Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA
Abstract
1. Introduction
2. Results
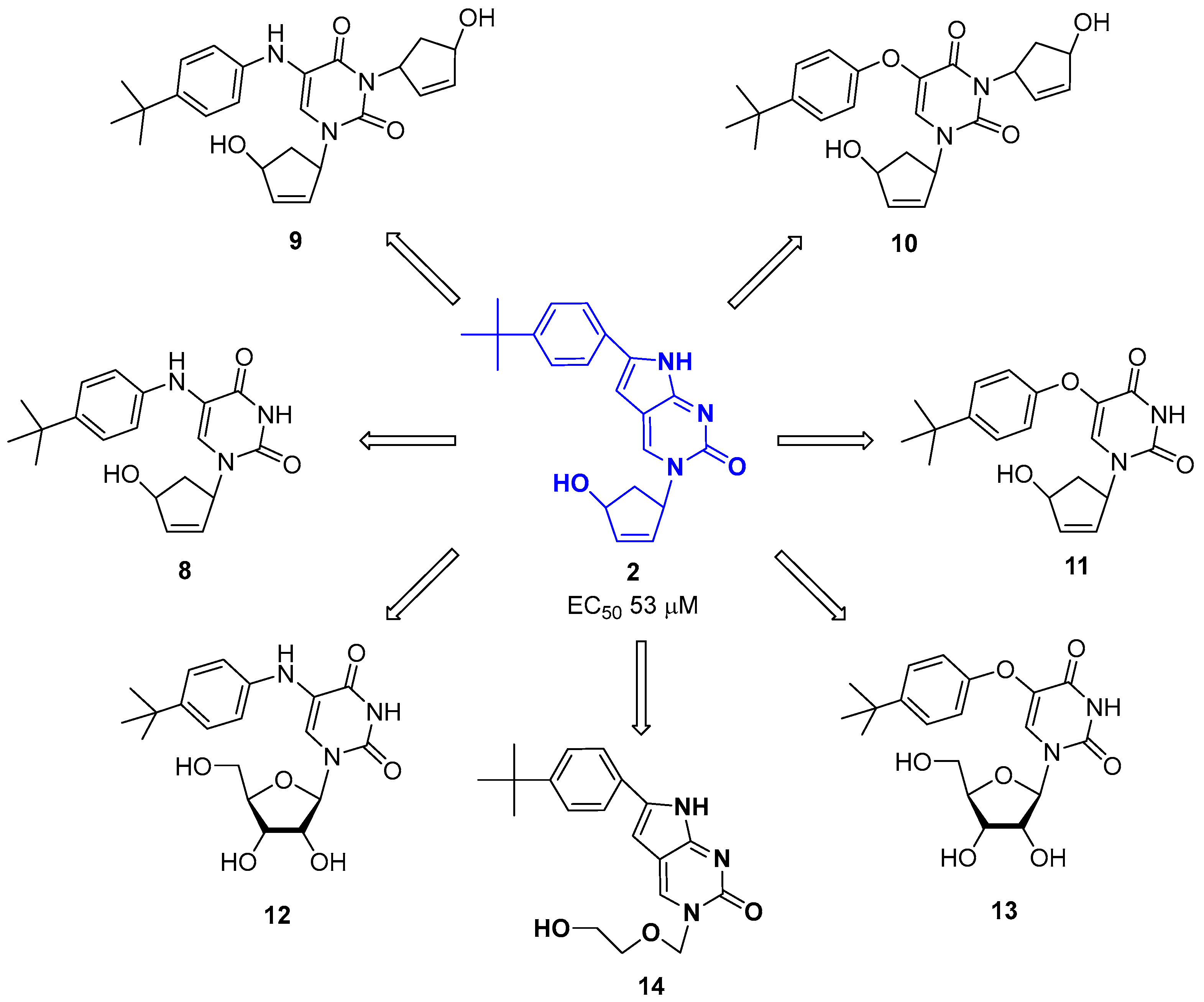
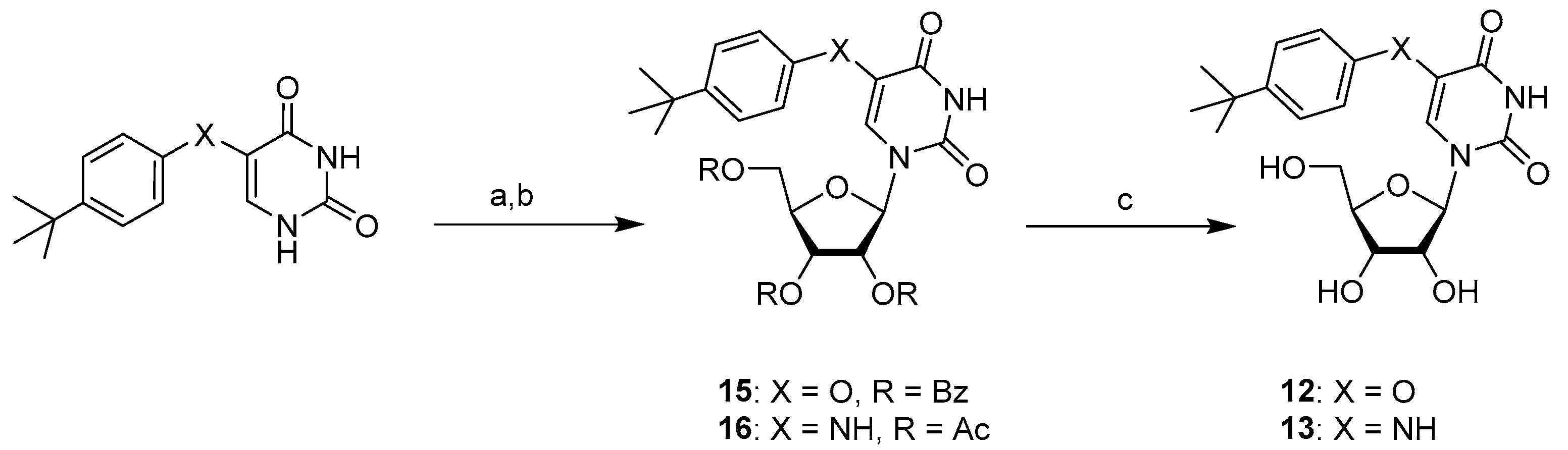
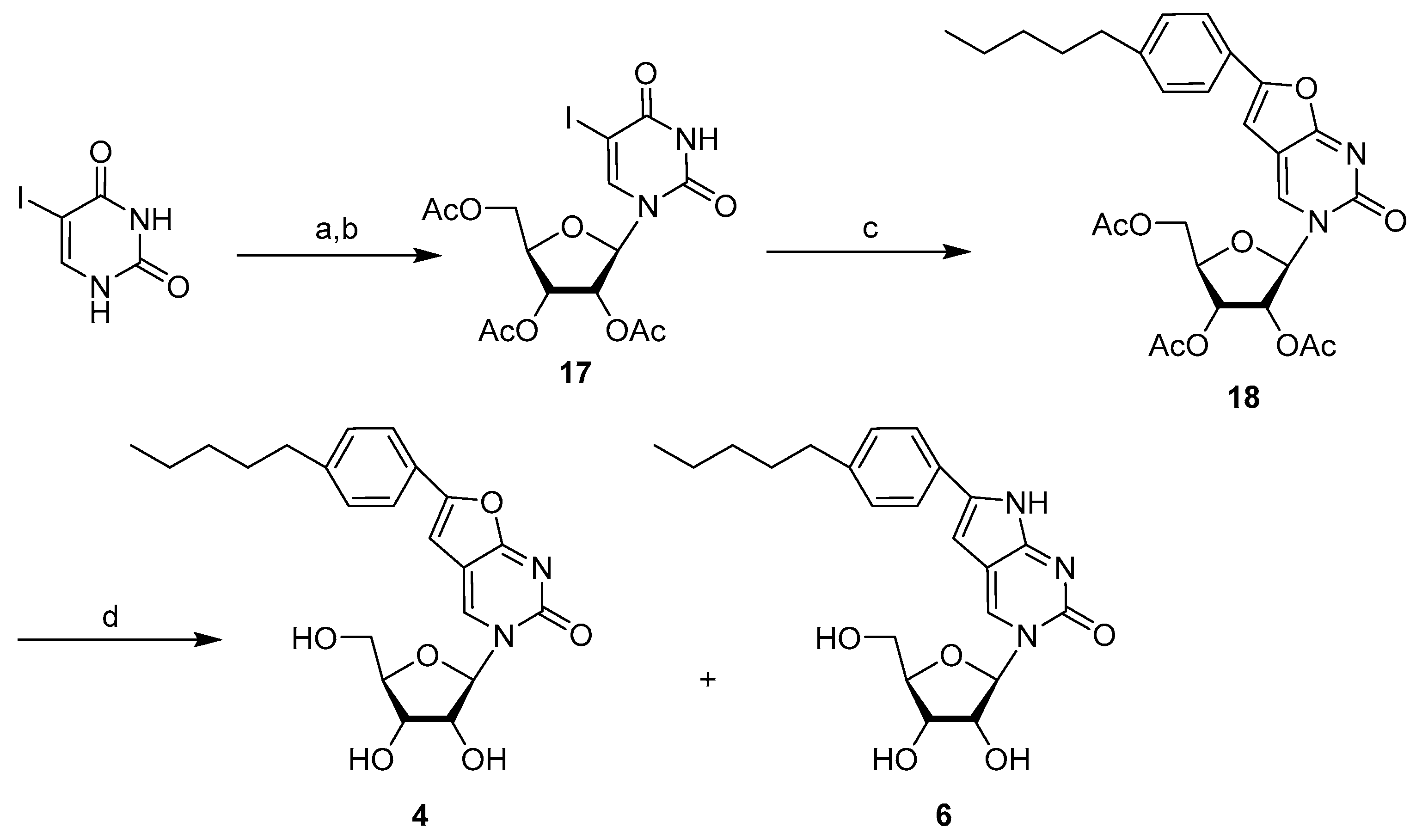
2.1. Chemistry
2.2. Biological Evaluation
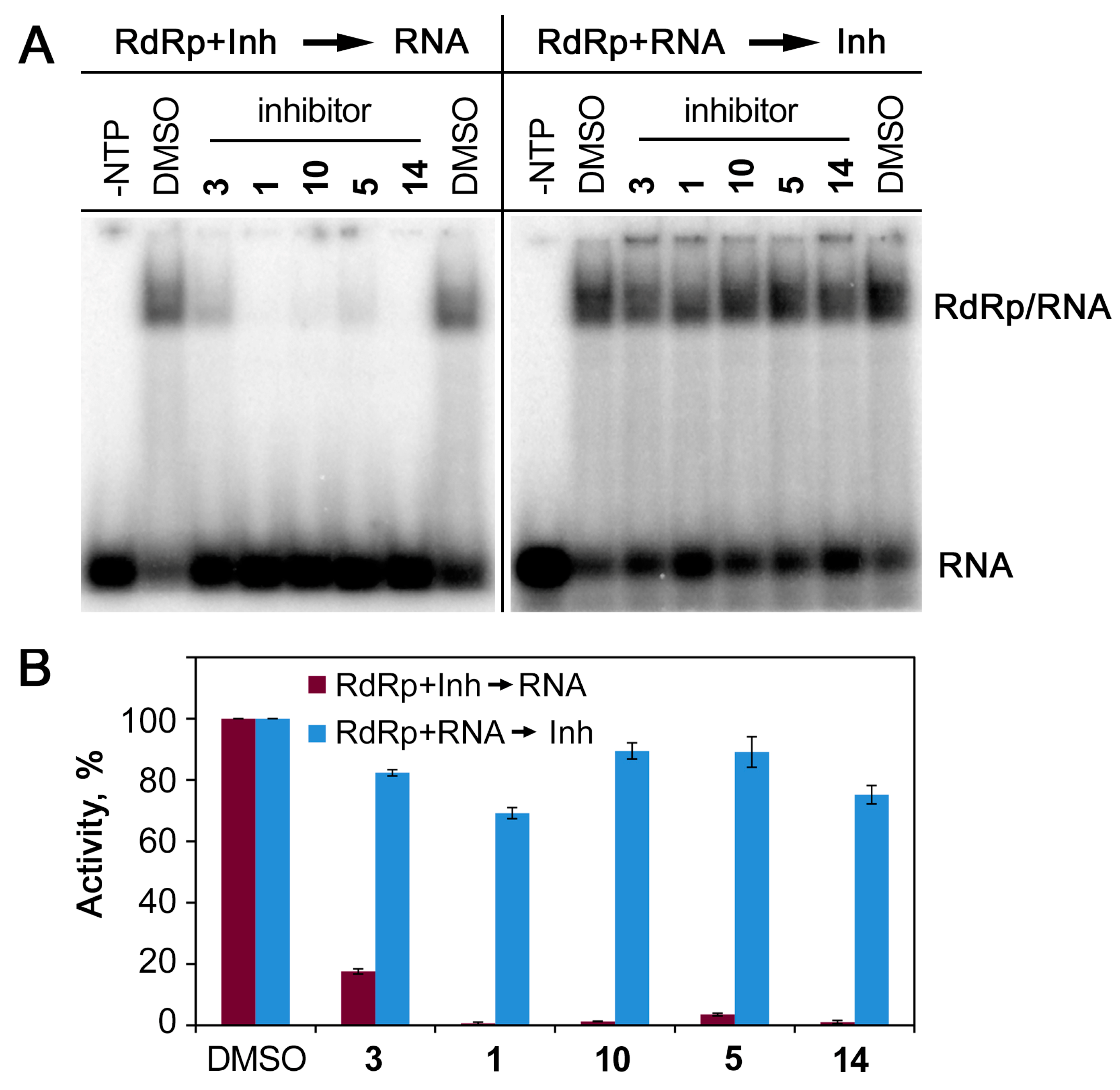
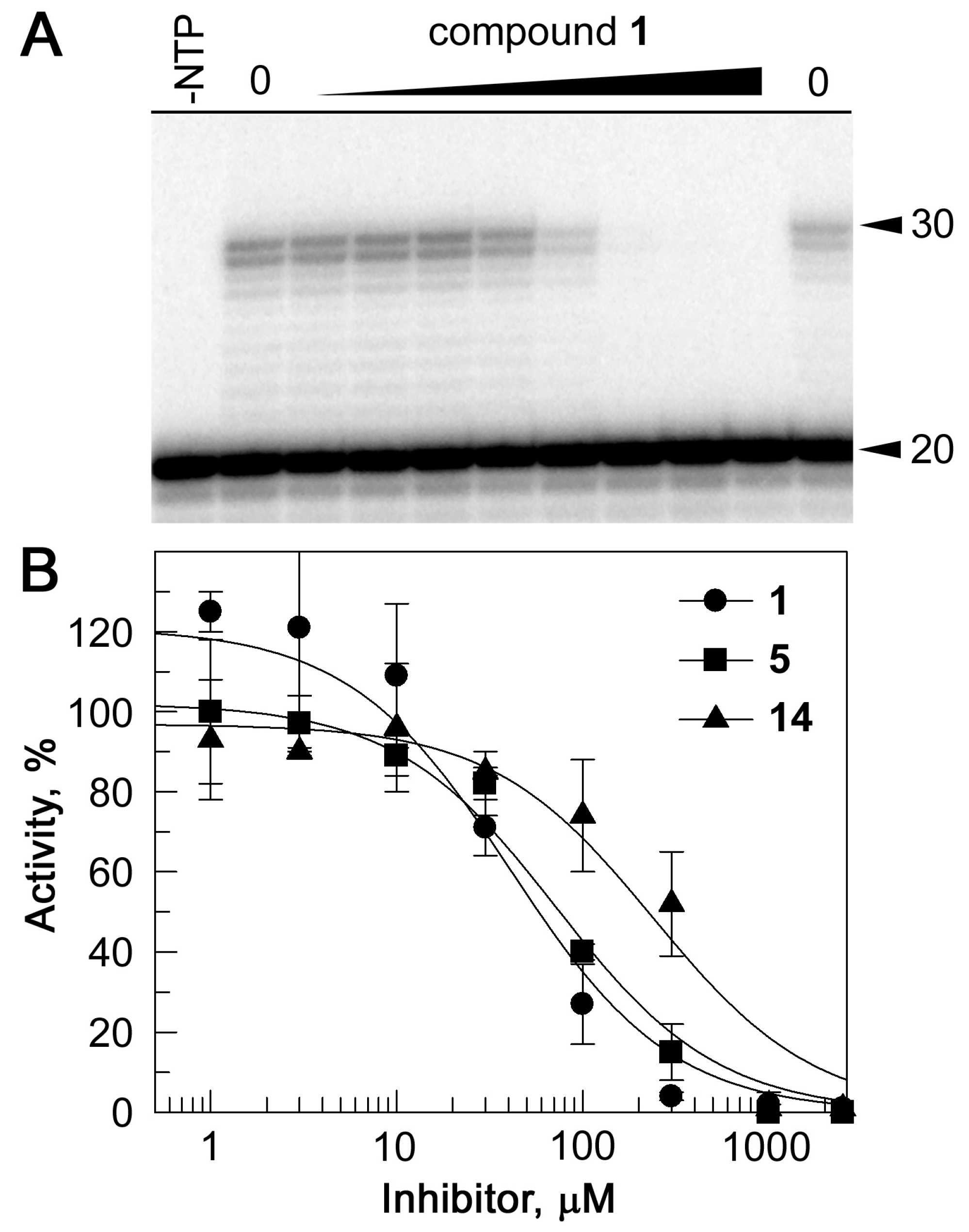
2.3. Inhibition of RdRp Activity In Vitro
3. Discussion
4. Materials and Methods
4.1. Chemistry Experimental
4.1.1. General
4.1.2. Compound Synthesis and Characterization
4.2. Virology Assays
4.2.1. General
4.2.2. SARS-CoV-2 Assays
4.2.3. Influenza Virus Assay
4.3. Biochemical Assays
4.3.1. RdRp and RNA Substrates
4.3.2. In Vitro Transcription Assay
4.3.3. Electrophoretic Mobility Shift Assay
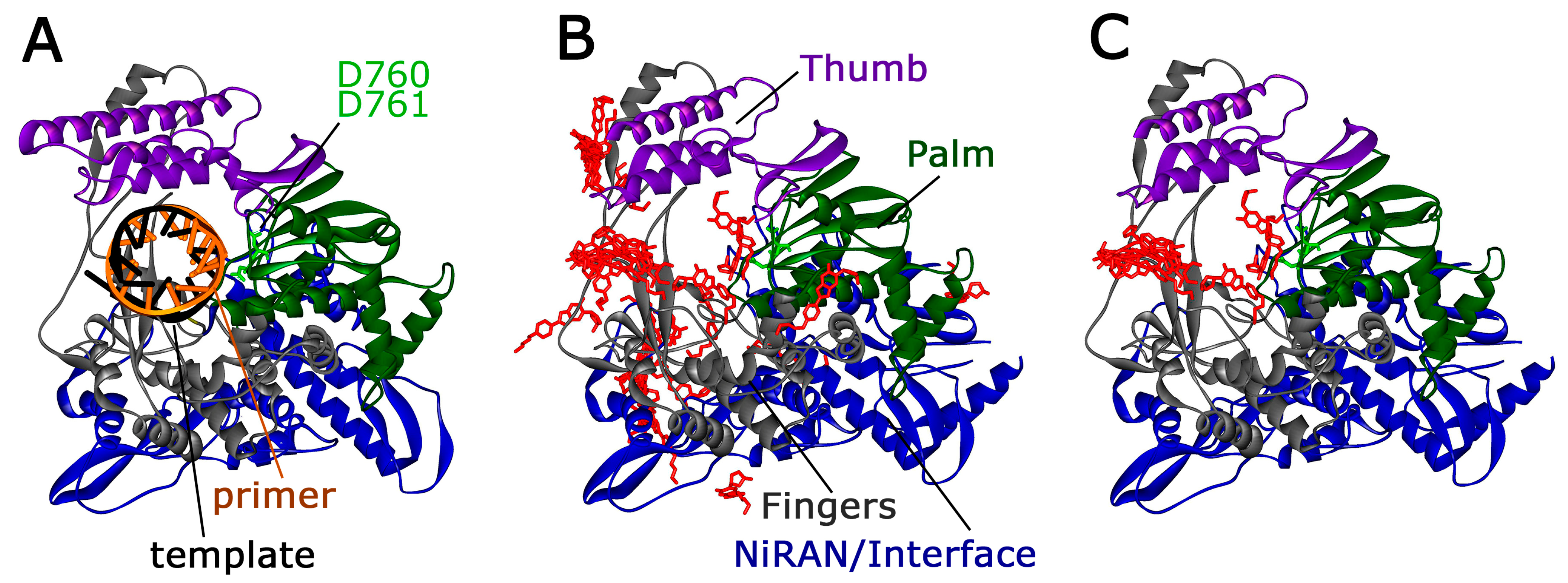
4.3.4. Molecular Docking
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Sinagra, E.; Shahini, E.; Crispino, F.; Macaione, I.; Guarnotta, V.; Marasa, M.; Testai, S.; Pallio, S.; Albano, D.; Facciorusso, A.; et al. COVID-19 and the pancreas: A narrative review. Life 2022, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Budinger, G.R.S.; Misharin, A.V.; Ridge, K.M.; Singer, B.D.; Wunderink, R.G. Distinctive features of severe SARS-CoV-2 pneumonia. J. Clin. Investig. 2021, 131, e149412. [Google Scholar] [CrossRef] [PubMed]
- Torres Acosta, M.A.; Singer, B.D. Pathogenesis of COVID-19-induced ards: Implications for an ageing population. Eur. Respir. J. 2020, 56, 2002049. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Keyhanian, K.; Umeton, R.P.; Mohit, B.; Davoudi, V.; Hajighasemi, F.; Ghasemi, M. SARS-CoV-2 and nervous system: From pathogenesis to clinical manifestation. J. Neuroimmunol. 2020, 350, 577436. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Groff, A.; Kavanaugh, M.; Ramgobin, D.; McClafferty, B.; Aggarwal, C.S.; Golamari, R.; Jain, R. Gastrointestinal manifestations of COVID-19: A review of what we know. Ochsner J. 2021, 21, 177–180. [Google Scholar] [CrossRef]
- Doroftei, B.; Ciobica, A.; Ilie, O.D.; Maftei, R.; Ilea, C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics 2021, 11, 579. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Otter, A.D.; Lin, K.M.; Munoz Sandoval, D.; Pieper, F.P.; Butler, D.K.; Liu, S.; Joy, G.; et al. Immune boosting by b.1.1.529 (omicron) depends on previous SARS-CoV-2 exposure. Science 2022, 377, eabq1841. [Google Scholar] [CrossRef] [PubMed]
- Acherjee, T.; Behara, A.; Saad, M.; Vittorio, T.J. Mechanisms and management of prothrombotic state in COVID-19 disease. Ther. Adv. Cardiovasc. Dis. 2021, 15, 17539447211053470. [Google Scholar] [CrossRef] [PubMed]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Atzeni, F.; Gerratana, E.; Giallanza, M.; La Corte, L.; Nucera, V.; Miceli, G.; Sangari, D.; Masala, I.F. The effect of drugs used in rheumatology for treating SARS-CoV-2 infection. Expert Opin. Biol. Ther. 2021, 21, 219–228. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; de Bono, S.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Saraiva, J.F.K.; Chakladar, S.; Marconi, V.C.; et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 327–336. [Google Scholar] [CrossRef]
- Marconi, V.; Ramanan, A.; de Bono, S.; Kartman, C.; Krishnan, V.; Liao, R.; Piruzeli, M.L.; Goldman, J.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418, Correction in Lancet. Respir. Med. 2021, 9, e102. [Google Scholar] [CrossRef]
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated hiv-positive individuals in the united states and canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis c research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- McCown, M.F.; Rajyaguru, S.; Le Pogam, S.; Ali, S.; Jiang, W.R.; Kang, H.; Symons, J.; Cammack, N.; Najera, I. The hepatitis c virus replicon presents a higher barrier to resistance to nucleoside analogs than to nonnucleoside polymerase or protease inhibitors. Antimicrob. Agents Chemother. 2008, 52, 1604–1612. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schafer, A.; Dinnon, K.H., 3rd; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19-final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and nirmatrelvir-ritonavir: Oral covid antiviral drugs. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 76, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Pruijssers, A.J.; George, A.S.; Schafer, A.; Leist, S.R.; Gralinksi, L.E.; Dinnon, K.H., 3rd; Yount, B.L.; Agostini, M.L.; Stevens, L.J.; Chappell, J.D.; et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric sars-cov expressing the SARS-CoV-2 rna polymerase in mice. Cell Rep. 2020, 32, 107940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. Beta-d-n4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J. Infect Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef]
- Ansems, K.; Grundeis, F.; Dahms, K.; Mikolajewska, A.; Thieme, V.; Piechotta, V.; Metzendorf, M.I.; Stegemann, M.; Benstoem, C.; Fichtner, F. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014962. [Google Scholar]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during hong kong’s omicron ba.2 wave: A retrospective cohort study. Lancet Infect Dis. 2022, 22, 1681–1693. [Google Scholar] [CrossRef]
- Matyugina, E.S.; Novikov, M.S.; Kozlovskaya, L.I.; Volok, V.P.; Shustova, E.Y.; Ishmukhametov, A.A.; Kochetkov, S.N.; Khandazhinskaya, A.L. Evaluation of the antiviral potential of modified heterocyclic base and 5′-norcarbocyclic nucleoside analogs against SARS-CoV-2. Acta Nat. 2021, 13, 78–81. [Google Scholar] [CrossRef]
- Klimenko, A.A.; Matyugina, E.S.; Logashenko, E.B.; Solyev, P.N.; Zenkova, M.A.; Kochetkov, S.N.; Khandazhinskaya, A.L. Novel 5′-norcarbocyclic derivatives of bicyclic pyrrolo- and furano[2,3-d]pyrimidine nucleosides. Molecules 2018, 23, 2654. [Google Scholar] [CrossRef]
- Kezin, V.A.; Matyugina, E.S.; Novikov, M.S.; Chizhov, A.O.; Snoeck, R.; Andrei, G.; Kochetkov, S.N.; Khandazhinskaya, A.L. New derivatives of 5-substituted uracils: Potential agents with a wide spectrum of biological activity. Molecules 2022, 27, 2866. [Google Scholar] [CrossRef]
- Miropolskaya, N.; Kozlov, M.; Petushkov, I.; Prostova, M.; Pupov, D.; Esyunina, D.; Kochetkov, S.; Kulbachinskiy, A. Effects of natural polymorphisms in SARS-CoV-2 rna-dependent rna polymerase on its activity and sensitivity to inhibitors in vitro. Biochimie, 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the rna-dependent rna polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. Swissdock, a protein-small molecule docking web service based on eadock dss. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Cannalire, R.; Mastrangelo, E.; Croci, R.; Querat, G.; Barreca, M.L.; Bolognesi, M.; Manfroni, G.; Cecchetti, V.; Milani, M. Targeting flavivirus rna dependent rna polymerase through a pyridobenzothiazole inhibitor. Antivir. Res. 2016, 134, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dejmek, M.; Konkolova, E.; Eyer, L.; Strakova, P.; Svoboda, P.; Sala, M.; Krejcova, K.; Ruzek, D.; Boura, E.; Nencka, R. Non-nucleotide rna-dependent rna polymerase inhibitor that blocks SARS-CoV-2 replication. Viruses 2021, 13, 1585. [Google Scholar] [CrossRef] [PubMed]
- Konkolova, E.; Krejcova, K.; Eyer, L.; Hodek, J.; Zgarbova, M.; Fortova, A.; Jirasek, M.; Teply, F.; Reyes-Gutierrez, P.E.; Ruzek, D.; et al. A helquat-like compound as a potent inhibitor of flaviviral and coronaviral polymerases. Molecules 2022, 27, 1894. [Google Scholar] [CrossRef]
- Yin, W.; Luan, X.; Li, Z.; Zhou, Z.; Wang, Q.; Gao, M.; Wang, X.; Zhou, F.; Shi, J.; You, E.; et al. Structural basis for inhibition of the SARS-CoV-2 rna polymerase by suramin. Nat. Struct. Mol. Biol. 2021, 28, 319–325. [Google Scholar] [CrossRef]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Smirnova, O.A.; Ivanova, O.N.; Fedyakina, I.T.; Yusubalieva, G.M.; Baklaushev, V.P.; Yanvarev, D.V.; Kechko, O.I.; Mitkevich, V.A.; Vorobyev, P.O.; Fedorov, V.S.; et al. SARS-CoV-2 establishes a productive infection in hepatoma and glioblastoma multiforme cell lines. Cancers 2023, 15, 632. [Google Scholar] [CrossRef]
- Golikov, M.V.; Karpenko, I.L.; Lipatova, A.V.; Ivanova, O.N.; Fedyakina, I.T.; Larichev, V.F.; Zakirova, N.F.; Leonova, O.G.; Popenko, V.I.; Bartosch, B.; et al. Cultivation of cells in a physiological plasmax medium increases mitochondrial respiratory capacity and reduces replication levels of rna viruses. Antioxidants 2022, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Petushkov, I.; Esyunina, D.; Kulbachinskiy, A. Effects of natural rna modifications on the activity of sars-cov-2 rna-dependent rna polymerase. FEBS J. 2022, 290, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. Ucsf chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; de Azevedo, W.F. Docking with swissdock. In Docking Screens for Drug Discovery; Springer: Berlin, Germany, 2019; pp. 189–202. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]



| Compound | SARS-CoV-2 | CD50 ± SD 3 Vero E6 (µM) | Influenza Virus | CD50 ± SD MDCK (µM) | |||
|---|---|---|---|---|---|---|---|
| Δlog10(RNA) 1 (at 50 µM) | Δlog10(TCID50) 2 (at 50 µM) | Δlog10(TCID50) (at 50 µM) | IC50 4 ± SD, (µM) | SI 5 | |||
| 1 | 1.5 * | 1.0 ** | 153 ± 5 | 0 | >50 | - | 89 ± 12 |
| 2 | 0.3 | 0.5 ** | 150 ± 15 | 1.5 | 1.37 ± 0.28 | 49.60 | 68 ± 5 |
| 3 | 1.3 | 0.5 ** | >500 | 0 | > 50 | - | 442 ± 21 |
| 4 | 0.2 | 0.5 ** | >500 | 1.0 | > 25 | - | 652 ± 77 |
| 5 | 0.3 | 1.0 | 400 ± 98 | 1.5 | 1.34 ± 0.33 | 511.17 | 686 ± 46 |
| 6 | 0.2 | 0 | 450 ± 166 | 0 | >50 | - | 614 ± 52 |
| 7 | 0.4 | 0 | 240 ± 19 | 0.5 | >50 | - | 74 ± 12 |
| 8 | 1.1 | 0.5 | >500 | 0.5 | >50 | - | 552 ± 35 |
| 9 | 0.8 | 0.5 | 220 ± 23 | 0.5 | >50 | - | 392 ± 9 |
| 10 | 0.8 | 0.5 *** | 500 ± 30 | -0.5 | >50 | - | 418 ± 42 |
| 11 | 0.7 | 1.0 ** | 440 ± 6 | -0.5 | >50 | - | >1000 |
| 12 | 0.3 | 0.5 ** | >500 | 0.75 | >40 | - | 657 ± 114 |
| 13 | 0.8 | 1.5 1.0 *** | >500 | 0.5 | >50 | - | >1000 |
| 14 | 0.4 | 1.0 | 310 ± 24 | 1.5 | 0.67 ± 0.12 | 110.61 | 74 ± 18 |
| NHC | 3.9 * | 80 ± 18 | |||||
| Oseltamivir | 0.61 ± 0.15 | 820 | 500 ± 82 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matyugina, E.; Petushkov, I.; Surzhikov, S.; Kezin, V.; Maslova, A.; Ivanova, O.; Smirnova, O.; Kirillov, I.; Fedyakina, I.; Kulbachinskiy, A.; et al. Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA. Int. J. Mol. Sci. 2023, 24, 3361. https://doi.org/10.3390/ijms24043361
Matyugina E, Petushkov I, Surzhikov S, Kezin V, Maslova A, Ivanova O, Smirnova O, Kirillov I, Fedyakina I, Kulbachinskiy A, et al. Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA. International Journal of Molecular Sciences. 2023; 24(4):3361. https://doi.org/10.3390/ijms24043361
Chicago/Turabian StyleMatyugina, Elena, Ivan Petushkov, Sergei Surzhikov, Vasily Kezin, Anna Maslova, Olga Ivanova, Olga Smirnova, Ilya Kirillov, Irina Fedyakina, Andrey Kulbachinskiy, and et al. 2023. "Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA" International Journal of Molecular Sciences 24, no. 4: 3361. https://doi.org/10.3390/ijms24043361
APA StyleMatyugina, E., Petushkov, I., Surzhikov, S., Kezin, V., Maslova, A., Ivanova, O., Smirnova, O., Kirillov, I., Fedyakina, I., Kulbachinskiy, A., Kochetkov, S., & Khandazhinskaya, A. (2023). Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA. International Journal of Molecular Sciences, 24(4), 3361. https://doi.org/10.3390/ijms24043361









