Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields
Abstract
1. Introduction
2. A Developmental Model of Endochondral Bone Formation That Allows for Exploration of Cell Differentiation and Phenotypic Expression of ECM Synthesis in Response to PEMFs
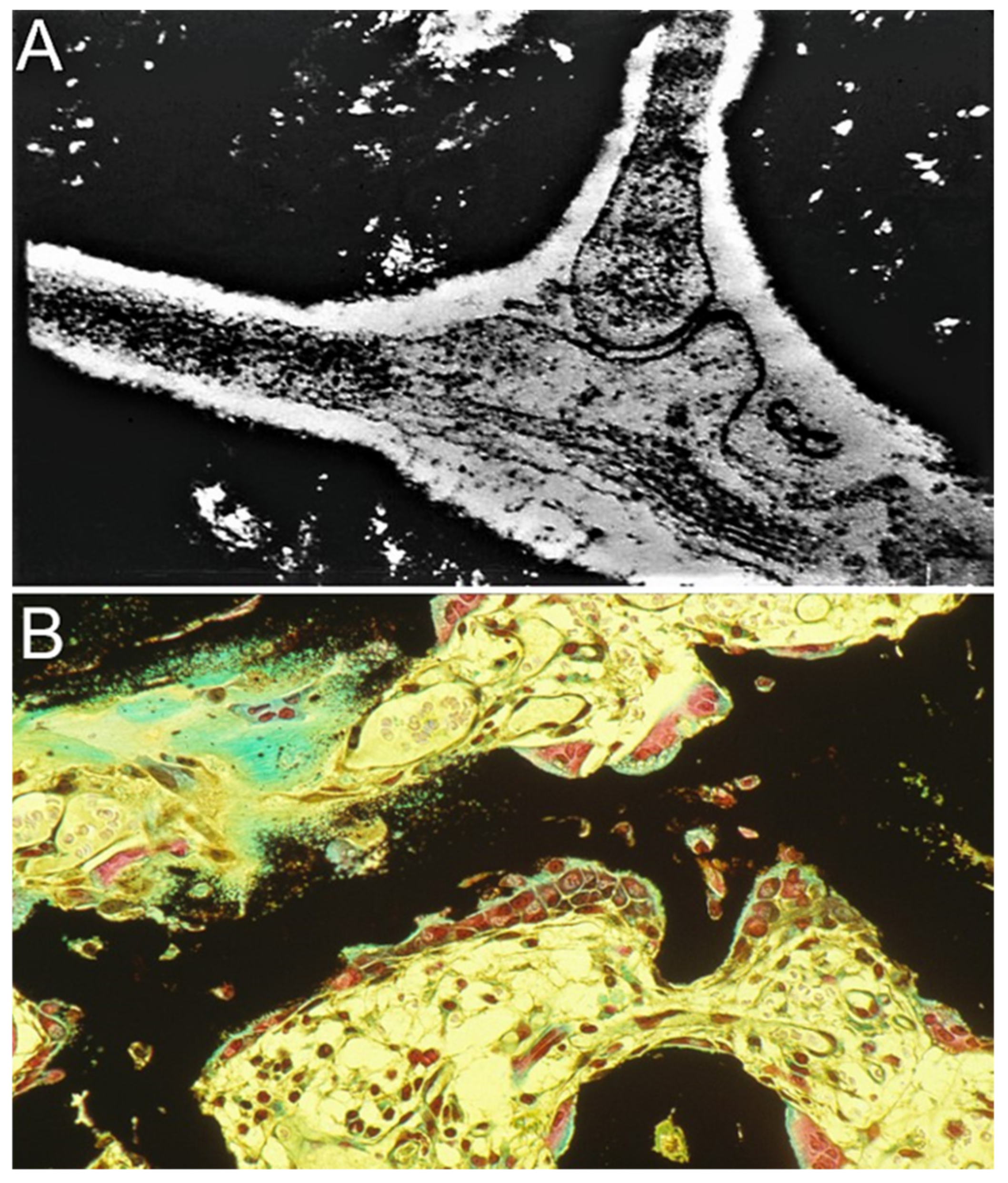
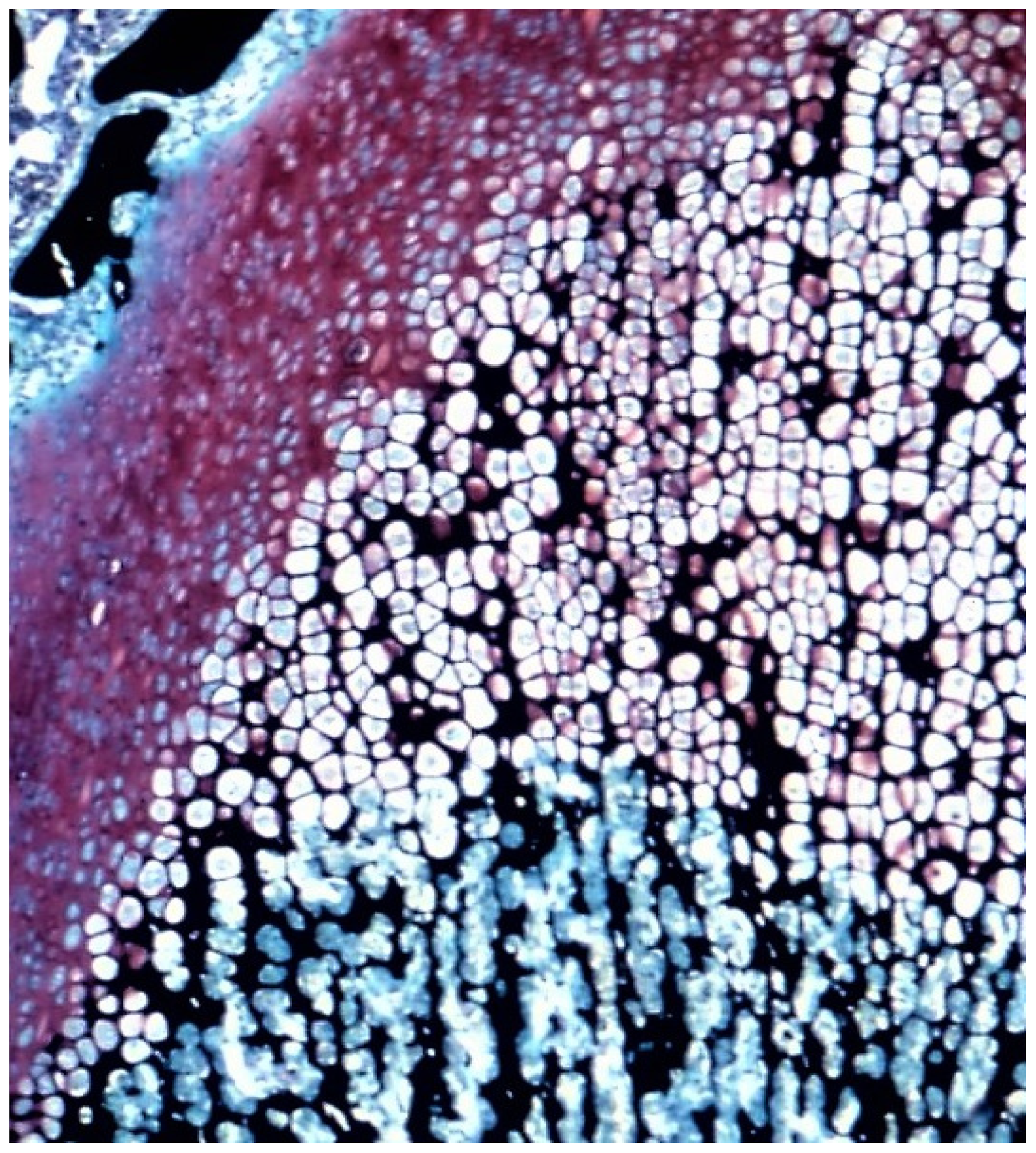
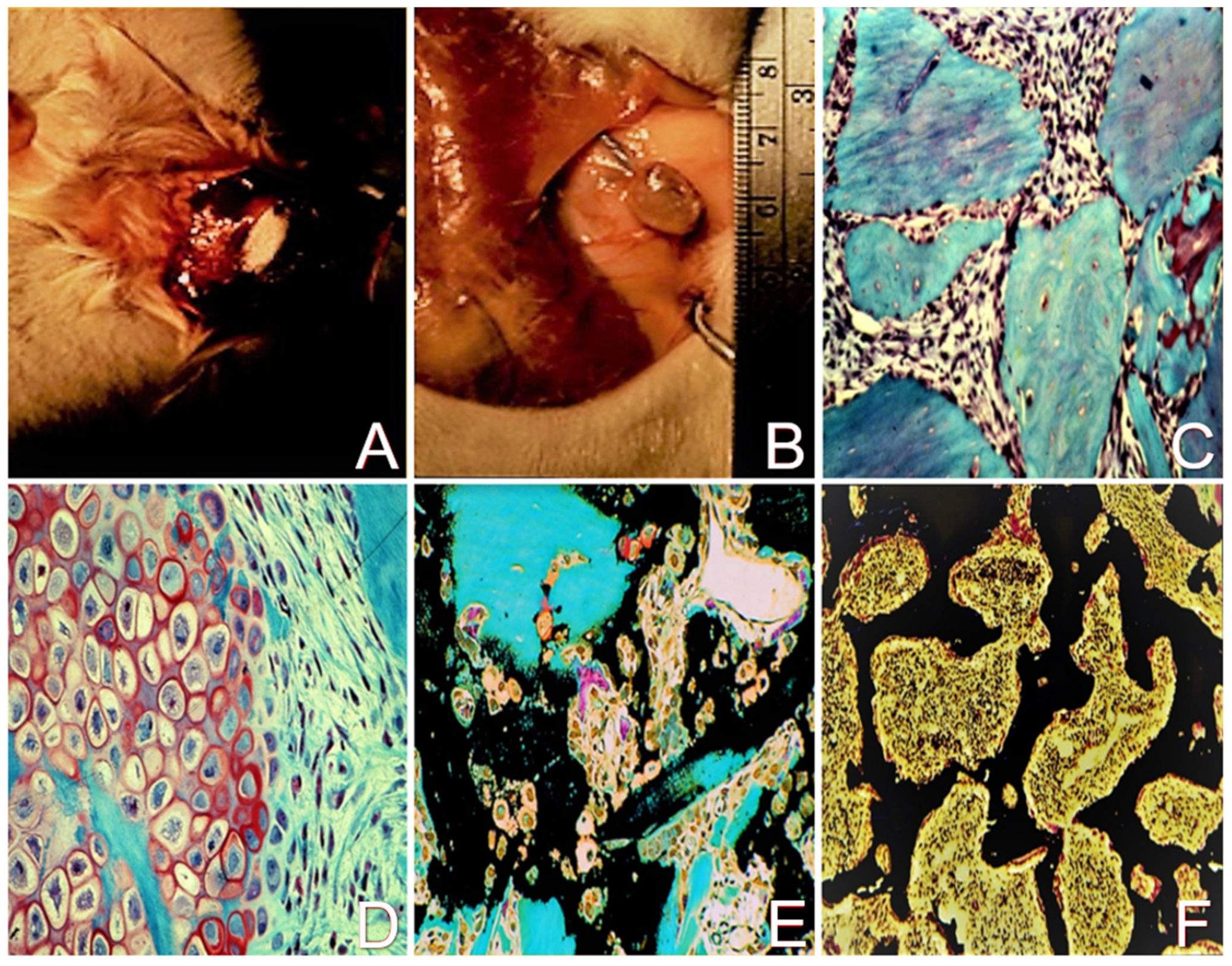
2.1. The Demineralized Bone Matrix-Induced Endochondral Bone Formation Model
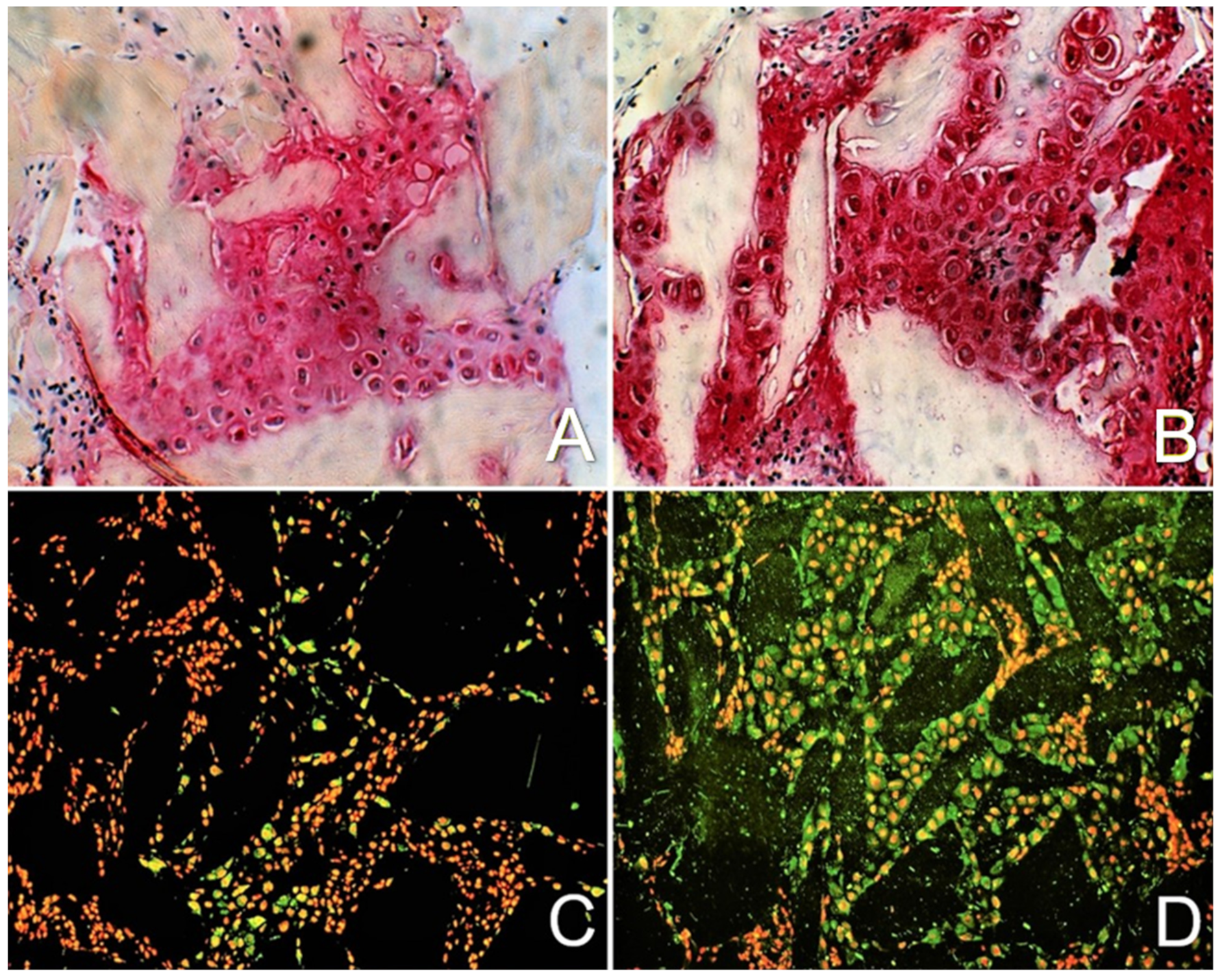
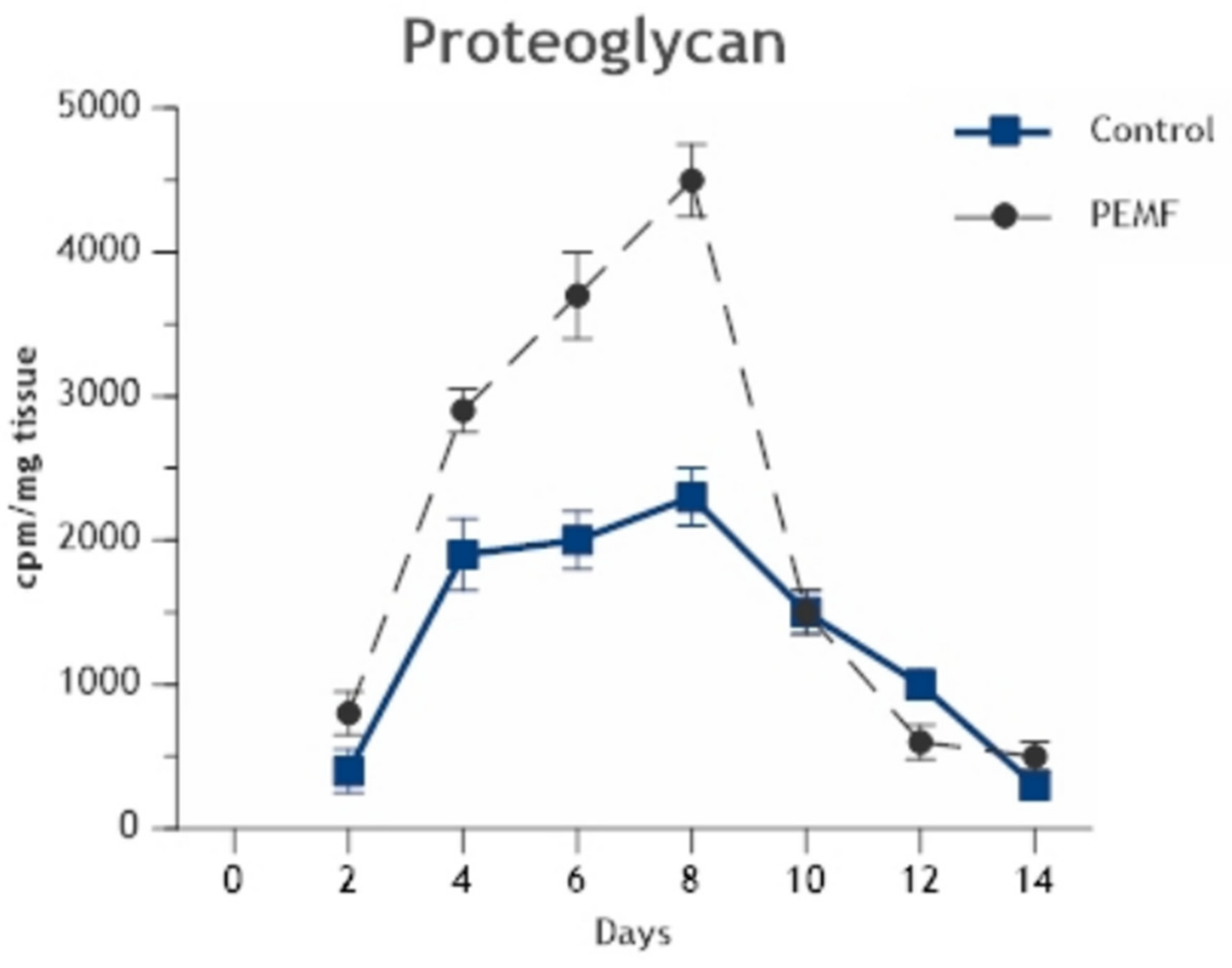
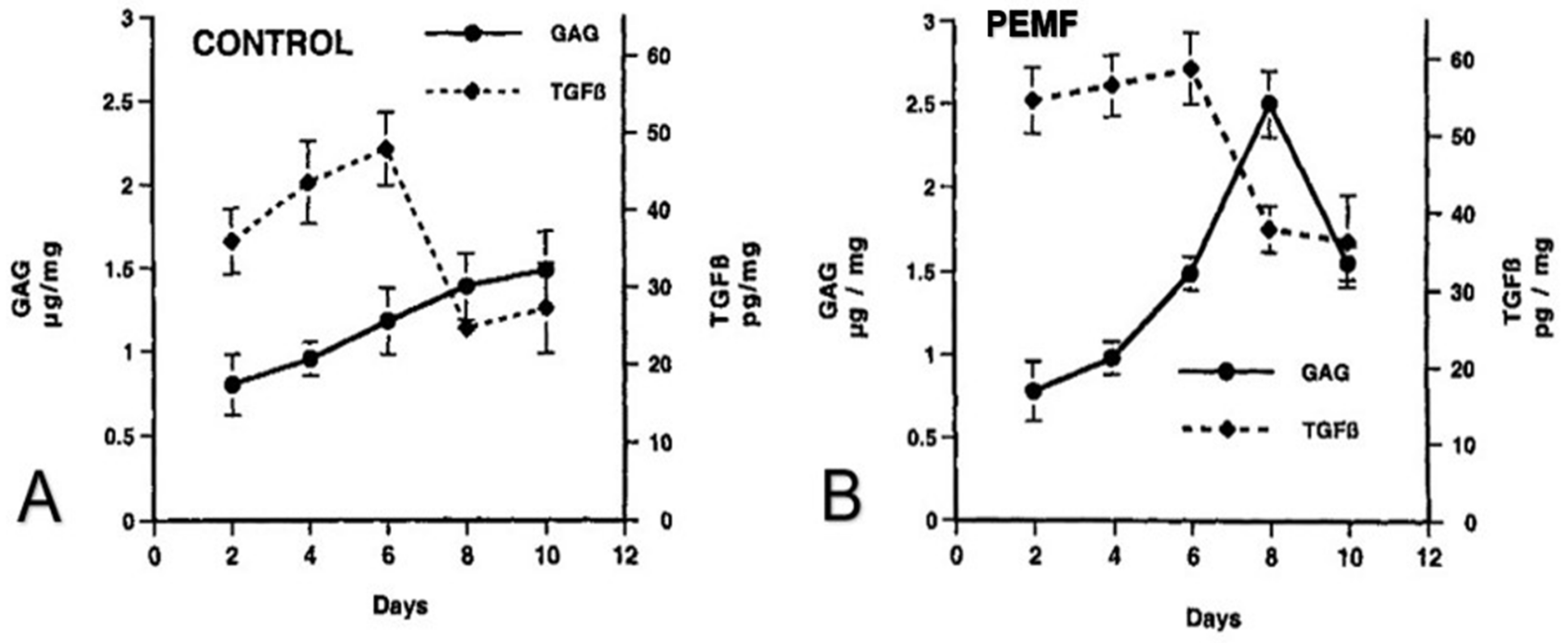
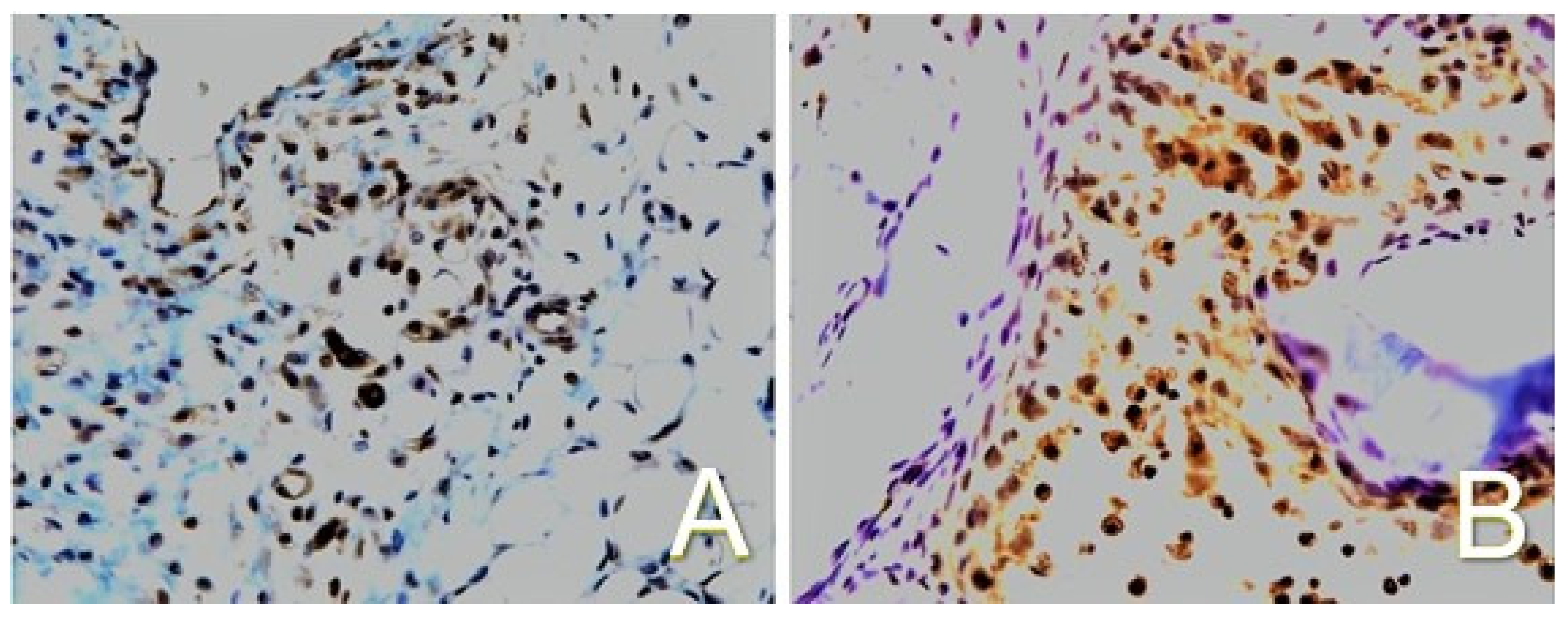
2.2. Enhancement of Cell Differentiation in the DBM-EO Model by PEMFs
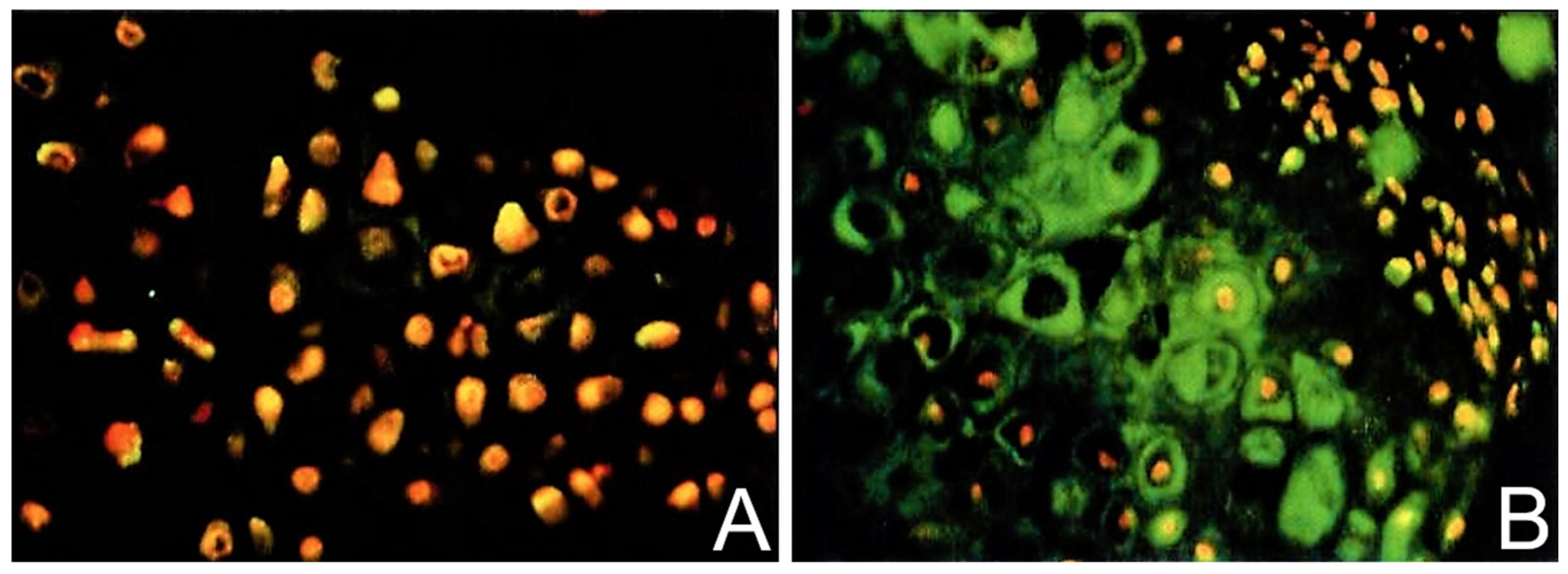
2.3. TGF-β Signaling under PEMF Stimulation in the DBM-EO Model
2.4. PEMF Influences on Signal Transduction, Intracellular Messaging, and Activation of TGF-β
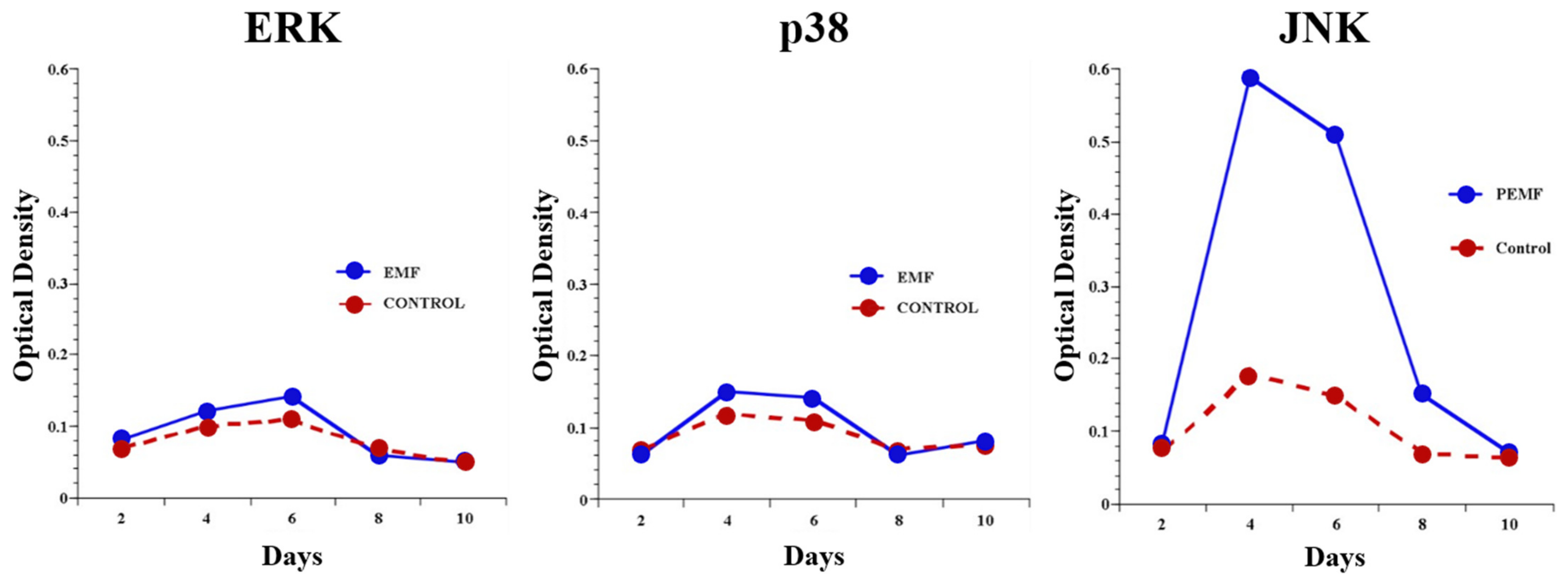
2.4.1. Signal Transduction
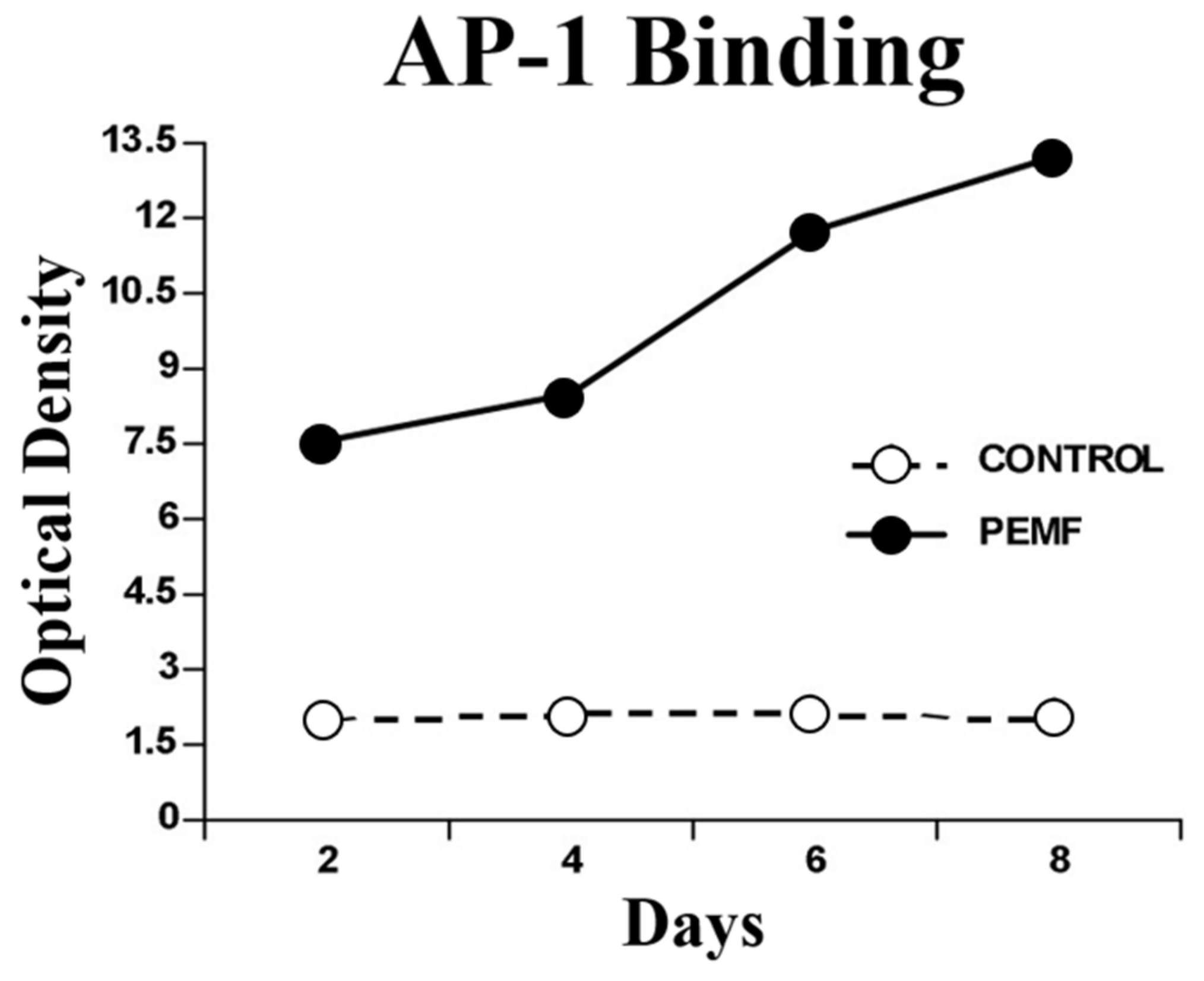
2.4.2. Intracellular Messaging
2.4.3. Activation of TGF-β
3. PEMF Dosimetry in the Regulation of Cell Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, C.T.; Racine-Avila, J.; Pellicore, M.J.; Aaron, R. Biophysical Modulation of Mesenchymal Stem Cell Differentiation in the Context of Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 3919. [Google Scholar] [CrossRef] [PubMed]
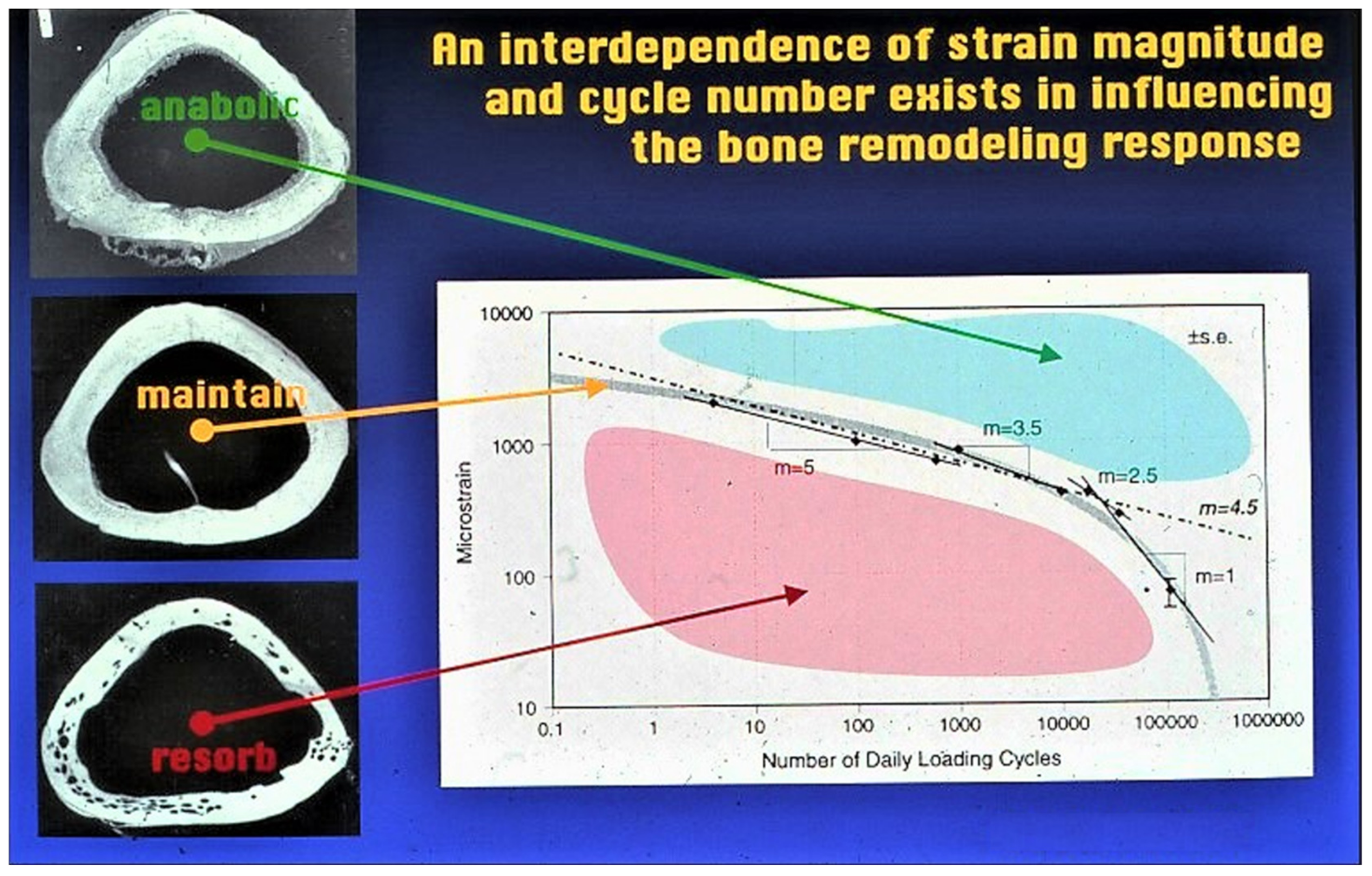
- Qin, Y.-X.; Rubin, C.T.; McLeod, K.J. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J. Orthop. Res. 1998, 16, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Testa, E.J.; Callanan, T.C.; Evans, A.R.; Aaron, R.K. Osteoporosis and Fragility Fractures. Rhode Isl. Med. J. 2022, 105, 15–21. [Google Scholar]
- Cadossi, R.; Massari, L.; Racine-Avila, J.; Aaron, R.K. Pulsed Electromagnetic Field Stimulation of Bone Healing and Joint Preservation: Cellular Mechanisms of Skeletal Response. JAAOS: Glob. Res. Rev. 2020, 4, e19.00155. [Google Scholar] [CrossRef] [PubMed]
- Aaron, R.K.; Jolly, G.; McK Ciombor, D.; Barrach, H.-J. A histochemical method for the demonstration of calcifying cartilage. Calcif. Tissue Int. 1988, 43, 244–249. [Google Scholar] [CrossRef]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell. Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
- Aaron, R.K.; Mc Ciombor, D.K. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J. Orthop. Res. 1996, 14, 582–589. [Google Scholar] [CrossRef]
- Aaron, R.K. “Electrical Stimulation of Bone Repair-Clinical Experiences”. Warren Alpert Medical School Scholarly Concentrations Program Gallery of Scholarly Work. Brown Digital Repository; Brown University Library: Providence, RI, USA, 2022. [Google Scholar] [CrossRef]
- Ciombor, D.M.; Lester, G.; Aaron, R.K.; Neame, P.; Caterson, B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J. Orthop. Res. 2002, 20, 40–50. [Google Scholar] [CrossRef]
- Aaron, R.K.; Wang, S.; Ciombor, D.M. Upregulation of basal TGFβ1 levels by EMF coincident with chondrogenesis–implications for skeletal repair and tissue engineering. J. Orthop. Res. 2002, 20, 233–240. [Google Scholar] [CrossRef]
- Aaron, R.K.; Ciombor, D.M.; Wang, S.; Simon, B. Clinical Biophysics: The Promotion of Skeletal Repair by Physical Forces. Ann. New York Acad. Sci. 2006, 1068, 513–531. [Google Scholar] [CrossRef]
- Govey, P.M.; Loiselle, A.E.; Donahue, H.J. Biophysical Regulation of Stem Cell Differentiation. Curr. Osteoporos. Rep. 2013, 11, 83–91. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Li, W.-J.; Tuan, R.S.; Chang, W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J. Orthop. Res. 2009, 27, 1169–1174. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Zhuang, H.; Wang, W.; Seldes, R.M.; Tahernia, A.D.; Fan, H.; Brighton, C.T. Electrical Stimulation Induces the Level of TGF-β1 mRNA in Osteoblastic Cells by a Mechanism Involving Calcium/Calmodulin Pathway. Biochem. Biophys. Res. Commun. 1997, 237, 225–229. [Google Scholar] [CrossRef]
- Guerkov, H.H.; Lohmann, C.; Liu, Y.; Dean, D.; Simon, B.J.; Heckman, J.D.; Schwartz, Z.; Boyan, B.D. Pulsed Electromagnetic Fields Increase Growth Factor Release by Nonunion Cells. Clin. Orthop. Relat. Res. 2001, 384, 265–279. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Schwartz, Z.; Liu, Y.; Guerkov, H.; Dean, D.D.; Simon, B.; Boyan, B.D. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J. Orthop. Res. 2000, 18, 637–646. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef]
- Yap, J.L.Y.; Tai, Y.K.; Fröhlich, J.; Fong, C.H.H.; Yin, J.N.; Foo, Z.L.; Ramanan, S.; Beyer, C.; Toh, S.J.; Casarosa, M.; et al. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB J. 2019, 33, 12853–12872. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediat. Inflamm. 2017, 2017, 2740963. [Google Scholar] [CrossRef]
- Bagheri, L.; Pellati, A.; Rizzo, P.; Aquila, G.; Massari, L.; De Mattei, M.; Ongaro, A. Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J. Tissue Eng. Regen. Med. 2018, 12, 304–315. [Google Scholar] [CrossRef]
- Gui, T.; Sun, Y.; Shimokado, A.; Muragaki, Y. The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-β-Induced Epithelial-Mesenchymal Transition. J. Signal Transduct. 2012, 2012, 289243. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, D.M.; Wang, S.; Keeping, H.S.; Simon, B.J.; Aaron, R.K. EMF fields activate the JNK MAPK pathway resulting in enhanced TGF-β gene ex-pression. Trans. Orthop. Res. Soc. 2004, 29, 0093. [Google Scholar]
- Poh, P.S.P.; Seeliger, C.; Unger, M.; Falldorf, K.; Balmayor, E.R.; Van Griensven, M. Osteogenic Effect and Cell Signaling Activation of Extremely Low-Frequency Pulsed Electromagnetic Fields in Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2018, 2018, 5402853. [Google Scholar] [CrossRef] [PubMed]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Lee, P.S.; Van Rienen, U.; Appali, R. A General Theoretical Framework to Study the Influence of Electrical Fields on Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 557447. [Google Scholar] [CrossRef]
- Anbarasan, S.; Baraneedharan, U.; Paul, S.F.D.; Kaur, H.; Rangaswami, S.; Bhaskar, E. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes. Indian J. Orthop. 2016, 50, 87–93. [Google Scholar] [CrossRef]
- Vinod, E.; Kachroo, U.; Rebekah, G.; Thomas, S.; Ramasamy, B. In vitro chondrogenic differentiation of human articular cartilage derived chondroprogenitors using pulsed electromagnetic field. J. Clin. Orthop. Trauma 2020, 14, 22–28. [Google Scholar] [CrossRef]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.P.; Franco-Obregón, A.; Yang, Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef]
- De Mattei, M.; Fini, M.; Setti, S.; Ongaro, A.; Gemmati, D.; Stabellini, G.; Pellati, A.; Caruso, A. Proteoglycan synthesis in bovine articular cartilage explants exposed to different low-frequency low-energy pulsed electromagnetic fields. Osteoarthr. Cartil. 2007, 15, 163–168. [Google Scholar] [CrossRef]
- Caplan, A.; Goldberg, V.M. Orthopedic Tissue Engineering. Clin. Orthop. Relat. Res. 1999, 367, S2–S4. [Google Scholar] [CrossRef]


| Control | PEMF | Percent Difference | p-Value | |
|---|---|---|---|---|
| mRNA aggrecan | 6.1 | 22.5 | 269 | 0.02 |
| mRNA type II collagen | 11.8 | 21.9 | 86 | 0.05 |
| 35SO4 incorporation (cpm/mg) | 2166 ± 387 | 4448 ± 293 | 105 | 0.005 |
| GAG content (μg/mg) | 1.4 ± 0.2 | 2.5 ± 0.2 | 79 | 0.01 |
| Cartilage area (mm2) | 24 ± 2.1 | 148 ± 11.7 | 517 | 0.001 |
| Chondrocytes (n) | 701 ± 227 | 3582 ± 675 | 411 | 0.005 |
| Chondrocyte/cartilage | 29.2 | 24.2 | n.s. |
| Aggrecan (kb) | α1 (II) Collagen | |||
|---|---|---|---|---|
| 8.0 | 7.4 | |||
| Day 6 | Control | 3.9 | 3.9 | 0 |
| Exposed | 13.3 * | 13.3 * | 0 | |
| Day 8 | Control | 6.2 | 6.1 | 11.8 |
| Exposed | 17.8 * | 22.5 * | 21.9 * | |
| Control | PEMF | Percent Difference | p-Value | |
|---|---|---|---|---|
| mRNA (OD/GAPDH) | 21.0 ± 4.4 | 54.1 ± 0.7 | 158 | 0.03 |
| Protein (pg/ng) | 47.9 ± 4.7 | 58.8 ± 3.2 | 23 | 0.05 |
| Immuno (+) cells (n) | 121.8 ± 43.8 | 540.0 ± 118.7 | 343 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Littman, J.; Aaron, R.K. Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields. Int. J. Mol. Sci. 2023, 24, 3275. https://doi.org/10.3390/ijms24043275
Littman J, Aaron RK. Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields. International Journal of Molecular Sciences. 2023; 24(4):3275. https://doi.org/10.3390/ijms24043275
Chicago/Turabian StyleLittman, Jake, and Roy K. Aaron. 2023. "Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields" International Journal of Molecular Sciences 24, no. 4: 3275. https://doi.org/10.3390/ijms24043275
APA StyleLittman, J., & Aaron, R. K. (2023). Stimulation of Chondrogenesis in a Developmental Model of Endochondral Bone Formation by Pulsed Electromagnetic Fields. International Journal of Molecular Sciences, 24(4), 3275. https://doi.org/10.3390/ijms24043275






