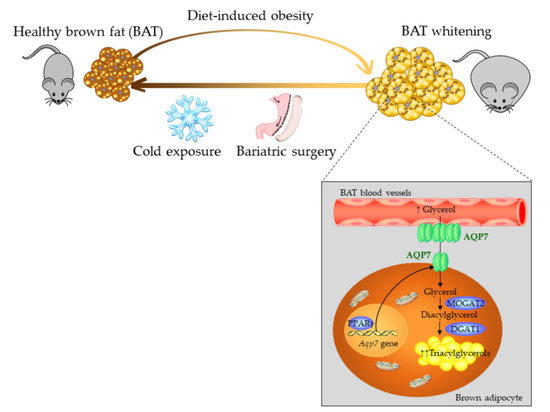
Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery
Abstract
:1. Introduction
2. Results
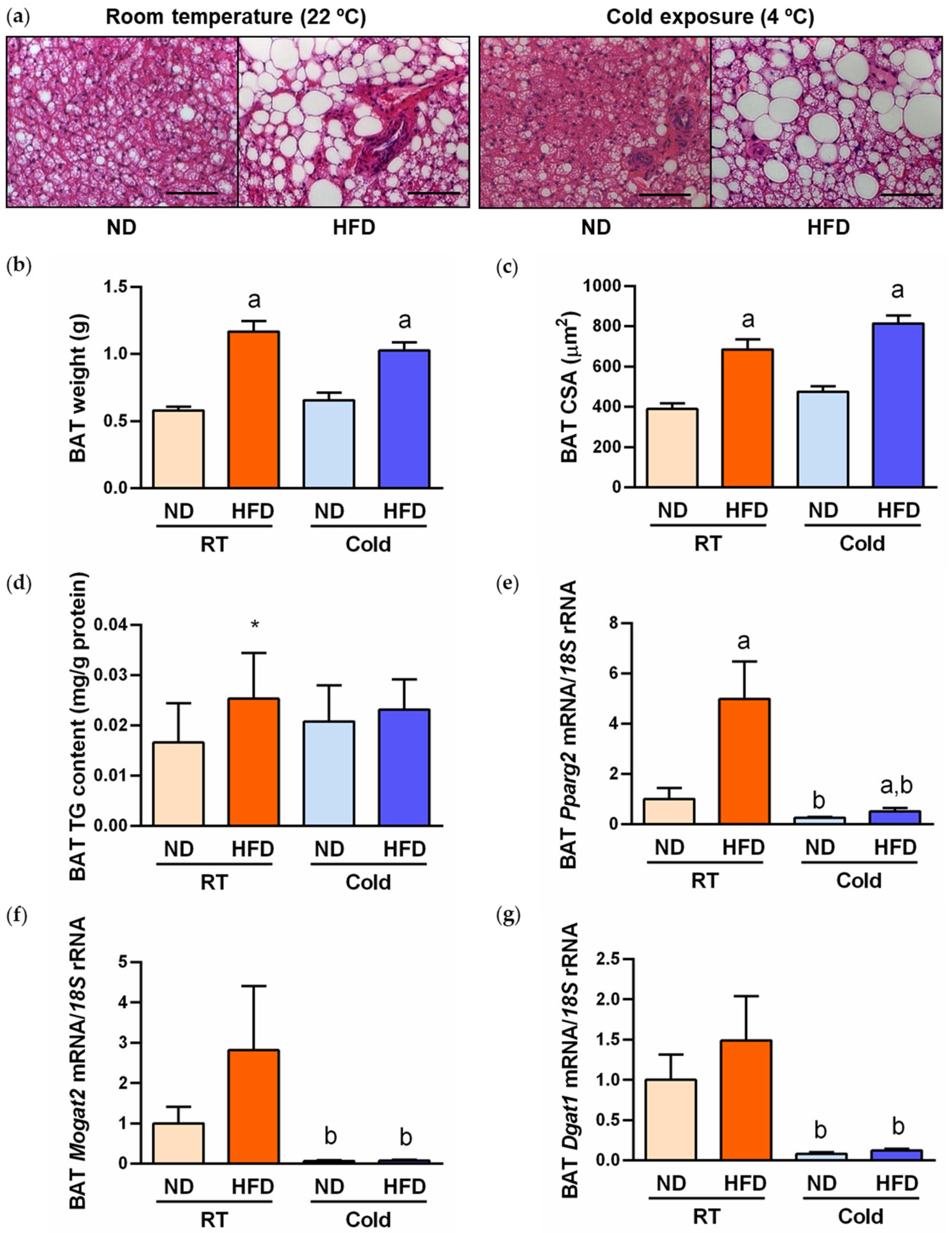
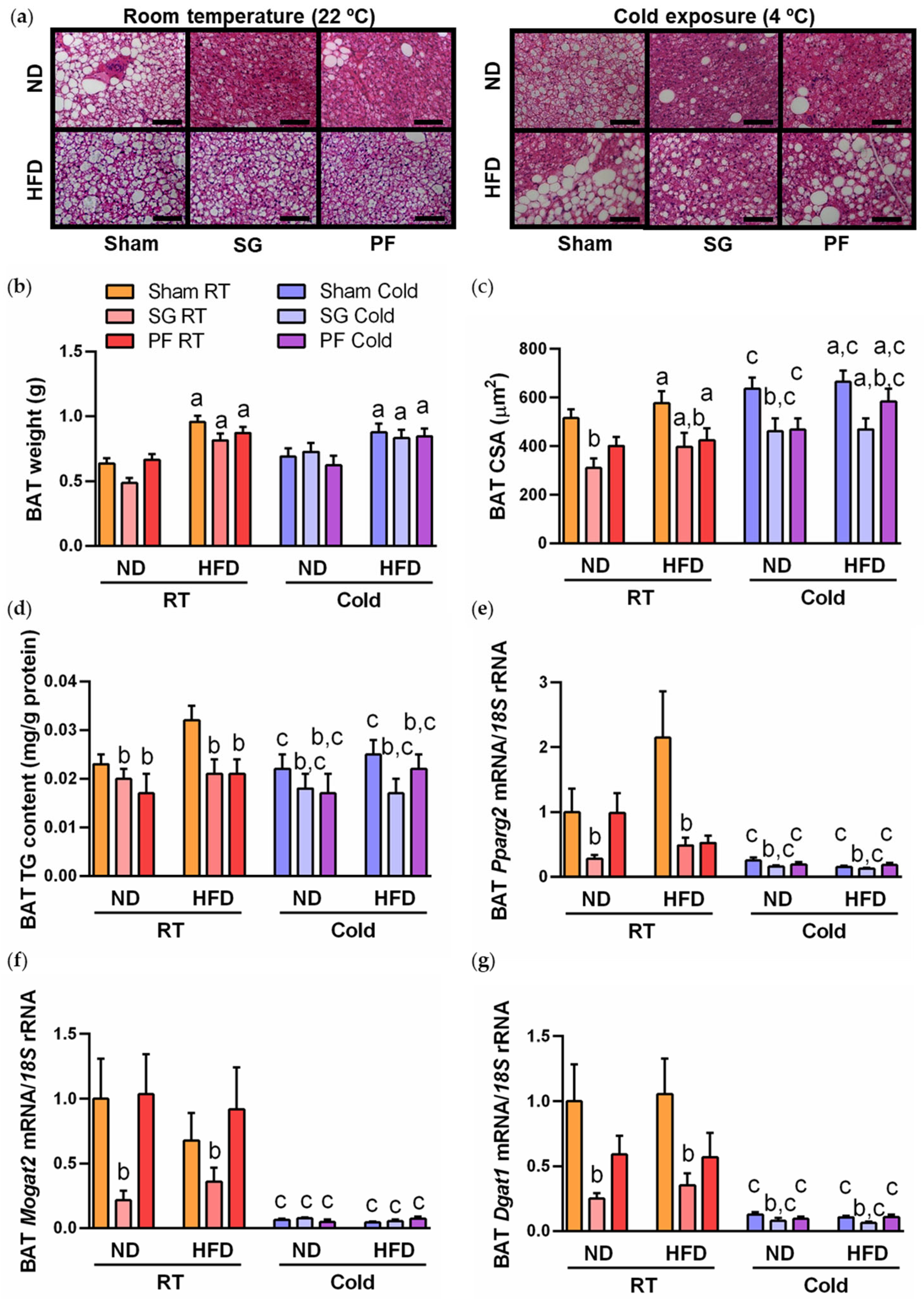
2.1. Cold Exposure Improved Metabolic Profile and BAT Whitening in DIO Rats
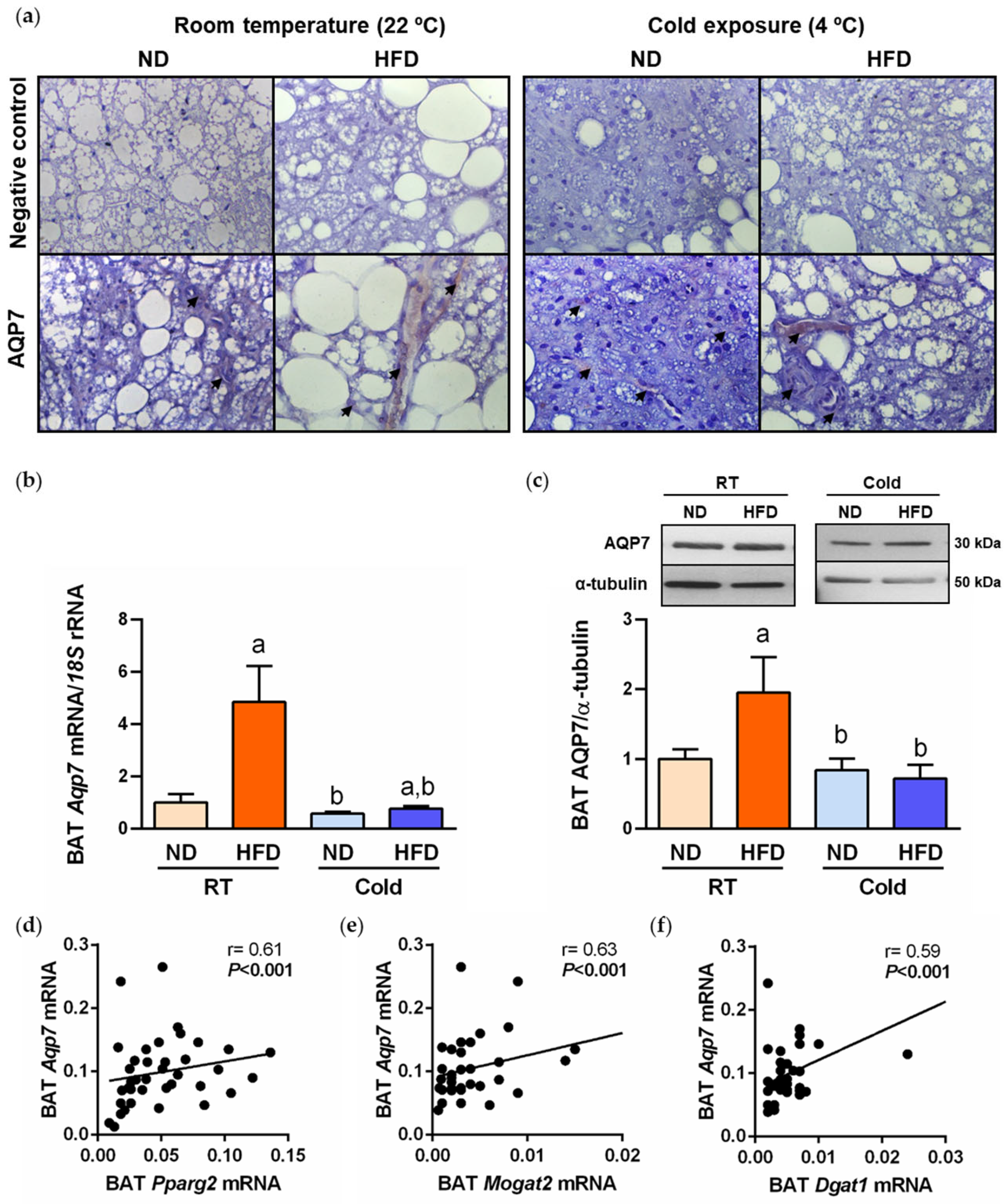
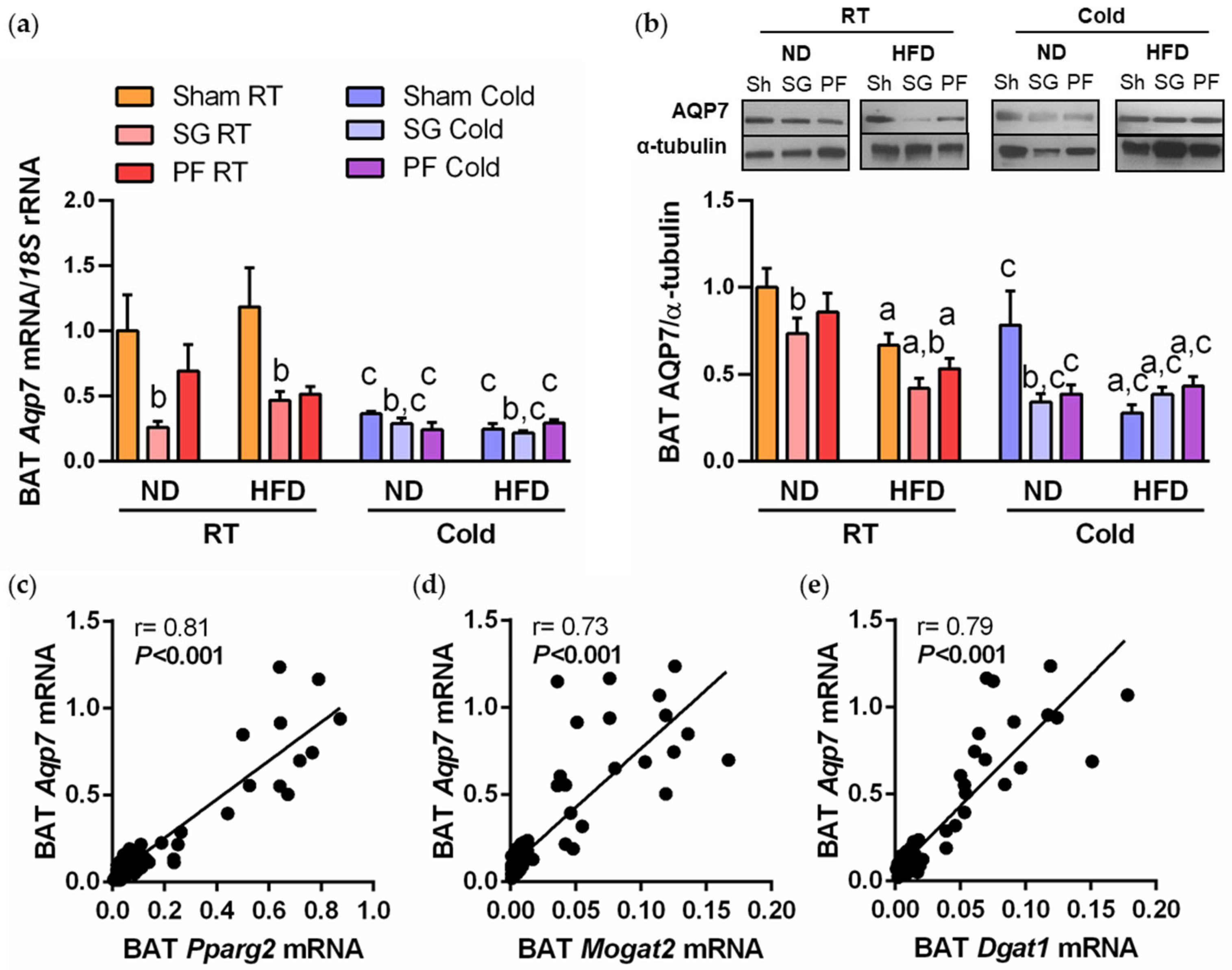
2.2. AQP7 Expression Is Increased during BAT Whitening and Downregulated by Cold Exposure
2.3. Sleeve Gastrectomy Ameliorates BAT Whitening under Both Thermic Conditions
2.4. A Reduction in AQP7 Expression Is Found after Weight Loss Achieved by Sleeve Gastrectomy
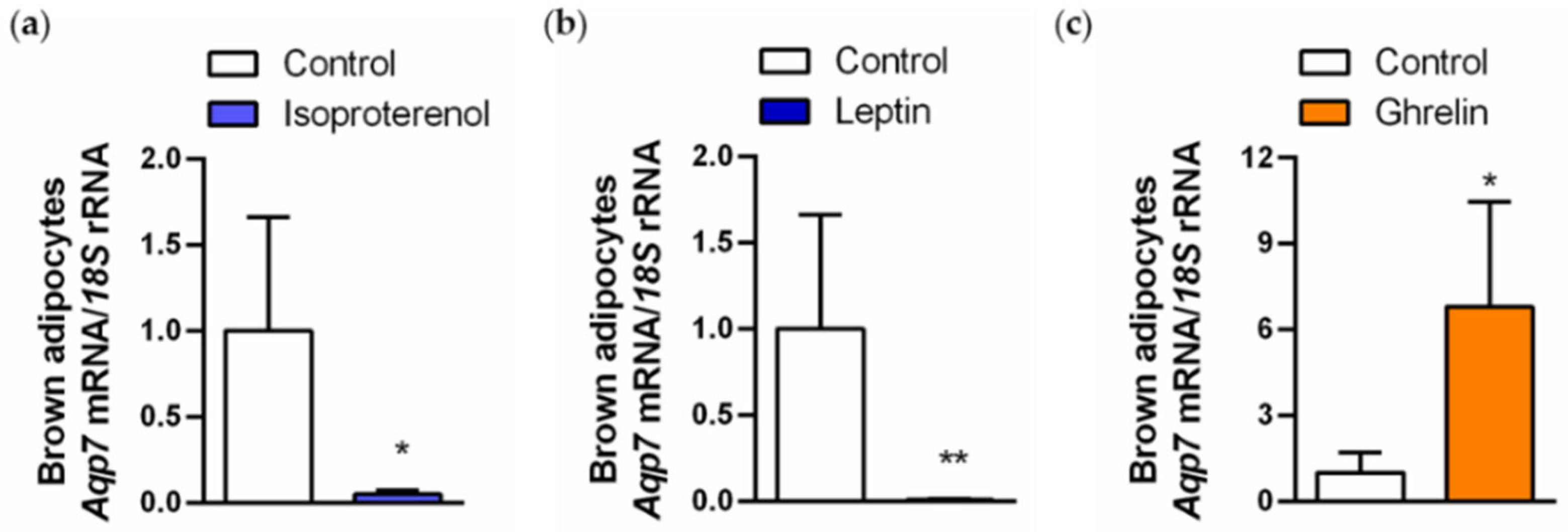
2.5. Regulation of AQP7 in Brown Adipocytes Is Regulated by Lipolytic and Lipogenic Factors
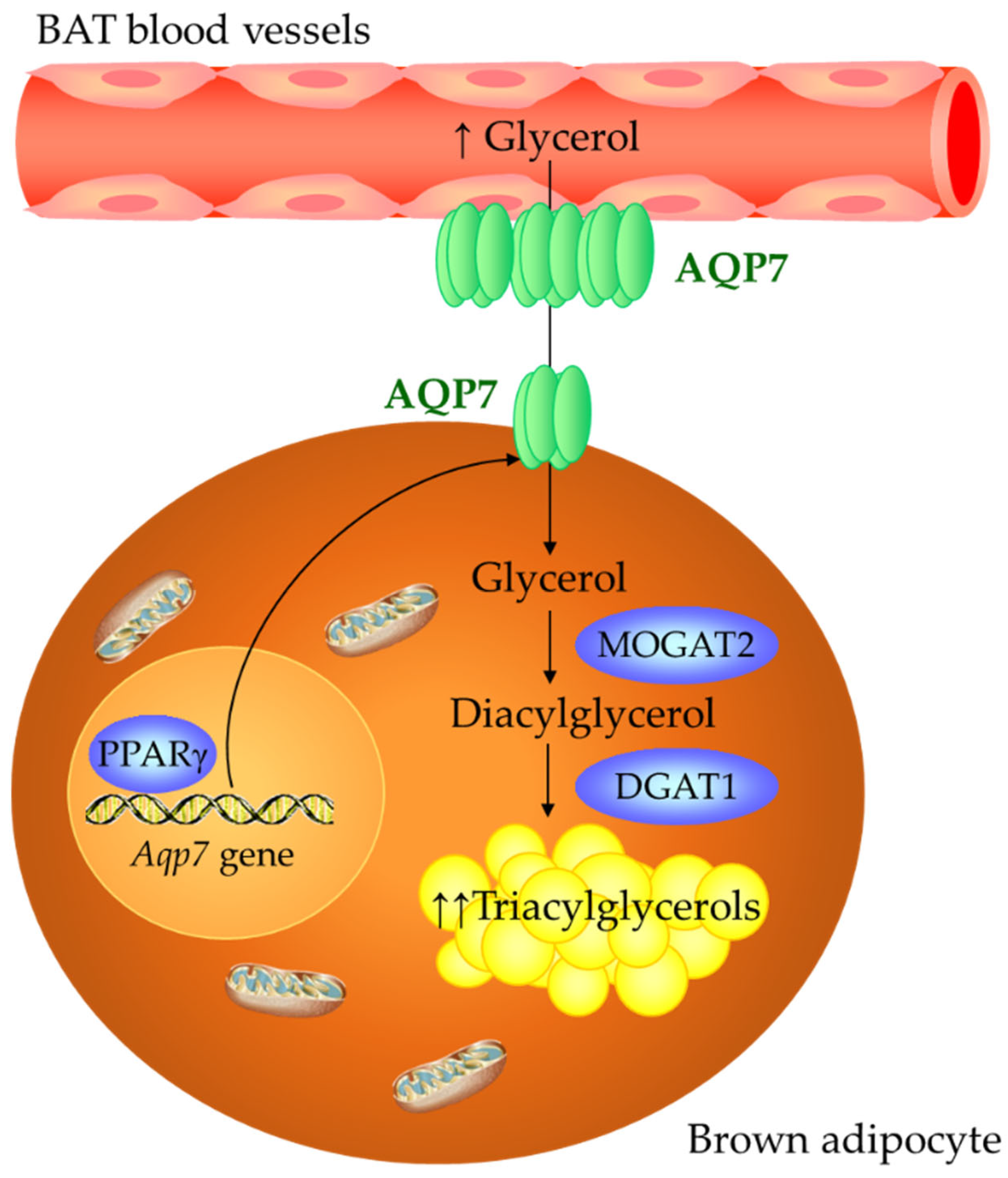
3. Discussion
4. Materials and Methods
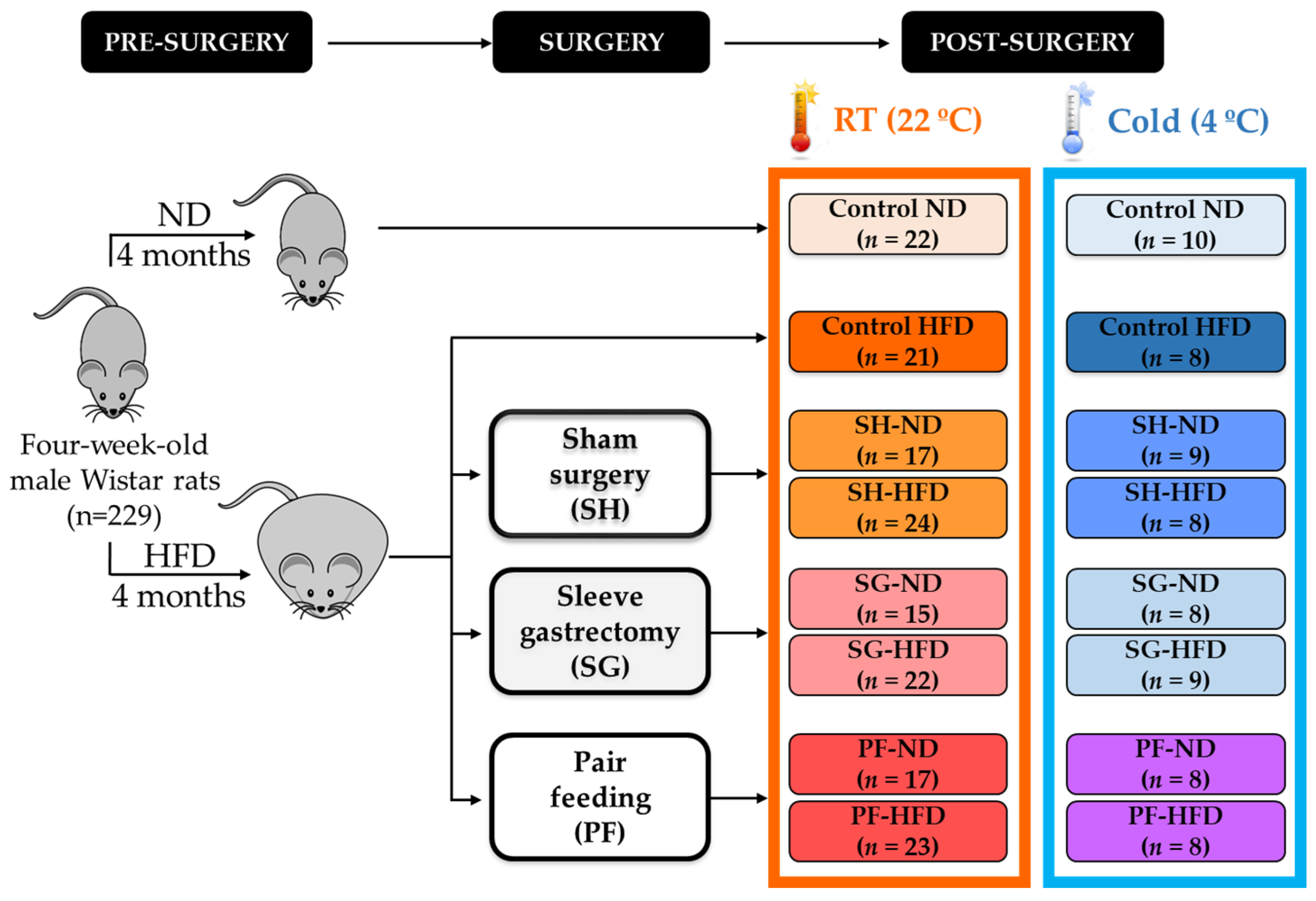
4.1. Experimental Animals and Study Design
4.2. Blood Analysis
4.3. Sample Handling
4.4. Brown Adipocyte Culture and Treatment
4.5. Real-Time PCR
4.6. Western Blot
4.7. Immunohistochemistry of AQP7
4.8. Measurement of BAT Intracellular Triacylglycerols
4.9. Determination of the Area of Brown Adipocytes
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cypess, A.M. Reassessing human adipose tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Becerril, S.; Hernández-Pardos, A.W.; Frühbeck, G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr. Opin. Pharmacol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Busetto, L.; Dicker, D.; Yumuk, V.; Goossens, G.H.; Hebebrand, J.; Halford, J.G.C.; Farpour-Lambert, N.J.; Blaak, E.E.; Woodward, E.; et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes. Facts 2019, 12, 131–136. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Arias-Loza, P.; Hayakawa, N.; Wakabayashi, H.; Werner, R.A.; Chen, X.; Shinaji, T.; Herrmann, K.; Pelzer, T.; Higuchi, T. Whitening and impaired glucose utilization of brown adipose tissue in a rat model of type 2 diabetes mellitus. Sci. Rep. 2017, 7, 16795. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Azevedo, C.; Santana-Oliveira, D.A.; Miranda, C.S.; Martins, F.F.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Progressive brown adipocyte dysfunction: Whitening and impaired nonshivering thermogenesis as long-term obesity complications. J. Nutr. Biochem. 2022, 105, 109002. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Li, Q.; Spalding, K.L. The regulation of adipocyte growth in white adipose tissue. Front. Cell Dev. Biol. 2022, 10, 1003219. [Google Scholar] [CrossRef]
- Nedergaard, J.; Petrovic, N.; Lindgren, E.M.; Jacobsson, A.; Cannon, B. PPARgamma in the control of brown adipocyte differentiation. Biochim. Biophys. Acta 2005, 1740, 293–304. [Google Scholar] [CrossRef]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef]
- Kishida, K.; Shimomura, I.; Nishizawa, H.; Maeda, N.; Kuriyama, H.; Kondo, H.; Matsuda, M.; Nagaretani, H.; Ouchi, N.; Hotta, K.; et al. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor g. J. Biol. Chem. 2001, 276, 48572–48579. [Google Scholar] [CrossRef]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Krintel, C.; Hernebring, M.; Haataja, T.J.; de Mare, S.; Wasserstrom, S.; Kosinska-Eriksson, U.; Palmgren, M.; Holm, C.; Stenkula, K.G.; et al. Perilipin 1 binds to aquaporin 7 in human adipocytes and controls its mobility via protein kinase A mediated phosphorylation. Metabolism 2016, 65, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Moreno, N.R.; Balaguer, I.; Méndez-Giménez, L.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef]
- Walker, C.G.; Holness, M.J.; Gibbons, G.F.; Sugden, M.C. Fasting-induced increases in aquaporin 7 and adipose triglyceride lipase mRNA expression in adipose tissue are attenuated by peroxisome proliferator-activated receptor alpha deficiency. Int. J. Obes. 2007, 31, 1165–1171. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Pastor, C.; Rotellar, F.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Vendrell, J.; et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008, 18, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Marrades, M.P.; Milagro, F.I.; Martínez, J.A.; Moreno-Aliaga, M.J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006, 339, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Becerril, S.; Moncada, R.; Valentí, V.; Ramirez, B.; Lancha, A.; Gurbindo, J.; Balaguer, I.; Cienfuegos, J.A.; Catalán, V.; et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes. Surg. 2015, 25, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Becerril, S.; Moncada, R.; Valentí, V.; Fernández, S.; Ramírez, B.; Catalán, V.; Gómez-Ambrosi, J.; Soveral, G.; Malagón, M.M.; et al. Gastric plication improves glycemia partly by restoring the altered expression of aquaglyceroporins in adipose tissue and the liver in obese rats. Obes. Surg. 2017, 27, 1763–1774. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Rodríguez, A.; Becerril, S.; Ramírez, B.; Gonçalves, I.O.; Beleza, J.; Frühbeck, G.; Ascensão, A.; Magalhaes, J. Physical exercise remodels visceral adipose tissue and mitochondrial lipid metabolism in rats fed a high-fat diet. Clin. Exp. Pharmacol. Physiol. 2017, 44, 386–394. [Google Scholar] [CrossRef]
- González-Davalos, L.; Álvarez-Pérez, M.; Quesada-López, T.; Cereijo, R.; Campderros, L.; Pina, E.; Shimada, A.; Villarroya, F.; Varela-Echavarria, A.; Mora, O. Glucocorticoid gene regulation of aquaporin-7. Vitam. Horm. 2020, 112, 179–207. [Google Scholar] [CrossRef]
- Lopes, P.A.; Martins, R.; da Silva, I.V.; Madeira, M.S.; Prates, J.A.M.; Soveral, G. Modulation of aquaporin gene expression by n-3 long-chain PUFA lipid structures in white and brown adipose tissue from hamsters. Br. J. Nutr. 2018, 120, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Yang, M.; Nickels, J.T. MOGAT2: A new therapeutic target for metabolic syndrome. Diseases 2015, 3, 176–192. [Google Scholar] [CrossRef]
- Yen, C.L.; Cheong, M.L.; Grueter, C.; Zhou, P.; Moriwaki, J.; Wong, J.S.; Hubbard, B.; Marmor, S.; Farese, R.V., Jr. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 2009, 15, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Díez Caballero, A.; Gil, M.J. Fundus functionality and ghrelin concentrations after bariatric surgery. N. Engl. J. Med. 2004, 350, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.A.; Fernández, S.; Rodríguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Avilés-Olmos, I.; Rodríguez, A.; Becerril, S.; Fernández-Formoso, J.A.; Kiortsis, D.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Time to consider the “exposome hypothesis” in the development of the obesity pandemic. Nutrients 2022, 14, 1597. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Ji, M.; Zhang, S. Global warming and obesity: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Farhat, G.; Aldiss, P.; Pope, M.; Budge, H. Brown adipose tissue and glucose homeostasis-the link between climate change and the global rise in obesity and diabetes. Adipocyte 2019, 8, 46–50. [Google Scholar] [CrossRef]
- Du, K.; Chen, G.H.; Bai, X.; Chen, L.; Hu, S.Q.; Li, Y.H.; Wang, G.Z.; He, J.W.; Lai, S.J. Dynamics of transcriptome and chromatin accessibility revealed sequential regulation of potential transcription factors during the brown adipose tissue whitening in rabbits. Front. Cell Dev. Biol. 2022, 10, 981661. [Google Scholar] [CrossRef]
- Jensen, M.O.; Tajkhorshid, E.; Schulten, K. The mechanism of glycerol conduction in aquaglyceroporins. Structure 2001, 9, 1083–1093. [Google Scholar] [CrossRef]
- Mosca, A.F.; de Almeida, A.; Wragg, D.; Martins, A.P.; Sabir, F.; Leoni, S.; Moura, T.F.; Prista, C.; Casini, A.; Soveral, G. Molecular basis of aquaporin-7 permeability regulation by pH. Cells 2018, 7, 207. [Google Scholar] [CrossRef]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef]
- Gotfryd, K.; Mosca, A.F.; Missel, J.W.; Truelsen, S.F.; Wang, K.; Spulber, M.; Krabbe, S.; Helix-Nielsen, C.; Laforenza, U.; Soveral, G.; et al. Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat. Commun. 2018, 9, 4749. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Fuchtbauer, E.M.; Frokiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: Implications in glycerol metabolism. Am. J. Physiol. Ren. Physiol. 2007, 292, F956–F965. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Nagelhus, E.A.; Amiry-Moghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J.; Ostergard, T.; Rojek, A.; Fuchtbauer, E.M.; Lund, S.; Nielsen, S.; Praetorius, J. Gender-specific effect of physical training on AQP7 protein expression in human adipose tissue. Acta Diabetol. 2012, 49 (Suppl. S1), S215–S226. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Yamamoto, H.; Abe, Y.; Yoshida, G.J.; Rojek, A.; Sohara, E.; Uchida, S.; Nielsen, S.; Yasui, M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett. 2015, 589, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.V.; Diaz-Saez, F.; Zorzano, A.; Guma, A.; Camps, M.; Soveral, G. Aquaglyceroporins are differentially expressed in beige and white adipocytes. Int. J. Mol. Sci. 2020, 21, 610. [Google Scholar] [CrossRef]
- Iena, F.M.; Kalucka, J.; Nielsen, L.; Sondergaard, E.; Nielsen, S.; Lebeck, J. Localization of aquaglyceroporins in human and murine white adipose tissue. Histochem. Cell Biol. 2022, 157, 623–639. [Google Scholar] [CrossRef]
- Miranda, M.; Escote, X.; Ceperuelo-Mallafre, V.; Alcaide, M.J.; Simon, I.; Vilarrasa, N.; Wabitsch, M.; Vendrell, J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: Differences and similarities between depots. J. Clin. Endocrinol. Metab. 2010, 95, 3470–3479. [Google Scholar] [CrossRef]
- Madeira, A.; Mosca, A.F.; Moura, T.F.; Soveral, G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 2015, 67, 54–60. [Google Scholar] [CrossRef]
- Frühbeck, G.; Balaguer, I.; Méndez-Giménez, L.; Valentí, V.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; Salvador, J.; Calamita, G.; et al. Aquaporin-11 contributes to TGF-beta1-induced endoplasmic reticulum stress in human visceral adipocytes: Role in obesity-associated inflammation. Cells 2020, 9, 1403. [Google Scholar] [CrossRef]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef]
- Frühbeck, G.; Becerril, S.; Sáinz, N.; Garrastachu, P.; García-Velloso, M.J. BAT: A new target for human obesity? Trends Pharm. Sci. 2009, 30, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Song, Z.; Yin, J.; Zhu, Y.; Miao, X.; Qian, H.; Wang, J.; Ye, L.; Zhou, L. Effect of acute cold exposure on energy metabolism and activity of brown adipose tissue in humans: A systematic review and meta-analysis. Front. Physiol. 2022, 13, 917084. [Google Scholar] [CrossRef] [PubMed]
- Marcher, A.B.; Loft, A.; Nielsen, R.; Vihervaara, T.; Madsen, J.G.; Sysi-Aho, M.; Ekroos, K.; Mandrup, S. RNA-seq and mass-spectrometry-based lipidomics reveal extensive changes of glycerolipid pathways in brown adipose tissue in response to cold. Cell Rep. 2015, 13, 2000–2013. [Google Scholar] [CrossRef] [PubMed]
- Becerril, S.; Tuero, C.; Cienfuegos, J.A.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Valenti, V.; Moncada, R.; Unamuno, X.; Gómez-Ambrosi, J.; et al. Improved adipose tissue function after single anastomosis duodeno-ileal bypass with sleeve-gastrectomy (SADI-S) in diet-induced obesity. Int. J. Mol. Sci. 2022, 23, 11641. [Google Scholar] [CrossRef]
- Moncada, R.; Becerril, S.; Rodríguez, A.; Méndez-Giménez, L.; Ramírez, B.; Catalán, V.; Gómez-Ambrosi, J.; Gil, M.J.; Fernández, S.; Cienfuegos, J.A.; et al. Sleeve gastrectomy reduces body weight and improves metabolic profile also in obesity-prone rats. Obes. Surg. 2016, 26, 1537–1548. [Google Scholar] [CrossRef]
- Piquer-García, I.; Cereijo, R.; Corral-Pérez, J.; Pellitero, S.; Martínez, E.; Taxerås, S.D.; Tarasco, J.; Moreno, P.; Balibrea, J.; Puig-Domingo, M.; et al. Use of Infrared Thermography to Estimate Brown Fat Activation After a Cooling Protocol in Patients with Severe Obesity That Underwent Bariatric Surgery. Obes. Surg. 2020, 30, 2375–2381. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Teule, G.J.; Brans, B.; Hoeks, J.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2012, 97, E1229–E1233. [Google Scholar] [CrossRef]
- Virtanen, K.A.; van Marken Lichtenbelt, W.D.; Nuutila, P. Brown adipose tissue functions in humans. Biochim. Biophys. Acta 2013, 1831, 1004–1008. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Nie, X.; Song, Z.; Gu, Y. Effects of bariatric surgery on change of brown adipocyte tissue and energy metabolism in obese mice. Obes. Surg. 2018, 28, 820–830. [Google Scholar] [CrossRef]
- Pimpão, C.; Wragg, D.; da Silva, I.V.; Casini, A.; Soveral, G. Aquaglyceroporin modulators as emergent pharmacological molecules for human diseases. Front. Mol. Biosci. 2022, 9, 845237. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Ibañez, P.; Vila, N.; Margall, M.A.; Moncada, R.; et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int. J. Obes. 2016, 40, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Klein, J.; Lossner, U.; Klier, M.; Kralisch, S.; Paschke, R. Suppression of aquaporin adipose gene expression by isoproterenol, TNFa, and dexamethasone. Horm. Metab. Res. 2003, 35, 222–227. [Google Scholar] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Gil, M.J.; Becerril, S.; Sáinz, N.; Silva, C.; Salvador, J.; Colina, I.; Frühbeck, G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int. J. Obes. 2009, 33, 541–552. [Google Scholar] [CrossRef]
- Shen, F.X.; Gu, X.; Pan, W.; Li, W.P.; Li, W.; Ye, J.; Yang, L.J.; Gu, X.J.; Ni, L.S. Over-expression of AQP7 contributes to improve insulin resistance in adipocytes. Exp. Cell Res. 2012, 318, 2377–2384. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Liu, X.; Li, J.; Gao, Q.; Shi, S.; Wang, T.; Ye, X.; Lu, Y.; Zhang, D.; et al. AQP7 mediates post-menopausal lipogenesis in adipocytes through FSH-induced transcriptional crosstalk with AP-1 sites. Reprod. Biomed. Online 2020, 41, 1122–1132. [Google Scholar] [CrossRef]
- Muruzabal, F.J.; Frühbeck, G.; Gómez-Ambrosi, J.; Archanco, M.; Burrell, M.A. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Rotellar, F.; Valentí, V.; Silva, C.; Mugueta, C.; Pulido, M.R.; Vázquez, R.; Salvador, J.; et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 2012, 55, 3038–3050. [Google Scholar] [CrossRef]
- Viengchareun, S.; Servel, N.; Feve, B.; Freemark, M.; Lombes, M.; Binart, N. Prolactin receptor signaling is essential for perinatal brown adipocyte function: A role for insulin-like growth factor-2. PLoS ONE 2008, 3, e1535. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gena, P.; Méndez-Giménez, L.; Rosito, A.; Valentí, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M.; et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014, 38, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
| RT (22 °C) | Cold (4 °C) | |||
|---|---|---|---|---|
| ND | HFD | ND | HFD | |
| Body weight (g) | 481 ± 13 | 619 ± 14 a | 474± 20 b | 551± 22 a,b |
| Total white adiposity (g) | 16.5 ± 2.1 | 38.7 ± 2.2 *** | 17.8 ± 2.3 | 28.9 ± 2.7 *,† |
| Glucose (mg/dL) | 80 ± 2 | 90 ± 2 a | 81 ± 3 | 87 ± 4 a |
| Insulin (µU/mL) | 1.8 ± 0.4 | 3.7 ± 0.4 a | 1.6 ± 0.6 b | 1.9 ± 0.7 a,b, |
| HOMA | 0.37 ± 0.10 | 0.84 ± 0.10 a | 0.32 ± 0.15 b | 0.42 ± 0.17 a,b |
| FFA (mmol/L) | 25.4 ± 1.7 | 21.0 ± 1.8 | 17.9 ± 2.4 b | 17.5 ± 2.5 b |
| Glycerol (mg/dL) | 28.8 ± 2.4 | 33.3 ± 2.5 a | 22.5 ± 3.4 | 34.4 ± 3.8 a |
| Triacylglycerol (mg/dL) | 119 ± 12 | 100 ± 12 | 40 ± 17 b | 44 ± 19 b |
| Total cholesterol (mg/dL) | 105 ± 7 | 115 ± 7 | 66 ± 10 b | 95 ± 11 b |
| Total ghrelin (ng/mL) | 0.9 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.1 b | 1.3 ± 0.2 b |
| Leptin (ng/mL) | 7.7 ± 1.1 | 17.1 ± 1.2 a | 6.4 ± 1.6 b | 10.1 ± 1.8 a,b |
| RT (22 °C) | ||||||
|---|---|---|---|---|---|---|
| ND | HFD | |||||
| Sham | SG | PF | Sham | SG | PF | |
| Body weight (g) | 503 ± 10 | 478 ± 11 b | 495± 11 | 549± 12 a | 514 ± 13 a,b | 554± 12 a |
| Total white adiposity (g) | 21.8 ± 1.5 | 17.5 ± 1.6 b | 24.2 ± 1.4 | 28.8 ± 2.0 a | 27.2 ± 2.3 a,b | 30.0 ± 2.3 a |
| Glucose (mg/dL) | 79 ± 3 | 78 ± 3 | 90 ± 3 | 101 ± 3 * | 103 ± 4 * | 111 ± 3 * |
| Insulin (µU/mL) | 2.2 ± 0.2 | 1.8 ± 0.2 b | 1.4 ± 0.3 | 2.2 ± 0.3 | 1.8 ± 0.3 b | 2.3 ± 0.3 |
| HOMA | 0.43 ± 0.05 | 0.21 ± 0.06 b | 0.43 ± 0.06 | 0.55 ± 0.6 a | 0.46 ± 0.7 a,b | 0.73 ± 0.06 a |
| FFA (mmol/L) | 19.6 ± 1.2 | 18.8 ± 1.3 b | 19.8 ± 1.2 | 22.3 ± 1.5 a | 19.7 ± 1.5 a,b | 21.3 ± 1.5 a |
| Glycerol (mg/dL) | 23.9 ± 1.6 | 21.4 ± 1.7 b | 24.2 ± 1.7 | 28.9 ± 2.0 a | 25.1 ± 2.0 a,b | 29.9 ± 2.0 |
| Triacylglycerol (mg/dL) | 101 ± 5 | 61 ± 5 b | 76 ± 5 | 69 ± 6 a | 53 ± 6 a,b | 68 ± 6 a |
| Total cholesterol (mg/dL) | 110 ± 3 | 101 ± 8 | 109 ± 3 | 93 ± 4 a | 82 ± 4 a | 88 ± 4 a |
| Total ghrelin (ng/mL) | 1.0 ± 0.7 | 0.5 ± 0.1 b | 1.1 ± 0.1 | 0.9 ± 0.1 a | 0.4 ± 0.1 a,b | 0.9 ± 0.1 a |
| Leptin (ng/mL) | 7.4 ± 0.7 | 5.0 ± 0.8 b | 9.0 ± 0.8 | 12.4 ± 0.9 a | 9.9 ± 1.0 a,b | 16.0 ± 0.9 a |
| Cold (4 °C) | ||||||
| ND | HFD | |||||
| Sham | SG | PF | Sham | SG | PF | |
| Body weight (g) | 503 ± 10 | 477 ± 19 b | 514± 13 a,b | 558± 18 a | 527± 17 a,b | 557± 21 a |
| Total white adiposity (g) | 20.5 ± 1.7 | 17.0 ± 2.3 b | 18.3 ± 1.8 | 29.2 ± 1.8 a | 25.1 ± 1.7 a,b | 27.8 ± 2.3 a |
| Glucose (mg/dL) | 88 ± 5 | 76 ± 5 | 77 ± 5 | 87 ± 5 | 75 ± 5 # | 81 ± 5 |
| Insulin (µU/mL) | 1.5 ± 0.3 c | 1.3 ± 0.4 c | 0.9 ± 0.4 c | 1.4 ± 0.4 c | 1.4 ± 0.4 c | 1.7 ± 0.4 c |
| HOMA | 0.33 ± 0.08 c | 0.23 ± 0.10 b | 0.16 ± 0.09 b | 0.31 ± 0.09 c | 0.25 ± 0.09 a,b,c | 0.34 ± 0.09 a,c |
| FFA (mmol/L) | 16.9 ± 2.0 c | 18.3 ± 2.1 c | 17.9 ± 2.1 c | 15.5 ± 2.1 c | 16.5 ± 2.0 c | 19.4 ± 2.1 c |
| Glycerol (mg/dL) | 18.2 ± 2.7 | 22.1 ± 2.8 | 22.4 ± 2.8 | 27.1 ± 2.8 a | 25.9 ± 2.7 a | 31.2 ± 2.8 a |
| Triacylglycerol (mg/dL) | 44 ± 8 c | 53 ± 8 c | 37 ± 8 c | 53 ± 6 c | 41 ± 7 c | 44 ± 8 c |
| Total cholesterol (mg/dL) | 68 ± 5 c | 81 ± 5 c | 68 ± 5 c | 88 ± 5 c | 74 ± 5 c | 72 ± 5 c |
| Total ghrelin (ng/mL) | 1.3 ± 1.1 c | 1.0 ± 0.1 b,c | 1.5 ± 0.1 c | 1.2 ± 0.1 a,c | 0.9 ± 0.1 a,b,c | 1.3 ± 0.1 c |
| Leptin (ng/mL) | 5.6 ± 1.3 c | 6.1± 1.3 c | 4.0 ± 1.4 c | 8.8 ± 1.3 a,c | 8.8 ± 1.3 a,c | 9.0 ± 1.3 a,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frühbeck, G.; Méndez-Giménez, L.; Becerril, S.; Ramírez, B.; Hernández-Pardos, A.W.; Cienfuegos, J.A.; Valentí, V.; Moncada, R.; Catalán, V.; Gómez-Ambrosi, J.; et al. Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery. Int. J. Mol. Sci. 2023, 24, 3412. https://doi.org/10.3390/ijms24043412
Frühbeck G, Méndez-Giménez L, Becerril S, Ramírez B, Hernández-Pardos AW, Cienfuegos JA, Valentí V, Moncada R, Catalán V, Gómez-Ambrosi J, et al. Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery. International Journal of Molecular Sciences. 2023; 24(4):3412. https://doi.org/10.3390/ijms24043412
Chicago/Turabian StyleFrühbeck, Gema, Leire Méndez-Giménez, Sara Becerril, Beatriz Ramírez, Ana Wenting Hernández-Pardos, Javier A. Cienfuegos, Víctor Valentí, Rafael Moncada, Victoria Catalán, Javier Gómez-Ambrosi, and et al. 2023. "Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery" International Journal of Molecular Sciences 24, no. 4: 3412. https://doi.org/10.3390/ijms24043412
APA StyleFrühbeck, G., Méndez-Giménez, L., Becerril, S., Ramírez, B., Hernández-Pardos, A. W., Cienfuegos, J. A., Valentí, V., Moncada, R., Catalán, V., Gómez-Ambrosi, J., da Silva, I. V., Soveral, G., & Rodríguez, A. (2023). Increased Aquaporin-7 Expression Is Associated with Changes in Rat Brown Adipose Tissue Whitening in Obesity: Impact of Cold Exposure and Bariatric Surgery. International Journal of Molecular Sciences, 24(4), 3412. https://doi.org/10.3390/ijms24043412