Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels
Abstract
1. Introduction
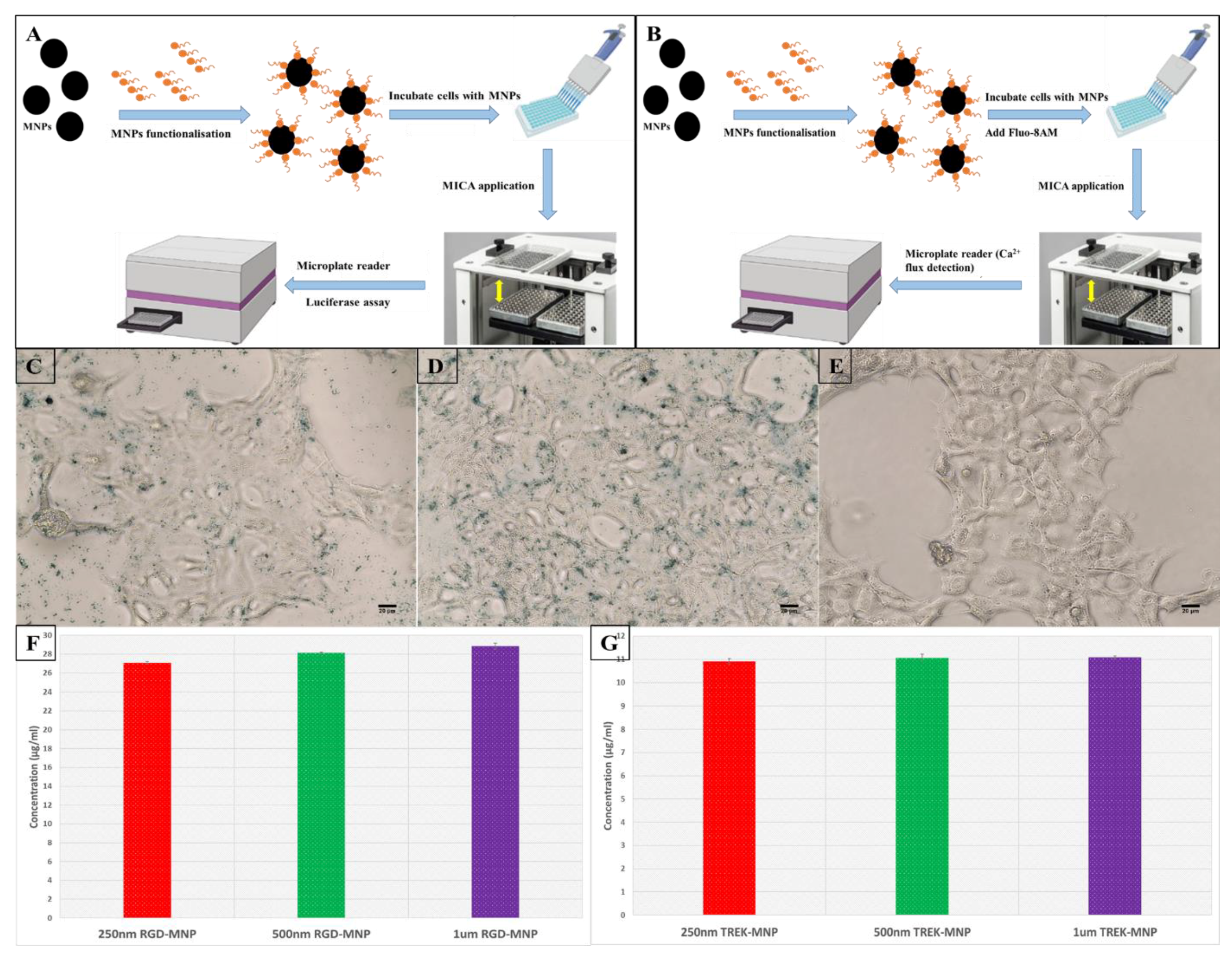
2. Results
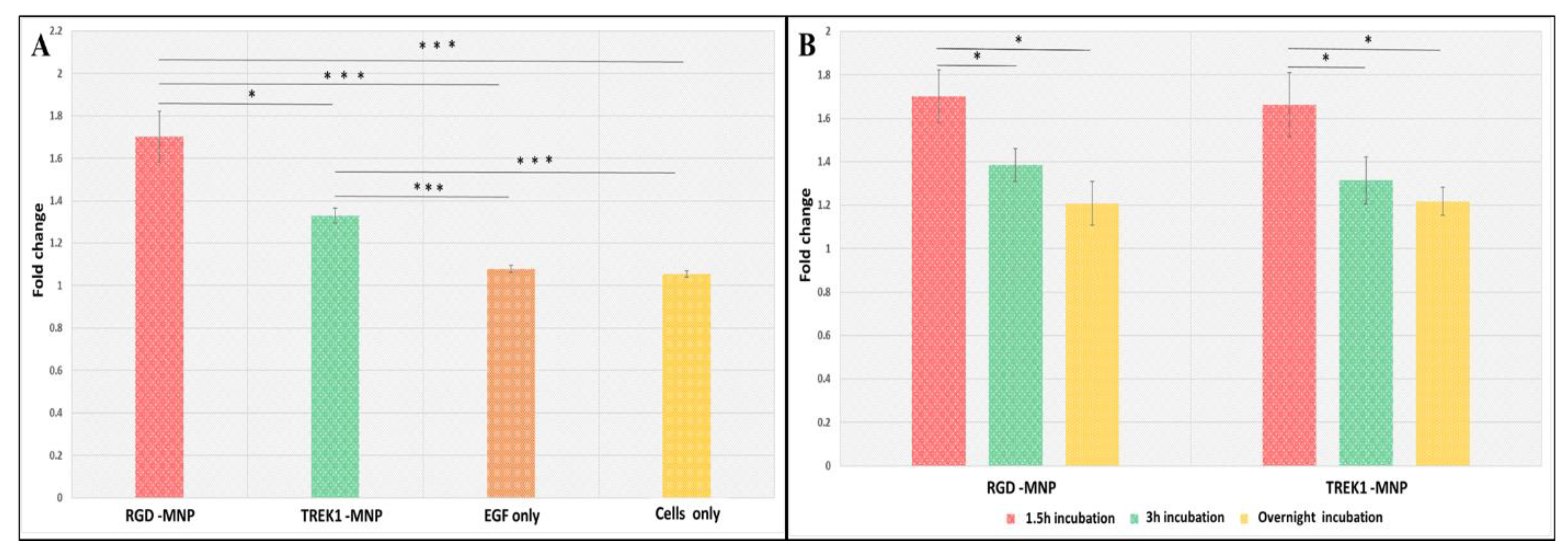
2.1. Influence of MNP Incubation Period
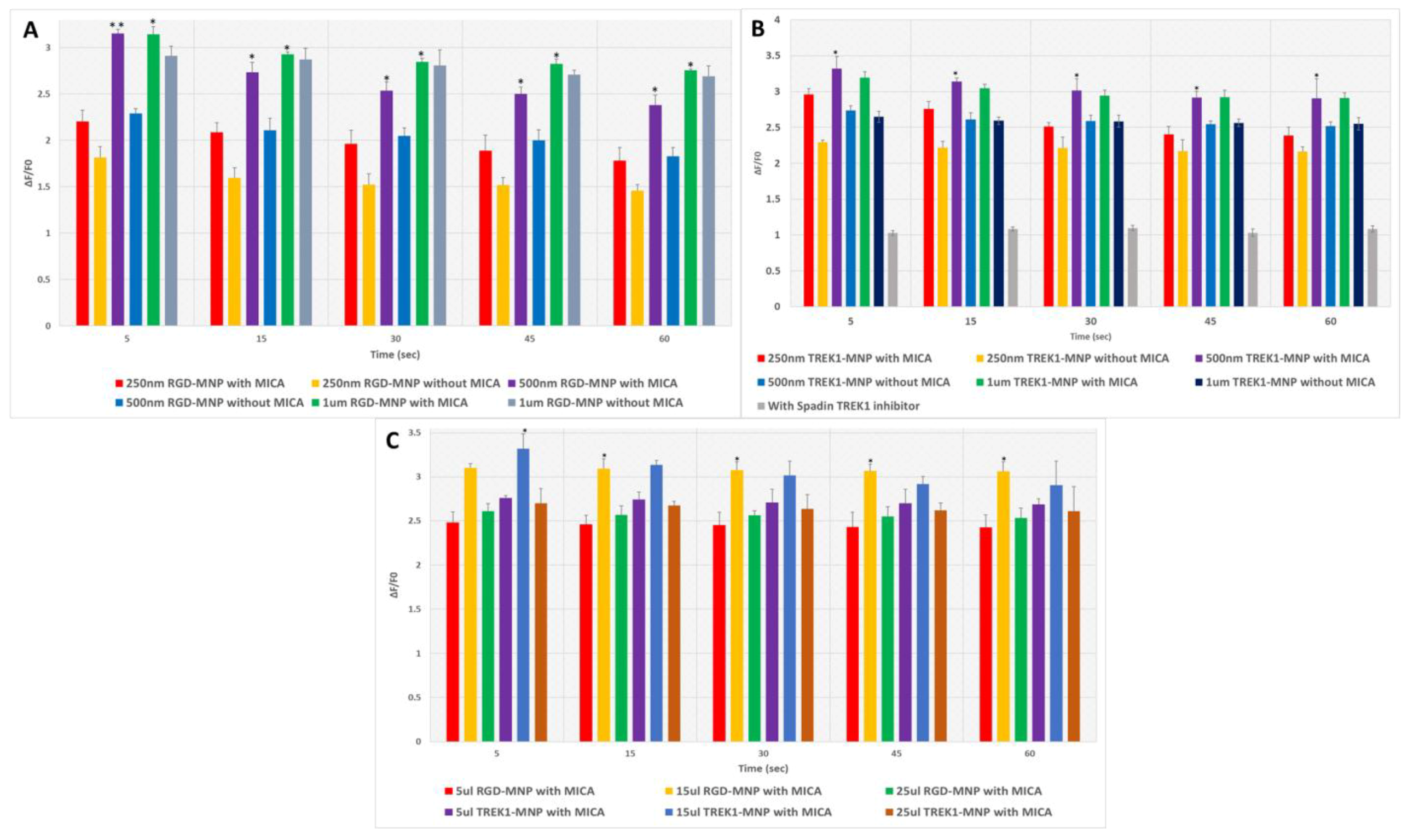
2.2. Impact of MNP Size in Fluo-8AM Response
2.3. Effect of MNP Concentration in Fluo-8AM Response
3. Materials and Methods
4. Conclusions
Statistical Testing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; GhavamiNejad, A.; Unnithan, A.R.; Thomas, R.G.; Moon, M.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. A smart magnetic nanoplatform for synergistic anticancer therapy: Manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale 2015, 7, 18119–18128. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.; Unnithan, A.; Thomas, R.; Ko, S.; Jeong, Y.; Park, C.; Kim, C. Multifaceted Implantable Anticancer Device for Potential Postsurgical Breast Cancer Treatment: A Single Platform for Synergistic Inhibition of Local Regional Breast Cancer Recurrence, Surveillance, and Healthy Breast Reconstruction. Adv. Funct. Mater. 2018, 28, 1704793. [Google Scholar] [CrossRef]
- Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 2008, 3, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Henstock, J.R.; Rotherham, M.; El Haj, A.J. Magnetic ion channel activation of TREK1 in human mesenchymal stem cells using nanoparticles promotes osteogenesis in surrounding cells. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Gahl, T.J.; Kunze, A. Force-Mediating Magnetic Nanoparticles to Engineer Neuronal Cell Function. Front. Neurosci. 2018, 12, 299. [Google Scholar] [CrossRef]
- Hu, K.H.; Butte, M.J. T cell activation requires force generation. J. Cell Biol. 2016, 213, 535–542. [Google Scholar] [CrossRef]
- Hughes, S.; McBain, S.; Dobson, J.; El Haj, A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface 2007, 5, 855–863. [Google Scholar] [CrossRef]
- Martinac, B. Mechanosensitive ion channels: Molecules of mechanotransduction. J. Cell Sci. 2004, 117, 2449–2460. [Google Scholar] [CrossRef]
- Jin, P.; Jan, L.Y.; Jan, Y.-N. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu. Rev. Neurosci. 2020, 43, 207–229. [Google Scholar] [CrossRef]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Dobson, J.; El Haj, A.J. Magnetic targeting of mechanosensors in bone cells for tissue engineering applications. J. Biomech. 2007, 40, S96–S104. [Google Scholar] [CrossRef] [PubMed]
- Djillani, A.; Mazella, J.; Heurteaux, C.; Borsotto, M. Role of TREK-1 in Health and Disease, Focus on the Central Nervous System. Front. Pharmacol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, J.; Kang, Y.; Lin, Y.; Li, D.; Shao, L. Interactions of nanomaterials with ion channels and related mechanisms. Br. J. Pharmacol. 2019, 176, 3754–3774. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Lee, D.K.; Hong, S.-G.; Han, J.; Kang, D. Activation of TREK-1, but Not TREK-2, Channel by Mood Stabilizers. Int. J. Mol. Sci. 2017, 18, 2460. [Google Scholar] [CrossRef]
- E Kennard, L.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.L.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef]
- Rotherham, M.; Moradi, Y.; Nahar, T.; Mosses, D.; Telling, N.; El Haj, A.J. Magnetic activation of TREK1 triggers stress signalling and regulates neuronal branching in SH-SY5Y cells. Front. Med Technol. 2022, 4. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Sasikala, A.R.K.; Shrestha, B.K.; Lincoln, A.; Thomson, T.; El Haj, A.J. Remotely Actuated Magnetic Nanocarpets for Bone Tissue Engineering: Non-Invasive Modulation of Mechanosensitive Ion Channels for Enhanced Osteogenesis. Adv. Funct. Mater. 2022, 32. [Google Scholar] [CrossRef]
- Hughes, S.; El Haj, A.J.; Dobson, J. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med Eng. Phys. 2005, 27, 754–762. [Google Scholar] [CrossRef]
- Kerekes, K.; Trexler, M.; Bányai, L.; Patthy, L. Wnt Inhibitory Factor 1 Binds to and Inhibits the Activity of Sonic Hedgehog. Cells 2021, 10, 3496. [Google Scholar] [CrossRef]
- Morrell, A.E.; Robinson, S.T.; Ke, H.Z.; Holdsworth, G.; Guo, X.E. Osteocyte mechanosensing following short-term and long-term treatment with sclerostin antibody. Bone 2021, 149, 115967. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; McLaren, J.S.; Telling, N.D.; Alom, N.; Al-Mutheffer, E.A.; Oreffo, R.O.C.; Zannettino, A.; Scammell, B.E.; White, L.J.; El Haj, A.J. Translation of remote control regenerative technologies for bone repair. npj Regen. Med. 2018, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-K. Recent developments in anti-cancer agents targeting the Ras/Raf/ MEK/ERK pathway. Recent Pat. Anti-Cancer Drug Discov. 2009, 4, 28–35. [Google Scholar] [CrossRef]
- Rotherham, M.; Henstock, J.R.; Qutachi, O.; El Haj, A.J. Remote regulation of magnetic particle targeted Wnt signaling for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 173–184. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Yarishkin, O.; Phuong, T.T.; Bretz, C.A.; Olsen, K.W.; Baumann, J.M.; Lakk, M.; Crandall, A.; Heurteaux, C.; Hartnett, M.E.; Križaj, D. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J. Gen. Physiol. 2018, 150, 1660–1675. [Google Scholar] [CrossRef]
- Yarishkin, O.; Baumann, J.M.; Križaj, D. Mechano-electrical transduction in trabecular meshwork involves parallel activation of TRPV4 and TREK-1 channels. Channels 2019, 13, 168–171. [Google Scholar] [CrossRef]
- Honoré, E. The neuronal background K2P channels: Focus on TREK1. Nat. Rev. Neurosci. 2007, 8, 251–261. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Vhora, I.; Patil, S.; Bhatt, P.; Misra, A. Protein- and Peptide-Drug Conjugates: An Emerging Drug Delivery Technology. Adv. Protein. Chem. Str. 2015, 98, 1–55. [Google Scholar]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.-J.; Mas-Moruno, C.; et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, A.K.; Carpenter, G.; Proctor, G. Measurement of intracellular calcium of submandibular glands using a high throughput plate reader. J. Biol. Methods 2017, 4, e74. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Putker, M.; Brancaccio, M.; O’Neill, J.S. In-depth Characterization of Firefly Luciferase as a Reporter of Circadian Gene Expression in Mammalian Cells. J. Biol. Rhythm. 2016, 31, 540–550. [Google Scholar] [CrossRef]
- Ling, K.; Jiang, H.; Li, Y.; Tao, X.; Qiu, C.; Li, F.-R. A self-assembling RNA aptamer-based graphene oxide sensor for the turn-on detection of theophylline in serum. Biosens. Bioelectron. 2016, 86, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Anna-Maria Pappa, H.-Y.L. Walther Traberg-Christensen, Quentin Thiburce, Achilleas Savva, Aimie Pavia, Alberto Salleo, Susan Daniel, Rois’ ín M. Owens, Optical and Electronic Ion Channel Monitoring from Native Human Membranes. ACS Nano 2020, 14, 12538–12545. [Google Scholar]
- Wang, Q.; Yang, Y.; Liang, X. LncRNA CTBP1-AS2 sponges miR-216a to upregulate PTEN and suppress endometrial cancer cell invasion and migration. J. Ovarian Res. 2020, 13. [Google Scholar] [CrossRef]
- Jones, L.; Haugland, R.; Singer, V. Development and characterization of the NanoOrange (R) protein quantitation assay: A fluorescence-based assay of proteins in solution. Biotechniques 2003, 34, 850. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Driskell, J.D. A fluorescence-based method to directly quantify antibodies immobilized on gold nanoparticles. Analyst 2016, 141, 3851–3857. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnithan, A.R.; Rotherham, M.; Markides, H.; El Haj, A.J. Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels. Int. J. Mol. Sci. 2023, 24, 3364. https://doi.org/10.3390/ijms24043364
Unnithan AR, Rotherham M, Markides H, El Haj AJ. Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels. International Journal of Molecular Sciences. 2023; 24(4):3364. https://doi.org/10.3390/ijms24043364
Chicago/Turabian StyleUnnithan, Afeesh Rajan, Michael Rotherham, Hareklea Markides, and Alicia J. El Haj. 2023. "Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels" International Journal of Molecular Sciences 24, no. 4: 3364. https://doi.org/10.3390/ijms24043364
APA StyleUnnithan, A. R., Rotherham, M., Markides, H., & El Haj, A. J. (2023). Magnetic Ion Channel Activation (MICA)-Enabled Screening Assay: A Dynamic Platform for Remote Activation of Mechanosensitive Ion Channels. International Journal of Molecular Sciences, 24(4), 3364. https://doi.org/10.3390/ijms24043364






