Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Search
2.2. Eligibility Criteria
2.3. Data Extraction and Statistical Analysis
3. Results
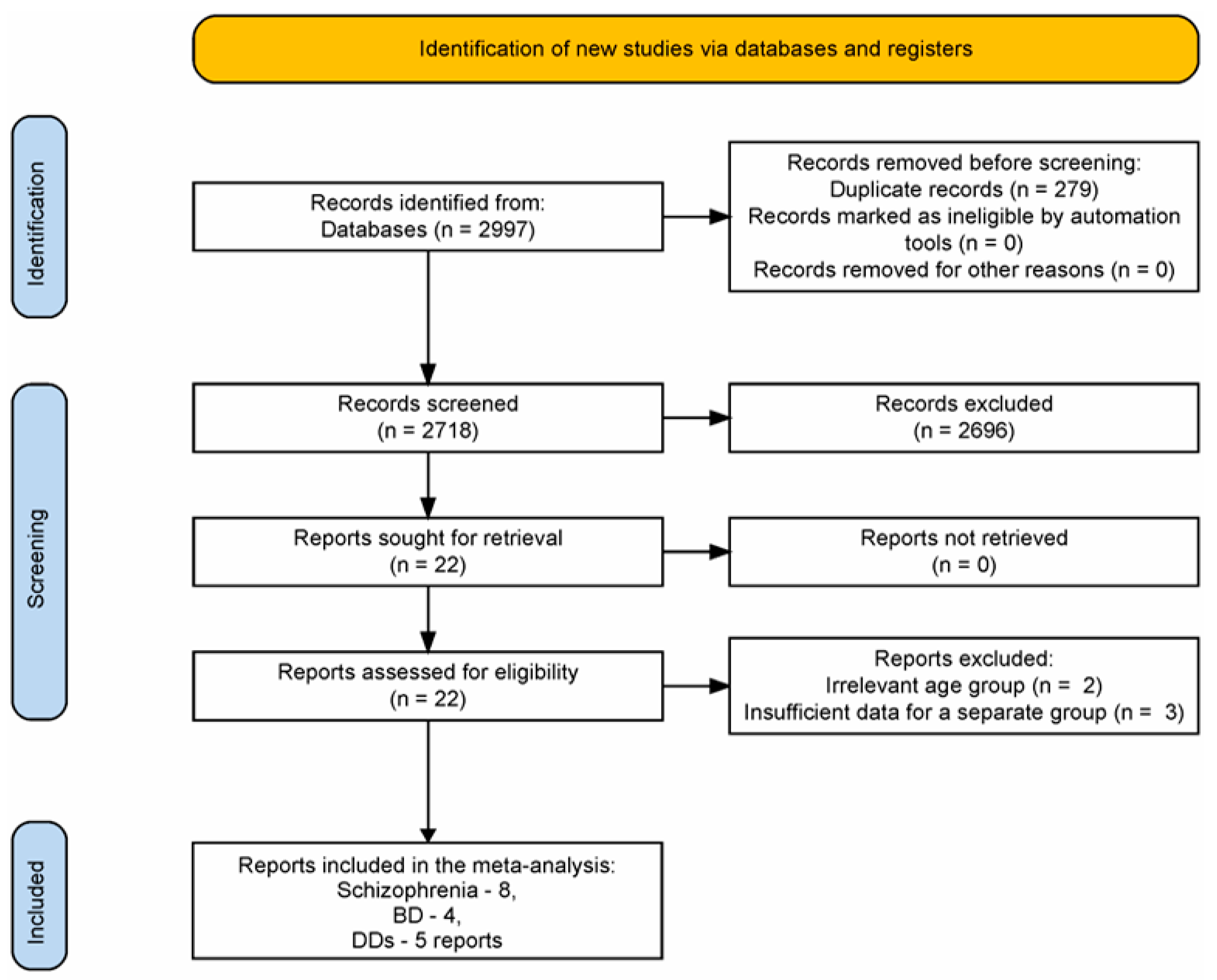
3.1. Reports Selection
3.2. Reports Characteristics
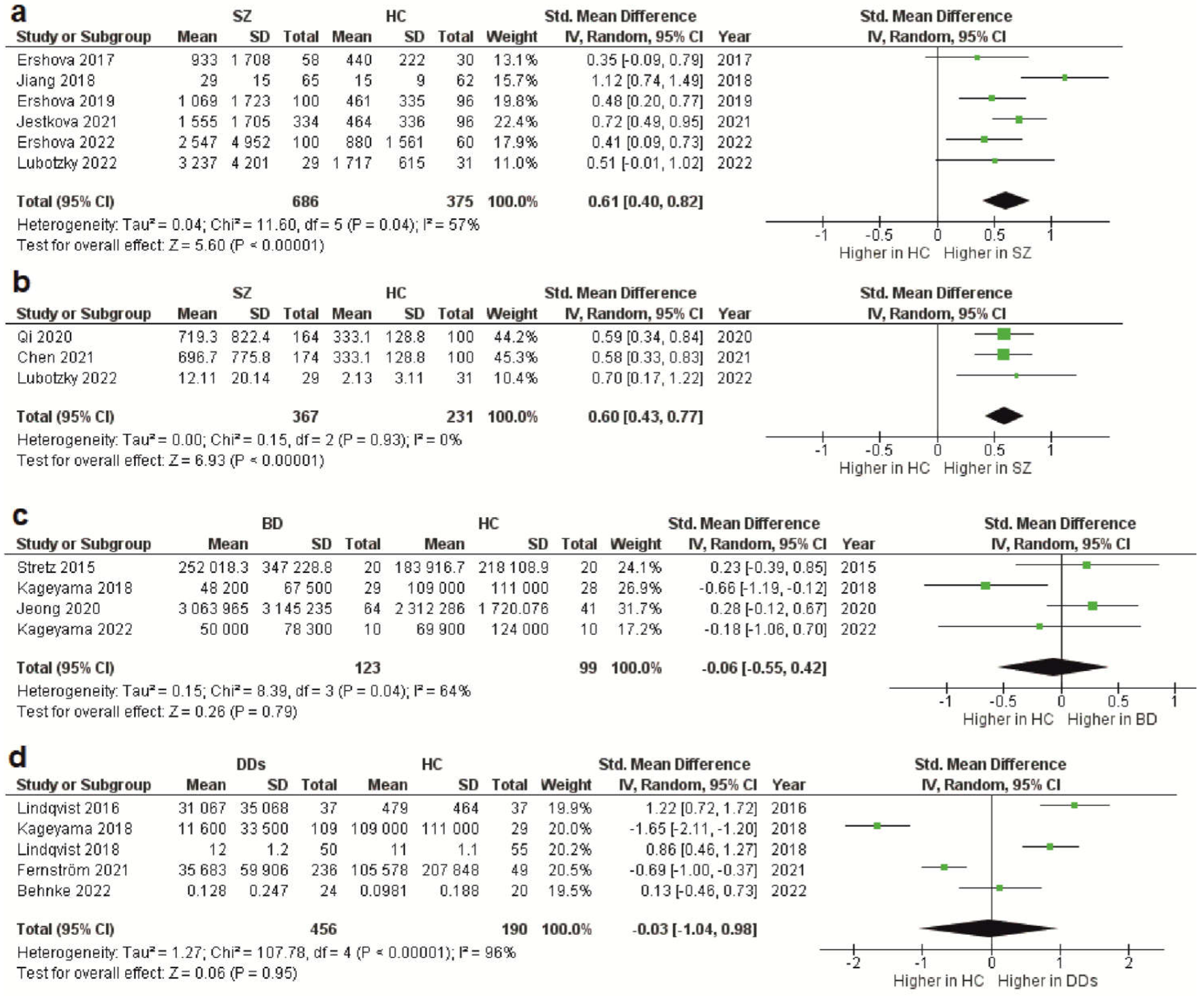
3.3. CfDNA Level in Schizophrenia
3.4. CfDNA Level in Bipolar Disorder
3.5. CfDNA Level in Depressive Disorders
4. Discussion
4.1. Results Overview
4.2. Association with Chronic Low-Grade Inflammation
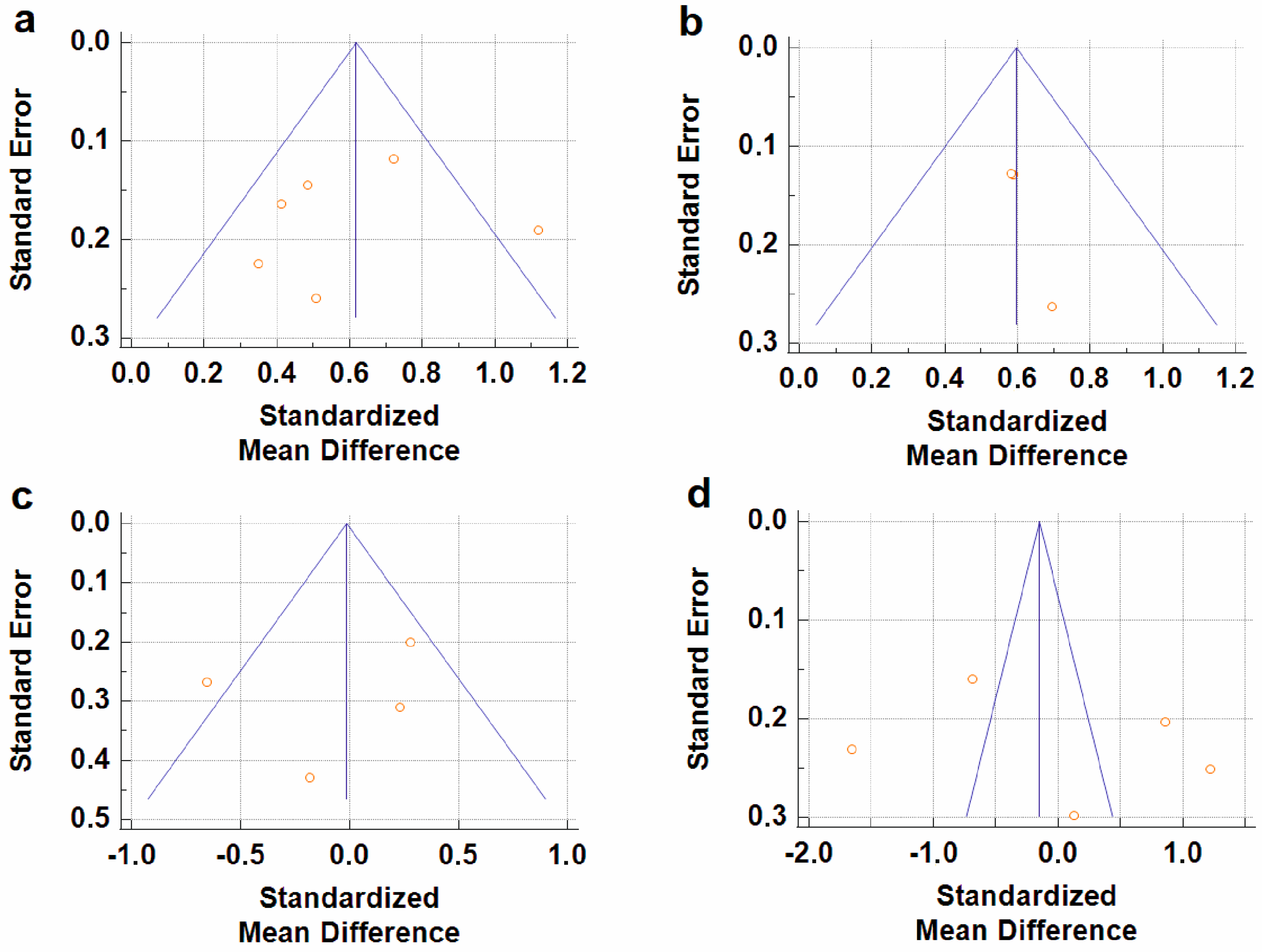
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aucamp, J.; Abel, J.B.; Christoffel, P.S.B. The diverse origins of circulating cell-free DNA in the human body: A critical re-evaluation of the literature. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1649–1683. [Google Scholar] [CrossRef] [PubMed]
- Labbe, M.; Petresco, M.; Fabrykant, M.L. Taux du phosphore sanguin et de ses differentes formes dans les leucemies et les anemies. J. Soc. Biol. 1931, 107, T2. [Google Scholar]
- Mandel, P.; Metais, P. Nuclear acids in human blood plasma. Comptes Rendus Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Bronkhorst, A.J.; Ungerer, V.; Diehl, F.; Anker, P.; Dor, Y.; Fleischhacker, M.; Gahan, P.B.; Hui, L.; Holdenrieder, S.; Thierry, A.R. Towards systematic nomenclature for cell-free DNA. Hum. Genet. 2021, 140, 565–578. [Google Scholar] [CrossRef]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating cell-free nucleic acids: Characteristics and applications. Eur. J. Hum. Genet. 2018, 26, 937–945. [Google Scholar] [CrossRef]
- Grabuschnig, S.; Bronkhorst, A.J.; Holdenrieder, S.; Rosales, R.I.; Schliep, K.P.; Schwendenwein, D.; Ungerer, V.; Sensen, C.W. Putative origins of cell-free DNA in humans: A review of active and passive nucleic acid release mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef]
- Heitzer, E.; Auinger, L.; Speicher, M.R. Cell-free DNA and apoptosis: How dead cells inform about the living. Trends Mol. Med. 2020, 26, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef]
- Van der Meij, K.R.M.; Sistermans, E.A.; Macville, M.V.E.; Stevens, S.J.C.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, E.M.J.; Boter, M.; Diderich, K.E.M.; et al. TRIDENT-2: National implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Azad, A.A. Cell-free DNA in cancer: Current insights. Cell. Oncol. (Dordr.) 2019, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Valantine, H.A.; Snyder, T.M.; Strehl, C.; Cohen, G.; Luikart, H.; Neff, N.F.; Okamoto, J.; Bernstein, D.; Weisshaar, D.; et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci. Transl. Med. 2014, 6, 241ra77. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2019, 15, 493–518. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 880568. [Google Scholar] [CrossRef]
- Pereira, A.C.; Oliveira, J.; Silva, S.; Madeira, N.; Pereira, C.M.F.; Cruz, M.T. Inflammation in Bipolar Disorder (BD): Identification of new therapeutic targets. Pharmacol. Res. 2021, 163, 105325. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Park, S.S.; Jeong, H.; Andreazza, A.C. Circulating cell-free mitochondrial DNA in brain health and disease: A systematic review and meta-analysis. World J. Biol. Psychiatry 2022, 23, 87–102. [Google Scholar] [CrossRef]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qi, J.; Xu, H.L.; Lin, X.Y.; Sun, Y.J.; Ju, S.Q. The Value of Serum Cell-Free DNA Levels in Patients with Schizophrenia. Front. Psychiatry 2021, 12, 637789. [Google Scholar] [CrossRef]
- Stertz, L.; Fries, G.R.; Rosa, A.R.; Kauer-Sant’anna, M.; Ferrari, P.; Paz, A.V.; Green, C.; Cunha, Â.B.; Dal-Pizzol, F.; Gottfried, C.; et al. Damage-associated molecular patterns and immune activation in bipolar disorder. Acta Psychiatr. Scand. 2015, 132, 211–217. [Google Scholar] [CrossRef]
- Weir, C.J.; Butcher, I.; Assi, V.; Lewis, S.C.; Murray, G.D.; Langhorne, P.; Brady, M.C. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: A systematic review. BMC Med. Res. Methodol. 2018, 18, 25. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Nikitina, S.G.; Ershova, E.S.; Chudakova, J.M.; Shmarina, G.V.; Veiko, N.N.; Martynov, A.V.; Kostuk, S.E.; Modestov, A.A.; Rozhnova, T.M.; Izhevskaya, V.L.; et al. Oxidative DNA Damage of Peripheral Blood Cells and Blood Plasma Сell-Free DNA as an Indicator of the Oxidative Stress Level in Children with Autism Spectrum Disorders and Schizophrenia. Psikhiatriya 2021, 19, 15–25. [Google Scholar] [CrossRef]
- Gonçalves, V.F.; Mendes-Silva, A.P.; Koyama, E.; Vieira, E.; Kennedy, J.L.; Diniz, B. Increased levels of circulating cell-free mtDNA in plasma of late life depression subjects. J. Psychiatr. Res. 2021, 139, 25–29. [Google Scholar] [CrossRef]
- Jylhävä, J.; Kotipelto, T.; Raitala, A.; Jylhä, M.; Hervonen, A.; Hurme, M. Aging is associated with quantitative and qualitative changes in circulating cell-free DNA: The Vitality 90+ study. Mech. Ageing. Dev. 2011, 132, 20–26. [Google Scholar] [CrossRef]
- Ouyang, H.; Huang, M.; Xu, Y.; Yao, Q.; Wu, X.; Zhou, D. Reduced Cell-Free Mitochondrial DNA Levels Were Induced by Antipsychotics Treatment in First-Episode Patients with Schizophrenia. Front. Psychiatry 2021, 12, 652314. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2018, 233, 15–20. [Google Scholar] [CrossRef]
- Qi, J.; Chen, L.Y.; Shen, X.J.; Ju, S.Q. Analytical Value of Cell-Free DNA Based on Alu in Psychiatric Disorders. Front. Psychiatry 2020, 10, 992. [Google Scholar] [CrossRef]
- Ershova, E.S.; Jestkova, E.M.; Martynov, A.V.; Shmarina, G.V.; Umriukhin, P.E.; Bravve, L.V.; Zakharova, N.V.; Kostyuk, G.P.; Saveliev, D.V.; Orlova, M.D.; et al. Accumulation of circulating cell-free CpG-enriched ribosomal DNA fragments on the background of high endonuclease activity of blood plasma in schizophrenic patients. Int. J. Genom. 2019, 2019, 8390585. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Sun, L.; Qing, Y.; Yang, X.; Hu, X.; Yang, C.; Xu, T.; Wang, J.; Wang, P.; et al. Analysis of the concentrations and size distributions of cell-free DNA in schizophrenia using fluorescence correlation spectroscopy. Transl. Psychiatry 2018, 8, 104. [Google Scholar] [CrossRef]
- Ershova, E.S.; Jestkova, E.M.; Chestkov, I.V.; Porokhovnik, L.N.; Izevskaya, V.L.; Kutsev, S.I.; Veiko, N.N.; Shmarina, G.; Dolgikh, O.; Kostyuk, S.V. Quantification of cell-free DNA in blood plasma and DNA damage degree in lymphocytes to evaluate dysregulation of apoptosis in schizophrenia patients. J. Psychiatr. Res. 2017, 87, 15–22. [Google Scholar] [CrossRef]
- Jestkova, E.M.; Ershova, E.S.; Martynov, A.V.; Zakharova, N.V.; Kostyuk, G.P.; Veiko, N.N.; Kostyuk, S.V. Concentration of Circulating Cell-Free DNA in the Peripheral Blood Plasma of Patients with Acute Endogenous and Exogenous Etiology Psychoses. Psikhiatria 2021, 19, 6–14. [Google Scholar] [CrossRef]
- Ershova, E.S.; Shmarina, G.V.; Martynov, A.V.; Zakharova, N.V.; Veiko, R.V.; Umriukhin, P.E.; Kostyuk, G.P.; Kutsev, S.I.; Veiko, N.N.; Kostyuk, S.V. NADPH-oxidase 4 gene over-expression in peripheral blood lymphocytes of the schizophrenia patients. PLoS ONE 2022, 17, e0269130. [Google Scholar] [CrossRef] [PubMed]
- Lubotzky, A.; Pelov, I.; Teplitz, R.; Neiman, D.; Smadja, A.; Zemmour, H.; Piyanzin, S.; Ochana, B.L.; Spalding, K.L.; Glaser, B.; et al. Elevated brain-derived cell-free DNA among patients with first psychotic episode—A proof-of-concept study. Elife 2022, 11, e76391. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Dimick, M.K.; Sultan, A.; Duong, A.; Park, S.S.; El Soufi El Sabbagh, D.; Goldstein, B.I.; Andreazza, A.C. Peripheral biomarkers of mitochondrial dysfunction in adolescents with bipolar disorder. J. Psychiatr. Res. 2020, 123, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Deguchi, Y.; Kasahara, T.; Tani, M.; Kuroda, K.; Inoue, K.; Kato, T. Intra-individual state-dependent comparison of plasma mitochondrial DNA copy number and IL-6 levels in patients with bipolar disorder. J. Affect. Disord. 2022, 299, 644–651. [Google Scholar] [CrossRef]
- Lindqvist, D.; Fernström, J.; Grudet, C.; Ljunggren, L.; Träskman-Bendz, L.; Ohlsson, L.; Westrin, Å. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: Associations with HPA-axis hyperactivity. Transl. Psychiatry 2016, 6, e971. [Google Scholar] [CrossRef]
- Fernström, J.; Ohlsson, L.; Asp, M.; Lavant, E.; Holck, A.; Grudet, C.; Westrin, Å.; Lindqvist, D. Plasma circulating cell-free mitochondrial DNA in depressive disorders. PloS ONE 2021, 16, e0259591. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Picard, M.; Ohlsson, L.; Bersani, F.S.; Fernström, J.; Westrin, Å.; Hough, C.M.; Lin, J.; Reus, V.I.; et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 2018, 43, 1557–1564. [Google Scholar] [CrossRef]
- Behnke, A.; Gumpp, A.M.; Rojas, R.; Sänger, T.; Lutz-Bonengel, S.; Moser, D.; Schelling, G.; Krumbholz, A.; Kolassa, I.T. Circulating inflammatory markers, cell-free mitochondrial DNA, cortisol, endocannabinoids, and N-acylethanolamines in female depressed outpatients. World J. Biol. Psychiatry 2023, 24, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the preanalytical conditions for analyzing circulating cell-free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Korzeneva, I.B.; Kostuyk, S.V.; Ershova, L.S.; Osipov, A.N.; Zhuravleva, V.F.; Pankratova, G.V.; Porokhovnik, L.N.; Veiko, N.N. Human circulating plasma DNA significantly decreases while lymphocyte DNA damage increases under chronic occupational exposure to low-dose gamma-neutron and tritium β-radiation. Mutat. Res. 2015, 779, 1–15. [Google Scholar] [CrossRef]
- Duvvuri, B.; Lood, C. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. Front. Immunol 2019, 10, 502. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative stress-related mechanisms in schizophrenia pathogenesis and new treatment perspectives. Oxid. Cell. Med. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Gassó, P.; Mas, S.; Molina, O.; Lafuente, A.; Bernardo, M.; Parellada, E. Increased susceptibility to apoptosis in cultured fibroblasts from antipsychotic-naïve first-episode schizophrenia patients. J. Psychiatr. Res. 2014, 48, 94–101. [Google Scholar] [CrossRef]
- Jarskog, L.F.; Selinger, E.S.; Lieberman, J.A.; Gilmore, J.H. Apoptotic proteins in the temporal cortex in schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation. Am. J. Psychiatry 2004, 161, 109–115. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Milas, G.P.; Michopoulos, I. Neutrophil-to-lymphocyte ratio in schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2019, 206, 4–12. [Google Scholar] [CrossRef]
- Mazza, M.G.; Lucchi, S.; Rossetti, A.; Clerici, M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World. J. Biol. Psychiatry 2020, 21, 326–338. [Google Scholar] [CrossRef]
- Jackson, A.J.; Miller, B.J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 2020, 142, 18–26. [Google Scholar] [CrossRef]
- Sisirak, V.; Sally, B.; D’Agati, V.; Martinez-Ortiz, W.; Özçakar, Z.B.; David, J.; Rashidfarrokhi, A.; Yeste, A.; Panea, C.; Chida, A.S.; et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 2016, 166, 88–101. [Google Scholar] [CrossRef]
- Santa, P.; Garreau, A.; Serpas, L.; Ferriere, A.; Blanco, P.; Soni, C.; Sisirak, V. The role of nucleases and nucleic acid editing enzymes in the regulation of self-nucleic acid sensing. Front. Immunol. 2021, 12, 629922. [Google Scholar] [CrossRef]
- Ezeoke, A.; Mellor, A.; Buckley, P.; Miller, B. A systematic, quantitative review of blood autoantibodies in schizophrenia. Schizophr. Res. 2013, 150, 245–251. [Google Scholar] [CrossRef]
- Kozłowska, E.; Brzezińska-Błaszczyk, E.; Agier, J.; Wysokiński, A.; Żelechowska, P. Alarmins (IL-33, sST2, HMGB1, and S100B) as potential biomarkers for schizophrenia. J. Psychiatr. Res. 2021, 138, 380–387. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mousa, R.F.; Al-Hakeim, H.K.; Maes, M. High mobility group protein 1 and dickkopf-related protein 1 in schizophrenia and treatment-resistant schizophrenia: Associations with interleukin-6, symptom domains, and neurocognitive impairments. Schizophr. Bull. 2021, 47, 530–541. [Google Scholar] [CrossRef]
- Kumar, V. The trinity of cGAS, TLR9, and ALRs guardians of the cellular galaxy against host-derived self-DNA. Front. Immunol. 2021, 11, 624597. [Google Scholar] [CrossRef] [PubMed]
- Bartok, E.; Hartmann, G. Immune sensing mechanisms that discriminate self from altered self and foreign nucleic acids. Immunity 2020, 53, 54–77. [Google Scholar] [CrossRef]
- Kostjuk, S.; Loseva, P.; Chvartatskaya, O.; Ershova, E.; Smirnova, T.; Malinovskaya, E.; Roginko, O.; Kuzmin, V.; Izhevskaia, V.; Baranova, A.; et al. Extracellular GC-rich DNA activates TLR9- and NF-kB-dependent signaling pathways in human adipose-derived mesenchymal stem cells (haMSCs). Expert Opin. Biol. Ther. 2012, 12, S99–S111. [Google Scholar] [CrossRef]
- Kostyuk, S.; Smirnova, T.; Kameneva, L.; Porokhovnik, L.; Speranskij, A.; Ershova, E.; Stukalov, S.; Izevskaya, V.; Veiko, N. GC-Rich Extracellular DNA Induces Oxidative Stress, Double-Strand DNA Breaks, and DNA Damage Response in Human Adipose-Derived Mesenchymal Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 782123. [Google Scholar] [CrossRef]
- Shmarina, G.V.; Ershova, E.S.; Simashkova, N.V.; Nikitina, S.G.; Chudakova, J.M.; Veiko, N.N.; Porokhovnik, L.N.; Basova, A.Y.; Shaposhnikova, A.F.; Pukhalskaya, D.A.; et al. Oxidized cell-free DNA as a stress-signaling factor activating the chronic inflammatory process in patients with autism spectrum disorders. J. Neuroinflamm. 2020, 17, 212. [Google Scholar] [CrossRef]
- Kostyuk, S.V.; Tabakov, V.J.; Chestkov, V.V.; Konkova, M.S.; Glebova, K.V.; Baydakova, G.V.; Ershova, E.S.; Izhevskaya, V.L.; Baranova, A.; Veiko, N.N. Oxidized DNA induces an adaptive response in human fibroblasts. Mutat. Res. 2013, 747–748, 6–18. [Google Scholar] [CrossRef]
- Ershova, E.S.; Shmarina, G.V.; Porokhovnik, L.N.; Zakharova, N.V.; Kostyuk, G.P.; Umriukhin, P.E.; Kutsev, S.I.; Sergeeva, V.A.; Veiko, N.N.; Kostyuk, S.V. In Vitro Analysis of Biological Activity of Circulating Cell-Free DNA Isolated from Blood Plasma of Schizophrenic Patients and Healthy Controls. Genes 2022, 13, 551. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, J.W.; Zhang, Y.; Leong, K.W.; Pisetsky, D.; Sullenger, B.A. Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl. Acad. Sci. USA 2011, 108, 14055–14060. [Google Scholar] [CrossRef]
| Study | Year | DNA Type | Sample | Extraction Method | Detection Method | Population |
|---|---|---|---|---|---|---|
| Schizophrenia | ||||||
| Ershova et al. [39] | 2017 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 58/HC 30 |
| Jiang et al. [38] | 2018 | Total CfDNA | Plasma | TIANamp Micro DNA Kit (spin-column) | FCS | SZ 65/HC 62 |
| Ershova et al. [37] | 2019 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 100/HC 96 |
| Jestkova et al. [40] | 2021 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 334/HC 95 |
| Ershova et al. [41] | 2022 | Total CfDNA | Plasma | Solvent extraction method | FL, PicoGreen dye | SZ 100/HC 60 |
| Lubotzky et al. [42] | 2022 | Total CfDNA and Cf-gDNA | Plasma | QIAsymphony DSP Circulating DNA Kit (magnetic particles) | FL, bisulfite DNA treatment, PCR amplification followed by NGS | FEP 29/HC 31 |
| Chen et al. [24] | 2021 | Cf-gDNA | Serum | TianLong DNA Kit (spin-column) | qPCR, target: Alu repeats | SZ 174/HC 100 |
| Qi et al. [36] | 2020 | Cf-gDNA | Serum | TianLong DNA Kit (spin-column) | qPCR, target: Alu repeats | SZ 164/HC 100 |
| Bipolar Disorder | ||||||
| Stertz et al. [25] | 2015 | Cf-mtDNA | Serum | QIAmp DNA Mini Kit (spin-column) | qPCR, target: MT-ATP8 gene | BD 20/HC 20 |
| Kageyama et al. [35] | 2018 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: MT-ND1 and MT-ND4 genes | BD 28/HC 29 |
| Jeong et al. [43] | 2020 | Cf-mtDNA | Serum | QIAmp DNA Mini Kit (spin-column) | qPCR, target: MT-ND1 gene | BD 64/HC 41 |
| Kageyama et al. [44] | 2022 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: MT-ND1 and MT-ND4 genes | BD 10/HC 10 |
| Depressive disorders | ||||||
| Lindqvist et al. [45] | 2016 | Cf-mtDNA | Plasma | QIAmp 96 DNA Blood Kit (spin-column) | qPCR, target: MT-ND2 gene | Suicide attempters 37/HC 37 |
| Kageyama et al. [35] | 2018 | Cf-mtDNA | Plasma | QIAamp DNA Blood Mini Kit (spin-column) | qPCR, target: MT-ND1 and MT-ND4 genes | MDD 109/HC 29 |
| Lindqvist et al. [47] | 2018 | Cf-mtDNA | Plasma | QIAmp 96 DNA Blood Kit (spin-column) | qPCR, target: MT-ND1 and MT-ND4 genes | MDD 50/HC 55 |
| Fernström et al. [46] | 2021 | Cf-mtDNA | Plasma | QIAmp DNA Blood Mini Kit (spin-column) | qPCR, target: MT-ND2 gene | Current depression 236/HC 49 |
| Behnke et al. [48] | 2022 | Cf-mtDNA | Serum | QIAamp DNA Micro Kit (spin-column) | qPCR with multiple target | MDD 24/HC 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melamud, M.M.; Buneva, V.N.; Ermakov, E.A. Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 3402. https://doi.org/10.3390/ijms24043402
Melamud MM, Buneva VN, Ermakov EA. Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(4):3402. https://doi.org/10.3390/ijms24043402
Chicago/Turabian StyleMelamud, Mark M., Valentina N. Buneva, and Evgeny A. Ermakov. 2023. "Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 4: 3402. https://doi.org/10.3390/ijms24043402
APA StyleMelamud, M. M., Buneva, V. N., & Ermakov, E. A. (2023). Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(4), 3402. https://doi.org/10.3390/ijms24043402







