3D Characterization of the Molecular Neighborhood of •OH Radical in High Temperature Water by MD Simulation and Voronoi Polyhedra
Abstract
:1. Introduction
2. Results
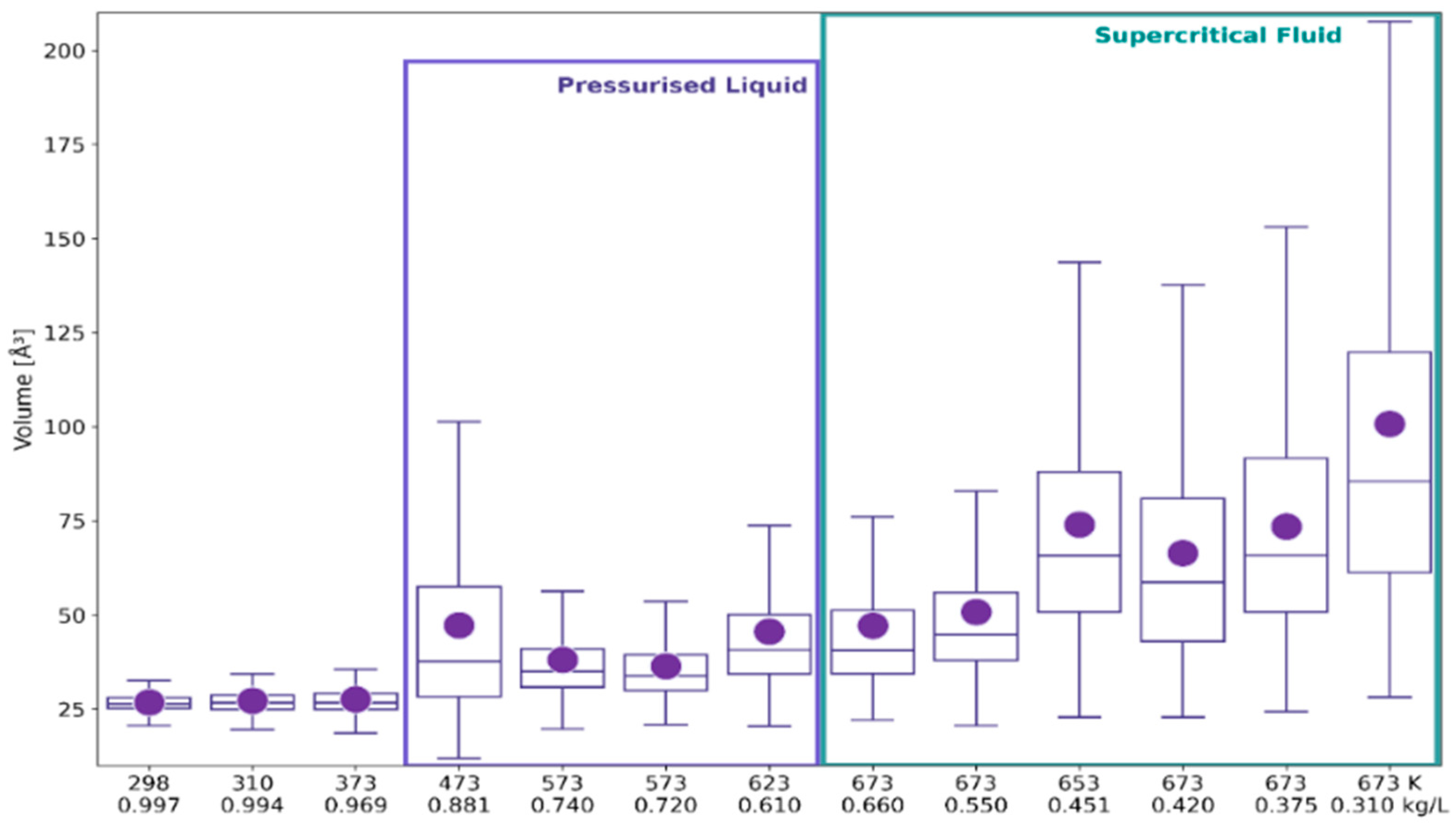
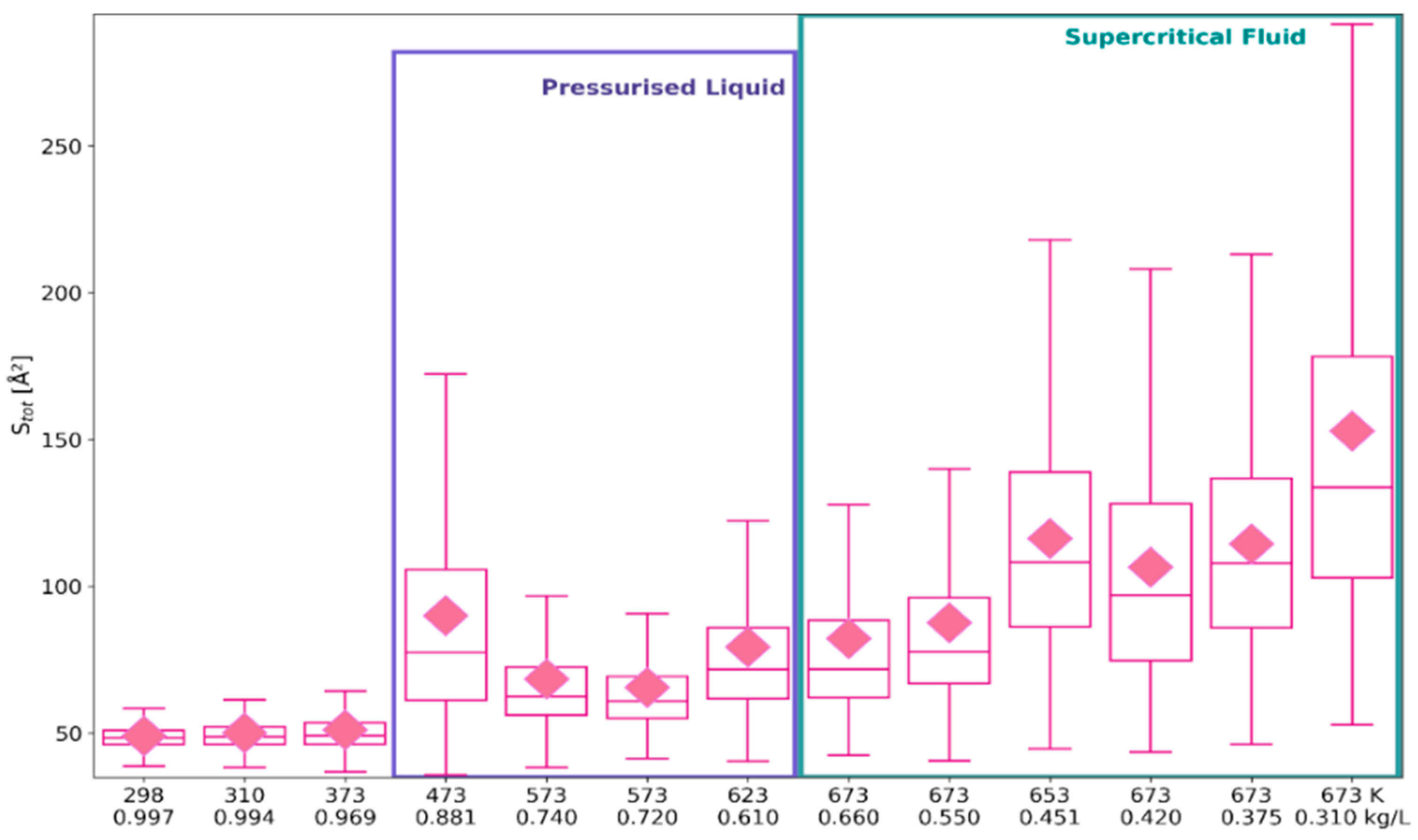
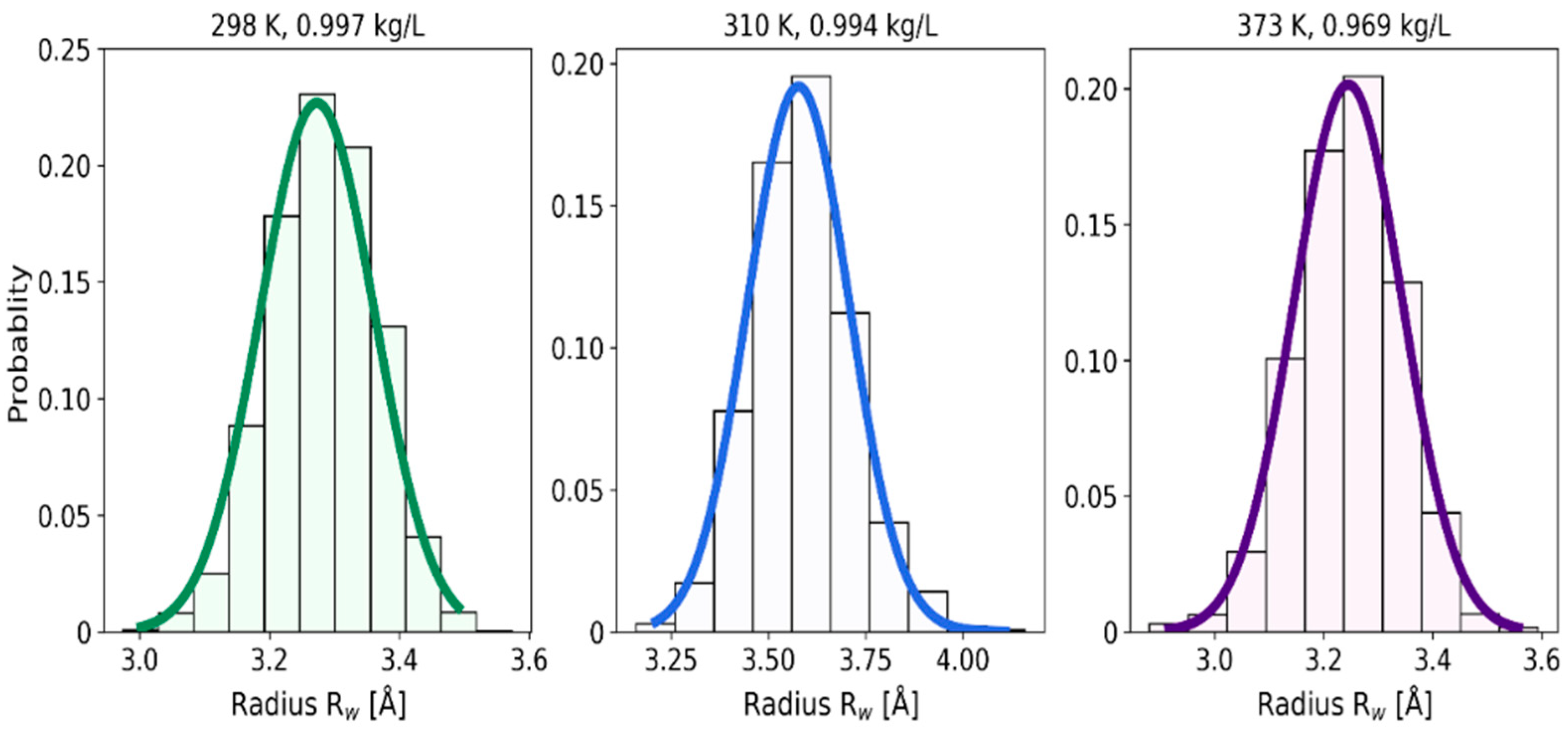
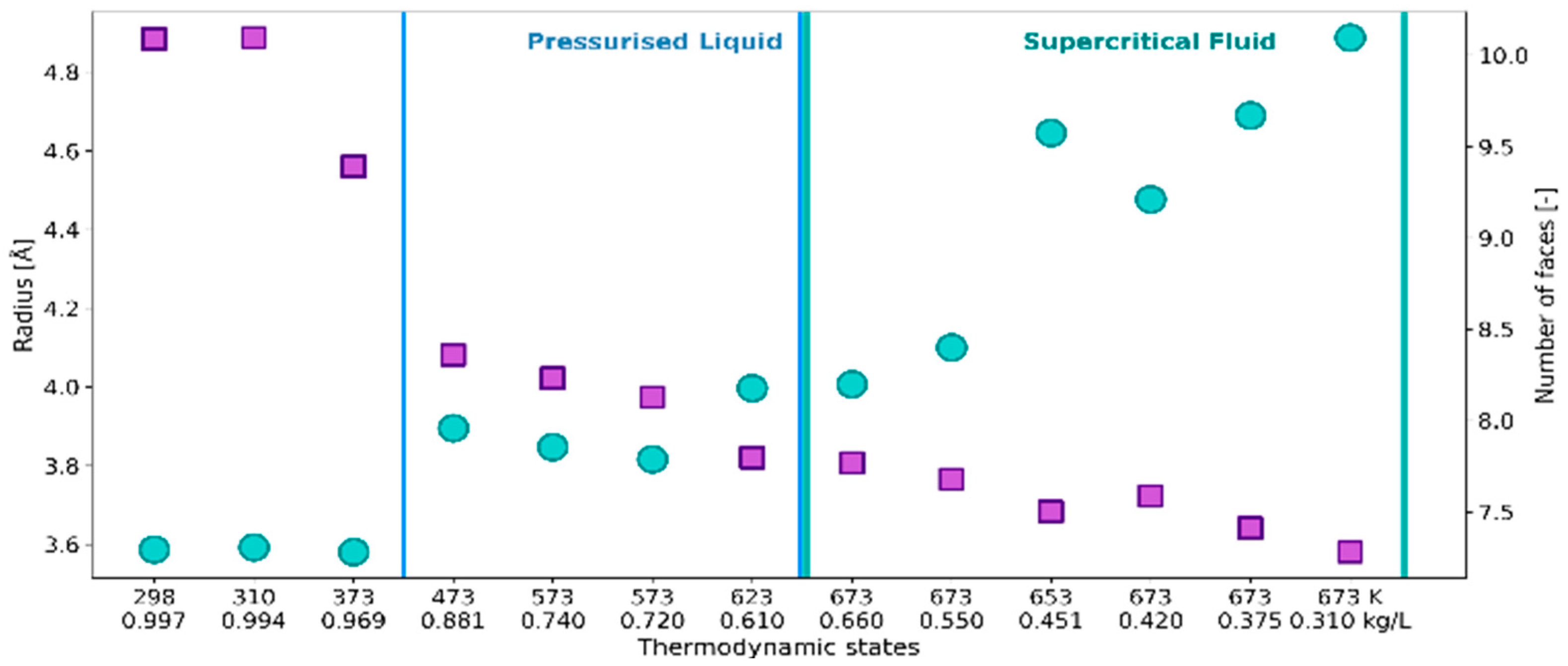
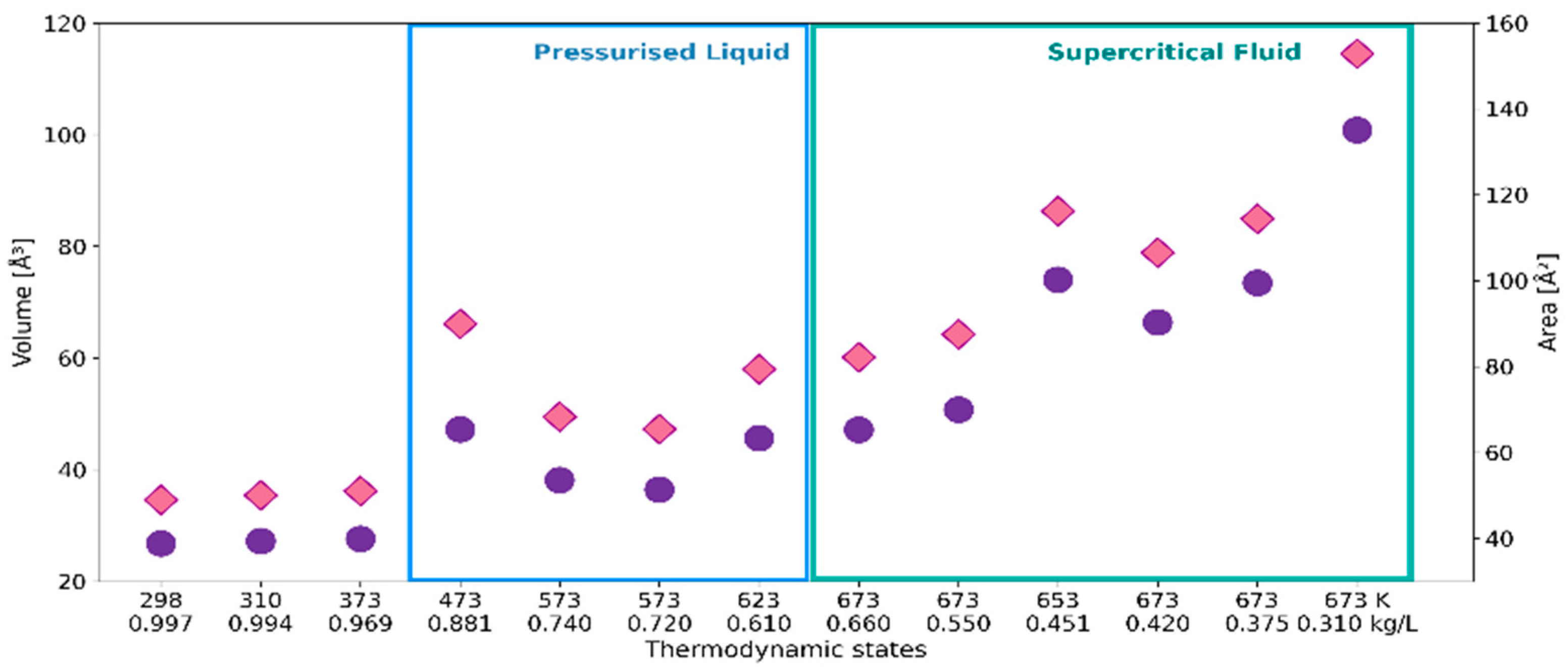
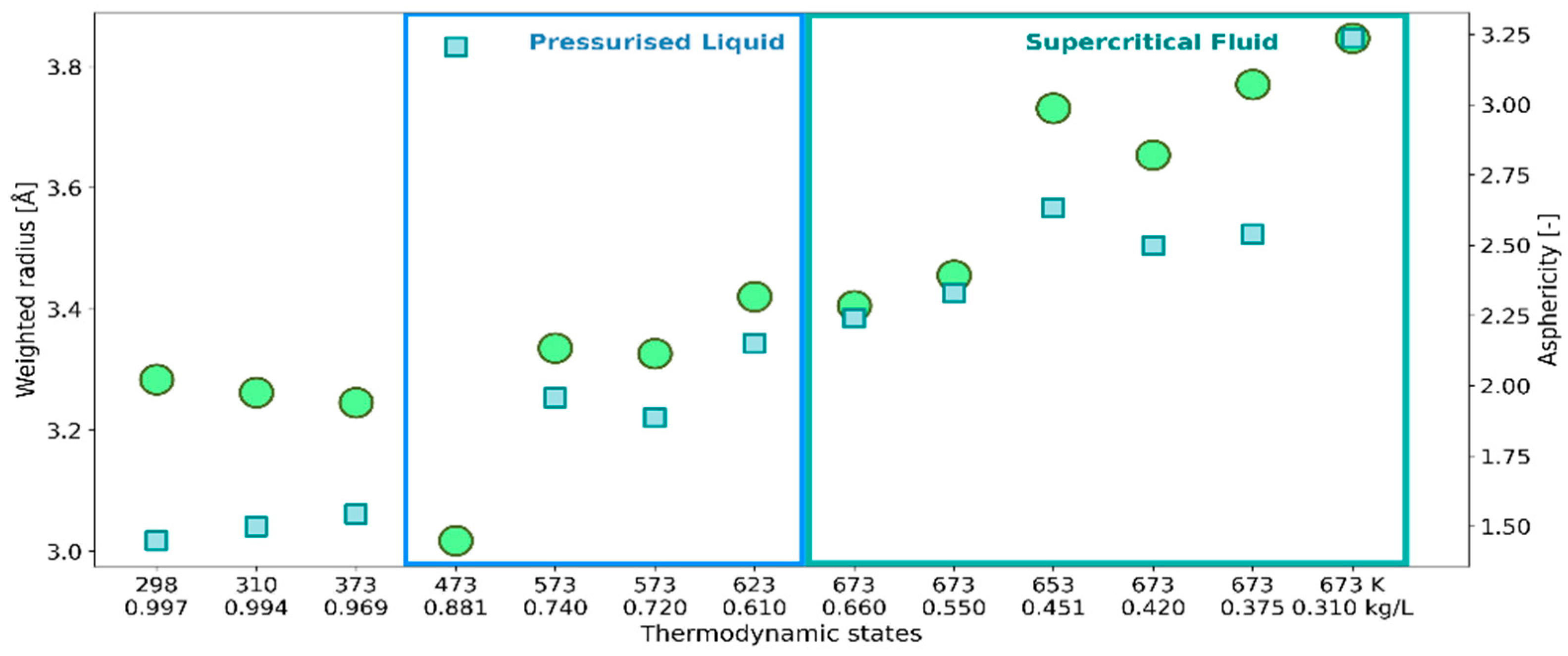
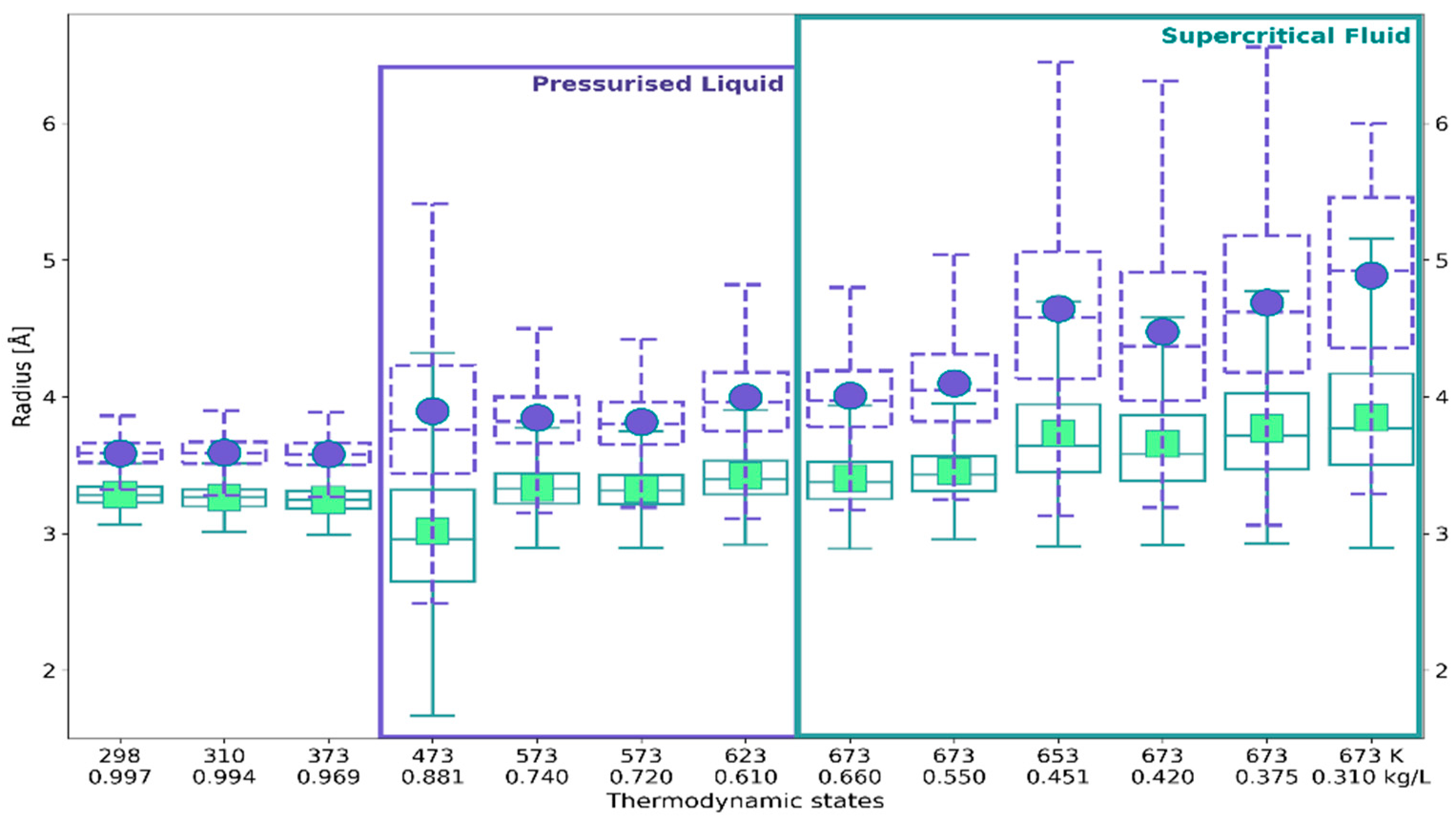
2.1. 3D Characteristics of •OHaq
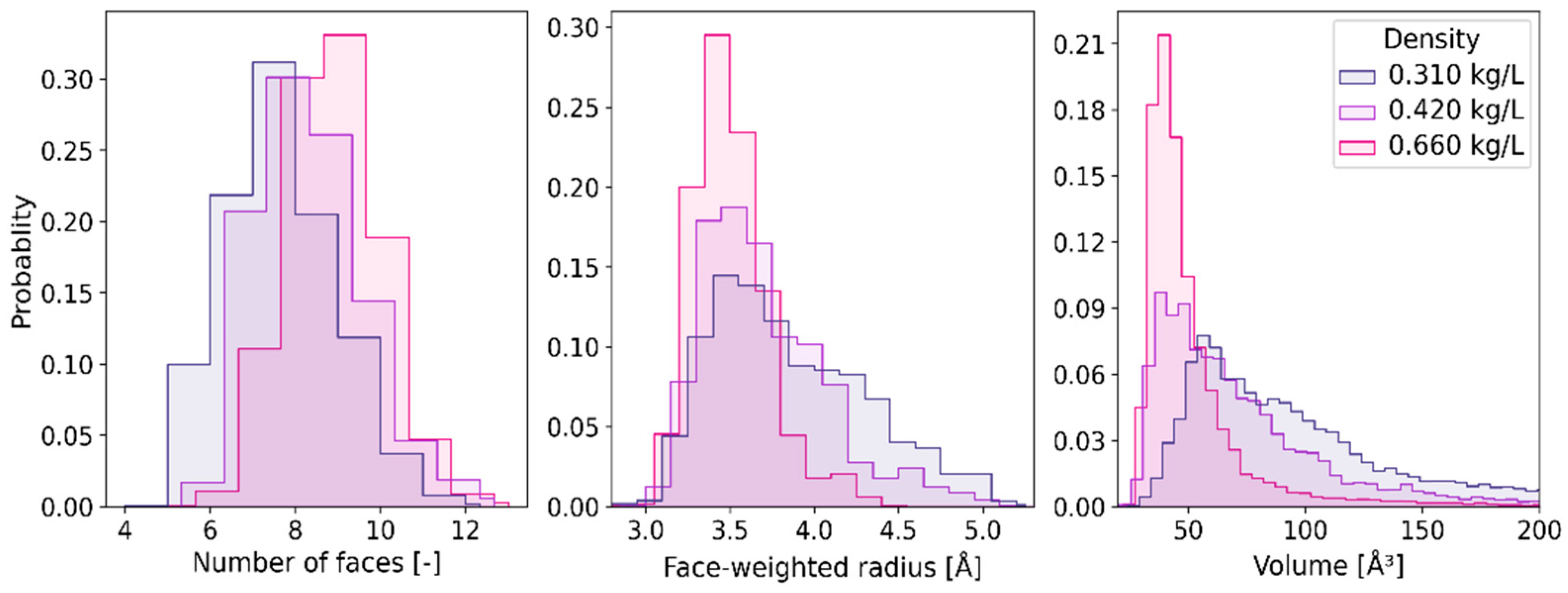
2.2. Statistical Distributions of Metric and Topological Properties of •OHaq
2.3. RDF-Based Description Versus 3D Characteristics of Solvation Shell
3. Discussion
4. Methods
4.1. MD Simulation
4.2. RDF-Based Approach
4.3. VP-Based Approach
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Swiatla-Wojcik, D. Role of water in radical reactions: Molecular simulation and modelling. In Reaction Rate Constant Computations: Theories and Applications; Han, K., Chu, T., Eds.; Theoretical and Computational Chemistry Series No. 6; RSC Publishing: Cambridge, UK, 2014; pp. 352–378. [Google Scholar]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef] [PubMed]
- Cabral do Couto, P.; Guedes, R.C.; Costa Cabral, B.J. The hydration of the OH radical: Microsolvation modeling and statistical mechanics simulation. J. Chem. Phys. 2003, 119, 7344–7355. [Google Scholar] [CrossRef]
- Vassilev, P.; Louwerse, M.J.; Baerends, E.J. Hydroxyl radical and hydroxide ion in liquid water: A comparative electron density functional study. J. Phys. Chem. B 2005, 109, 23605–23610. [Google Scholar] [CrossRef]
- VandeVondele, J.; Sprik, M. A molecular dynamics study of the hydroxyl radical in solution applying self-interaction corrected density functional methods. Phys. Chem. Chem. Phys. 2005, 7, 1363–1367. [Google Scholar] [CrossRef]
- Khalack, J.M.; Lyubartsev, A.P. Solvation structure of hydroxyl radical by Car-Parrinello molecular dynamics. J. Phys. Chem. A 2005, 109, 378–386. [Google Scholar] [CrossRef]
- Svishchev, I.M.; Plugatyr, A.Y. Hydroxyl radical in aqueous solution: Computer simulation. J. Phys. Chem. B 2005, 109, 4123–4128. [Google Scholar] [CrossRef]
- D’Auria, R.; Kuo, I.-F.W.; Tobias, D.J. Ab initio molecular dynamics simulation of the OH• radical in liquid water. J. Phys. Chem. A 2008, 112, 4644–4650. [Google Scholar] [CrossRef] [PubMed]
- Chipman, D.M. Hemibonding between hydroxyl radical and water. J. Phys. Chem. A 2011, 115, 1161–1171. [Google Scholar] [CrossRef]
- Codorniu-Hernandez, E.; Kusalik, P.G. Mobility mechanism of hydroxyl radicals in aqueous solution via hydrogen transfer. J. Am. Chem. Soc. 2012, 134, 532–538. [Google Scholar] [CrossRef]
- Pabis, A.; Szala-Bilnik, J.; Swiatla-Wojcik, D. Molecular dynamics study of the hydration of the hydroxyl radical at body temperature. Phys. Chem. Chem. Phys. 2011, 13, 9458–9468. [Google Scholar] [CrossRef]
- Szala-Bilnik, J.; Swiatla-Wojcik, D. Hydration of OH radical in high temperature water. J. Mol. Liq. 2011, 164, 34–38. [Google Scholar] [CrossRef]
- Swiatla-Wojcik, D.; J. Szala-Bilnik, J. Mechanism of OH radical hydration: A comparative computational study of liquid and supercritical solvent. J. Chem. Phys. 2012, 136, 064510. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, L.; Swiatla-Wojcik, D. Voronoi polyhedra probing of hydrated OH radical. RSC Adv. 2014, 4, 41812–41818. [Google Scholar] [CrossRef]
- Apostolidou, C. OH radical in water from ab initio molecular dynamics simulation employing hybrid functionals. J. Chem. Phys. 2019, 151, 064111. [Google Scholar] [CrossRef]
- Swiatla-Wojcik, D.; Szala-Bilnik, J. High temperature aqueous solvent effect on stretching vibrations of the hydroxyl radical—MD simulation study of spectral shifts and hydrogen bond statistics. J. Supercrit. Fluids 2019, 143, 126–133. [Google Scholar] [CrossRef]
- Swiatla-Wojcik, D.; Szala-Bilnik, J. High temperature aqueous solvent effect on translational and hydrogen bond dynamics of the hydroxyl radical—MD simulation study. J. Supercrit. Fluids 2019, 145, 103–110. [Google Scholar] [CrossRef]
- Rana, B.; Herbert, J.M. Role of hemibonding in the structure and ultraviolet spectroscopy of the aqueous hydroxyl radical. Phys. Chem. Chem. Phys. 2020, 22, 27829–27844. [Google Scholar] [CrossRef] [PubMed]
- Berens, P.H.; Wilson, K.R. Molecular dynamics and spectra. I. Diatomic rotation and vibration. J. Chem. Phys. 1981, 74, 4872–4882. [Google Scholar] [CrossRef]
- Ashton, L.; Buxton, G.V.; Stuart, C.R. Temperature dependence of the rate of reaction of OH with some aromatic compounds in aqueous solution. Evidence for the formation of a π-complex intermediate? J. Chem. Soc. Faraday Trans. 1995, 91, 1631–1633. [Google Scholar] [CrossRef]
- Elliot, A.J.; McCracken, D.R.; Buxton, G.V.; Wood, N.D. Estimation of rate constants for near-diffusion-controlled reactions in water at high temperatures. J. Chem. Soc. Faraday Trans. 1990, 86, 1539–1547. [Google Scholar] [CrossRef]
- Feng, J.; Aki, S.N.V.K.; Chateauneuf, J.; Brennecke, J. Hydroxyl radical reactivity with nitrobenzene in subcritical and supercritical water. J. Am. Chem. Soc. 2002, 124, 6304–6311. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, A.; Karlström, G.; Nelander, B. The water–hydroxyl radical complex: A matrix isolation study. J. Chem. Phys. 2003, 118, 7797–7802. [Google Scholar] [CrossRef]
- Tsuji, K.; Shibuya, K. Infrared spectroscopy and quantum chemical calculations of OH-(H2O)n complexes. J. Phys. Chem. 2009, 113, 9945–9951. [Google Scholar] [CrossRef] [PubMed]
- Kallikragas, D.; Plugatyr, A.; Svishchev, I. High temperature diffusion coefficients for O2, H2, and OH in water and for pure water. J. Chem. Eng. Data 2014, 59, 1964–1968. [Google Scholar] [CrossRef]
- Kjellsson, L.; Nanda, K.D.; Rubensson, J.-E.; Doumy, G.; Southworth, S.H.; Ho, P.J.; March, A.M.; Al Haddad, A.; Kumagai, Y.; Tu, M.-F.; et al. Resonant inelastic x-ray scattering reveals hidden local transitions of the aqueous OH radical. Phys. Rev. Lett. 2020, 124, 236001. [Google Scholar] [CrossRef] [PubMed]
- David, E.E.; David, C.W. Voronoi polyhedra as a tool for studying solvation structure. J. Chem. Phys. 1982, 76, 4611–4614. [Google Scholar] [CrossRef]
- Swiatla-Wojcik, D.; Szala-Bilnik, J. Transition from patchlike to clusterlike inhomogeneity arising from hydrogen bonding in water. J. Chem. Phys. 2011, 134, 54121. [Google Scholar] [CrossRef]
- Sun, C.Q. Unprecedented O:⇔:O compression and H↔H fragilization in Lewis solutions. Phys. Chem. Chem. Phys. 2019, 21, 2234–2250. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; Huang, Y.; Zhang, X.; Ma, Z.; Wang, B. The physics behind water irregularity. Phys. Rep. 2023, 998, 1–68. [Google Scholar] [CrossRef]
- Bopp, P.; Jancsó, G.; Heinzinger, K. An improved potential for non-rigid water molecules in the liquid phase. Chem. Phys. Lett. 1983, 98, 129–133. [Google Scholar] [CrossRef]
- DuToit, S.H.C.; Steyn, A.G.W.; Stumpf, R.H. Graphical Exploratory Data Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Hintze, J.L.; Nelson, R.D. Violin Plots: A Box Plot-Density Trace Synergism. Am. Stat. 1998, 52, 181–184. [Google Scholar]

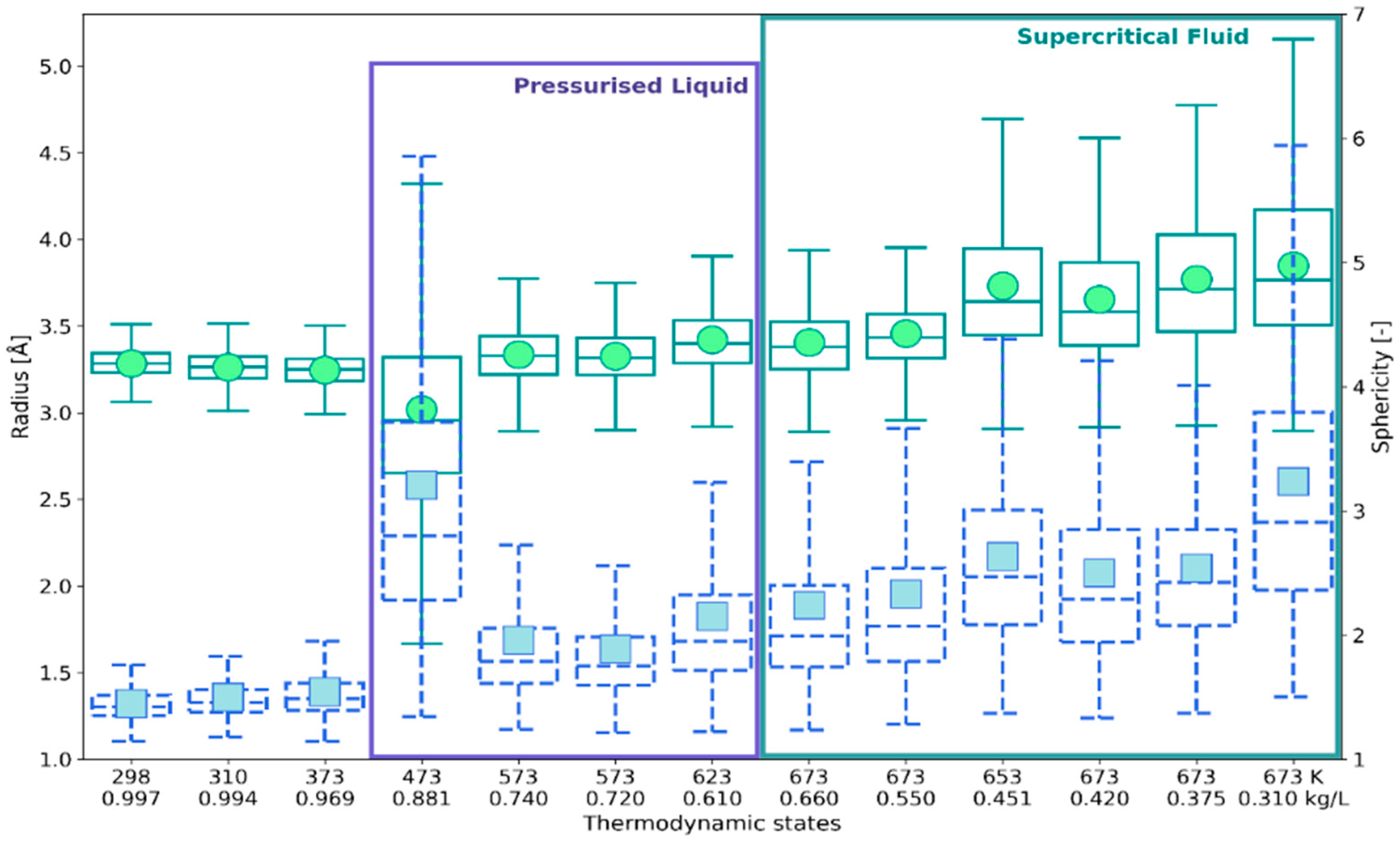
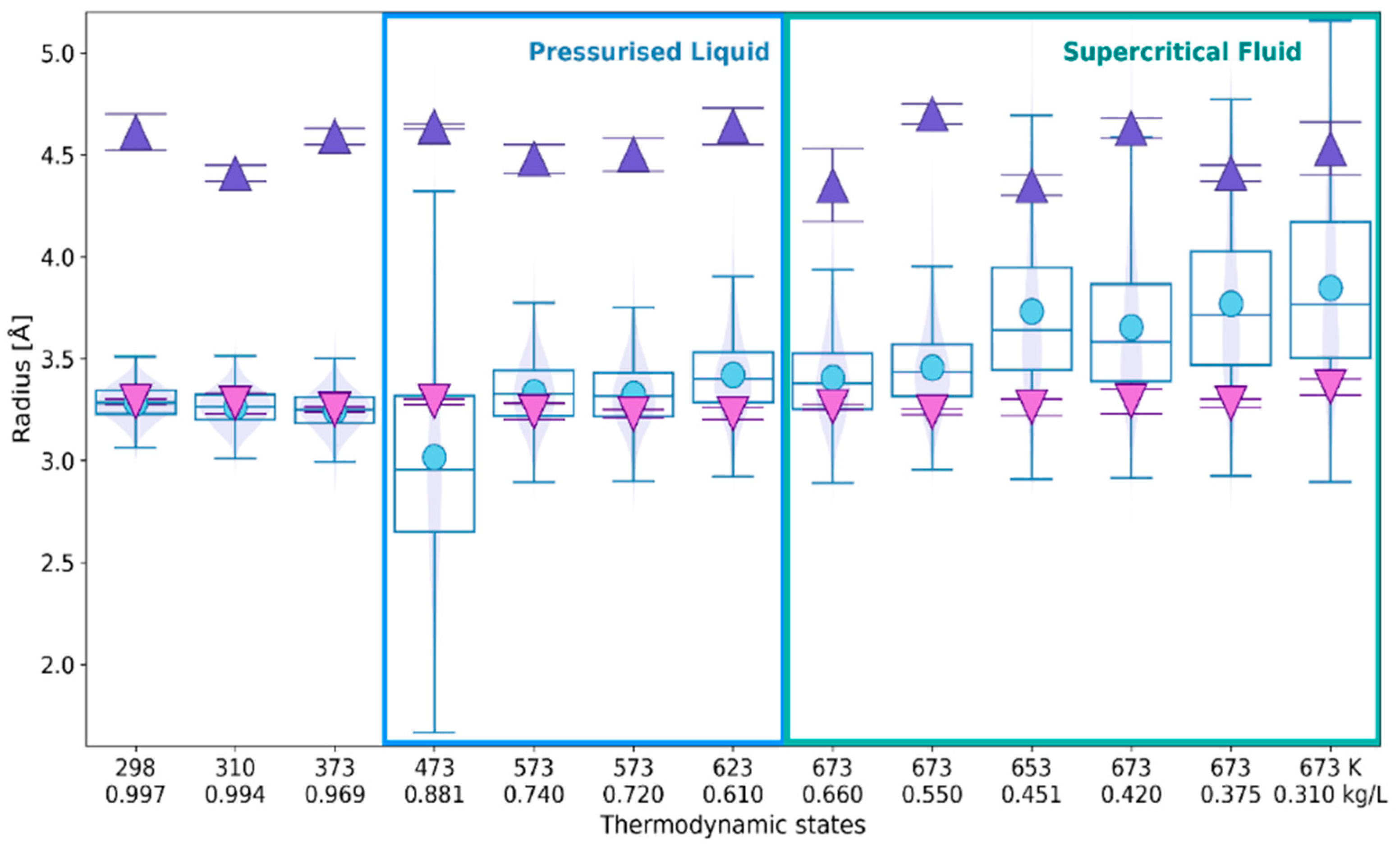
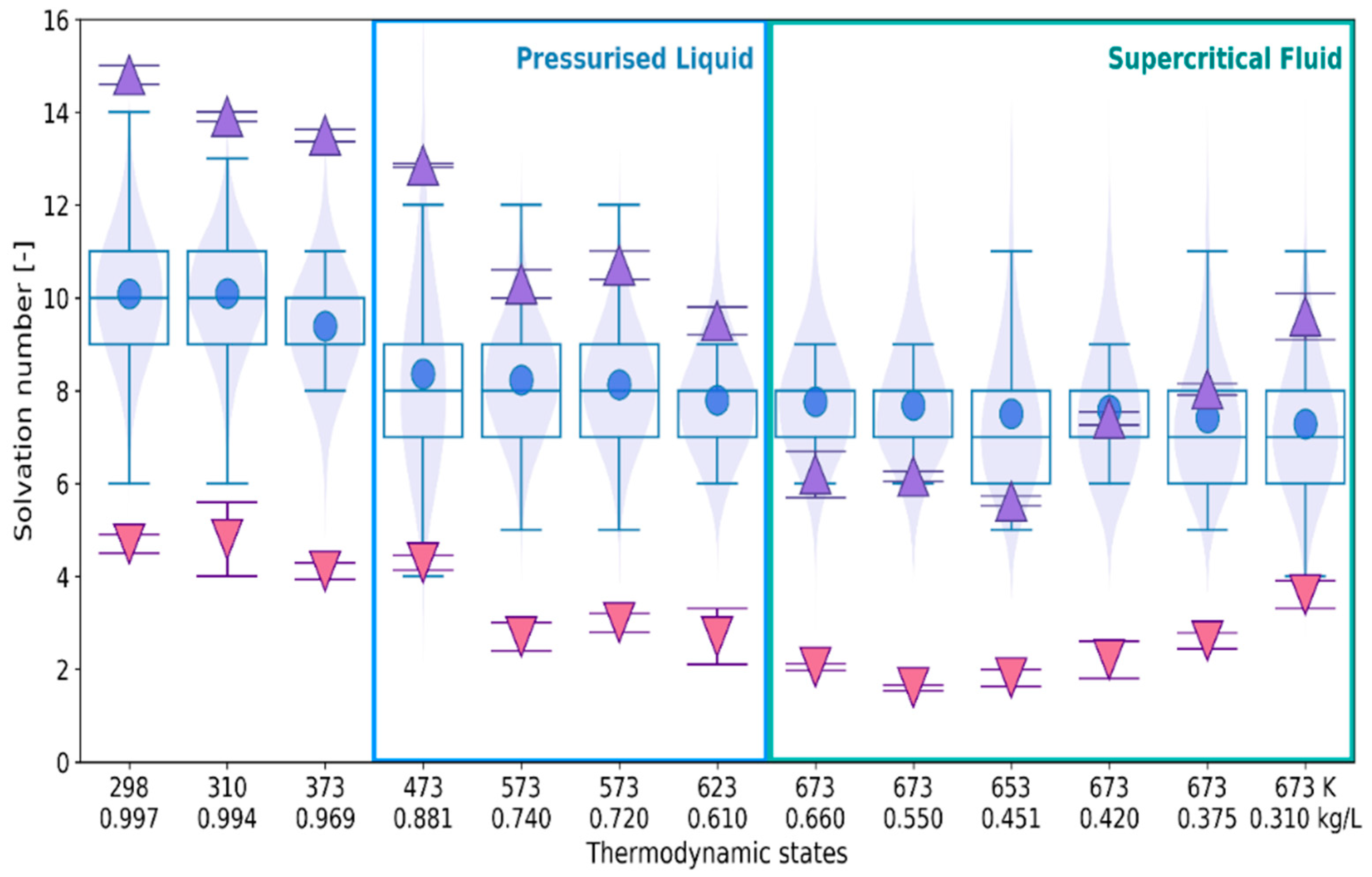
| Temperature [K] | Density [kg/L] | Number of Probes |
|---|---|---|
| 298 | 0.997 | 24,996 |
| 310 | 0.994 | 25,000 |
| 373 | 0.969 | 24,999 |
| 473 | 0.881 | 21,036 |
| 573 | 0.740 | 24,493 |
| 573 | 0.720 | 24,843 |
| 623 | 0.610 | 24,577 |
| 673 | 0.660 | 24,376 |
| 673 | 0.550 | 24,353 |
| 653 | 0.451 | 22,613 |
| 673 | 0.420 | 23,020 |
| 673 | 0.375 | 19,929 |
| 673 | 0.310 | 19,358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazmierczak, L.; Szala-Rearick, J.; Swiatla-Wojcik, D. 3D Characterization of the Molecular Neighborhood of •OH Radical in High Temperature Water by MD Simulation and Voronoi Polyhedra. Int. J. Mol. Sci. 2023, 24, 3294. https://doi.org/10.3390/ijms24043294
Kazmierczak L, Szala-Rearick J, Swiatla-Wojcik D. 3D Characterization of the Molecular Neighborhood of •OH Radical in High Temperature Water by MD Simulation and Voronoi Polyhedra. International Journal of Molecular Sciences. 2023; 24(4):3294. https://doi.org/10.3390/ijms24043294
Chicago/Turabian StyleKazmierczak, Lukasz, Joanna Szala-Rearick, and Dorota Swiatla-Wojcik. 2023. "3D Characterization of the Molecular Neighborhood of •OH Radical in High Temperature Water by MD Simulation and Voronoi Polyhedra" International Journal of Molecular Sciences 24, no. 4: 3294. https://doi.org/10.3390/ijms24043294
APA StyleKazmierczak, L., Szala-Rearick, J., & Swiatla-Wojcik, D. (2023). 3D Characterization of the Molecular Neighborhood of •OH Radical in High Temperature Water by MD Simulation and Voronoi Polyhedra. International Journal of Molecular Sciences, 24(4), 3294. https://doi.org/10.3390/ijms24043294







