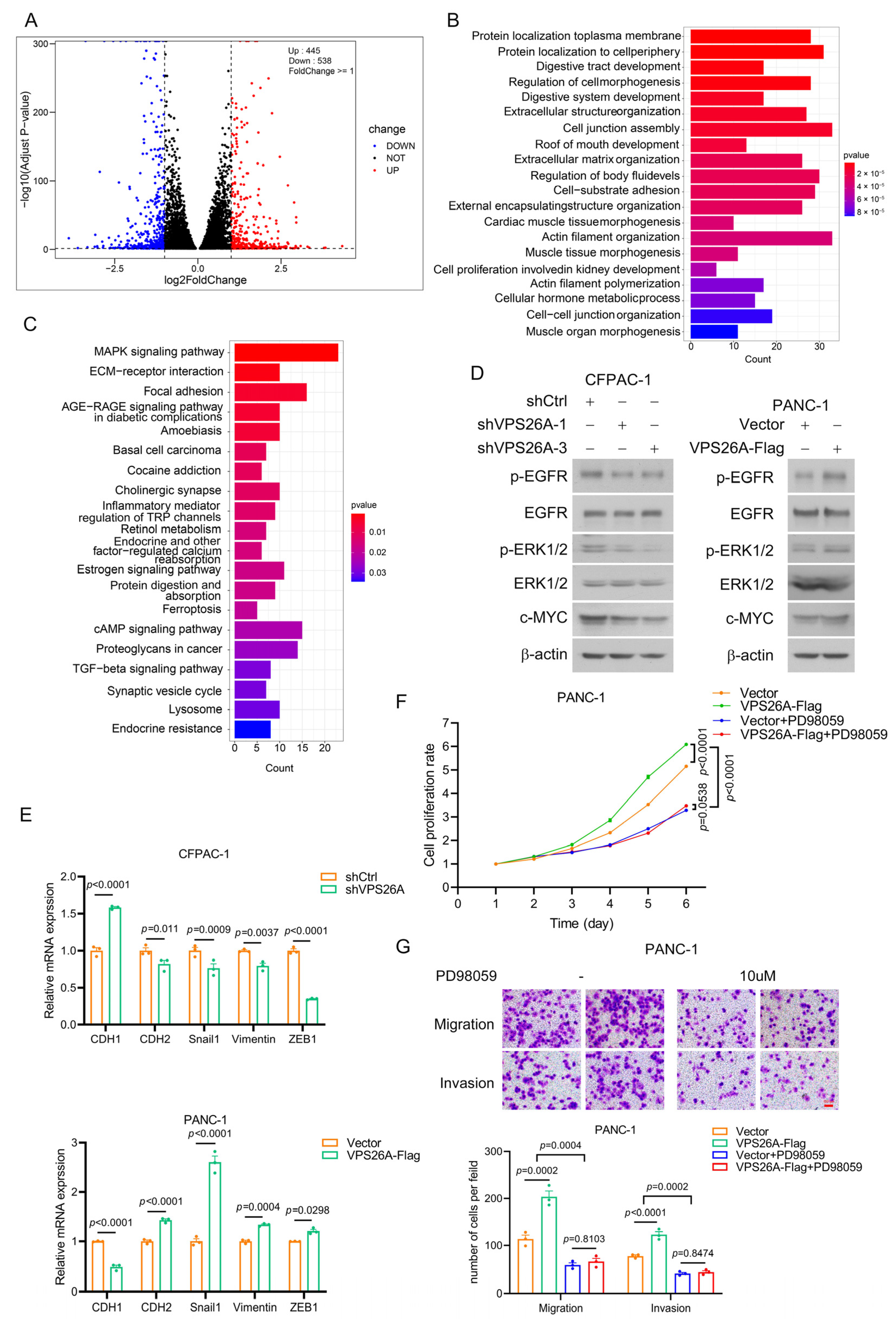
2.8. VPS26A Activated EGFR-ERK Signaling to Regulate the Proliferation, Migration and Invasion of PAAD Cells
3. Discussion
4. Materials and Methods
4.1. Gene Expression Mutation Analysis
4.2. Diagnostic and Prognostic Analysis
4.3. Gene Functional Enrichment Analysis
4.4. Immunology Analysis
4.5. Patients and Samples
4.6. Cell Culture and Western Blot
4.7. Real-Time PCR
4.8. Lentivirus-Mediated Stable Knockdown and Overexpression
4.9. Cell Proliferation Assay
4.10. Transwell Migration and Invasion Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Krempley, B.D.; Yu, K.H. Preclinical models of pancreatic ductal adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 25. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Andersson, R.; Haglund, C.; Seppanen, H.; Ansari, D. Pancreatic cancer-the past, the present, and the future. Scand. J. Gastroenterol. 2022, 57, 1169–1177. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Follett, J.; Bugarcic, A.; Collins, B.M.; Teasdale, R.D. Retromer’s Role in Endosomal Trafficking and Impaired Function in Neurodegenerative Diseases. Curr. Protein Pept. Sci. 2017, 18, 687–701. [Google Scholar] [CrossRef]
- Bugarcic, A.; Zhe, Y.; Kerr, M.C.; Griffin, J.; Collins, B.M.; Teasdale, R.D. Vps26A and Vps26B subunits define distinct retromer complexes. Traffic 2011, 12, 1759–1773. [Google Scholar] [CrossRef]
- Cullen, P.J.; Korswagen, H.C. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 2011, 14, 29–37. [Google Scholar] [CrossRef]
- Lorenowicz, M.J.; Macurkova, M.; Harterink, M.; Middelkoop, T.C.; de Groot, R.; Betist, M.C.; Korswagen, H.C. Inhibition of late endosomal maturation restores Wnt secretion in Caenorhabditis elegans vps-29 retromer mutants. Cell Signal. 2014, 26, 19–31. [Google Scholar] [CrossRef]
- Choi, S.-A.; Kim, Y.-H.; Park, Y.-H.; Yang, H.-J.; Jeong, P.-S.; Cha, J.-J.; Yoon, S.-B.; Kim, J.-S.; Song, B.-S.; Lee, J.-H.; et al. Novel crosstalk between Vps26a and Nox4 signaling during neurogenesis. Cell Death Differ. 2019, 26, 1582–1599. [Google Scholar] [CrossRef]
- Lin, T.B.; Lai, C.Y.; Hsieh, M.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Chen, G.D.; Peng, H.Y. VPS26A-SNX27 Interaction-Dependent mGluR5 Recycling in Dorsal Horn Neurons Mediates Neuropathic Pain in Rats. J. Neurosci. 2015, 35, 14943–14955. [Google Scholar] [CrossRef]
- Shannon, B.; Soto-Ortolaza, A.; Rayaprolu, S.; Cannon, H.D.; Labbé, C.; Benitez, B.A.; Choi, J.; Lynch, T.; Boczarska-Jedynak, M.; Opala, G.; et al. Genetic variation of the retromer subunits VPS26A/B-VPS29 in Parkinson’s disease. Neurobiol. Aging 2014, 35, 1958.e1–1958.e2. [Google Scholar] [CrossRef]
- Chae, C.W.; Choi, G.E.; Jung, Y.H.; Lim, J.R.; Cho, J.H.; Yoon, J.H.; Han, H.J. High glucose-mediated VPS26a down-regulation dysregulates neuronal amyloid precursor protein processing and tau phosphorylation. Br. J. Pharmacol. 2022, 179, 3934–3950. [Google Scholar] [CrossRef]
- Koschmidder, E.; Mollenhauer, B.; Kasten, M.; Klein, C.; Lohmann, K. Mutations in VPS26A are not a frequent cause of Parkinson’s disease. Neurobiol. Aging 2014, 35, 1512.e1–1512.e2. [Google Scholar] [CrossRef]
- Small, S.A.; Kent, K.; Pierce, A.; Leung, C.; Kang, M.S.; Okada, H.; Honig, L.; Vonsattel, J.P.; Kim, T.W. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann. Neurol. 2005, 58, 909–919. [Google Scholar] [CrossRef]
- Xu, W.; Guo, W.; Lu, P.; Ma, D.; Liu, L.; Yu, F. Identification of an autophagy-related gene signature predicting overall survival for hepatocellular carcinoma. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Althubiti, M.; Lezina, L.; Carrera, S.; Jukes-Jones, R.; Giblett, S.M.; Antonov, A.; Barlev, N.; Saldanha, G.S.; Pritchard, C.A.; Cain, K.; et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 2014, 5, e1528. [Google Scholar] [CrossRef]
- Sheng, W.; Chen, C.; Dong, M.; Wang, G.; Zhou, J.; Song, H.; Li, Y.; Zhang, J.; Ding, S. Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis. 2017, 8, e3147. [Google Scholar] [CrossRef]
- Bian, Y.; Yu, Y.; Wang, S.; Li, L. Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer. Biochem. Biophys. Res. Commun. 2015, 463, 612–617. [Google Scholar] [CrossRef]
- Eguchi, H.; Nakachi, K. Smoking as a risk factor for pancreatic cancer. Nihon. Rinsho. 2006, 64 (Suppl. S1), 10–13. [Google Scholar]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Lawlor, R.T.; Mattiolo, P.; Mafficini, A.; Hong, S.M.; Piredda, M.L.; Taormina, S.V.; Malleo, G.; Marchegiani, G.; Pea, A.; Salvia, R.; et al. Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Pancreatic Cancer: Systematic Review and Still-Open Questions. Cancers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Wang, X.; Ricciuti, B.; Alessi, J.V.; Nguyen, T.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Smoking History as a Potential Predictor of Immune Checkpoint Inhibitor Efficacy in Metastatic Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2021, 113, 1761–1769. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Huang, C.F.; Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Sun, Z.J. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 116. [Google Scholar] [CrossRef]
- Ansari, D.; Ohlsson, H.; Althini, C.; Bauden, M.; Zhou, Q.; Hu, D.; Andersson, R. The Hippo Signaling Pathway in Pancreatic Cancer. Anticancer Res. 2019, 39, 3317–3321. [Google Scholar] [CrossRef]
- Liu, J.; Kang, L.; Ratnayake, I.; Ahrenkiel, P.; Smith, S.; Wang, C. Targeting cancer cell adhesion molecule, CD146, with low-dose gold nanorods and mild hyperthermia disrupts actin cytoskeleton and cancer cell migration. J. Colloid Interface Sci. 2021, 601, 556–569. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Zhang, Y.M.; Liu, Y.H.; Liu, Y. Actin Cytoskeleton-Disrupting and Magnetic Field-Responsive Multivalent Supramolecular Assemblies for Efficient Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 13709–13717. [Google Scholar] [CrossRef]
- Sun, L.; Kees, T.; Almeida, A.S.; Liu, B.; He, X.Y.; Ng, D.; Han, X.; Spector, D.L.; McNeish, I.A.; Gimotty, P.; et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 2021, 39, 1361–1374.e9. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Wang, D.; Lin, Y.; Hou, J.; Xu, X.; Wu, J.; Zhong, L.; Zhou, Y.; Shen, J.; et al. The HNF4alpha-BC200-FMR1-Positive Feedback Loop Promotes Growth and Metastasis in Invasive Mucinous Lung Adenocarcinoma. Cancer Res. 2021, 81, 5904–5918. [Google Scholar] [CrossRef]
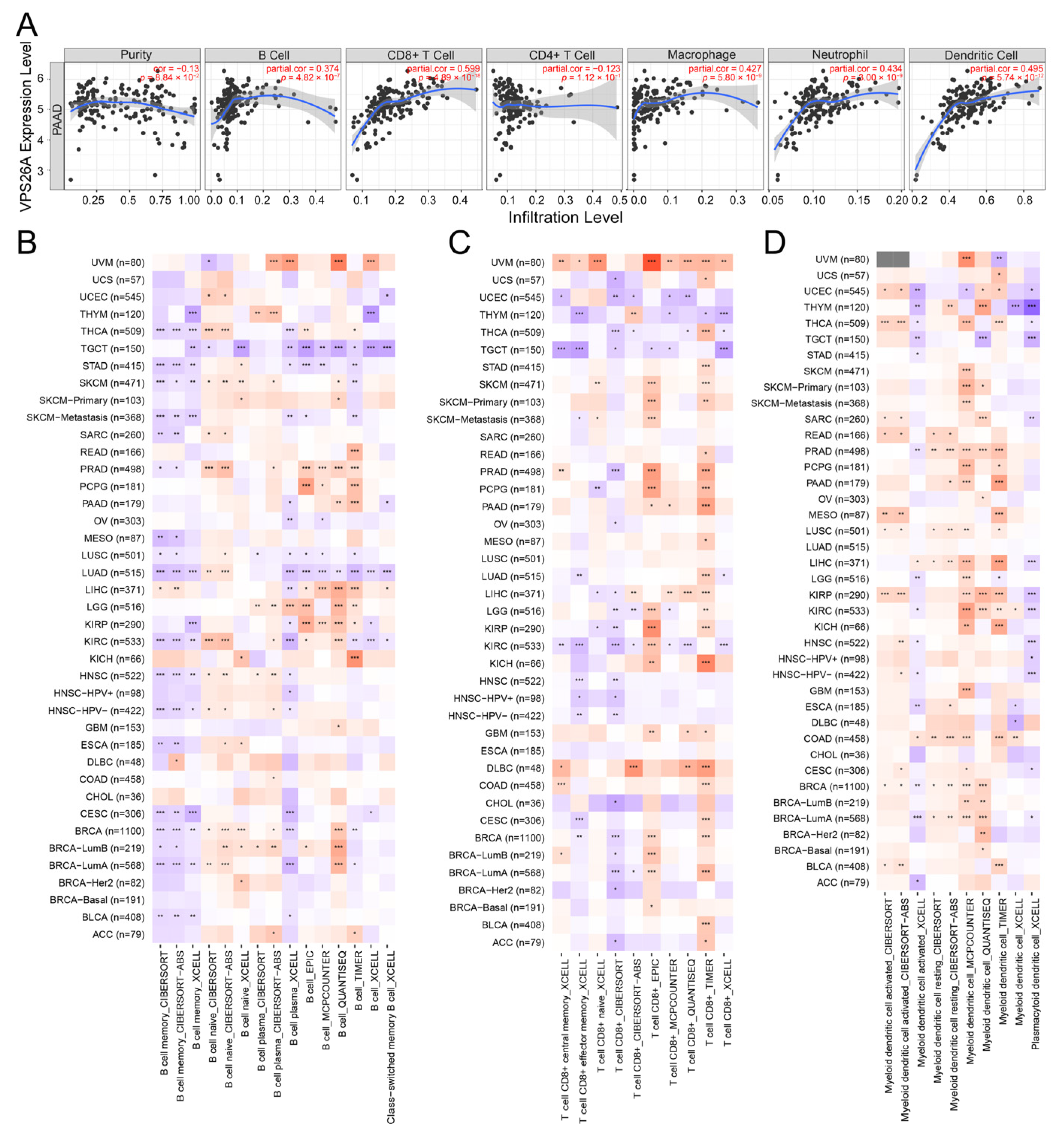

| Immune Cell Types | Markers | R Value | p-Value |
|---|---|---|---|
| B Cell | CD19 | 0.13 | 0.072 |
| CD79A | 0.14 | 0.06 | |
| CD8+ T Cell | ADRM1 | 0.081 | 0.28 |
| AHSA1 | 0.38 | 1.7 × 10−7 | |
| CD37 | 0.2 | 0.0063 | |
| CD3D | 0.16 | 0.034 | |
| CD8A | 0.25 | 0.00092 | |
| CETN3 | 0.36 | 1 × 10−6 | |
| CSE1L | 0.45 | 1.8 × 10−10 | |
| IL2RB | 0.33 | 8.4 × 10−6 | |
| MPZL1 | 0.63 | 8 × 10−21 | |
| CD4+ T Cell | AIM2 | 0.22 | 0.0036 |
| CCL4 | 0.18 | 0.016 | |
| CCNB1 | 0.33 | 7.9 × 10−6 | |
| EXO1 | 0.25 | 6 × 10−4 | |
| KIF11 | 0.54 | 5.1 × 10−15 | |
| KNTC1 | 0.12 | 0.12 | |
| NUF2 | 0.31 | 2 × 10−5 | |
| PRC1 | 0.36 | 8.4 × 10−7 | |
| RTKN2 | 0.45 | 3.5 × 10−10 | |
| M1 macrophage | NOS2 | 0.22 | 0.0025 |
| IRF5 | 0.24 | 0.00098 | |
| PTGS2 | 0.43 | 2.4 × 10−9 | |
| M2 macrophage | CD163 | 0.33 | 8.1 × 10−6 |
| VSIG4 | 0.32 | 1.1 × 10−5 | |
| MS4A4A | 0.37 | 4.3 × 10−7 | |
| Neutrophil | CEACAM8 | 0.28 | 0.00018 |
| ITGAM | 0.33 | 7.6 × 10−6 | |
| CCR7 | 0.16 | 0.033 | |
| Dendritic Cell | HLA-DPB1 | 0.27 | 0.00023 |
| HLA-DQB1 | 0.16 | 0.028 | |
| HLA-DRA | 0.4 | 4.4 × 10−8 | |
| HLA-DPA1 | 0.37 | 2.4 × 10−7 | |
| CD1C | 0.18 | 0.015 | |
| NRP1 | 0.53 | 1.6 × 10−14 | |
| ITGAX | 0.17 | 0.023 |
| Gene | HR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| PICALM | 1.58 | 1.07 | 2.32 | 0.02 |
| DPH1 | 0.53 | 0.39 | 0.72 | 0 |
| VTI1A | 1.29 | 0.79 | 2.08 | 0.31 |
| MCMBP | 1.64 | 1.01 | 2.65 | 0.05 |
| SMNDC1 | 1.53 | 0.93 | 2.52 | 0.09 |
| ST3GAL1 | 1.34 | 1.07 | 1.68 | 0.01 |
| PDCD10 | 2.2 | 1.39 | 3.49 | 0 |
| XRCC5 | 1.56 | 1.02 | 2.4 | 0.04 |
| RAB6A | 1.61 | 1.09 | 2.4 | 0.02 |
| STX6 | 1.44 | 0.97 | 2.13 | 0.07 |
| SP100 | 1.59 | 1.18 | 2.15 | 0 |
| SERPINB5 | 1.33 | 1.17 | 1.52 | 0 |
| ZW10 | 1.81 | 1.11 | 2.95 | 0.02 |
| TOM1L2 | 0.57 | 0.43 | 0.76 | 0 |
| G6PC3 | 0.53 | 0.37 | 0.76 | 0 |
| HNRNPF | 1.68 | 1.1 | 2.56 | 0.02 |
| ARPC2 | 1.49 | 1.02 | 2.16 | 0.04 |
| UGP2 | 1.66 | 1.03 | 2.68 | 0.04 |
| RPE | 1.69 | 1.2 | 2.38 | 0 |
| GDI2 | 1.14 | 0.71 | 1.82 | 0.6 |
| KPNA4 | 2.24 | 1.39 | 3.59 | 0 |
| HK1 | 1.4 | 1.04 | 1.9 | 0.03 |
| SLK | 1.59 | 1.2 | 2.11 | 0 |
| NET1 | 1.42 | 1.14 | 1.77 | 0 |
| GPRC5A | 1.32 | 1.15 | 1.52 | 0 |
| CAB39 | 1.66 | 1.16 | 2.37 | 0.01 |
| MKS1 | 0.53 | 0.38 | 0.76 | 0 |
| ITGA6 | 1.45 | 1.16 | 1.8 | 0 |
| FKRP | 0.6 | 0.44 | 0.82 | 0 |
| SFN | 1.27 | 1.12 | 1.45 | 0 |
| NIP7 | 1.84 | 1.22 | 2.76 | 0 |
| ABI1 | 1.32 | 0.9 | 1.95 | 0.16 |
| VCL | 1.52 | 1.14 | 2.02 | 0.01 |
| WAPL | 1.31 | 0.87 | 1.97 | 0.19 |
| ACTR3 | 1.5 | 1.05 | 2.14 | 0.03 |
| GART | 1.7 | 1.14 | 2.55 | 0.01 |
| NRBF2 | 1.48 | 0.92 | 2.39 | 0.11 |
| SDR39U1 | 0.65 | 0.5 | 0.84 | 0 |
| BBS5 | 0.56 | 0.41 | 0.76 | 0 |
| TRIM29 | 1.21 | 1.09 | 1.35 | 0 |
| YAP1 | 1.57 | 1.21 | 2.05 | 0 |
| PRPF40A | 1.62 | 1.05 | 2.49 | 0.03 |
| DDX18 | 1.58 | 1.08 | 2.32 | 0.02 |
| UNK | 0.55 | 0.41 | 0.74 | 0 |
| CAPG | 1.37 | 1.12 | 1.68 | 0 |
| VPS26A | 1.93 | 1.24 | 3.01 | 0 |
| “MARCH5” | 1.67 | 1.02 | 2.75 | 0.04 |
| REEP3 | 1.5 | 1.13 | 2 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Wu, H.; Xu, B.; Shang, J.; Xu, X.; Li, G.; Zhang, H.; Zhang, W.; Deng, Y.; Hong, X.; et al. The Prognostic Value and the Oncogenic and Immunological Roles of Vacuolar Protein Sorting Associated Protein 26 A in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 3486. https://doi.org/10.3390/ijms24043486
Hou J, Wu H, Xu B, Shang J, Xu X, Li G, Zhang H, Zhang W, Deng Y, Hong X, et al. The Prognostic Value and the Oncogenic and Immunological Roles of Vacuolar Protein Sorting Associated Protein 26 A in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2023; 24(4):3486. https://doi.org/10.3390/ijms24043486
Chicago/Turabian StyleHou, Jihuan, Han Wu, Beibei Xu, Jin Shang, Xuechun Xu, Guixia Li, Haoran Zhang, Wenqing Zhang, Yabin Deng, Xiaoting Hong, and et al. 2023. "The Prognostic Value and the Oncogenic and Immunological Roles of Vacuolar Protein Sorting Associated Protein 26 A in Pancreatic Adenocarcinoma" International Journal of Molecular Sciences 24, no. 4: 3486. https://doi.org/10.3390/ijms24043486
APA StyleHou, J., Wu, H., Xu, B., Shang, J., Xu, X., Li, G., Zhang, H., Zhang, W., Deng, Y., Hong, X., Hu, T., Zhang, M., & Zhan, Y. (2023). The Prognostic Value and the Oncogenic and Immunological Roles of Vacuolar Protein Sorting Associated Protein 26 A in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences, 24(4), 3486. https://doi.org/10.3390/ijms24043486







