Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress
Abstract
1. Introduction
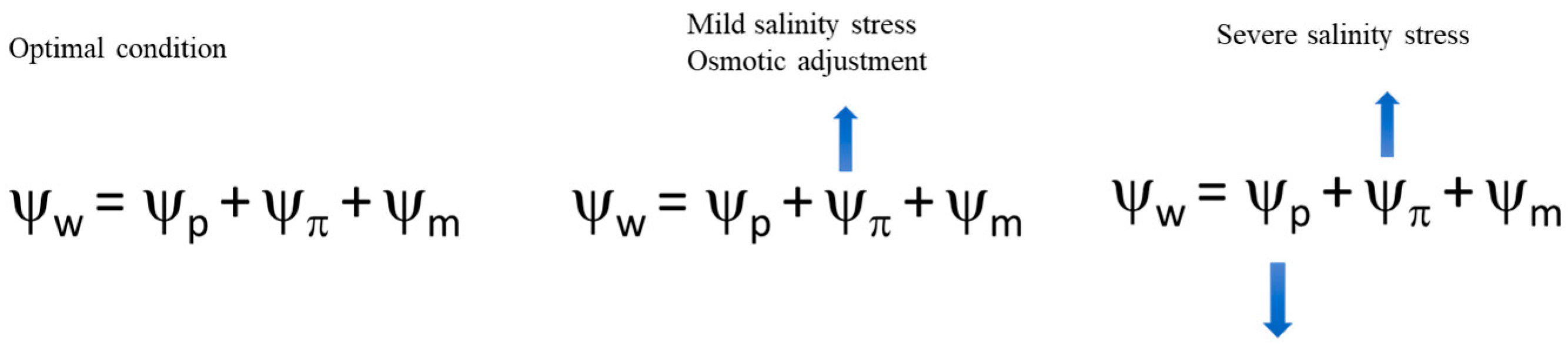
2. Osmotic Regulation Mechanism
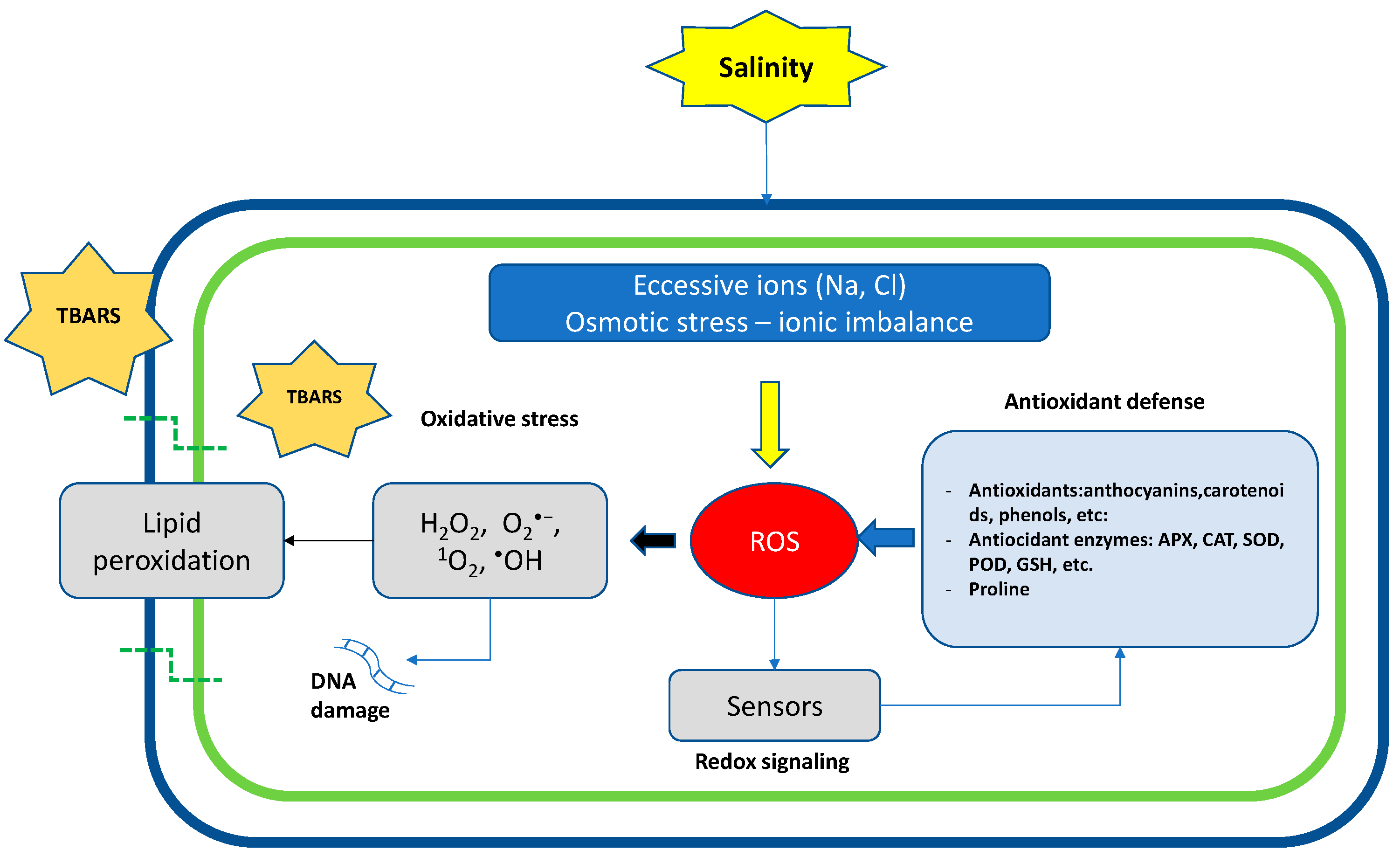
3. Reactive Oxygen Species Metabolism
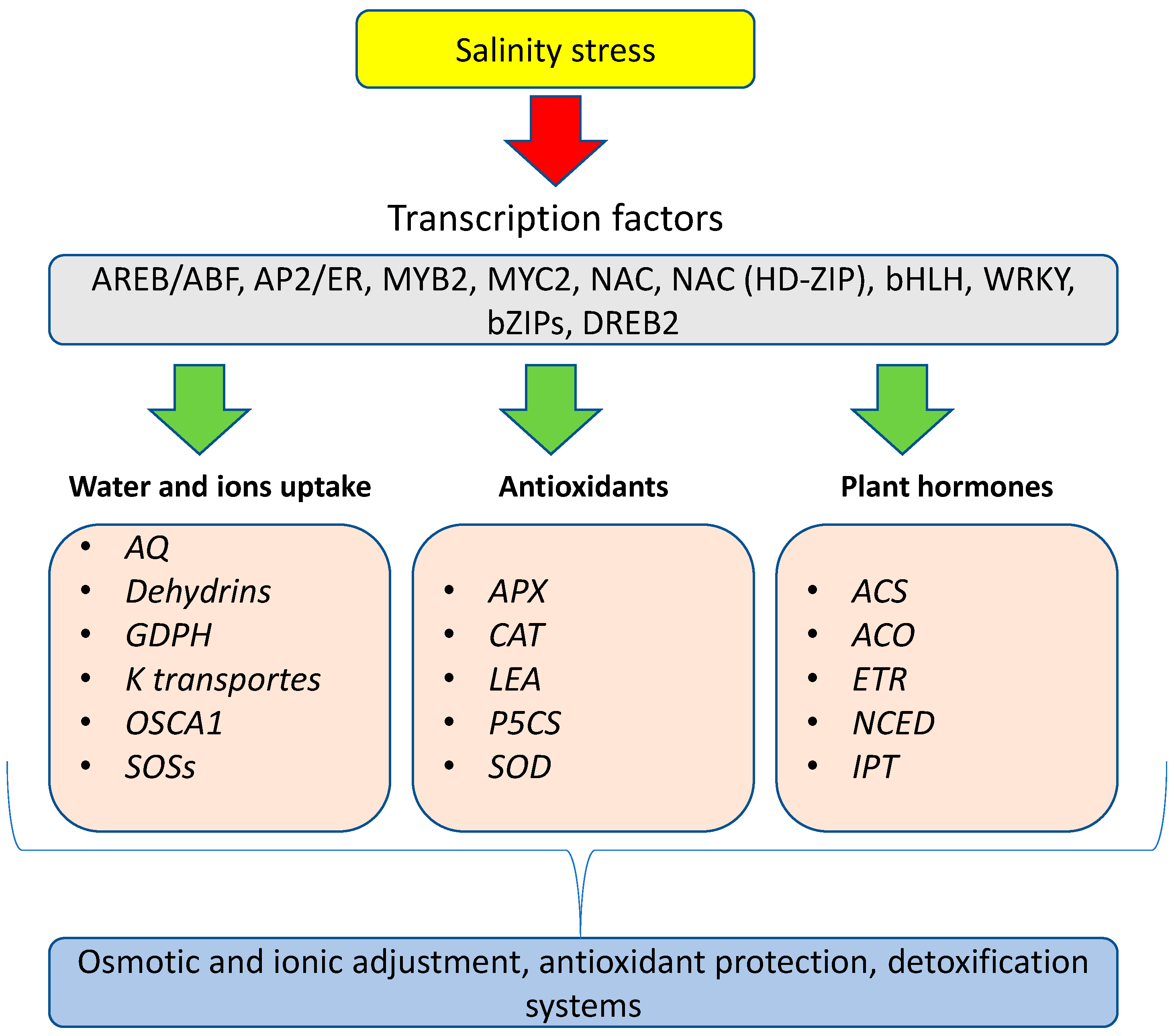
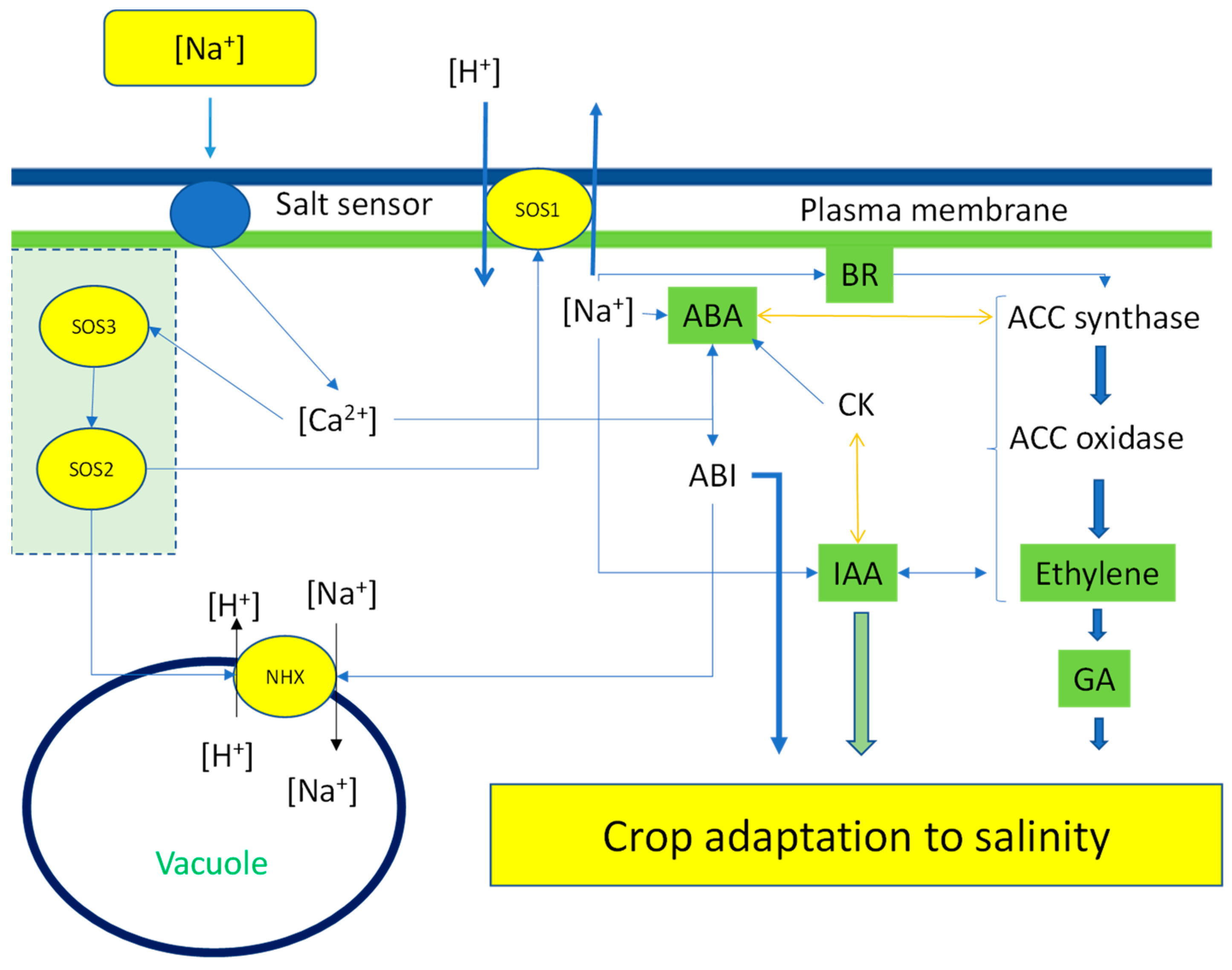
4. Mechanism of Signal Transduction and the Development of Salinity Stress
5. Salinity-Induced Proteins, Amino Acids, and Enzymes
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Malvankar, M.R.; Karle, S.B.; Kumar, K. Reactive nitrogen species: Paradigms of cellular signaling and regulation of salt stress in plants. Environ. Exp. Bot. 2019, 161, 86–97. [Google Scholar] [CrossRef]
- Chen, J.-T.; Aroca, R.; Romano, D. Molecular aspects of plant salinity stress and tolerance. Int. J. Mol. Sci 2021, 22, 4918. [Google Scholar] [CrossRef]
- Agarwal, P.; Agarwal, P.K.; Gohil, D. Transcription Factor-Based Genetic Engineering for Salinity Tolerance in Crops. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 185–211. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Baniasadi, F.; Saffari, V.R.; Moud, A.A.M. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic. 2018, 234, 312–317. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Cassaniti, C.; Romano, D.; Hop, M.E.C.M.; Flowers, T.J. Growing floricultural crops with brackish water. Environ. Exp. Bot. 2013, 92, 165–175. [Google Scholar] [CrossRef]
- Bañón, S.; Fernández, J.A.; Ochoa, J.; Sánchez-Blanco, M.J. Paclobutrazol as an aid to reduce some effects of salt stress in oleander seedlings. Eur. J. Hortic. Sci. 2005, 70, 43–49. [Google Scholar]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Stevens, R.M.; Pech, J.M.; Grigson, G.J. A short non-saline sprinkling increases the tuber weights of saline sprinkler irrigated potatoes. Agronomy 2017, 7, 4. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. Afr. J. Bot. 2017, 1, 261–266. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Jiang, D.; Yang, F.; Zhou, Y. Physio-biochemical responses of three Aquilegia species seedlings to salt stress. Agronomy 2022, 12, 2841. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Hernandez, M.; Casado-Vela, J.; Bru, R.; Elortza, F.; Hedden, P.; Olmos, E. Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ. 2011, 34, 821–836. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Jia, J.; Liang, Y.; Gou, T.; Hu, Y.; Zhu, Y.; Huo, H.; Guo, J.; Gong, H. The expression response of plasma membrane aquaporins to salt stress in tomato plants. Environ. Exp. Bot. 2020, 178, 104190. [Google Scholar] [CrossRef]
- Lacramioara, O.; Grigore, M.N.; Vochita, G. Impact of saline stress on growth and biochemical indices of Calendula officinalis seedlings. Rom. Biotechnol. Lett. 2015, 20, 11007. [Google Scholar]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2019, 10, 26. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Alam, M.W.; Ali, Q.; Ayyub, C.M.; Haider, M.S.; Ashraf, M.I.; Mahboob, W. Triacontanol regulates morphological traits and enzymatic activities of salinity affected hot pepper plants. Sci. Rep. 2022, 12, 3736. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A.; et al. Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanzen 2022, 74, 423–433. [Google Scholar] [CrossRef]
- Gengmao, Z.; Yu, H.; Xing, S.; Shihui, L.; Quanmei, S.; Changhai, W. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crops Prod. 2015, 64, 175–181. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vanlal, R.; Anand, P.; Kumar, G.; Tiwari, A.K. Effect of saline stress on growth and biochemical indices of chrysanthemum (Chrysanthemum morifolium) germplasm. Indian J. Agric. Sci. 2019, 89, 41–45. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Chen, C.; Zou, L.; Yin, S.; Liu, S.; Yuan, J.; Wu, N.; Liu, X. Comparative transcriptome analysis reveals variations of bioactive constituents in Lonicera japonica flowers under salt stress. Plant Physiol. Biochem. 2022, 173, 87–96. [Google Scholar] [CrossRef]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef]
- Sarwar, M.; Anjum, S.; Ali, Q.; Alam, M.W.; Haider, M.S.; Mehboob, W. Triacontanol modulates salt stress tolerance in cucumber by altering the physiological and biochemical status of plant cells. Sci. Rep. 2021, 11, 24504. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Hegarat, E.; Jiménez-Lao, M.; Lao, M.T. Effects of exogenous application of osmotic adjustment substances on growth, pigment concentration, and physiological parameters of Dracaena sanderiana Sander under different levels of salinity. Agronomy 2020, 10, 125. [Google Scholar] [CrossRef]
- Samadi, S.; Habibi, G.; Vaziri, A. Exogenous trehalose alleviates the inhibitory effects of salt stress in strawberry plants. Acta Physiol. Plant. 2019, 41, 112. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A.; et al. Strigolactone (GR24) Application positively regulates photosynthetic attributes, stress-related metabolites and antioxidant enzymatic activities of ornamental sunflower (Helianthus annuus cv. Vincent’s Choice) under salinity stress. Agriculture 2023, 13, 50. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Lavandula multifida response to salinity: Growth, nutrient uptake, and physiological changes. J. Plant. Nutr. Soil Sci. 2017, 180, 96–104. [Google Scholar] [CrossRef]
- Shahzadi, A.K.; Bano, H.; Ogbaga, C.C.; Ayyaz, A.; Parveen, R.; Zafar, Z.U.; Ashraf, M. Coordinated impact of ion exclusion, antioxidants and photosynthetic potential on salt tolerance of ridge gourd [Luffa acutangula (L.) Roxb.]. Plant Physiol. Biochem. 2021, 167, 517–528. [Google Scholar] [CrossRef]
- Loko, B.; Montcho, K.D.H.; Agbossékpé, F.; Mensah, A.C.G.; Assogba Komlan, F.; Lutts, S.; Gandonou, C.B. Response of African Basil (Ocimum gratissimum L.) to salt stress under tropical conditions in the Republic of Benin: Growth, ions and organic solutes accumulation. Int. J. Soil Sci. 2022, 34, 47–60. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Javed, T.; Fraz Ali, M.; Alhomrani, M.; Alamri, A.S. Proline-Induced Modifications in Morpho-Physiological, Biochemical and Yield Attributes of Pea (Pisum sativum L.) Cultivars under Salt Stress. Sustainability 2022, 14, 13579. [Google Scholar] [CrossRef]
- Shahzad, S.; Ali, S.; Ahmad, R.; Ercisli, S.; Anjum, M.A. Foliar application of silicon enhances growth, flower yield, quality and postharvest life of tuberose (Polianthes tuberosa L.) under saline conditions by improving antioxidant defense mechanism. Silicon 2022, 14, 1511–1518. [Google Scholar] [CrossRef]
- Borsai, O.; Hassan, M.A.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to salt stress in Portulaca: Insight into its tolerance mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef]
- Omidi, M.; Khandan-Mirkohi, A.; Kafi, M.; Zamani, Z.; Ajdanian, L.; Babaei, M. Biochemical and molecular responses of Rosa damascena Mill. cv. Kashan to salicylic acid under salinity stress. BMC Plant Biol. 2022, 22, 373. [Google Scholar] [CrossRef]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Mittova, V.; Theodoulou, F.L.; Kiddle, G.; Gómez, L.; Volokita, M.; Tal, M.; Foyer, C.H.; Guy, M. Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett. 2003, 554, 417–421. [Google Scholar] [CrossRef]
- Ikuyinminu, E.; Goñi, O.; O’Connell, S. Enhancing irrigation salinity stress tolerance and increasing yield in tomato using a precision engineered protein hydrolysate and Ascophyllum nodosum-derived biostimulant. Agronomy 2022, 12, 809. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Qadir, A.; Anwar, M.; Shakoor, A.; Hayat, F. Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol. Plant. 2020, 64, 604–615. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, J.; Meng, S.; Liu, Y.; Mostafa, I.; Qi, M.; Li, T. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate–glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; Hsiao, T.C.; Silk, W.K. Growth of the maize primary root at low water potentials: II. Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol. 1990, 93, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dai, W. Plant response to salinity stress. In Stress Physiology of Woody Plants; Day, W., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 155–173. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, K.; Goto, Y.; Asatsuma, S.; Koizumi, M.; Mitsui, T.; Matsuoka, K. A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 2009, 21, 1212–1229. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; de Almeida Viégas, R.; da Rocha, I.M.A.; de Oliveira Monteiro Moreira, A.C.; de Azevedo Moreira, R.; Oliveira, J.T.A. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Physiol. 2003, 160, 115–123. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.H. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Yun, B.W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, K.I.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Czégény, G.; Wu, M.; Dér, A.; Eriksson, L.A.; Strid, Å.; Hideg, É. Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Lett. 2014, 588, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, B.; Yaşar, F.; Ozpay, T.; Tuerkoezue, D.; Terziodlu, O.; Tamkoc, A. Variations in response to salt stress among field pea genotypes (Pisum sativum sp. arvense L.). J. Anim. Veter. Adv. 2008, 7, 907–910. Available online: https://medwelljournals.com/abstract/?doi=javaa.2008.907.910 (accessed on 2 December 2022).
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Baek, K.H. Physiological and biochemical perspectives of non-salt tolerant plants during bacterial interaction against soil salinity. Plant Physiol. Biochem. 2017, 116, 116–126. [Google Scholar] [CrossRef]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Kahrizi, S.; Sedighi, M.; Sofalian, O. Effect of salt stress on proline and activity of antioxidant enzymes in ten durum wheat cultivars. Ann. Biol. Res. 2012, 3, 3870–3874. [Google Scholar]
- Chookhampaeng, S. The effect of salt stress on growth, chlorophyll content proline content and antioxidative enzymes of pepper (Capsicum annuum L.) seedling. Eur. J. Res. 2011, 49, 103–109. [Google Scholar]
- Sevengor, S.; Yasar, F.; Kusvuran, S.; Ellialtioglu, S. The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. Afr. J. Agric. Res. 2011, 6, 4920–4924. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jeleel, C.A.; Azooz, M.M.; Nabi, G. Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot. Res. Intern. 2009, 2, 11–20. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidative defence system, lipid peroxidation, proline metabolizing enzymes and biochemical activity in two genotypes of Morus alba L. subjected to NaCl stress. Russ. J. Plant. Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Xue, Y.F.; Liu, Z.P. Antioxidant enzymes and physiological characteristics in two Jerusalem artichoke cultivars under salt stress. Russ. J. Plant Physiol. 2008, 55, 776–781. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Bybordi, A. Exogenous silicium and zinc increase antioxidant enzyme activity and alleviate salt stress in leaves of sunflower. J. Food Agric. Environ. 2011, 9, 422–427. [Google Scholar]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res. Commun. 2018, 46, 67–78. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 387–394. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—Drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Shelake, R.M.; Kadam, U.S.; Kumar, R.; Pramanik, D.; Singh, A.K.; Kim, J.Y. Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: Targets, tools, challenges, and perspectives. Plant Commun. 2022, 3, 100417. [Google Scholar] [CrossRef]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001, 160, 265–272. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of vacuolar membrane transport systems in plant salinity tolerance. J. Plant Growth Regul. 2022, 1–38. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Histo-anatomical strategies of Chenopodiaceae halophytes: Adaptive, ecological and evolutionary implications. WSEAS Trans. Biol. Biomed. 2007, 4, 204–218. [Google Scholar]
- Qiu, Q.S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef]
- Quintero, F.J.; Ohta, M.; Shi, H.; Zhu, J.K.; Pardo, J.M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 2002, 99, 9061–9066. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Dong, Q.; Wallrad, L.; Almutairi, B.O.; Kudla, J. Ca2+ signaling in plant responses to abiotic stresses. J. Integr. Plant Biol. 2022, 64, 287–300. [Google Scholar] [CrossRef]
- Amirbakhtiar, N.; Ismaili, A.; Ghaffari, M.R.; Nazarian Firouzabadi, F.; Shobbar, Z.S. Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS ONE 2019, 14, e0213305. [Google Scholar] [CrossRef]
- Kashyap, S.P.; Kumari, N.; Mishra, P.; Moharana, D.P.; Aamir, M.; Singh, B.; Prasanna, H.C. Transcriptional regulation-mediating ROS homeostasis and physio-biochemical changes in wild tomato (Solanum chilense) and cultivated tomato (Solanum lycopersicum) under high salinity. Saudi J. Biol. Sci. 2020, 27, 1999–2009. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Ferrante, A. Transcriptional regulation in rocket leaves as affected by salinity. Plants 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ak, B.E.; Higgs, D. Response of salt-stressed strawberry plants to supplementary calcium nitrate and/or potassium nitrate. J. Plant Nutr. 2003, 26, 543–560. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Shannon, M.C.; Rhoades, J.D.; Draper, J.H.; Scardaci, S.C.; Spyres, M.D. Assessment of salt tolerance in rice cultivars in response to salinity problems in California. Crop Sci. 1998, 38, 394–398. [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Kumar, A.; Das, S.K.; Singh, R.K.; Zinta, G.; et al. Salinity responses and tolerance mechanisms in underground vegetable crops: An integrative review. Planta 2022, 255, 68. [Google Scholar] [CrossRef]
- Zhang, G.C.; Zhu, W.L.; Gai, J.Y.; Zhu, Y.L.; Yang, L.F. Enhanced salt tolerance of transgenic vegetable soybeans resulting from overexpression of a novel Δ1-pyrroline-5-carboxylate synthetase gene from Solanum torvum Swartz. Hortic. Environ. Biotechnol. 2015, 56, 94–104. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. C. R. Biol. 2007, 330, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Jampeetong, A.; Brix, H. Effects of NaCl salinity on growth, morphology, photosynthesis and proline accumulation of Salvinia natans. Aquat. Bot. 2009, 91, 181–186. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Martínez-Pérez, A.; Ferrández-Ayela, A.; Albacete, A.; Martínez-Melgarejo, P.A.; Dodd, I.C.; Thompson, A.J.; Pérez-Pérez, J.M.; Pérez-Alfocea, F. Impact of overexpression of 9-cis-epoxycarotenoid dioxygenase on growth and gene expression under salinity stress. Plant Sci. 2020, 295, 110268. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Martínez-Pérez, A.; Albacete, A.; Martínez-Melgarejo, P.A.; Dodd, I.C.; Thompson, A.J.; Mohareb, F.; Estelles-Lopez, L.; Kevei, Z.; Ferrández-Ayela, A.; et al. Overproduction of ABA in rootstocks alleviates salinity stress in tomato shoots. Plant Cell Environ. 2021, 44, 2966–2986. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- El-Keltawi, N.E.; Croteau, R. Salinity depression of growth and essential oil formation in spearmint and marjoram and its reversal by foliar applied cytokinin. Phytochemistry 1987, 26, 1333–1334. [Google Scholar] [CrossRef]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an inflorescence meristem-specific cytokinin oxidase–OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Jiroutova, P.; Oklestkova, J.; Strnad, M. Crosstalk between brassinosteroids and ethylene during plant growth and under abiotic stress conditions. Int. J. Mol. Sci. 2018, 19, 3283. [Google Scholar] [CrossRef] [PubMed]

| Plant Species and Cultivar | Salt Stress Level | Effect of Salt Stress on Plants | References |
|---|---|---|---|
| Aquilegia oxysepala Trautv. & C.A.Mey., A. parviflora Ledeb., and A. viridiflora Pall. | 5.0 ± 0.2 dS m−1 and 10.0 ± 0.2 dS m−1 | Increase in MDA and proline; increased activity of SOD (5.0 dS m−1); A. parviflora POD increase; and A. viridiflora POD increase (10.0 dS m−1) | [14] |
| Brassica oleracea L. | 80 mM | POX increase | [15] |
| Brassica oleracea L. | 50, 100, 150, and 200 mM NaCl | Increase in CAT and POX activity, increase in proline | [16] |
| Brassica rapa L. subsp. rapa ‘Qiamagu’ | 50, 100, 150, and 200 mM NaCl | Increased activity of SOD (50, 100, 150, and 200 mM), POD (100, 150, and 200 mM), CAT (150 mM), and APX (200 mM); increase in MDA (100, 150, 200 mM) | [17] |
| Brassica rapa L. subsp. rapa ‘Wenzhoupancai’ | 50, 100, 150, and 200 mM NaCl | Increased activity in SOD and APX (200 mM), POD and CAT (100, 150, and 200 mM); increase in MDA (100, 150 mM) | [17] |
| Calendula officinalis L. | 50–100 mM NaCl, 36 d | Increase in proline | [18] |
| Calendula officinalis L. | 1, 5, and 9 dSm−1 | Increase in MDA in leaves and roots, increase in proline in leaves (9 dS m−1), and increase in CAT activity; decrease in POD activity | [6] |
| Capsicum annuum L. | 2000 and 4000 ppm NaCl | Increased activity of CAT and POX; increase in proline | [19] |
| Capsicum annuum L. | 75 mM NaCl | Increase in SOD, POX, and CAT activity; increase in MDA | [20] |
| Capsicum annuum L. ‘Candy Apple’ | 35, 70, and 105 mM | Increase in APX and PPO activity | [21] |
| Carthamus tinctorius L. | 50, 100, and 150 mM NaCl | Increased activity of CAT (50 mM), SOD (100 mM), and POD (50, 100, and 150 mM) | [22] |
| Catharanthus roseus (L.) G. Don | 150 mM NaCl | MDA increase in vegetative and flowering stage; increase in CAT, GPX, and GR activity in vegetative and flowering stage | [23] |
| Chrysanthemum L. cvs. (‘Garden Beauty’, ‘Shanti’, ‘Red Stone’, ‘Basanti’, ‘Yellow Reflex’, ‘Ravi Kiran’, ‘Anmol’, ‘Mother Teresa’, ‘Sweta Singar’, and ‘Jaya’) | 150 mM NaCl | Increase in proline | [24] |
| Cornus florida L. and C. hongkongensis subsp. elegans (Fang & Hsieh) Q.Y.Xiang | 0.2%, 0.3%, 0.4%, and 0.45% salt solution | Increase in MDA, SOD activity (0.2%, 0.3%, 0.4%, and 0.45% salt solution) and proline (0.3%, 0.4%, and 0.45% salt solution) | [25] |
| Cucumis melo L. | 30, 60, and 90 mM NaCl | Increase in proline, MDA, APX, CAT, SOD, and POD | [26] |
| Cucumis sativus L. (‘Green long’, ‘Marketmore’, ‘Summer green’, and ‘20252’) | NaCl 50 mM L−1 | Increase in proline | [27] |
| Dracaena braunii Engl. | 2.0 and 7.5 dS m−1 | Increase in proline | [28] |
| Fragaria ×ananassa (Duchesne ex Weston) Duchesne ex Rozier ‘Gaviota’ | 50 mM | Increase in MDA | [29] |
| Helianthus annuus L. (ornamental sunflower) | 150 mM NaCl | Increased activity of CAT and POD; increase of proline | [30] |
| Lavandula multifida L. | 10–200 mM NaCl, 60 d | Increase in soluble sugars concentration | [31] |
| Luffa acutangula Roxb. | 75 and 150 mM | Increase in proline | [32] |
| Ocimum gratissimum L. (African basil) | 30, 60, 90, 120 mM | Increase in proline in leaves (120 mM) and root (90 and 120 mM) | [33] |
| Oenanthe javanica DC. ‘V11E0022’ and ‘V11E0135’ | 50 and 100 mM NaCl | Increase in leaves and roots of MDA and proline | [34] |
| Pisum sativum L. ‘L-888’ and ‘Round’ | 150 mM | ‘L-888’ increase in CAT activity; ‘Round’ increase in proline and decrease in SOD activity | [35] |
| Polianthes tuberosa L. | 50 and 100 mM NaCl | Increase in SOD, POD (100 mM), GR, and APX; increase in proline (100 mM) | [36] |
| Portulaca oleracea subsp. oleracea L., P. grandiflora Hook., P. halimoides L., and P. oleracea ‘Toucan Scarlet Shades’ | 100, 200, and 400 mM | Increase in proline in leaves and roots | [37] |
| Rosa damascena Mill. ‘Kashan’ | 4, 8, and 12 dS m−1 | Increase in MDA (8 mM); increase in proline (8, 12 mM); and increase of CAT activity | [38] |
| Solanum lycopersicum L. | 50 μM S-nitroso-N-acetyl penicillamine (SNAP) 200 | Increased activity of APX, glutathione reductase (GR), peroxidase and rise in proline content | [39] |
| Solanum lycopersicum L. | 100 mM | Mn-SOD, MDHAR, and GR decrease | [40] |
| Solanum lycopersicum L. | 300 mM NaCl | Increase in proline | [41] |
| Solanum lycopersicum L. | 150 mM NaCl | Increase in MDA content; increase in SOD and CAT activity | [42] |
| Solanum lycopersicum L. ‘Liaoyuanduoli’ | 150 mM NaCl | Increase in MDA and SOD, APX, GPX, GR, MDHAR, and DHAR activity | [43] |
| Solanum lycopersicum L. ‘Pusa Ruby’ | 150 mM NaCl | Increase in proline and MDA; increase activity of APX, MDHAR, DHAR, GR, SOD, CAT, GPX, and GST | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; Romano, D.; Ferrante, A. Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress. Int. J. Mol. Sci. 2023, 24, 3190. https://doi.org/10.3390/ijms24043190
Toscano S, Romano D, Ferrante A. Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress. International Journal of Molecular Sciences. 2023; 24(4):3190. https://doi.org/10.3390/ijms24043190
Chicago/Turabian StyleToscano, Stefania, Daniela Romano, and Antonio Ferrante. 2023. "Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress" International Journal of Molecular Sciences 24, no. 4: 3190. https://doi.org/10.3390/ijms24043190
APA StyleToscano, S., Romano, D., & Ferrante, A. (2023). Molecular Responses of Vegetable, Ornamental Crops, and Model Plants to Salinity Stress. International Journal of Molecular Sciences, 24(4), 3190. https://doi.org/10.3390/ijms24043190








