Inhibitors against Two PDZ Domains of MDA-9 Suppressed Migration of Breast Cancer Cells
Abstract
1. Introduction
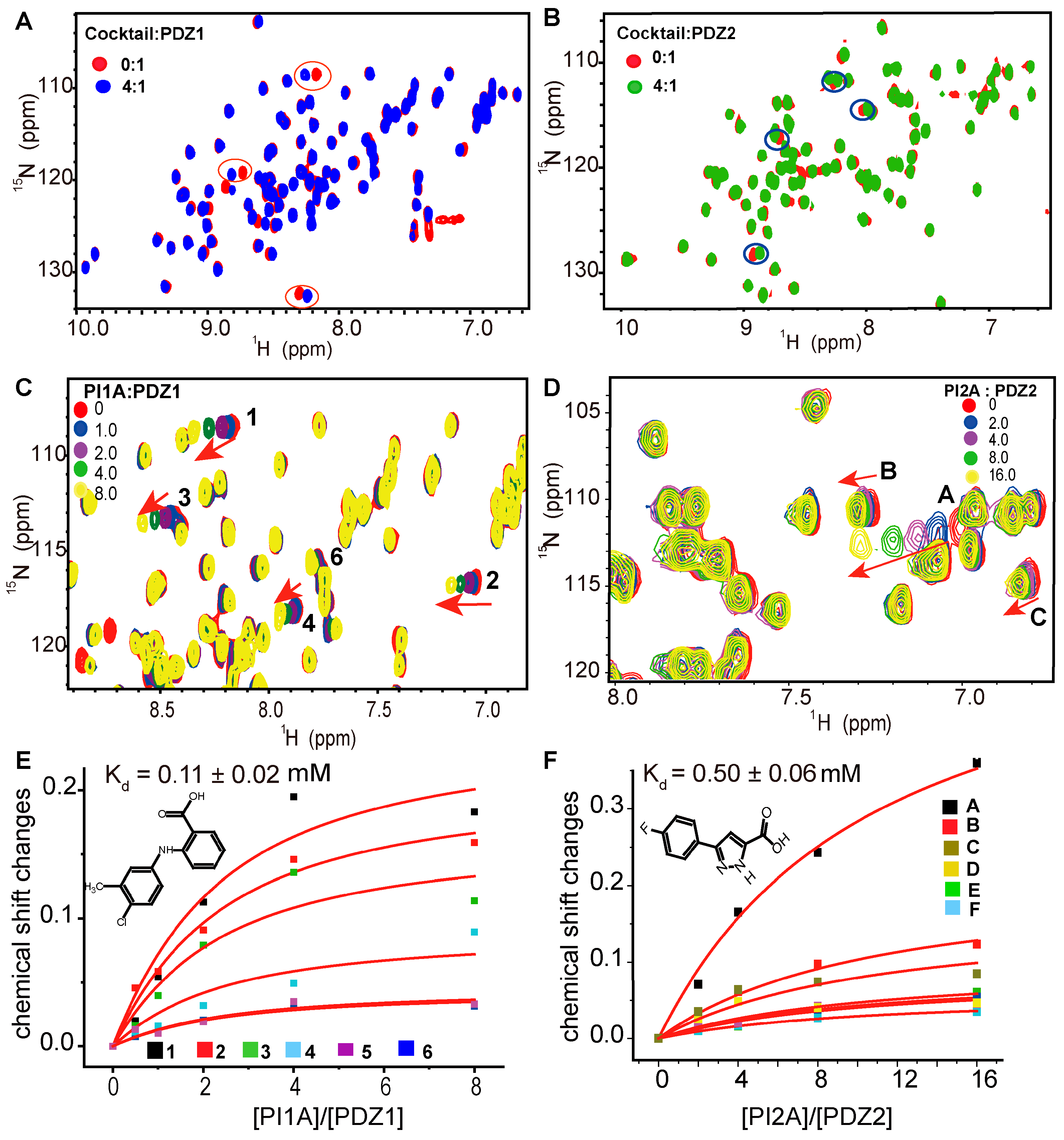
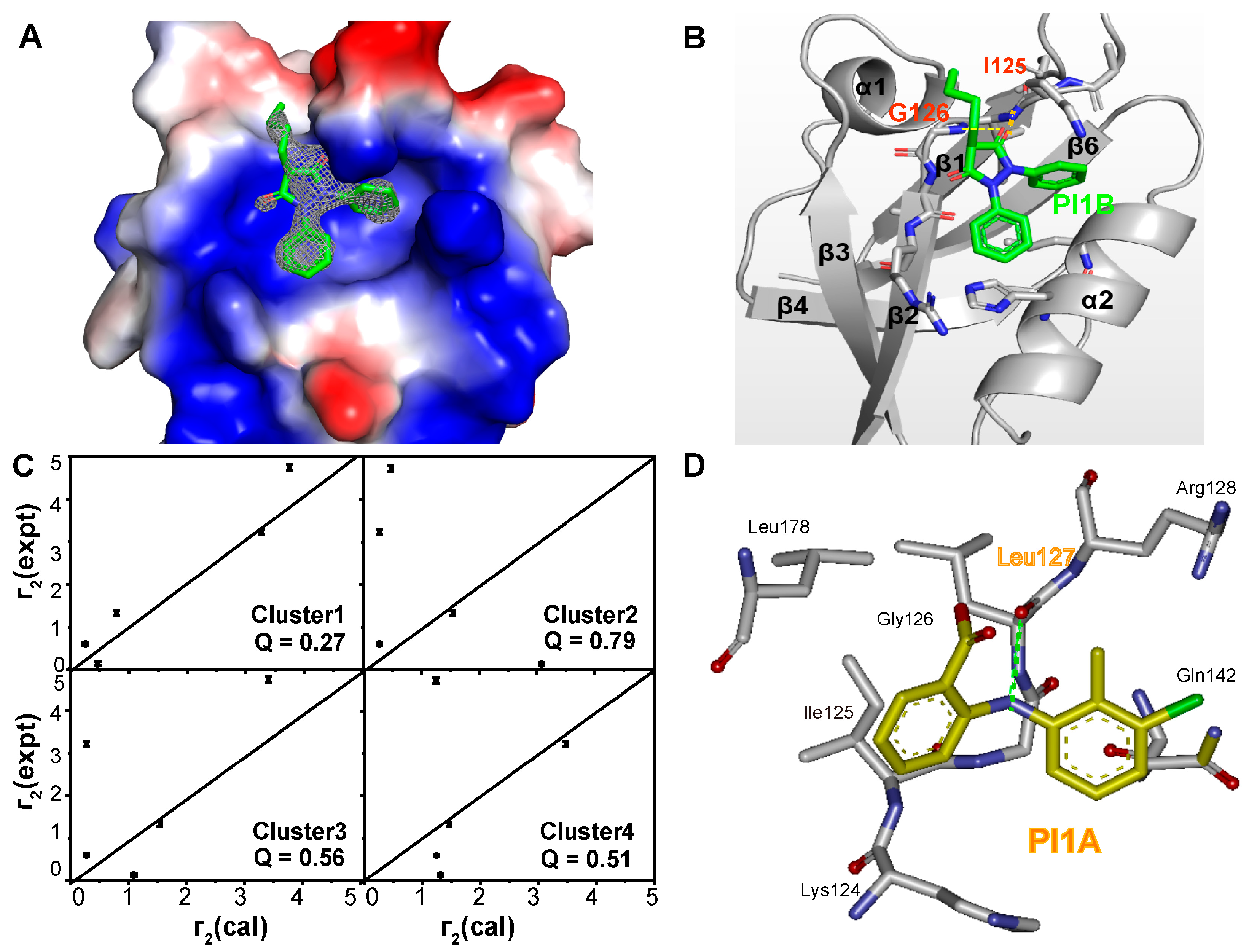
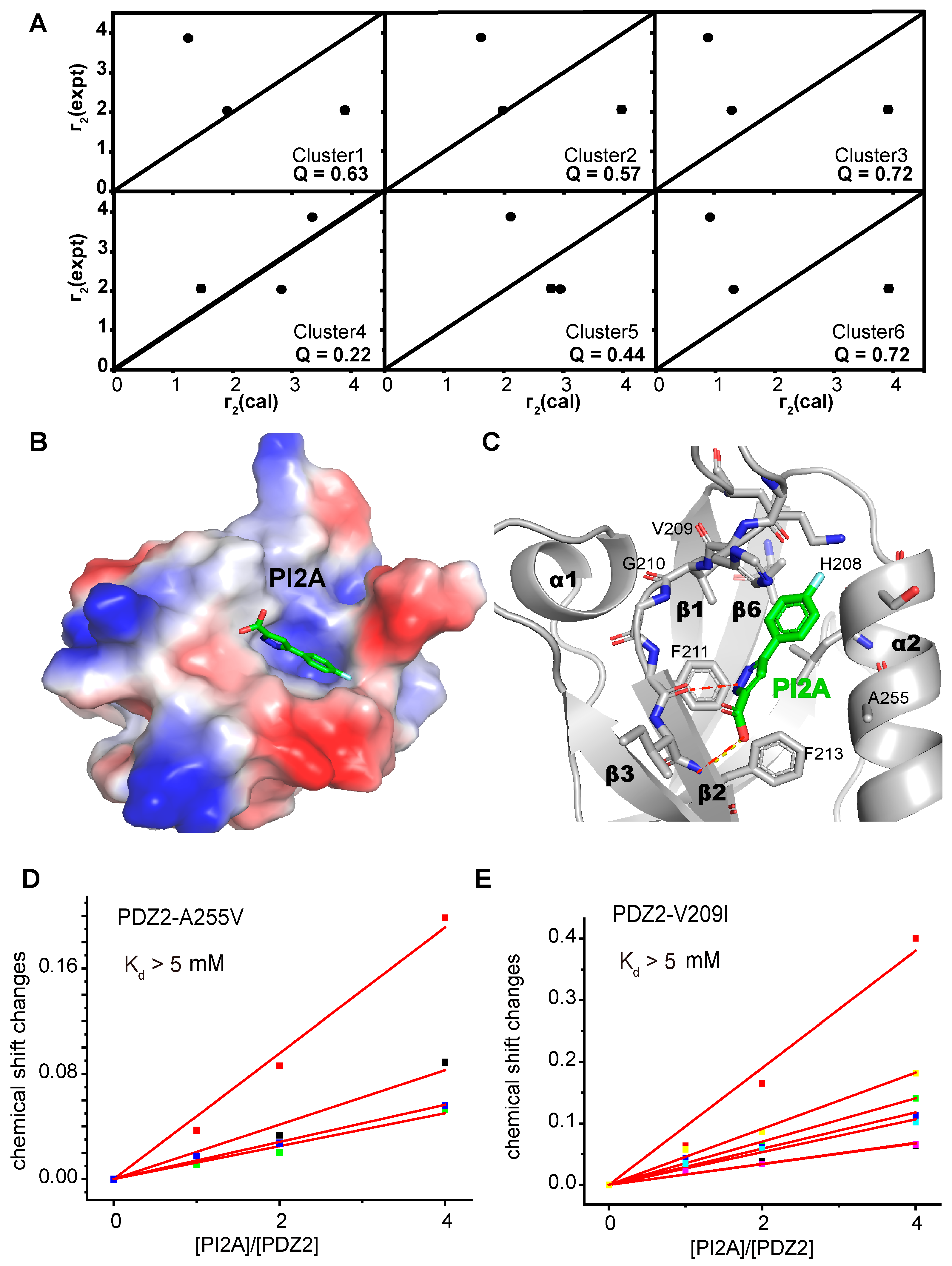
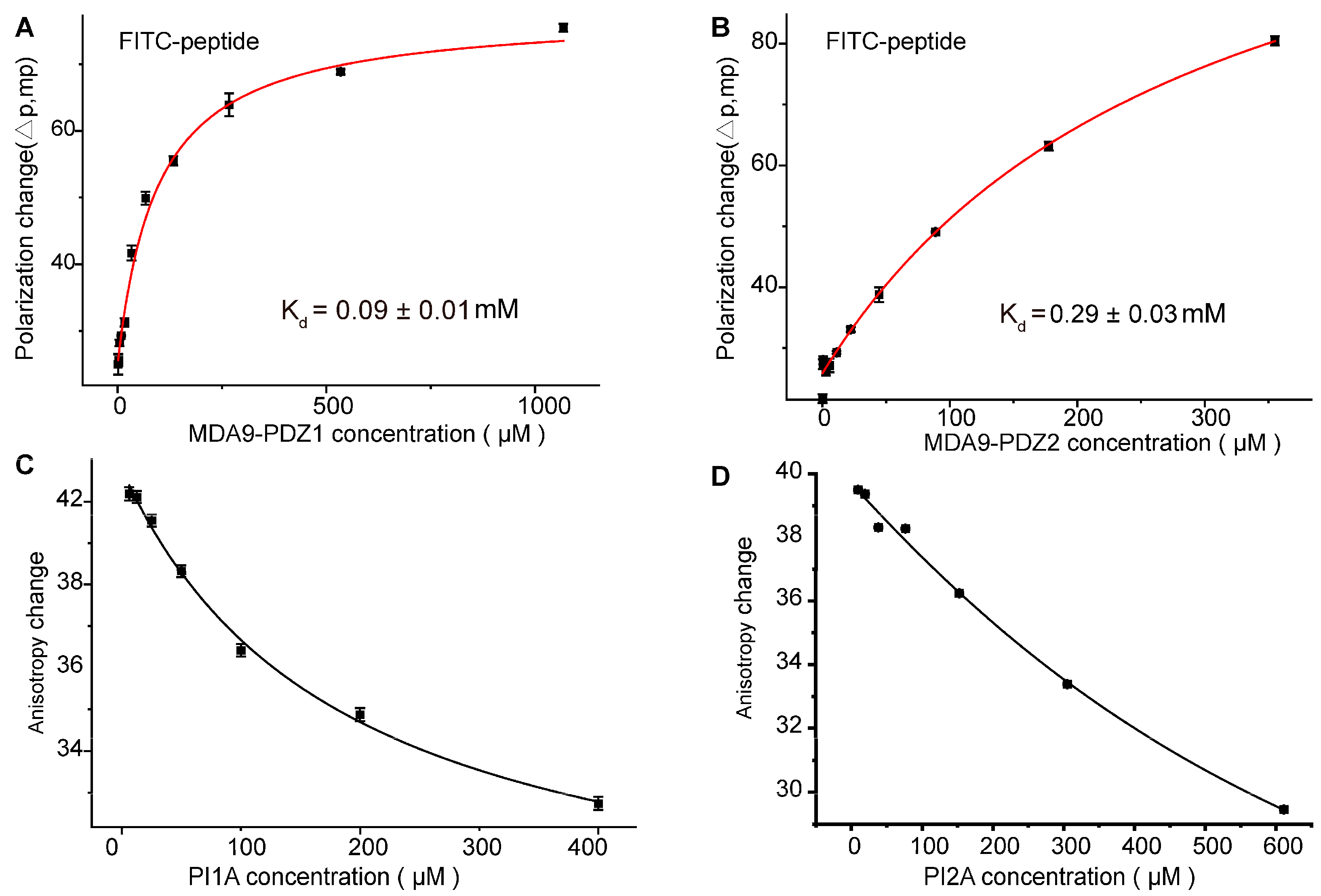
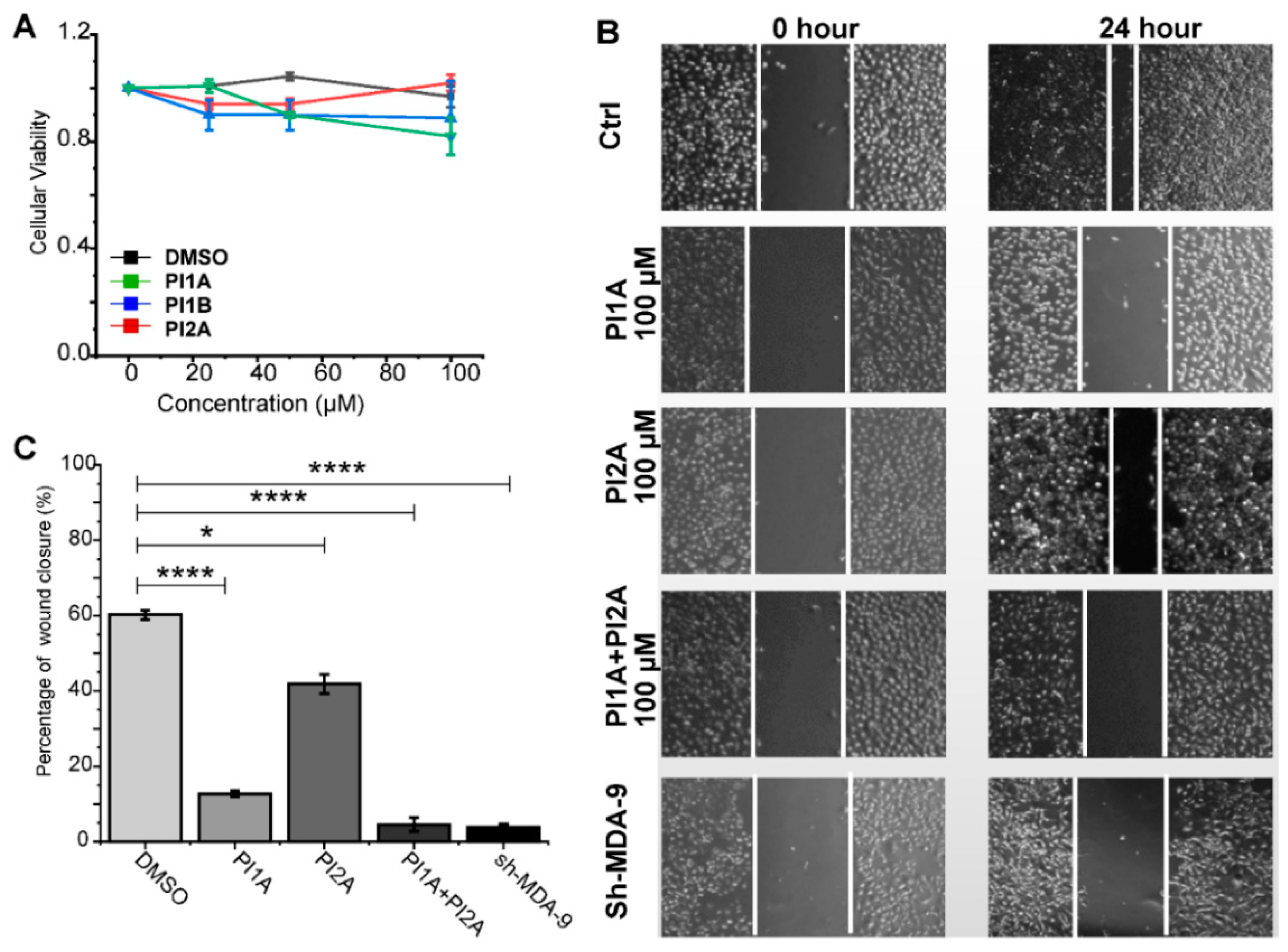
2. Results
3. Materials and Methods
3.1. Cloning, Expression, and Protein Purification
3.2. NMR Fragment-Based Screening
3.3. NMR Chemical Shift Perturbation
3.4. Crystallization, Data Collection, and Structure Determination
3.5. Molecular Docking
3.6. Transferred PRE
3.7. Fluorescence Polarization (FP)
3.8. Cell Viability Assay
3.9. Wound-Healing Assay
3.10. Quantitative Real-Time PCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDA-9 | Melanoma differentiation-associated gene 9 |
| FBLD | Fragment-based lead discovery |
| CSPs | Chemical shift perturbations |
| HSQC | Heteronuclear single-quantum coherence |
| NMR | Nuclear Magnetic Resonance |
| PRE | Paramagnetic relaxation enhancement |
| FP | Fluorescence depolarization |
| FITC | Fluorescein isothiocyanate |
| CCK8 | Cell counting kit-8 |
| sh-MDA-9 | MDA-9 knockdown |
References
- Li, T.; Wernersson, R.; Hansen, R.B.; Horn, H.; Mercer, J.; Slodkowicz, G.; Workman, C.T.; Rigina, O.; Rapacki, K.; Staerfeldt, H.H.; et al. A scored human protein-protein interaction network to catalyze genomic interpretation. Nat. Methods 2017, 14, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Nardella, C.; Visconti, L.; Malagrino, F.; Pagano, L.; Bufano, M.; Nalli, M.; Coluccia, A.; La Regina, G.; Silvestri, R.; Gianni, S.; et al. Targeting PDZ domains as potential treatment for viral infections, neurodegeneration and cancer. Biol. Direct 2021, 16, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, S.; Wang, K.; Wan, X. A PDZ Protein MDA-9/Syntenin: As a Target for Cancer Therapy. Comput. Struct. Biotechnol. J. 2019, 17, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Pradhan, A.K.; Bhoopathi, P.; Talukdar, S.; Shen, X.-N.; Sarkar, D.; Emdad, L.; Fisher, P.B. The MDA-9/Syntenin/IGF1R/STAT3 Axis Directs Prostate Cancer Invasion. Cancer Res. 2018, 78, 2852–2863. [Google Scholar] [CrossRef]
- Das, S.K.; Sarkar, D.; Cavenee, W.K.; Emdad, L.; Fisher, P.B. Rethinking Glioblastoma Therapy: MDA-9/Syntenin Targeted Small Molecule. ACS Chem. Neurosci. 2019, 10, 1121–1123. [Google Scholar] [CrossRef]
- Kegelman, T.P.; Wu, B.; Das, S.K.; Talukdar, S.; Beckta, J.M.; Hu, B.; Emdad, L.; Valerie, K.; Sarkar, D.; Furnari, F.B.; et al. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc. Natl. Acad. Sci. USA 2017, 114, 370–375. [Google Scholar] [CrossRef]
- Subbaiah, V.K.; Kranjec, C.; Thomas, M.; Banks, L. PDZ domains: The building blocks regulating tumorigenesis. Biochem. J. 2011, 439, 195–205. [Google Scholar] [CrossRef]
- Halaoui, R.; McCaffrey, L. Rewiring cell polarity signaling in cancer. Oncogene 2015, 34, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Boukerche, H.; Aissaoui, H.; Prevost, C.; Hirbec, H.; Das, S.K.; Su, Z.Z.; Sarkar, D.; Fisher, P.B. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene 2010, 29, 3054–3066. [Google Scholar] [CrossRef]
- Philley, J.V.; Kannan, A.; Dasgupta, S. MDA-9/Syntenin Control. J. Cell. Physiol. 2016, 231, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Devedjiev, Y.; Derewenda, U.; Derewenda, Z.S. The PDZ2 Domain of Syntenin at Ultra-high Resolution: Bridging the Gap Between Macromolecular and Small Molecule Crystallography. J. Mol. Biol. 2004, 338, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kegelman, T.P.; Das, S.K.; Emdad, L.; Hu, B.; Menezes, M.E.; Bhoopathi, P.; Wang, X.-Y.; Pellecchia, M.; Sarkar, D.; Fisher, P.B. Targeting tumor invasion: The roles of MDA-9/Syntenin. Expert Opin. Ther. Targets 2014, 19, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 2016, 7, 80175–80189. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Stoorvogel, W. Resolving sorting mechanisms into exosomes. Cell Res. 2015, 25, 531–542. [Google Scholar] [CrossRef]
- Friand, V.; David, G.; Zimmermann, P. Syntenin and syndecan in the biogenesis of exosomes. Biol. Cell 2015, 107, 331–341. [Google Scholar] [CrossRef]
- Bhoopathi, P.; Pradhan, A.K.; Bacolod, M.D.; Emdad, L.; Sarkar, D.; Das, S.K.; Fisher, P.B. Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene 2019, 38, 6781–6793. [Google Scholar] [CrossRef]
- Koo, T.H.; Lee, J.J.; Kim, E.M.; Kim, K.W.; Kim, H.D.; Lee, J.H. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene 2002, 21, 4080–4088. [Google Scholar] [CrossRef]
- Ohno, K.; Koroll, M.; El Far, O.; Scholze, P.; Gomeza, J.; Betz, H. The neuronal glycine transporter 2 interacts with the PDZ domain protein syntenin-1. Mol. Cell. Neurosci. 2004, 26, 518–529. [Google Scholar] [CrossRef]
- Ro, Y.T.; Jang, B.K.; Shin, C.Y.; Park, E.U.; Kim, C.G.; Yang, S.I. Akt regulates the expression of MafK, synaptotagmin I, and syntenin-1, which play roles in neuronal function. J. Biomed. Sci. 2010, 17, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Kashyap, R.; Barral, K.; Egea-Jimenez, A.L.; Kovalskyy, D.; Feracci, M.; Garcia, M.; Derviaux, C.; Betzi, S.; Ghossoub, R.; et al. Pharmacological inhibition of syntenin PDZ2 domain impairs breast cancer cell activities and exosome loading with syndecan and EpCAM cargo. J. Extracell. Vesicles 2020, 10, 12039–12055. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Pradhan, A.K.; Bhoopathi, P.; Shen, X.N.; August, L.A.; Windle, J.J.; Sarkar, D.; Furnari, F.B.; Cavenee, W.K.; Das, S.K.; et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc. Natl. Acad. Sci. USA 2018, 115, 5768–5773. [Google Scholar] [CrossRef]
- Das, S.K.; Bhutia, S.K.; Kegelman, T.P.; Peachy, L.; Oyesanya, R.A.; Dasgupta, S.; Sokhi, U.K.; Azab, B.; Dash, R.; Quinn, B.A.; et al. MDA-9/syntenin: A positive gatekeeper of melanoma metastasis. Front. Biosci. 2012, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Menezes, M.E.; Das, S.K.; Emdad, L.; Janjic, A.; Bhatia, S.; Mukhopadhyay, N.D.; Shao, C.; Sarkar, D.; Fisher, P.B. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin. Cancer Res. 2013, 19, 4621–4633. [Google Scholar] [CrossRef]
- Boukerche, H.; Su, Z.Z.; Prévot, C.; Sarkar, D.; Fisher, P.B. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc. Natl. Acad. Sci. USA 2008, 105, 15914–15919. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171–4188. [Google Scholar] [CrossRef]
- Das, S.K.; Bhutia, S.K.; Azab, B.; Kegelman, T.P.; Peachy, L.; Santhekadur, P.K.; Dasgupta, S.; Dash, R.; Dent, P.; Grant, S.; et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013, 73, 844–854. [Google Scholar] [CrossRef]
- Zhong, D.; Ran, J.H.; Tang, W.Y.; Zhang, X.D.; Tan, Y.; Chen, G.J.; Li, X.S.; Yan, Y. Mda-9/syntenin promotes human brain glioma migration through focal adhesion kinase (FAK)-JNK and FAK-AKT signaling. Asian Pac. J. Cancer Prev. 2012, 13, 2897–2901. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.Y.; Jeon, Y.K.; Chung, D.H.; Kim, Y.G.; Kim, C.W. Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1. Exp. Mol. Med. 2014, 46, e90. [Google Scholar] [CrossRef]
- Hirbec, H.; Martin, S.; Henley, J.M. Syntenin is involved in the developmental regulation of neuronal membrane architecture. Mol. Cell. Neurosci. 2005, 28, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.J.; Zimmermann, P.; Reekmans, G.; Smets, A.; Degeest, G.; Dürr, J.; David, G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA 1997, 94, 13683–13688. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Maji, S.; Wechman, S.L.; Bhoopathi, P.; Pradhan, A.K.; Talukdar, S.; Sarkar, D.; Landry, J.; Guo, C.; Wang, X.Y.; et al. MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol. Res. 2020, 155, 104695–104732. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Kegelman, T.P.; Pradhan, A.K.; Shen, X.N.; Bhoopathi, P.; Talukdar, S.; Maji, S.; Sarkar, D.; Emdad, L.; Fisher, P.B. Suppression of Prostate Cancer Pathogenesis Using an MDA-9/Syntenin (SDCBP) PDZ1 Small-Molecule Inhibitor. Mol. Cancer Ther. 2019, 18, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.; Drant, J.; Williamson, M.P. The measurement of binding affinities by NMR chemical shift perturbation. J. Biomol. NMR 2022, 76, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Clore, G.M. Practical Aspects of Paramagnetic Relaxation Enhancement in Biological Macromolecules. Methods Enzymol. 2015, 564, 485–497. [Google Scholar] [PubMed]
- Kang, B.S.; Cooper, D.R.; Devedjiev, Y.; Derewenda, U.; Derewenda, Z.S. Molecular Roots of Degenerate Specificity in Syntenin’s PDZ2 Domain. Structure 2003, 11, 845–853. [Google Scholar] [CrossRef]
- Roehrl, M.H.; Wang, J.Y.; Wagner, G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein-protein interactions by fluorescence polarization. Biochemistry 2004, 43, 16056–16066. [Google Scholar] [CrossRef]
- Gao, J.; Liang, E.; Ma, R.; Li, F.; Liu, Y.; Liu, J.; Jiang, L.; Li, C.; Dai, H.; Wu, J.; et al. Fluorine Pseudocontact Shifts Used for Characterizing the Protein-Ligand Interaction Mode in the Limit of NMR Intermediate Exchange. Angew. Chem. Int. Ed. 2017, 56, 12982–12986. [Google Scholar] [CrossRef]
- Lv, M.; Gao, J.; Li, M.; Ma, R.; Li, F.; Liu, Y.; Liu, M.; Zhang, J.; Yao, X.; Wu, J.; et al. Conformational Selection in Ligand Recognition by the First Tudor Domain of PHF20L1. J. Phys. Chem. Lett. 2020, 11, 7932–7938. [Google Scholar] [CrossRef]
- Helgren, T.R.; Hagen, T.J. Demonstration of AutoDock as an Educational Tool for Drug Discovery. J. Chem. Educ. 2017, 94, 345–349. [Google Scholar] [CrossRef] [PubMed]
| Amplicon | Forward Primer | Reverse Primer |
|---|---|---|
| MDA-9 | TTGAACATATTATTAAGCGGATG | TGAACAAGGTATCAGATGAAAGAG |
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Wang, L.; Li, S.; Wei, X.; Lv, M.; Zhong, F.; Liu, Y.; Liu, J.; Fu, B.; Zhu, Q.; et al. Inhibitors against Two PDZ Domains of MDA-9 Suppressed Migration of Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 3431. https://doi.org/10.3390/ijms24043431
Tang H, Wang L, Li S, Wei X, Lv M, Zhong F, Liu Y, Liu J, Fu B, Zhu Q, et al. Inhibitors against Two PDZ Domains of MDA-9 Suppressed Migration of Breast Cancer Cells. International Journal of Molecular Sciences. 2023; 24(4):3431. https://doi.org/10.3390/ijms24043431
Chicago/Turabian StyleTang, Heng, Lei Wang, Shuju Li, Xiaoli Wei, Mengqi Lv, Fumei Zhong, Yaqian Liu, Jiuyang Liu, Bangguo Fu, Qizhi Zhu, and et al. 2023. "Inhibitors against Two PDZ Domains of MDA-9 Suppressed Migration of Breast Cancer Cells" International Journal of Molecular Sciences 24, no. 4: 3431. https://doi.org/10.3390/ijms24043431
APA StyleTang, H., Wang, L., Li, S., Wei, X., Lv, M., Zhong, F., Liu, Y., Liu, J., Fu, B., Zhu, Q., Wang, D., Liu, J., Ruan, K., Gao, J., & Xu, W. (2023). Inhibitors against Two PDZ Domains of MDA-9 Suppressed Migration of Breast Cancer Cells. International Journal of Molecular Sciences, 24(4), 3431. https://doi.org/10.3390/ijms24043431






