Abstract
Plant transcriptome contains an enormous amount of non-coding RNAs (ncRNAs) that do not code for proteins but take part in regulating gene expression. Since their discovery in the early 1990s, much research has been conducted to elucidate their function in the gene regulatory network and their involvement in plants’ response to biotic/abiotic stresses. Typically, 20–30 nucleotide-long small ncRNAs are a potential target for plant molecular breeders because of their agricultural importance. This review summarizes the current understanding of three major classes of small ncRNAs: short-interfering RNAs (siRNAs), microRNA (miRNA), and transacting siRNAs (tasiRNAs). Furthermore, their biogenesis, mode of action, and how they have been utilized to improve crop productivity and disease resistance are discussed here.
1. Introduction
Two major kingdoms in taxonomy, kingdom Animalia and kingdom Plantae, divide this whole universe, and among these two kingdoms, Plantae is non-motile. Hence, evading stress or constantly dynamic environmental conditions has posed a constant challenge for plants. Avoidance, tolerance, or resistance to abiotic and biotic stress factors can substantially minimize yield loss, which could be a turning point to increase food security for millions. Researchers are seeking solutions and a permanent way out from these harsh and demanding situations; non-coding RNAs (ncRNAs) have come to the rescue. Their dramatic development as a rescue agent among the food and cash crops has emerged as a windfall in the plant science field. These ncRNAs are classified as significant bioactive molecules and are substantial players in generating diverse genotypes and phenotypes. It is a well-established fact that 90% of the genome in eukaryotes is transcribed into mRNA, from which only 2% are translated and finally produce proteins [1,2]. Other than this essential 2%, the rest is made up of ncRNAs. These RNAs were condemned as useless because they could not deliver the signature function of RNA, which is characterized by its protein-coding capacity and also imperfectly conserved sequences [1,3]. Over the years, it has been established experimentally and by high-throughput sequencing that ncRNAs are linked to a plethora of gene regulatory functions categorized as epigenetic, transcriptional, or post-transcriptional fields [4,5,6].
Soon after their discovery, the ncRNAs were labeled into classes based on their origin, biogenesis, and mode of action. There are (a) housekeeping RNAs and (b) regulatory RNAs. The list of regulatory ncRNAs has two members, namely small non-coding RNAs (Table 1), which include microRNAs (miRNAs); short interfering RNAs (siRNAs); piwi-interacting RNAs (piRNAs) (in animal only); transacting siRNA (tasiRNA); and long non-coding RNAs (lncRNAs). This category of RNA follows the usual route of being transcribed from DNA, but they do not carry out the translation process and produce proteins [7]. They generally specialize in performing other crucial activities related to the growth and development of plants and, most essentially, bring about responses to various abiotic stress factors both transcriptionally and post-transcriptionally [8].
It has been inferred from modern research that ncRNAs play a vital role in the regulation of gene expression (transcriptional and post-transcriptional) during development and stress response (abiotic and biotic) in plants [9]. In this review, we delve into a detailed discussion about the biogenesis and structure of small ncRNAs (miRNA, siRNA, and tasiRNA) along with an in-depth analysis of their mode of action (how they repress the target gene expression) and about the wide variety of roles choreographed by them in plant gene silencing.

Table 1.
Classes of small non-coding RNAs involved in gene silencing in plants.
Table 1.
Classes of small non-coding RNAs involved in gene silencing in plants.
| Class | Mechanism | Length (nt) | References |
|---|---|---|---|
| Short interfering RNA (siRNA) | Targeted mRNA cleavage | 21–24 | [10] |
| MicroRNA (miRNA) | Translational silencing, mRNA cleavage | 20–24 | [11] |
| Trans-acting siRNA (tasiRNA) | mRNA cleavage | 21–22 | [12] |
| Phased secondary small interfering RNAs (phasiRNAs) | mRNA cleavage, function in development | 20–21 | [13] |
2. Short Interfering RNAs
RNA interference (RNAi) has inarguably been a revolutionary discovery in the field of biology in the last several decades. The discovery of small (20–30 nucleotide long) non-coding RNAs that can regulate genes and the genome completely transformed RNA biology. These small RNAs can guide effector proteins targeting any complementary nucleotide sequence through the RNAi pathway, thereby downregulating its expression level. Napoli and Jorgensen first reported small RNA-mediated gene regulation in plants while working with chalcone synthase (CHS) in petunia [14]. It was referred to as co-suppression/ post-transcriptional gene silencing (PTGS). Later in 1992, a similar phenomenon was reported in a fungus (Neurospora crassa), which is termed quelling [15], and in Caenorhabditis elegans by Guo and Kemphues (sense/anti-sense mRNA) in 1995 [16]. Then, in 1998, Fire and Mello explained for the first time that double-stranded RNAs (dsRNAs) were the actual reason behind such silencing of endogenes while working with Caenorhabditis elegans [17]. Andrew Fire and Craig Mello were awarded the prestigious Nobel prize in 2006 in the category of Physiology/Medicine for discovering RNAi: gene silencing by dsRNAs. It has led to extensive research on how small RNAs can regulate several essential aspects of a plant’s life, such as transcription, RNA processing, RNA stability, chromosome segregation, etc.
2.1. Biogenesis of Short Interfering RNAs
dsRNA-mediated post-transcriptional gene silencing (PTGS) symbolizes the cellular defense mechanism protecting it from foreign nucleic acids of invading viruses or transposons [18,19]. Such integration of alien genes produces dsRNAs, which act as a guide for sequence-specific RNA degradation and are believed to be associated with maintaining the silencing process for a long time [20,21]. The processing of these long dsRNAs into 21–24 nt long short-interfering RNAs (siRNAs) is facilitated by Dicer, a ribonuclease III enzyme [22] (Figure 1A). The presence of these siRNAs was first reported by Hamilton and Baulcombe in plant tissues that showed virus-induced PTGS [23]. These siRNAs were later found to be present also in Drosophila melanogaster embryo lysate [24], where the added synthetic 20–22 nt RNA duplexes can efficiently target and cleave mRNAs at 21 nt intervals [25]. That is why these 21 nt long RNAs were called siRNAs or silencing RNAs. Dicer-mediated processing is coupled with other co-factors, and these siRNAs are finally loaded onto the RNA-induced silencing complex (RISC), a member of the argonaute protein family [26]. During incorporation within the RISC, one strand called the “passenger strand” is dissociated by the activity of AGO2, which is encoded by the gene AGO2 (argonaute RISC catalytic component 2), whereas the other “guide strand” that serves as a guide for RNA-directed sequence-specific silencing stays within the complex, forming the mature RISC [27,28]. Targeted mRNA with sequence complementarity with the 21 nt long guide-siRNA within the mature RISC is cleaved between the 10th and 11th nucleotide (from the 5′ end) by the PIWI domain of the AGO2 protein, generating products containing 5′-monophosphate and 3′-hydroxyl termini [29,30]. These cleaved products are rapidly degraded by the endogenous exonuclease activity due to the lack of 5′ capping or a 3′poly(A) tail [31]. Apart from the above-mentioned RNAi, plants [20,32], fungi [33], and C. elegans [21,34] possess a special enzyme called RNA-dependent RNA polymerases (RdRPs), which can produce additional dsRNAs for amplifying the RNAi response. Such dsRNAs are synthesized in a primer-independent manner using the targeted mRNA as a template. It is subsequently processed by Dicer to produce more siRNAs, thereby facilitating the recycling of the RISC complex [35] (Figure 1B).
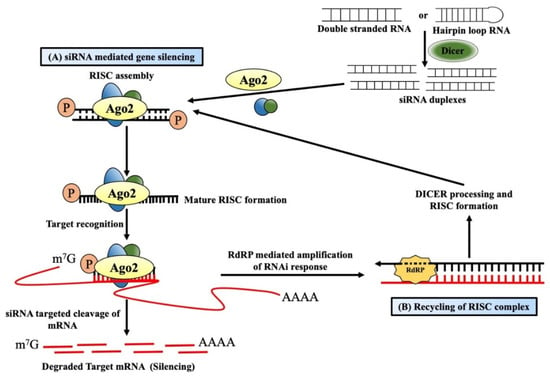
Figure 1.
Mechanism of short-interfering RNA-mediated gene silencing. (A) Long double-stranded RNAs/hairpin loop RNAs from alien genes are processed into short interfering RNAs (siRNAs) by the Dicer/TRBP (the human immunodeficiency virus transactivating response RNA binding domain) complex and finally become incorporated into RNA-induced silencing complex (RISC). One strand (passenger strand) is degraded from the RNA duplex, and the other strand (guide strand), along with argonaute 2, forms the active RISC. The guide strand guides the active RISC to target and cleaves the complementary mRNAs into the cytosol, resulting in gene silencing. (B) RNAi response in plants and worms generally becomes amplified by the RNA-dependent RNA polymerase enzymes (RdRPs). RdRPs and RISC use targeted mRNAs as a template to generate double-stranded RNAs dsRNAs, which then are processed by the Dicer into secondary siRNAs. These siRNAs eventually amplify the RNAi effect in the system.
2.2. RNA-Induced Silencing Complex: The Versatile Gene Silencing Complex
Although there are diverse ways to regulate gene expressions using RISC, two significant incidents are common for all types. Firstly, every RISC should comprise one argonaute protein family member, and secondly, at its core, a small RNA should guide the RISC to target mRNAs through Watson–Crick base pairing [36]. Each class of small RNAs in eukaryotes, such as siRNA/miRNA/piRNA (only in animals), together with the AGO protein family, forms the ribonucleoprotein RISC. Our current understanding of RISC (from birth to death) has been summarized here.
The Argonaute family of proteins is at the heart of RISC-mediated gene regulation [37,38]. There are four functional domains in AGO proteins: PIWI-AGO-Zwille (PAZ), Middle (MID), N-terminal (N), and PIWI (Figure 2) [37,39]. There are two linkers (L1, L2), out of which L1 connects the N and PAZ domains, whereas L2 supports the N-L1-PAZ structure connecting it to the MID-PIWI lobe [40,41]. The PAZ domain of AGO proteins contains a pocket that interacts with and binds the guide strand from the 3′ end [42]. A conserved sequence of a catalytic tetrad (Asp-Glu-Asp-His/Asp) can be found in the PIWI domain of some AGO proteins that are responsible for the target mRNA degradation. This catalytic domain is also responsible for the cleavage of some passenger strands before it gets ejected [39]. In the initial phase of RISC assembly, a small RNA duplex is loaded onto an empty AGO protein with the help of Hsp70/Hsp90 chaperons and forms the pre-RISC [43]. One of the strands, which is less stable and likely to be adenine/uridine (AU) rich, is preferably able to be the guide strand. This type of strand selection is asymmetric and generally depends on the difference in the thermodynamic stability of two ends of the RNA duplex [44,45]. At the initial stage of passenger strand separation, the N domain disrupts the 3′ end base-pairing of the guide strand to help open the RNA duplex [46]. Then, the passenger strand is sliced at a position opposite the guide strand’s 10th and 11th nucleotide (g10 and g11) by the catalytic activity of the AGO protein [27,28,47]. After passenger strand separation, the mature RISC binds to the targeted mRNA guided by the guide strand and either slices it directly or induces translational repression by recruiting necessary proteins [48,49]. Although AGO and small RNAs are short-lived, once the RISC is formed, those two tend to be long-lived [50,51]. Generally blank/unloaded AGO proteins are degraded by the autophagy pathway [52,53], whereas the target RNA-directed miRNA degradation (TDMD) targeted RISC are degraded by the ubiquitin–protease system [54,55].
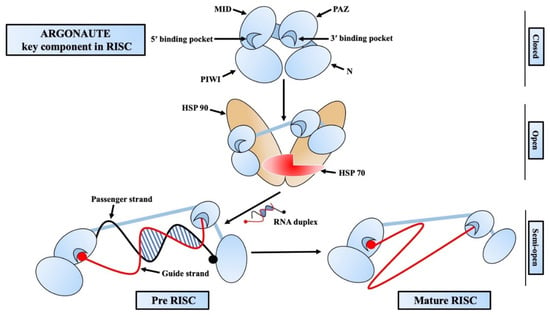
Figure 2.
Mechanism of RNA-induced silencing complex (RISC) assembly. Argonaute has four functional domains: PIWI-AGO-Zwille (PAZ), Middle (MID), N-terminal (N), and PIWI (Piwi/Argonaute/Zwille). An empty argonaute loads an RNA duplex with the help of HSP70/HSP90 and forms the pre-RISC. The passenger strand (black strand) is ejected from the pre-RISC, and the guide strand (red strand) and argonaute form the mature/active RISC that start targeting the complimentary mRNAs to assert gene silencing.
2.3. Short Interfering RNA Mediated Silencing in Plants
The siRNA-mediated gene silencing mechanism has been exploited extensively over the years for crop protection against biotic stresses and as a platform for overall crop improvement. RNAi-mediated silencing requires the design of a hairpin loop structure containing both the sense and anti-sense strand of the targeted gene separated by an intron sequence. Such a construct will create hpRNAs in plants that are cleaved by Dicer and form siRNAs, which trigger the RNAi pathway to silence the targeted gene [56]. Pests such as insects, nematodes, viruses, and bacteria pose a severe threat to agricultural produce. RNAi has been used to make pest-resistant crops by targeting essential genes in the pests and silencing them by host plant-mediated RNAi (host-induced gene silencing—HIGS) [57].
The two-spotted spider mite is one of the deadliest plant pests; it can attack more than three thousand crops and feeds mainly on Chinese cabbage. RNAi-mediated targeting against the COPB2 gene of this pest resulted in an almost 100% mortality rate [58]. Similarly, transgenic potato plants expressing the molting-associated EcR gene showed enhanced resistance against the deadly Colorado potato beetle [59]. The root-knot nematode Meloidogyne incognita causes colossal damage to agricultural products worldwide. RNAi-mediated simultaneous silencing of Mi-flp1, Mi-flp12, and Mi-flp18 genes resulted in enhanced resistance against this nematode [60]. An extensive list of such siRNA-mediated gene silencing to improve desirable traits in plants is listed in Table 2. Because RNAi-derived plants have been categorized as genetically modified crops (GM crops) and have been the subject of bitter controversy, the exogenous application of RNAi-inducing dsRNA-based bio-pesticides is gaining popularity, as it provides a non-transgenic approach [61]. Tenllado et al. first reported the effective foliar application of dsRNAs targeting the Alfalfa mosaic virus (AMV), Tobacco etch virus (TEV), and Pepper mild mottle virus (PMMoV) in 2001 [62]. However, the authors did mention that the commercial success of such topical application of dsRNAs will depend on two critical parameters: cost-effectiveness and an optimized mode of delivery. In recent years, several studies have been conducted to deliver such topical solutions of dsRNAs with impeccable efficiency and target specificity [62,63,64]. Topically applied dsRNA-based biopesticides have high species specificity, low levels of toxicity, and a minimal environmental effect compared to traditional pesticides. If their distribution and usage can be regulated in a precautionary way, dsRNA-based biopesticides can revolutionize the integrated pest-management system [65].
2.4. Tweaking the siRNAs to Improve Desirable Traits in Plants
siRNAs are well known for their silencing role in the case of viral RNAs. They play a master role in regulating plant defense machinery against potential pathogens such as bacteria, viruses, fungi, oomycetes, and other parasitic plants. Recent research has provided evidence of the ability of siRNAs to suppress fungus and oomycetes by silencing specific pathogen genes related to pathogenesis. Thus, scientists have precisely concluded that siRNAs are a potential concoction of diverse gene sequences and are used as a “shotgun” which targets random genes of the pathogen with great efficiency [66]. After discovering the antiviral factor, virus-derived siRNAs (vsiRNAs) in tobacco infected with potato virus, there has been a breakthrough in plant immunity. These viral dsRNAs are directly targeted by plant DCLs, resulting in 21–24 nt long primary vsiRNAs. It has been established that these 21 nt long vsiRNAs specifically silence the detrimental viral RNAs through Post Transcriptional Gene Silencing (PTGS) [67]. The functions of vsiRNAs can also be categorized into two parts. One part is when the vsiRNAs degrade the intruder viral genome and render antiviral tolerance to plants. On the other side, some vsiRNAs silence host gene expression and manipulate host resistance towards viral attack. During tomato yellow leaf curl virus (TYLCV) infection, it has been observed that vsiRNAs are utilized by TYLCV to silence SILNR1, a long noncoding RNA (lncRNA) associated with antiviral defense. vsiRNAs obtained from wheat yellow mosaic virus (WYMV) down-regulate host genes and activated broad-spectrum host immunity [68]. Another category of siRNA, which was first seen in Arabidopsis, which also participates in antiviral defense, is virus-activated siRNA (vasiRNA) [69]. In the case of antiviral defense, the vsiRNAs are generated from the viral genome, which safeguards the plants by destroying the viral RNA. However, in the case of non-viral plant pathogens, endogenous siRNA-orchestrated gene silencing is instantly triggered to alter the gene expression associated with plant immunity [66]. The components of the siRNA pathway interact among themselves and others to orchestrate plant immunity. RDR6 is essential for plant immunity because it aids in producing secondary siRNAs and silencing signals. For example, in rice, shl2-rol, which is a mutant line of the rice gene OsRDR6 results in severe infection symptoms when the plant is attacked by Xanthomonas oryzae PV. oryzae, thus proving the importance of RDR6-dependent siRNAs in rendering tolerance against bacteria [70].
Artificial siRNAs are produced in plants via Host-Induced Gene Silencing (HIGS) to silence the deadly pathogen genes causing infection. A well-tested example is transgenic barley and wheat, which express artificial siRNAs that target gene Avra10 and display increased resistance to Blumeria graminis, the causative agent of powdery mildew disease [71]. HIGS via engineered dsRNA resists parasitic plants. This strategy has been demonstrated in transgenic tobacco that expresses dsRNA against transcription factors controlling haustoria development and reduced vigor in Cuscuta patagonia. Similarly, Orobanche aegyptiaca has been recorded to grow and feed on tomatoes. The above examples highlight host-produced synthetic siRNAs’ significant role in controlling parasitic plants. An extensive list of such siRNA-mediated gene silencing to improve desirable traits in plants is listed in Table 2.

Table 2.
Genetic alteration of siRNAs in plants to improve the desirable traits.
Table 2.
Genetic alteration of siRNAs in plants to improve the desirable traits.
| Plant | Targeted Gene | Traits Involved | References |
|---|---|---|---|
| Wheat | SBE IIa and SBE IIb | Increased amylose content | [72] |
| Rice | OsDWARF4 | Improved biomass | [73] |
| Cotton | Δ 9-desaturase and oleoyl- phosphatidylcholine γ6-desaturase | Improved stearic- and oleic- fatty acid content | [74] |
| Onion | Lachrymatory factor synthase (LFS) | Tearless onion | [75] |
| Rice | OsGA20ox2 | Improved grain yield | [76] |
| Cotton | Delta-cadinene synthase | Reduced toxic terpenoid gossypol | [77] |
| Rice | OsSSI2 | Resistance against Magnaporthe grisea and Xanthomonas oryzae | [78] |
| Rice | GluB | Decreased glutelin content | [79] |
| Rice | OsFAD7 and OsFAD8 | Resistance against Magnaporthe grisea | [80] |
| Tomato | SlNCED1 | Increased concentration of β-Carotene and lycopene | [81] |
| Potato | SYR1 | Resistance against Phytophthora infestans | [82] |
| Tomato | DET1 | Increased concentration of Carotenoid and flavonoid | [83] |
| Wheat | MLO | Resistance against Blumeria graminis f. sp. tritici | [84] |
| Tomato | α-Man/β-Hex | Increased fruit shelf-life | [85] |
| Arabidopsis | 16D10 | Resistance against Meloidogyne incognita | [86] |
| Tomato | ACC synthase (ACS) | Decreased content of ethylene | [87] |
| Tobacco | Splicing factor and integrase | Resistance against Meloidogyne incognita | [88] |
| Tomato | Chalcone synthase | Production of Seedless fruit | [89] |
| Rice | PNS12 | Resistance against Rice Dwarf Virus (RDV) | [90] |
| Arabidopsis | HC-Pro | Turnip Mosaic Virus (TuMV) | [91] |
| Canola | Farnesyl transferase | Increased drought tolerance | [92] |
| Rice | OsDIS1 | Increased drought tolerance | [93] |
| Sugarcane | CP | Enhanced resistance against sugarcane mosaic virus | [94] |
| Potato | eIF4E | Enhanced resistance against potato virus Y | [95] |
| Soybean | AC2 | Improved resistance against mungbean yellow mosaic India virus | [96] |
| Soybean | CP | Improved resistance against mungbean yellow mosaic India virus | [97] |
| Soybean | P3 cistron | Enhanced resistance to soybean mosaic virus | [98] |
| Rice | S7-2 | Improved resistance against rice black streak dwarf virus | [99] |
| Cotton | IR | Improved resistance against cotton leaf curl Rajasthan virus | [100] |
3. microRNA: Structure and Biogenesis
MicroRNAs are extremely well-known members of molecular biology as a group of tiny 20–24 nt (as opposed to siRNA, Table 3) endogenous and non-coding RNAs that mastermind the regulation of gene expression [101]. Researchers worldwide have investigated plants and proved a labyrinthine molecular pathway regarding miRNA biogenesis and their various modes of action. The hub of miRNA biogenesis and activity are clandestine subcellular locations that have been established after decades of research [102].
A multitude of MIRNA genes (MIR genes) are encoded by the plant genome, and it has been observed that a vast majority of them survive as clusters [103,104]. There is a plethora of complexities and regulations throughout the miRNA biogenesis pathway [102] (Figure 3). miRNAs have been classified based on their position inside the genome and are “intronic” or “intergenic.” The intronic miRNAs are prepared from the introns present in the host transcript [104]. On the other hand, the intergenic miRNAs connect two protein-coding genes, and their transcription occurs in separate independent units by RNA polymerase II (Pol II). Because they are certified Pol II products, the primary transcripts of the MIR genes, also called the pri-miRNAs, have a typical 5′ capping and a 3′ Poly A tail with splicing [105]. Subsequent folding of the pri-miRNAs takes place, which results in a hairpin-like structure comprising an upper stem, a terminal loop, the miRNA zone, a lower stem portion, and a number of arms that are perceived, handled, and fixed by Dicer-like RNase III endonucleases (DCLs). The number of DCL proteins varies across the immense range of plants. For example, in Arabidopsis thaliana, the number of DCL proteins is four. DCL1 is the leader protein, and they expedite the generation of the majority of miRNAs with the aid of accessory proteins such as double-stranded RNA-binding protein Hyponastic Leaves 1 (HYL1) along with Serrate (SE), which is a zinc finger protein [106]. Many other DCLs are also engaged in the production of miRNA; for example, miR822 and miR839 are produced by AtDCL4, and OsDCL3a is responsible for producing 24-nt long miRNAs that administer DNA methylation, e.g., hc-siRNAs [107]. In the process of miRNA biogenesis, there are multiple other factors that participate in the process, such as THO2, SE, and RCF3. They create structures called splicing speckles and stay adjacent to dicing bodies [108,109]. Other factors such as MOS2, PINP1, and NOT2 display scattered nucleoplasmic design, and they too remain partially abutting the dicing bodies [110,111,112]. The detailed analysis of the subcellular localization of all the above-discussed factors is highly suggestive that they actively participate in miRNA biogenesis [102]. A remarkably noteworthy feature that has unfolded from the work of [102] is that the pri-miRNAs are processed, folded, and finally modified co-transcriptionally. This concept was hypothesized earlier, but the above studies act as solid evidence for this fact. Firstly, DCL1 is closely connected with the MIR genes via their chromatin region [113]. Secondly, the plethora of regulatory proteins (NOT2, CDC5, and ELP2) play a vital role in miRNA transcription and link up with the miRNA machinery functioning proteins [114]. mRNA adenosine methylase (MTA) layers and coats m6A on the surface of pri-miRNAs and might also have a profound impact on miRNA biogenesis. In plants, the structure of the pri-miRNAs is comparatively variable in terms of length, which starts from 60nt and might stretch over to 500 nt. In terms of structure, they display more complexity compared to their animal counterparts (70 nt long) [114], and they can be fixed in two ways: the loop-proximal site to the loop-distal site and conversely as well [115,116,117,118,119,120,121].
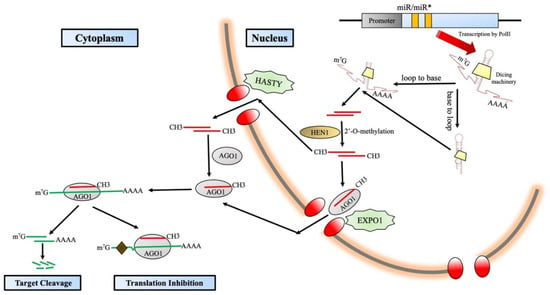
Figure 3.
RNA Polymerase II (Pol II) transcribes MIR(micro-RNA) genes and forms stem–loop structure by folding to form pri-miRNAs. These are processed by DCL1 either in a “base-to-loop” or “loop-to-base” track. Small RNA methyltransferase HEN1 methylates the newly formed miRNA/miRNA*duplexes. RISC takes place either in the nucleus or cytoplasm. The basic mode of action of miRNAs (gene silencing) takes place via target cleavage or translation inhibition.
The DCL-umpired fixation of the nascent miRNA/miRNA* duplex has 2-nt 3′ overhangs on both strands with a phosphate group at the 5′ end and two hydroxyl groups at the 3′ end. These two hydroxyl groups are of extreme necessity, and the 2′-OH site undergoes methylation orchestrated by small RNA methyltransferase HUA Enhancer (HEN1) [122,123]. For years, the prejudice existed that methylated miRNA/miRNA* duplexes were transported out by the animal Exportin 5 (EXPO5) homologue hasty (HST) protein [124]. The assembly of RISC was an enigma to scientists for decades. Later, in 2018, Bologna et al. established the fact that RISC is predominantly assembled in the nucleus and then exported out to the cytoplasm by the EXPO1 protein. Some more research also throws light on the fact that some miRNAs might be exported out of the nucleus in their original duplex form and fabricated in the cytosol altogether. The guide strand, miRNA of the miRNA/miRNA*complex, is fabricated inside the argonaute (AGO) protein, and the passenger strand (miRNA*) is degraded. In Arabidopsis thaliana, 10 AGO proteins are present, and AGO1 is the key performer for miRNAs and is designated the chief effector protein [125].

Table 3.
Major differences between siRNAs and miRNAs in plants.
Table 3.
Major differences between siRNAs and miRNAs in plants.
| Properties | siRNA | miRNA | References |
|---|---|---|---|
| Discovery | 1999 | 1993 | [29,126] |
| Definition | Cell’s defense mechanism against foreign nucleic acids | Regulator of endogenous genes | [127] |
| Length | 21–24 nt | 20–22 nt | [128] |
| Precursor | Long dsRNAs | Hairpin-shaped ssRNAs | [129] |
| Gene regulation mechanism | Transcriptional and post-transcriptional | Post-transcriptional only | [128] |
| Mode of action | Histone modification, DNA methylation, and mRNA degradation | Translational repression and mRNA degradation | [128] |
| Argonaute requirement | AGO1, AGO4, AGO6, AGO7 | AGO1, AGO10 | [130,131] |
| Nature of complementation with target | Fully complementary | Fully/partially complementary | [127] |
| Functions | Defense against viruses, transposons | Biotic/abiotic stress response, cell development, and differentiation | [128,132,133] |
miRNAs mediate gene silencing by guiding the RISC complex to attack the target genes through complementary base pairing, but the primary route they follow is that of either target cleavage or/and inhibition of translation. With extensive research by researchers globally, it has been established that some miRNAs (miR390, miR173, and miR845) have the potential to commence the mass manufacture of secondary siRNAs called the phasiRNAs which also are known as easiRNAs [134,135,136]. In plants, an extremely inflexible base-pairing rule is engaged. It has been observed that an almost impeccable base-pairing happens in the 5′ region, with a maximum mismatch number being one, and a less stringent pairing happens in the 3′ end, with a maximum of four mismatches and very small bulges [137,138]. In plants, the target gene enormity is, to a large extent, lesser than what is found in animals. Apparently, translation inhibition seems to be the most widely accepted route, but practically, target cleavage holds immense importance because of its mandatory participation in the post-germination development of the plant [139].
3.1. Mode of Action of microRNA
The elemental phenomenon which dictates miRNA activity is its export from the nucleus to the cytoplasm [105]. In the model plant, Arabidopsis thaliana duplexes of miRNA/miRNA* are inferred to be cut off from the pri-miRNAs inside the nucleus itself because the activity of DCL1 is nucleus-specific, and the shifting from the nucleus to the cytoplasm is carried out by HASTY [124]. There is also another functional complex called the THO/TREX complex, which plays some role in miRNA biogenesis [108]. The above-mentioned complex is the mediator of the transcription-coupled export of mRNA through the nuclear pore complex, so scientists have inferred that the THO/TREX complex also participates in the miRNA transport [140].
The primary function of miRNA is to regulate post-transcriptional gene modification of the target genes through two major operational pieces of machinery: (1) transcript cleavage, and (2) translation repression [103,141,142]. The conventional, general rule that dictates the mode of action of the small RNAs is the level of sequence complementarity between them and their corresponding targets because the small RNAs, which are associated with transcript cleavage, require maximum sequence complementarity [143]. There was a tremendous misconception regarding the mode of action of the plant miRNAs, which happened due to the impeccable sequence complementarity between the plant miRNAs and their corresponding mRNAs [141,142]. It is true that a good amount of sequence complementarity is favorable for RNA cleavage, but it is not that stubborn toward translational repression. It has been confirmed experimentally by many research groups that a high degree of sequence complementarity is present between the miRNAs and their targets to finally bring about miRNA-mediated inhibition of translation [144,145,146]. Citable examples of target genes and their corresponding miRNAs (which downregulate the degree of protein translation) are APETALA2 (AP2)/miR172, SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 3 (SPL3)/miR156, COPPER/ZINC SUPEROXIDE DISMUTASE 2 (Cu/ZnSOD)/miR398, and SCARECROW-LIKE PROTEIN 4 (SCL4)/miR171 [144,147,148,149].
The above discussion validates the point that sequence complementarity is not a mandatory criterion for the mode of action of miRNAs in plants. Recent research has also established that the miRNA targets (mRNAs) are stuck on the ribosomes in the endoplasmic reticulum (ER) [150,151,152], which implies that translational inhibition might be a major instrument that is operational on a larger percentage of miRNA targets than what is expected. Because plant miRNAs show a significant level of sequence complementarity with their corresponding target mRNAs, the use of bioinformatics tools has proved to be extremely useful in predicting and subsequently validating the miRNA targets in plants [153]. As stated above, with regard to the three types of operational machinery used by miRNA to perform its function, here we discuss both of them chronologically.
3.1.1. Transcript Cleavage
The phenomenon of miRNA-directed RNA cleavage is popularly named slicing. This phenomenon occurs at a predetermined location in the targeted mRNA [154]. RNAs were identified throughout the genome with a 5′ monophosphate. It was observed that quite a large number of miRNA targets face transcript cleavage [155]. Transcript cleavage is achieved by the PIWI domain of the AGO proteins, which orients itself similar to the folding pattern of RNase-H and possesses endonuclease activity. The AGO1 protein, which acts as the primary effector in Arabidopsis thaliana, undergoes cleavage in the above-stated manner, and this activity also occurs with a plethora of other effectors such as AGO2, AGO4, AGO7, and AGO10 [156,157,158,159,160,161]. In Arabidopsis, the 5′-to-3′ exonuclease is designated as EXORIBONUCLEASE 4 (XRN4), and it degrades the 3′ fragments [162]. The same happens with the 5′ cleavage fragments, which are almost undetectable in the wild-type species because of their speedy degradation. Chlamydomonas reinhardtii shows an interesting phenomenon in which the nucleotidyl transferase MUT68 first polyadenylates the 5′ fragments, and then degradation takes place via the cytoplasmic exosome [163]. The Arabidopsis homolog of MUT68, called HEN SUPPRESSOR 1 (HESO1), along with its paralogue UTP: RNA URIDYLYLTRANSFERASE 1(URT1) carry out polyuridylation of the 5′ fragments both in vitro and in vivo [164,165], and RISC-INTERACTING CLEARING 3′-5′ EXORIBONUCLEASE 1(RICE1) degrades the uridylated 5′ pieces. If these fragments are left undegraded, then they might stock-pile in plants and result in the formation of a catalytically inactive variety of RICE1 [166]. The potential role played by cytoplasmic exosomes is also significant because the subunits {SUPERKILLER 2, (SKI2), SKI3, SKI8,} of the cofactor are essential for the breakdown of the RISC-bread 5′ shreds [167].
3.1.2. Translation Inhibition
The phenomenon of translation inhibition is not as common in plants as is transcript cleavage because miRNA-guided cleavage is active all over; in addition, there are bottlenecks in estimating the protein levels because of the lack of worthwhile antibodies. Some examples can be cited that show miRNA-induced translation inhibition, and those are in plants in which AP2 is regulated by miR172, and SPL3 is regulated by miR156/7 [147,148,149]. The necessary components required to carry out miRNA-mediated translation inhibition involve the microtubule-serving enzyme KATANIN1 (KTN1) [144]; the processing body (P body) component VARICOSE (VCS) [144]; SUO protein, which is a GW repeat [145]; and the ALTERED MERISTEM PROGRAM1 (AMP1), which is the ER membrane protein [146]. It had been established that mutation in any of these above-stated genes could significantly hinder the process of miRNA-guided translation repression, and these events vividly indicate that transcript cleavage and translation inhibition are two independent lines of action with no link with each other. Genome-wide RNA profiling with 5′ monophosphates in Arabidopsis thaliana is performed to obtain an idea of the RNA degradation phenomenon, which clearly indicates that co-translational mRNA breakdown takes place for the majority of genes, which involves a plethora of miRNA targets [150,152]. A notable inference has been drawn from the entire study, i.e., even if the mode of action of the miRNAs is “RNA cleavage,” then also the potential targets are the translating miRNAs. Although the entire molecular mechanism of translation inhibition is yet unclear, in vitro studies hint that plant miRNA has the capacity to hinder the activity and operations of the ribosomes [168]. However, an immeasurable amount of information and knowledge has been gained about all the lead role players participating in miRNA biogenesis, mode of action etc. Nonetheless, uncertainty still persists about the subcellular locations where these activities are carried out. Activities such as the process of formation of D-bodies containing the dicing complexes are yet unknown. Questions such as the method of association of AGO1 with the Endoplasmic Reticulum (ER) and membrane-bound polysomes or how membrane-bound polysome affects miRNA-guided phasiRNA biogenesis are still in a haze.
Since AGO1 has a dual behavior of associating not only with miRNAs but also with siRNAs from endogenous sequences such as transposons, phasiRNA loci, and exogenous sequences such as viruses, transgenes, etc. The subcellular division and distribution of AGO1 in the middle of the cytosol, endomembrane compartments, the nucleus, and the cytoplasm have a significant impact on the scheme of action of various small RNAs. The lack of a complete and clear understanding and updated knowledge about the subcellular locations of miRNA biogenesis, and activity, deprive us of a vivid picture of miRNAs and their crosstalk with siRNAs [169].
3.2. microRNA Mediated Silencing in Plants
RNAi technology, especially the small non-coding RNAs involving miRNA, has been proven to possess immense potential to act as an attractive tool for researchers worldwide in the design and generation of plants with refined and upgraded traits by tailoring both advantageous and disadvantageous genes. The functions of individual genes can be comprehended with this powerful tool and have proven to be of immense utility to molecular crop breeders to generate improved varieties. Non-coding small RNAs have been widely used in silencing genes of interest by the RNAi technique. Adopting the method of overexpression of miRNA is especially prevalent, along with the process of introducing artificially synthesized miRNA targeting the gene of interest.
Small non-coding RNAs have been of immense use in the field of agricultural biotechnology, and their mass utilization has led to food safety because the crop cultivars thus generated have improved agronomic traits such as higher yield and nutritional value. Interactions of miRNAs with their targets is an interesting domain of research, and it has thrown light on the procedures of post-transcriptional gene regulation and a labyrinth of signaling cascades that have complete control of the plant stress responses. Though there are immense benefits of small non-coding RNAs in improving agriculture, there are certain drawbacks as well. Modification of the expression of a certain gene or its corresponding miRNA might rebound, and certain pleiotropic changes might crop up in the plant’s morphology and its development. Therefore, a complete understanding of the mechanism of miRNAs is mandatory before designing transgenic strategies.
miRNA strategies are somewhat confusing when implemented into application. The strategy which has proved fruitful for a certain plant species might not achieve success for another species, and this phenomenon is attributed to the various types of regulation of the evolutionarily conserved miRNA across different species [170]. Synthetically prepared miRNAs are of immense use in the market because they can overcome the potential drawbacks and limitations posed by siRNAs given that they bring about gene silencing with the utmost precision and finesse. The chances of off-target effects are significantly lesser with miRNA-based-RNAi compared to siRNAs because they require lesser nucleotides (single 21/22 nt sequence) to identify the target sequence. This trait of miRNAs makes miRNA-based RNAi an extremely popular one because it becomes a cakewalk to target one specific gene in a family of closely related genes. Genetically engineered crops which contain constructs that encode for a heterologous protein or overexpress a certain protein are constitutionally dissimilar from the transgenics which are prepared by RNAi technology because the gene suppression cassettes express non-coding RNAs only. Hence, it can be inferred that the transgenic crops with traits introduced by RNA-based methods are secure for human and animal consumption and do not demand intense research on the digestibility issues of the newly launched RNA component inside the cell. A point of concern regarding the biosafety of the RNAi transgenics is that gene silencing by chromatin alteration and recast might have adverse effects, which could be passed on through progenies. Although these hindrances and limitations regarding the mindset of scientists are relatively small in number, it has been proven successfully that strategies executed based on small non-coding RNAs have immense and immeasurable potential to bring about a tremendous surge in crop productivity and a significant soar in their nutritional value.
3.3. Tweaking the miRNAs to Improve Desirable Traits in Plants
The miRNAs play a vital role in plant growth and development during stress resistance, whether biotic or abiotic (Table 4). These categories of RNAs are established regulators of various complex networks. Several research and review articles have vouched for the role of miRNAs in plant growth and development [171,172,173]. In unraveling the complex regulatory mechanisms that control numerous developmental stages in a plant, a plethora of miRNAs and their complex genetic networks came into the spotlight [172]. Different research groups have identified miRNA target modules, such as miR156-SQUMOSA PROMOTER BINDING PROTEIN-LIKE (SPL), miR172-APETALA2 (AP2), and miR159-MYELOBLASTOSIS (MYB), which regulate the various key transition phases during plant development [172,174]. To cite an example, in the model plant Arabidopsis, the miR156-SPL module is a potential negative regulator during the germination–vegetative growth–reproductive phase transition. A drop in the level of miR156 prompts SPL expression, which subsequently accelerates changes, whereas miR172-AP2 works precisely the opposite way [175]. miRNAs multitask, as they act as coordinators and connect the regulatory networks with various phytohormones such as gibberellic acid (GA) and abscisic acid (ABA), thus exercising a tight grip over germination and dormancy in plants [176,177,178]. Extensive global research has clearly established that miRNA acts as an efficient mediator in numerous signaling pathways governing plant development. Apart from these, miRNAs are adept at performing via an integrative mode controlling a solo function [173]. It has also been established that the various existing isoforms of a single miRNA family can play an active part in regulating different physiological functions via the same or various other genes [179].
The literature demonstrates that miRNAs regulate gene expression in dealing with various stress responses, both biotic and abiotic. Their versatile role has been demonstrated in the model plant Arabidopsis and other cereal crops such as rice, wheat, maize, and barley [175]. In many plants, it has been clearly witnessed that a wide range of miRNA expression exists to fight out various stress factors. However, the anomaly observed is that only a handful of miRNA-target modules can regulate the expression pattern of the target genes responsible for the specific stress response. It has also been observed that this pattern is conserved throughout various species of plants [180]. The role of these conserved miRNA-target modules is also to confer stress resistance to plants by participating in different metabolic pathways. Research has proved that numerous well-established miRNA-target modules, a citable example being mi-R398-COPPER/ZINC SUPEROXIDE DISMUTASE (CSD), can mitigate the detrimental effects of various stress factors. miRNAs interact with transcription factors (TFs) to regulate the signaling of stress-related hormones such as Auxin, Ethylene, ABA, and GA during drought conditions [181]. In a similar case, it is seen that the miR169-NFY module regulates plant stress response during water stress. Expression of NF-Y is increased in stomatal guard cells to control their aperture, which consequently facilitates drought tolerance [182]. In Arabidopsis, the enhanced heat tolerance is attributed to the increased expression of miR398, which downregulates the specific targets (CSD1, CSD2, and COP-PER CHAPERONE OF CSD (CCD) [183]. The highly conserved miR394-LCR module is an active participant in cold-stress response in plants, and overexpressed miR394a Arabidopsis plants exhibit cold tolerance because they downregulate the LCR gene [184]. In bentgrass (Agrostis stolonifera), tolerance towards saline stress is conferred by the overexpression of the osa-miR319a module [185].
The miRNA-targeted modules regulate tolerance towards biotic stress factors such as an attack of bacteria, fungi, virus, and various pests [180]. In Arabidopsis, miR393-TRANSPORT INHIBITOR RESPONSE1 (TIR1), AUXIN SIGNALING F-BOX1 (AFB2), and AFB3 are the modules responsible for the defense response against Pseudomonas syringae PV. tomato DC3000. The authors of one study [186] also worked on the miR773-METHYLTRANSFERASE2 (MET2) module, for which enhanced resistance was observed as a part of pathogen-associated triggered immunity in the case of fungus. In the case of viral defense, a typical example is rice, in which the miR528-ASCORBATE OXIDASE (AO) module facilitates the aggregation of reactive oxygen species (ROS). When the rice stripe virus (RSV) strikes, miR528 covered by AGO 18 comes into action, leading to the rise in ROS levels which render antiviral defense.

Table 4.
Genetic alteration of miRNAs in plants to improve the desirable traits.
Table 4.
Genetic alteration of miRNAs in plants to improve the desirable traits.
| Plant | miRNA | Targeted Towards | Traits Involved | References |
|---|---|---|---|---|
| Tomato | miR482e-3p | NBS-LRR class proteins | Improved fungal resistance | [187] |
| Tomato | miR482/2118 | Leucine-rich repeat protein | Improved bacterial resistance | [188] |
| Arabidopsis | miR827 | Nitrogen limitation adaptation | Improved nematode resistance | [189] |
| Cotton | miR166b | Mitochondrial ATP synthase of Bemisia tabaci | Improved insect resistance | [190] |
| Arabidopsis | miR773 | Methyltransferase 2 | Improved fungal resistance | [186] |
| Arabidopsis | miR408 | uclacyanin | Improved biomass grain yield | [191] |
| Rice | miR396 | GRF6 | Yield increase | [192] |
| Arabidopsis | miR319 | TCP transcription factors | Delayed flowering | [193] |
| Camelina sativa | miR159 | Fatty acyl-ACP thioesterases | Improved seed quality | [194] |
| Salvia miltiorrhiza | miR160 | ARF10, 16, 17 | Improved biomass | [195] |
| Tomato | miR858 | SlMYB7-like | Increased anthocyanin | [196] |
| Arabidopsis | miR398 | CSD1 and CSD2 | Improved resistance against salt and heavy metal | [197] |
| Wheat | miR408 | Phosphate transporter | Improved biomass and Pi acquisition | [198] |
| Rice, tobacco | miR444 | OsMADS23,27a,27b,57 and Tobacco genes (NRTs/AEEs) | Improved N and Pi acquisition | [199,200] |
| Rice | miR528 | L-ascorbate oxidase | Improved resistance against RBSDV | [201] |
| Rice | miR444 | MIKCC-type MADS-box proteins | Improved resistance against RSV | [202] |
| Arabidopsis | miR156 | SPL9 | Improved resistance against insect | [203] |
| Rice | miR396 | GRF8 | Improved resistance against insect and fungi | [204,205] |
| Arabidopsis | miR159 | sex lethal (Sxl) protein, acetylcholinesterase (AChE) and orcokinin (Orc) | Improved resistance against insect | [206] |
| Tomato, alfalfa | miR156 | SPL | Improved tolerance against drought | [207,208] |
| Arabidopsis | miR402 | DEMETER-LIKE Protein 3 | Improved tolerance against salinity | [209] |
| Cotton | miR160 | ARF10, ARF16, ARF17 | Improved tolerance against Heat | [210] |
| Sunflower | miR396 | HaWRKY6 | Improved tolerance against heat | [211] |
| Rice | miR166 | HD-Zip family proteins | Improved tolerance against Cd | [212] |
| Arabidopsis | miR402 | DEMETER-LIKE Protein 3 | Improved tolerance against salinity and increased seed germination | [209] |
| Tomato | miR169 | SlNF-YA1/2/3, SlMRP1 | Improved resistance against drought | [170] |
| Wheat | miR408 | NAC domain protein and protein phosphatase | Increased uptake of Potassium | [213] |
4. Trans-Acting siRNA
Gene silencing in eukaryotes was observed many years ago and has been a fascinating topic for researchers, but the mechanism behind this phenomenon was quite unclear then. Hence, this process of silencing (s-RNA) was called and identified by various names, viz., RNA interference, co-suppression, quelling, etc. are among the plethora of short sRNAs identified, mainly miRNA and siRNA rampant in plants. siRNA has been categorized into various types and among them, one is trans-acting siRNA (tasiRNA), which is being studied in great detail. tasiRNAs originate from non-coding RNA (ncRNA) precursors, and the most discerning feature of tasiRNA biogenesis lies in the need for a miRNA-dependent pathway for the production of a single-stranded RNA (ssRNA) precursor [214,215]. Arabidopsis thaliana, the universal model plant, first gave substantial evidence of the miRNA-activated secondary siRNA generating loci. In this case, the non-coding RNA molecules rampantly produced siRNAs that repressed the gene expression of all those genes which were unassociated with their precursor molecules; hence, the nomenclature of trans-acting siRNAs developed (tasiRNAs). Arabidopsis thaliana has four families of tasiRNA-generating loci (TAS1-4). miR173 targets TAS1 and TAS2, whereas the biogenesis of TAS3 and TAS4 happens in the presence of miR390 and miR828, respectively [216,217,218]. Extra TAS genes such as TAS5-10 have been speculated to exist in other plants as well; hence, global research focusing on secondary siRNAs is being performed by various research groups [219,220,221,222].
4.1. Biogenesis and Structure
tasiRNAs are 21-nt structures that are generated from non-protein-coding primary TAS transcripts. These primary TAS transcripts are capped and polyadenylated as per the general rule, and it has been observed that the generation of tasiRNAs is set off by the breakup of these primary TAS transcripts (pri-TASs) by the specific mRNAs, and these transcripts possess a binding site predominantly for 22-nt miRNA. tasiRNAs are proactive in the methylation process of the TAS DNA, but they do not play any role in the generation of the TAS transcripts [223]. The cleaved product formed by the miRNAs is brought into a steady state by the suppressor of gene silencing 3 (SGS3) [12] and is transformed into a dsRNA by RDR6. This transitional state is then acted upon by DCL4 and dsRNA binding protein 4 (DRB4) to form 21 nt siRNAs. These intermediatory siRNAs are integrated into the AGO-RISC complex, the aim of which is to target complementary sequences [12,215,216]. Research and observation have led to the inference that AGO1 and AGO7 proteins are linked with the activities of tasiRNAs. Irrespective of the type of small non-coding RNA, 3′-end methylation of all is carried out by HUA ENHANCER 1 (HEN1) (Figure 4).
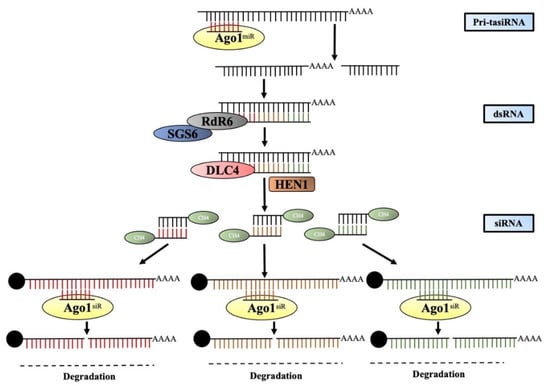
Figure 4.
The genomic TAS locus produces the pri-tasi transcripts. They are cut by miRNA by AGO1 and transformed into a double-strand by SGS3 and RDR6 jointly. Moreover, 21-nt-diced siRNA is produced and is methylated by HEN1. Finally, the target transcript is degraded by the 21-nt siRNA in a sequence-specific manner.
In the universal model plant Arabidopsis thaliana, there are eight tasiRNA-generating loci, and all of them have been classified into four TAS groups (TAS1-TAS4). Two 22 nt complexes, namely miR173::AGO1 RISC and miRNA::AGO1 RISC, start the generation of tasiRNA from TAS1a, b, c, TAS2 RNAs, and TAS4, respectively [157,224,225]. A dazzling exception exists in this entire scenario of tasiRNA generation, which concerns TAS3 because it is from that group that the generation of tasiRNA commences by miR390:AGO7 RISCs, each of which are 21 nt long.
Here too lies a hindrance similar to that of all reactions. Only the 3′ closest site of TAS3 could be cut, but the 5′ end fails to cleave the target because a high degree of sequence mismatch with miR390 has been found [157]. The most interesting fact that has been noticed by scientists is that the 3′ miR390 target site is not a mandatory part of the biogenesis process of tasiRNAs. In place of tasiRNAs, phasiRNAs can be generated. The miR390 can be replaced by many other miRNAs provided that they continue the cleavage [157]. Secondary siRNA generation is totally stopped on certain occasions, such as when any type of undesired change takes place in the miR390-binding site. Thus, the TAS3 5′ region is to be protected at any cost because it triggers the production of tasiRNAs on a large scale. Apart from these four families in the model plant Arabidopsis thaliana, many other plant sources that generate tasiRNAs similarly have been detected. Some solid examples are the following: TAS5 was first identified and reported in tomatoes, three TAS6 genes have been reported in moss, and a long list of TAS genes (TAS7-9) have been reported in grapevine (Sanan-Mishra et al. 2021) [226]. Much research is being conducted worldwide, yet the tasiRNAs belonging to TAS7-10 genes are yet to be fully characterized. The most interesting fact is that a plethora of non-coding RNAs generates tasiRNAs when given sufficient trigger by the initiator miRNAs [226].
4.2. tasiRNA Mediated Silencing in Plants
The function of the tasiRNAs is quite unique. The target site of the TASI tasiRNAs is the pentatricopeptide repeat which comprises the pentatricopeptide-repeat protein (PPR) genes and one or two other genes as well [216]. On the other hand, the TAS2-tasiRNA attacks solely the PPR genes. Findings reveal that Arabidopsis contains a count of almost 500 PPR genes, the majority of which are associated with the abiotic stress response, out of which only a selected few are the potential targets of TASI tasiRNAs. TASI controls and fine-tunes several heat stress-related transcription factors [227]. In Arabidopsis thaliana, an elevated thermotolerance level is achieved by the increased magnitude of “heat-induced TASI target 1 and 2 (HTT1, HTT2)” due to the reduced quantity of TASI-tasiRNA on being subjected to high heat. Kume et al. 2010 identified the reason behind chilling tolerance. He obtained a clear picture in which TASI-derived tasiRNAs gathered in significantly lesser amounts at 4 °C, so as a consequence of such a trigger, the gene expression of target genes such as At151670, At4g29760, and At5g18040 peaked in response in order to survive the severe cold [228]. TAS3-tasiRNAs are the conserved group of tasiRNAs, and they target the auxin response factors (ARF), thus guaranteeing a tight grip over a wide range of biological activities. In Arabidopsis thaliana, TAS3 can self-propagate and give rise to a minimum number of nine tasiRNAs. Out of these nine, two generally aim for two ARFs such as ARF3 and ARF4, whereas the remaining seven might target other ARFs. The association of ARF3 with tasiRNA predominantly determines the nature of the adaxial surface of the leaves.
Root architecture, leaf morphology, flower architecture, stress responses, hormonal crosstalk, developmental transition, embryo development, and other characteristics are controlled by the tasiRNA–ARF complex. It was noted that defective biogenesis of TAS3-tasiRNA resulted in unnatural floral development such that the development of juvenile to fully mature flowers was too fast [229]. This tasiRNA–ARF complex is also in charge in various other plants (Medicago tranculata, Lotus japonicus, Zea mays, Pyrus serotina, etc.) [136]. This module plays a role in auxin signaling and nitrogen sensitivity in the moss Physcomitrella patens, which clearly indicates that this module was coopted in the evolutionary process of lower plants [230]. tasiRNAs are equally responsible for AP2 transcription factors that are present in bryophytes.
Around 5047 tasiRNAs are found to be active in Arabidopsis thaliana and to downregulate the myb genes, just about nine tasiRNAs are begotten by TAS4 when activated by the 22-nt miR828. The most prepotent tasiRNAs {TAS4-tasiRNA81(-)} cut MYB-90 (PAP2), MYB-75 (PAP1), and MYB-113 [218], all of which are associated with the anthocyanin buildup pathway and the origin of trichomes in Arabidopsis leaves. Eventually, these tasiRNAs are negative regulators of trichome initiation [231]. miR828 actively regulates MYB2D/MYB2A genes in cotton and, subsequently, these genes give rise to tasiRNAs which also hinder the formation of cotton fiber. The miR828–TAS4 complex is also present in numerous dicot plants as per reports (not reported in monocots), and participation of miR828-TAS4 has been observed in apples as well [232].
4.3. Tweaking the tasiRNAs to Improve the Desirable Traits in Plants
TasiRNAs, a subcategory of siRNA (broad category sncRNAs), have a prominent role in plant vegetative growth and development [233]. The miR390-AGO7 complex gives rise to the phased TAS3-tasiRNAs, which target the ARF gene family and govern numerous biological processes in the plant. TAS3-tasiRNAs form a regulatory complex with ARF (TAS3-tasiRNAs–ARF), and this is considered to be the most conserved complex which plays a crucial role in the regulation of biological processes such as developmental transitions, root structure, embryo development, shoot apical meristem development, root structure, and leaf morphology along with flower and phytohormone crosstalk [173]. In the model plant Arabidopsis, miR828 targets the MYB genes (PRODUCTION OF ANTHOCYANIN PIGMENT1, PAP1, PAP2, and MYB113), which in turn triggers the TAS4-tasiRNAs and facilitates the anthocyanin biosynthetic pathway [234].
TasiRNAs have also been reported to be actively involved in regulating the plant stress response. A classic example of thermotolerance was reported in the model plant Arabidopsis, where miRNA173 is formed by the participation of numerous factors such as HEAT-INDUCED TAS1 TARGET1 (HTT1), HTT2 (both are targets of TAS1 (trans-acting siRNA precursor 1)-derived tasiRNAs) and the plants that had been overexpressed with genes HTT1 and HTT2 showed increased resistance towards thermal stress. In contrast, plants with high TASI-siRNAs and low HTT gene expression levels displayed susceptibility to heat stress [227]. Similarly, to combat biotic stress overexpressing two tasiRNAs originating from TAS1 and TAS2 loci rendered resistance to plants against the deadly fungus Botrytis cinerea [235].
5. Present Challenges Regarding Non-Coding RNAs
Traditional methods for the identification and prediction of ncRNAs include microarray, RNA sequencing, or fluorescence in situ hybridization (FISH) although paired-end strand-specific RNA sequencing may provide better transcript information in the near future. New and improved prediction tools for the tissue-specificity and chromosome location of ncRNAs are required on an urgent basis. For instance, to better predict the chromosomal location of ncRNAs, single-molecule RNA FISH (smFISH) was invented to aid the visualization of individual RNAs using target specific fluorescently labelled oligonucleotide probes [236]. With the increasing advancement of tools and technologies, methods of mapping the knowledge gained from ncRNAs in the animal system to the plant system could be possible. Apart from the small non-coding RNAs in plants, deciphering of the detailed mechanism of biogenesis and mode of action of long non-coding RNAs (lncRNA), which is still in its early phase, needs to be performed. With the advent of new technologies such as CRISPR/Cas and large-scale RNA profiling, more and more new classes of ncRNAs are coming to light, such as the discovery of a novel small nucleolar RNA (snoRNA) or tRNA-ended lncRNAs, which are not yet found in plants [237]. Moreover, to efficiently implement the existing knowledge of ncRNAs for creating improved engineered variety of crops, it is a matter of priority to develop a trait-specific candidate ncRNA catalogue.
6. Concluding Remarks and Future Prospects
miRNAs have long been associated with the cellular regulatory network, but with the emergence of new research, other non-coding RNAs such as siRNAs, tasiRNAs, or long non-coding RNAs have also been proven to be significant regulatory molecules. Over the years, comprehensive research on the biogenesis and mode of action of siRNAs/miRNAs proved valuable because that information is rapidly translated into plant–pathogen interaction studies to develop biotic stress-resilient crops. Some newly discovered non-coding RNAs, such as tasiRNAs, and phasiRNAs are still in their infancy and need thorough research to decipher how they are actively involved in the plant’s overall development. With the increasing population crisis, disease-resistant smart crops with enhanced productivity would be a top priority to feed the teeming millions. Exploiting non-coding RNAs might be the key to bringing out the solution because they are involved both in the developmental and immunity aspects of a plant’s life cycle.
Author Contributions
K.H. and A.C. conceptualized the idea; K.H. and A.C. prepared the original draft; final review and editing performed by K.H., A.C., M.Z.A. and A.D.; A.D. overall supervised and acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by both National Institute of Plant Genome Research, New Delhi, India and Department of Biotechnology, Ministry of Science & Technology, Government of India (No-BT/PR34369/AGIII/103/1176/2019). K.H. received INSPIRE fellowship from Department of Science and Technology, Ministry of Science and Technology, Govt. of India.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non Coding RNAs Regulation in Embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.I.; Alam, M.; Lightfoot, D.A.; Gurha, P.; Afzal, A.J. Classification and Experimental Identification of Plant Long Non-Coding RNAs. Genomics 2019, 111, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.; Romero-Barrios, N.; Jégu, T.; Benhamed, M.; Crespi, M. Battles and Hijacks: Noncoding Transcription in Plants. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The Noncoding RNA Revolution—Trashing Old Rules to Forge New Ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Peschansky, V.J.; Wahlestedt, C.W.C. Non-Coding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Ponjavic, J.; Ponting, C.P.; Lunter, G. Functionality or Transcriptional Noise? Evidence for Selection within Long Noncoding RNAs. Genome Res. 2007, 17, 556–565. [Google Scholar] [CrossRef]
- Brosnan, C.A.; Voinnet, O. The Long and the Short of Noncoding RNAs. Curr. Opin. Cell Biol. 2009, 21, 416–425. [Google Scholar] [CrossRef]
- D’Ario, M.; Griffiths-Jones, S.; Kim, M. Small RNAs: Big Impact on Plant Development. Trends Plant Sci. 2017, 22, 1056–1068. [Google Scholar] [CrossRef]
- Campalans, A.; Kondorosi, A.; Crespi, M. Enod40, a Short Open Reading Frame-Containing MRNA, Induces Cytoplasmic Localization of a Nuclear RNA Binding Protein in Medicago Truncatula. Plant Cell 2004, 16, 1047–1059. [Google Scholar] [CrossRef]
- Guleria, P.; Mahajan, M.; Bhardwaj, J.; Yadav, S.K. Plant Small RNAs: Biogenesis, Mode of Action and Their Roles in Abiotic Stresses. Genom. Proteom. Bioinform. 2011, 9, 183–199. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Chen, S.; Shi, M.; Jiang, X.; He, Z.; Gao, S. Mechanisms of MicroRNA Biogenesis and Stability Control in Plants. Front. Plant Sci. 2022, 13, 844149. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Peragine, A.; Mee, Y.P.; Poethig, R.S. A Pathway for the Biogenesis of Trans-Acting SiRNAs in Arabidopsis. Genes Dev. 2005, 19, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teng, C.; Xia, R.; Meyers, B.C. PhasiRNAs in Plants: Their Biogenesis, Genic Sources, and Roles in Stress Responses, Development, and Reproduction. Plant Cell 2020, 32, 3059–3080. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Romano, N.; Macino, G. Quelling: Transient Inactivation of Gene Expression in Neurospora Crassa by Transformation with Homologous Sequences. Mol. Microbiol. 1992, 6, 3343–3353. [Google Scholar] [CrossRef]
- Guo, S.; Kemphues, K.J. Par-1, a Gene Required for Establishing Polarity in C. Elegans Embryos, Encodes a Putative Ser/Thr Kinase That Is Asymmetrically Distributed. Cell 1995, 81, 611–620. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Fire, A. RNA-Triggered Gene Silencing. Trends Genet. 1999, 15, 358–363. [Google Scholar] [CrossRef]
- Carthew, R.W. Gene Silencing by Double-Stranded RNA. Curr. Opin. Cell Biol. 2001, 13, 244–248. [Google Scholar] [CrossRef]
- Dalmay, T.; Hamilton, A.; Rudd, S.; Angell, S.; Baulcombe, D.C. An RNA-Dependent RNA Polymerase Gene in Arabidopsis Is Required for Posttranscriptional Gene Silencing Mediated by a Transgene but Not by a Virus. Cell 2000, 101, 543–553. [Google Scholar] [CrossRef]
- Smardon, A.; Spoerke, J.M.; Stacey, S.C.; Klein, M.E.; MacKin, N.; Maine, E.M. EGO-1 Is Related to RNA-Directed RNA Polymerase and Functions in Germ-Line Development and RNA Interference in C. Elegans. Curr. Biol. 2000, 10, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Baulcombe, D.C. A Species of Small Antisense RNA in Posttranscriptional Gene Silencing in Plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-Stranded RNA Directs the ATP-Dependent Cleavage of MRNA at 21 to 23 Nucleotide Intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA Interference Is Mediated by 21- and 22-Nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Boettcher, S.; Caudy, A.A.; Kobayashi, R.; Hannon, G.J. Argonaute2, a Link between Genetic and Biochemical Analyses of RNAi. Science 2001, 293, 1146–1150. [Google Scholar] [CrossRef]
- Matranga, C.; Tomari, Y.; Shin, C.; Bartel, D.P.; Zamore, P.D. Passenger-Strand Cleavage Facilitates Assembly of SiRNA into Ago2-Containing RNAi Enzyme Complexes. Cell 2005, 123, 607–620. [Google Scholar] [CrossRef]
- Rand, T.A.; Petersen, S.; Du, F.; Wang, X. Argonaute2 Cleaves the Anti-Guide Strand of SiRNA during RISC Activation. Cell 2005, 123, 621–629. [Google Scholar] [CrossRef]
- Tomari, Y.; Zamore, P.D. Perspective: Machines for RNAi. Genes Dev. 2005, 19, 517–529. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21±nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells Sayda. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Martinez, J.; Patkaniowska, A.; Lendeckel, W.; Tuschl, T. Functional Anatomy of SiRNAs for Mediating Efficient RNAi in Drosophila Melanogaster Embryo Lysate. EMBO J. 2001, 20, 6877–6888. [Google Scholar] [CrossRef]
- Mourrain, P.; Béclin, C.; Elmayan, T.; Feuerbach, F.; Godon, C.; Morel, J.B.; Jouette, D.; Lacombe, A.M.; Nikic, S.; Picault, N.; et al. Arabidopsis SGS2 and SGS3 Genes Are Required for Posttranscriptional Gene Silencing and Natural Virus Resistance. Cell 2000, 101, 533–542. [Google Scholar] [CrossRef]
- Cogoni, C.; Macino, G. Gene Silencing in Neurospora Crassa Requires a Protein Homologous to RNA-Dependent RNA Polymerase. Nature 1999, 399, 166–169. [Google Scholar] [CrossRef]
- Brown, R.D.; Mattoccia, E.; Tocchini-Valentini, G.P. On the Role of RNA in Gene Amplification. Acta Endocrinol. Suppl. 1972, 168, 307–318. [Google Scholar] [CrossRef]
- Mello, C.C.; Conte, J.D. Revealing the World of RNA Interference. Nature 2004, 431, 338–342. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-Induced Silencing Complex: A Versatile Gene-Silencing Machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Meister, G. Argonaute Proteins: Mediators of RNA Silencing. Mol. Cell 2007, 26, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Meister, G. Argonaute Proteins: Functional Insights and Emerging Roles. Nat. Rev. Genet 2013, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Weinberg, D.E.; Bartel, D.P.; Patel, D.J. Structure of Yeast Argonaute with Guide RNA. Nature 2012, 486, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, E.; Kuhn, C.D.; Tocilj, A.; Haase, A.D.; Greene, E.M.; Hannon, G.J.; Joshua-Tor, L. The Structure of Human Argonaute-2 in Complex with MiR-20a. Cell 2012, 150, 100–110. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, G.; Juranek, S.; Tuschl, T.; Patel, D.J. Structure of the Guide-Strand-Containing Argonaute Silencing Complex. Nature 2008, 456, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Roe, S.M.; Barford, D. Structural Insights into MRNA Recognition from a PIWI Domain-SiRNA Guide Complex. Nature 2005, 434, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Kobayashi, M.; Yoda, M.; Sakaguchi, Y.; Katsuma, S.; Suzuki, T.; Tomari, Y. Hsc70/Hsp90 Chaperone Machinery Mediates ATP-Dependent RISC Loading of Small RNA Duplexes. Mol. Cell 2010, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, A.I.; Donchenko, G.V.; Stepanenko, S.P. Effect of Withdrawal of Phenazepam and Nicotinamide on the State of the Systems of Reception of Benzodiazepines and NAD. Ukr Biokhim Zh 1996, 68, 24–25. [Google Scholar]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional SiRNAs and MiRNAs Exhibit Strand Bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef]
- Wang, Y.; Juranek, S.; Li, H.; Sheng, G.; Wardle, G.S.; Tuschl, T.; Patel, D.J. Nucleation, Propagation and Cleavage of Target RNAs in Ago Silencing Complexes. Nature 2009, 461, 754–761. [Google Scholar] [CrossRef]
- Miyoshi, K.; Tsukumo, H.; Nagami, T.; Siomi, H.; Siomi, M.C. Slicer Function of Drosophila Argonautes and Its Involvement in RISC Formation. Genes Dev. 2005, 19, 2837–2848. [Google Scholar] [CrossRef]
- Djuranovic, S.; Nahvi, A.; Green, R. MiRNA-Mediated Gene Silencing by Translational Repression Followed by MRNA Deadenylation and Decay. Science 2012, 336, 237–240. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, Action, and Degradation of RNA-Induced Silencing Complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Winter, J.; Diederichs, S. Argonaute Proteins Regulate MicroRNA Stability: Increased MicroRNA Abundance by Argonaute Proteins Is Due to MicroRNA Stabilization. RNA Biol. 2011, 8, 1149–1157. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The Action of ARGONAUTE1 in the MiRNA Pathway and Its Regulation by the MiRNA Pathway Are Crucial for Plant Development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Derrien, B.; Baumberger, N.; Schepetilnikov, M.; Viotti, C.; De Cillia, J.; Ziegler-Graff, V.; Isono, E.; Schumacher, K.; Genschik, P. Degradation of the Antiviral Component ARGONAUTE1 by the Autophagy Pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 15942–15946. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Shoji, K.; Kiyokawa, K.; Negishi, L.; Tomari, Y. VCP Machinery Mediates Autophagic Degradation of Empty Argonaute. Cell Rep. 2019, 28, 1144–1153.e4. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Kingston, E.R.; Kleaveland, B.; Lin, D.H.; Stubna, M.W.; Bartel, D.P. The ZSWIM8 Ubiquitin Ligase Mediates Target-Directed MicroRNA Degradation. Science 2020, 370, eabc9359. [Google Scholar] [CrossRef]
- Han, J.; Lavigne, C.A.; Jones, B.T.; Zhang, H.; Gillett, F.; Mendell, J.T. A Ubiquitin Ligase Mediates Target-Directed MicroRNA Decay Independently of Tailing and Trimming. Science 2020, 370, eabc9546. [Google Scholar] [CrossRef]
- Rajam, M.V. RNA Silencing Technology: A Boon for Crop Improvement. J. Biosci. 2020, 45, 118. [Google Scholar] [CrossRef]
- Ghag, S.B. Host Induced Gene Silencing, an Emerging Science to Engineer Crop Resistance against Harmful Plant Pathogens. Physiol. Mol. Plant Pathol. 2017, 100, 242–254. [Google Scholar] [CrossRef]
- Shin, Y.H.; Lee, S.H.; Park, Y.D. Development of Mite (Tetranychus Urticae)-Resistant Transgenic Chinese Cabbage Using Plant-Mediated RNA Interference. Hortic. Environ. Biotechnol. 2020, 61, 305–315. [Google Scholar] [CrossRef]
- Hussain, T.; Aksoy, E.; Çalışkan, M.E.; Bakhsh, A. Transgenic Potato Lines Expressing Hairpin RNAi Construct of Molting-Associated EcR Gene Exhibit Enhanced Resistance against Colorado Potato Beetle (Leptinotarsa Decemlineata, Say). Transgenic. Res. 2019, 28, 151–164. [Google Scholar] [CrossRef]
- Banakar, P.; Hada, A.; Papolu, P.K.; Rao, U. Simultaneous RNAi Knockdown of Three FMRFamide-like Peptide Genes, Mi-Flp1, Mi-Flp12, and Mi-Flp18 Provides Resistance to Root-Knot Nematode, Meloidogyne incognita. Front. Microbiol. 2020, 11, 573916. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. RNA Interference for Improving Disease Resistance in Plants and Its Relevance in This Clustered Regularly Interspaced Short Palindromic Repeats-Dominated Era in Terms of DsRNA-Based Biopesticides. Front. Plant Sci. 2022, 13, 885128. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Díaz-Ruíz, J.R. Double-Stranded RNA-Mediated Interference with Plant Virus Infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; De Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and Application of Exogenous DsRNA Confers Plant Protection against Sclerotinia Sclerotiorum and Botrytis Cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of Virus Resistance by Exogenous Application of Double-Stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Höfle, L.; Biedenkopf, D.; Werner, B.T.; Shrestha, A.; Jelonek, L.; Koch, A. Study on the Efficiency of DsRNAs with Increasing Length in RNA-Based Silencing of the Fusarium CYP51 Genes. RNA Biol. 2020, 17, 463–473. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Le, B.H.; He, W.; Hou, Y. The Master Role of SiRNAs in Plant Immunity. Mol. Plant Pathol. 2022, 23, 1565–1574. [Google Scholar] [CrossRef]
- Wang, X.B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.X.; Chen, X.; Yu, J.L.; Ding, S.W. RNAi-Mediated Viral Immunity Requires Amplification of Virus-Derived SiRNAs in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Zhang, F.; Xu, M.; Ye, Z.; Wang, K.; Liu, S.; Han, X.; Cheng, Y.; Zhong, K.; et al. A Virus-Derived SiRNA Activates Plant Immunity by Interfering with ROS Scavenging. Mol. Plant 2021, 14, 1088–1103. [Google Scholar] [CrossRef]
- Cao, M.; Du, P.; Wang, X.; Yu, Y.Q.; Qiu, Y.H.; Li, W.; Gal-On, A.; Zhou, C.; Li, Y.; Ding, S.W. Virus Infection Triggers Widespread Silencing of Host Genes by a Distinct Class of Endogenous SiRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14613–14618. [Google Scholar] [CrossRef]
- Wagh, S.G.; Alam, M.M.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Toriba, T.; Hirano, H.Y.; Nishiguchi, M. Analysis of Rice RNA-Dependent RNA Polymerase 6 (OsRDR6) Gene in Response to Viral, Bacterial and Fungal Pathogens. J. Gen. Plant Pathol. 2016, 82, 12–17. [Google Scholar] [CrossRef]
- Nowara, D.; Schweizer, P.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J. HIGS: Host-Induced Gene Silencing in the Obligate Biotrophic Fungal Pathogen Blumeria Graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-Amylose Wheat Generated by RNA Interference Improves Indices of Large-Bowel Health in Rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, K.A. Steroid Regulation Improves Crop Yield. Nat. Biotechnol. 2006, 24, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Singh, S.P.; Green, A.G. High-Stearic and High-Oleic Cottonseed Oils Produced by Hairpin RNA-Mediated Post-Transcriptional Gene Silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Eady, C.C.; Kamoi, T.; Kato, M.; Porter, N.G.; Davis, S.; Shaw, M.; Kamoi, A.; Imai, S. Silencing Onion Lachrymatory Factor Synthase Causes a Significant Change in the Sulfur Secondary Metabolite Profile. Plant Physiol. 2008, 147, 2096–2106. [Google Scholar] [CrossRef]
- Qiao, F.; Yang, Q.; Wang, C.L.; Fan, Y.L.; Wu, X.F.; Zhao, K.J. Modification of Plant Height via RNAi Suppression of OsGA20ox2 Gene in Rice. Euphytica 2007, 158, 35–45. [Google Scholar] [CrossRef]
- Sunilkumar, G.; Campbell, L.A.M.; Puckhaber, L.; Stipanovic, R.D.; Rathore, K.S. Engineering Cottonseed for Use in Human Nutrition by Tissue-Specific Reduction of Toxic Gossypol. Proc. Natl. Acad. Sci. USA 2006, 103, 18054–18059. [Google Scholar] [CrossRef]
- Jiang, C.J.; Shimono, M.; Maeda, S.; Inoue, H.; Mori, M.; Hasegawa, M.; Sugano, S.; Takatsuji, H. Suppression of the Rice Fatty-Acid Desaturase Gene OsSSI2 Enhances Resistance to Blast and Leaf Blight Diseases in Rice. Mol. Plant-Microbe Interact. 2009, 22, 820–829. [Google Scholar] [CrossRef]
- Kusaba, M.; Miyahara, K.; Iida, S.; Fukuoka, H.; Takano, T.; Sassa, H.; Nishimura, M.; Nishio, T. Low Glutelin Content1: A Dominant Mutation That Suppresses the Glutelin Multigene Family via RNA Silencing in Rice. Plant Cell 2003, 15, 1455–1467. [Google Scholar] [CrossRef]
- Yara, A.; Yaeno, T.; Hasegawa, M.; Seto, H.; Montillet, J.L.; Kusumi, K.; Seo, S.; Iba, K. Disease Resistance against Magnaporthe Grisea Is Enhanced in Transgenic Rice with Suppression of ω-3 Fatty Acid Desaturases. Plant Cell Physiol. 2007, 48, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, B.; Zhang, M.; Wang, L.; Cui, M.; Wang, Q.; Leng, P. Fruit-Specific RNAi-Mediated Suppression of SlNCED1 Increases Both Lycopene and β-Carotene Contents in Tomato Fruit. J. Exp. Bot. 2012, 63, 3097–3108. [Google Scholar] [CrossRef]
- Eschen-Lippold, L.; Landgraf, R.; Smolka, U.; Schulze, S.; Heilmann, M.; Heilmann, I.; Hause, G.; Rosahl, S. Activation of Defense against Phytophthora Infestans in Potato by Down-Regulation of Syntaxin Gene Expression. New Phytol. 2012, 193, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Davuluri, G.R.; Van Tuinen, A.; Fraser, P.D.; Manfredonia, A.; Newman, R.; Burgess, D.; Brummell, D.A.; King, S.R.; Palys, J.; Uhlig, J.; et al. Fruit-Specific RNAi-Mediated Suppression of DET1 Enhances Carotenoid and Flavonoid Content in Tomatoes. Nat. Biotechnol. 2005, 23, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Riechen, J. Establishment of Broad-Spectrum Resistance against Blumeria Graminis f.Sp. Tritici in Triticum Aestivum by RNAi-Mediated Knock-down of MLO. J. Für Verbrauch. Und Lebensm. 2007, 2, 120. [Google Scholar] [CrossRef]
- Meli, V.S.; Ghosh, S.; Prabha, T.N.; Chakraborty, N.; Chakraborty, S.; Datta, A. Enhancement of Fruit Shelf Life by Suppressing N-Glycan Processing Enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 2413–2418. [Google Scholar] [CrossRef]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering Broad Root-Knot Resistance in Transgenic Plants by RNAi Silencing of a Conserved and Essential Root-Knot Nematode Parasitism Gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, R.K.; Rajam, M.V. Delayed Ripening and Improved Fruit Processing Quality in Tomato by RNAi-Mediated Silencing of Three Homologs of 1-Aminopropane-1-Carboxylate Synthase Gene. J. Plant Physiol. 2013, 170, 987–995. [Google Scholar] [CrossRef]
- Yadav, B.C.; Veluthambi, K.; Subramaniam, K. Host-Generated Double Stranded RNA Induces RNAi in Plant-Parasitic Nematodes and Protects the Host from Infection. Mol. Biochem. Parasitol. 2006, 148, 219–222. [Google Scholar] [CrossRef]
- Schijlen, E.G.W.M.; De Vos, C.H.R.; Martens, S.; Jonker, H.H.; Rosin, F.M.; Molthoff, J.W.; Tikunov, Y.M.; Angenent, G.C.; Van Tunen, A.J.; Bovy, A.G. RNA Interference Silencing of Chalcone Synthase, the First Step in the Flavonoid Biosynthesis Pathway, Leads to Parthenocarpic Tomato Fruits. Plant Physiol. 2007, 144, 1520–1530. [Google Scholar] [CrossRef]
- Shimizu, T.; Yoshii, M.; Wei, T.; Hirochika, H.; Omura, T. Silencing by RNAi of the Gene for Pns12, a Viroplasm Matrix Protein of Rice Dwarf Virus, Results in Strong Resistance of Transgenic Rice Plants to the Virus. Plant Biotechnol. J. 2009, 7, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.W.; Lin, S.S.; Reyes, J.L.; Chen, K.C.; Wu, H.W.; Yeh, S.D.; Chua, N.H. Expression of Artificial MicroRNAs in Transgenic Arabidopsis Thaliana Confers Virus Resistance. Nat. Biotechnol. 2006, 24, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beaith, M.; Chalifoux, M.; Ying, J.; Uchacz, T.; Sarvas, C.; Griffiths, R.; Kuzma, M.; Wan, J.; Huang, Y. Shoot-Specific down-Regulation of Protein Farnesyltransferase (α-Subunit) for Yield Protection against Drought in Canola. Mol. Plant 2009, 2, 191–200. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Zhao, M.; Tian, Q.; Zhang, W.-H. Identification of Drought-Responsive MicroRNAs in Medicago Truncatula by Genome-Wide High- Throughput Sequencing. BMC Genom. 2011, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Widyaningrum, S.; Pujiasih, D.R.; Sholeha, W.; Harmoko, R.; Sugiharto, B. Induction of Resistance to Sugarcane Mosaic Virus by RNA Interference Targeting Coat Protein Gene Silencing in Transgenic Sugarcane. Mol. Biol. Rep. 2021, 48, 3047–3054. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Timerbaev, V.; Okuneva, A.; Klementyeva, A.; Sidorova, T.; Pushin, A.; Dolgov, S. Enhancement of Resistance to PVY in Intragenic Marker-Free Potato Plants by RNAi-Mediated Silencing of EIF4E Translation Initiation Factors. Plant Cell Tissue Organ. Cult. 2020, 140, 691–705. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Shivakumar, M.; Praveen, S.; Chouhan, B.S.; Chand, S. Expression of Short Hairpin RNA (ShRNA) Targeting AC2 Gene of Mungbean Yellow Mosaic India Virus (MYMIV) Reduces the Viral Titre in Soybean. 3 Biotech 2019, 9, 334. [Google Scholar] [CrossRef]
- Kumari, A.; Hada, A.; Subramanyam, K.; Theboral, J.; Misra, S.; Ganapathi, A.; Malathi, V.G. RNAi-Mediated Resistance to Yellow Mosaic Viruses in Soybean Targeting Coat Protein Gene. Acta Physiol. Plant 2018, 40, 32. [Google Scholar] [CrossRef]
- Yang, X.; Niu, L.; Zhang, W.; Yang, J.; Xing, G.; He, H.; Guo, D.; Du, Q.; Qian, X.; Yao, Y.; et al. RNAi-Mediated SMV P3 Cistron Silencing Confers Significantly Enhanced Resistance to Multiple Potyvirus Strains and Isolates in Transgenic Soybean. Plant Cell Rep. 2018, 37, 103–114. [Google Scholar] [CrossRef]
- Ahmed, M.M.S.; Bian, S.; Wang, M.; Zhao, J.; Zhang, B.; Liu, Q.; Zhang, C.; Tang, S.; Gu, M.; Yu, H. RNAi-Mediated Resistance to Rice Black-Streaked Dwarf Virus in Transgenic Rice. Transgenic Res. 2017, 26, 197–207. [Google Scholar] [CrossRef]
- Khatoon, S.; Kumar, A.; Sarin, N.B.; Khan, J.A. RNAi-Mediated Resistance against Cotton Leaf Curl Disease in Elite Indian Cotton (Gossypium Hirsutum) Cultivar Narasimha. Virus Genes 2016, 52, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, J.; Ren, G. Plant MicroRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, M.; Miura, S.; Nei, M. Origins and Evolution of MicroRNA Genes in Plant Species. Genome Biol. Evol. 2012, 4, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Akpinar, B.A. Plant MiRNAs: Biogenesis, Organization and Origins. Funct. Integr. Genom. 2015, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.; Chen, X. Biogenesis, Turnover, and Mode of Action of Plant MicroRNAs. Plant Cell 2013, 25, 2383–2399. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Han, M.H.; Fedoroff, N. The RNA-Binding Proteins HYL1 and SE Promote Accurate in Vitro Processing of Pri-MiRNA by DCL1. Proc. Natl. Acad. Sci. USA 2008, 105, 9970–9975. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, H.; Zhang, Q.; Zhang, J.; Ni, F.; Liu, C.; Qi, Y. DNA Methylation Mediated by a MicroRNA Pathway. Mol. Cell 2010, 38, 465–475. [Google Scholar] [CrossRef]
- Francisco-Mangilet, A.G.; Karlsson, P.; Kim, M.H.; Eo, H.J.; Oh, S.A.; Kim, J.H.; Kulcheski, F.R.; Park, S.K.; Manavella, P.A. THO2, a Core Member of the THO/TREX Complex, Is Required for MicroRNA Production in Arabidopsis. Plant J. 2015, 82, 1018–1029. [Google Scholar] [CrossRef]
- Karlsson, P.; Christie, M.D.; Seymour, D.K.; Wang, H.; Wang, X.; Hagmann, J.; Kulcheski, F.; Manavella, P.A. KH Domain Protein RCF3 Is a Tissue-Biased Regulator of the Plant MiRNA Biogenesis Cofactor HYL1. Proc. Natl. Acad. Sci. USA 2015, 112, 14096–14101. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Gu, L.; Li, X.; Cao, S.; Chu, C.; Cui, X.; Chen, X.; Cao, X. NOT2 Proteins Promote Polymerase II—Dependent Transcription and Interact with Multiple MicroRNA Biogenesis Factors in Arabidopsis. Plant Cell 2013, 25, 715–727. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Y.; Li, J.; Xu, L.; Fang, Y.; Li, X.; Qi, Y. A Role for the RNA-Binding Protein MOS2 in MicroRNA Maturation in Arabidopsis. Cell Res. 2013, 23, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Shi, J.; Zhai, Y.; Hou, Y.; Ma, W. Phytophthora Effector Targets a Novel Component of Small RNA Pathway in Plants to Promote Infection. Proc. Natl. Acad. Sci. USA 2015, 112, 5850–5855. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cui, Y.; Li, Y.; Qi, Y. Transcription and Processing of Primary MicroRNAs Are Coupled by Elongator Complex in Arabidopsis. Nat. Plants 2015, 1, 15075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, M.; Ren, G.; Yu, B. CDC5, a DNA Binding Protein, Positively Regulates Posttranscriptional Processing and / or Transcription of Primary MicroRNA Transcripts. Proc. Natl. Acad. Sci. USA 2013, 110, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Bologna, N.G.; Voinnet, O. The Diversity, Biogenesis, and Activities of Endogenous Silencing Small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 2014, 65, 473–503. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Snyder, J.A.; Park, Y.B.; Li, Y.F.; Sunkar, R.; Axtell, M.J. Sliced MicroRNA Targets and Precise Loop-First Processing of MIR319 Hairpins Revealed by Analysis of the Physcomitrella Patens Degradome. RNA 2009, 15, 2112–2121. [Google Scholar] [CrossRef]
- Bologna, N.G.; Mateos, J.L.; Bresso, E.G.; Palatnik, J.F. A Loop-to-Base Processing Mechanism Underlies the Biogenesis of Plant MicroRNAs MiR319 and MiR159. EMBO J. 2009, 28, 3646–3656. [Google Scholar] [CrossRef]
- Bologna, N.G.; Schapire, A.L.; Zhai, J.; Chorostecki, U.; Boisbouvier, J.; Meyers, B.C.; Palatnik, J.F. Multiple RNA Recognition Patterns during MicroRNA Biogenesis in Plants. Genome Res. 2013, 23, 1675–1689. [Google Scholar] [CrossRef]
- Mateos, J.L.; Bologna, N.G.; Chorostecki, U.; Palatnik, J.F. Identification of MicroRNA Processing Determinants by Random Mutagenesis of Arabidopsis MIR172a Precursor. Curr. Biol. 2010, 20, 49–54. [Google Scholar] [CrossRef]
- Song, L.; Axtell, M.J.; Fedoroff, N.V. RNA Secondary Structural Determinants of MiRNA Precursor Processing in Arabidopsis. Curr. Biol. 2010, 20, 37–41. [Google Scholar] [CrossRef]
- Werner, S.; Wollmann, H.; Schneeberger, K.; Weigel, D. Structure Determinants for Accurate Processing of MiR172a in Arabidopsis Thaliana. Curr. Biol. 2010, 20, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, Z.; Li, J.; Minakhina, S.; Yang, M.; Padgett, R.W.; Steward, R.; Chen, X. Methylation as a Crucial Step in Plant MicroRNA Biogenesis Bin. Science 2005, 307, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ebright, Y.W.; Yu, B.; Chen, X. HEN1 Recognizes 21-24 Nt Small RNA Duplexes and Deposits a Methyl Group onto the 2′ OH of the 3′ Terminal Nucleotide. Nucleic Acids Res. 2006, 34, 667–675. [Google Scholar] [CrossRef]
- Mee, Y.P.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear Processing and Export of MicroRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, R.; Meyers, B.C.; Walbot, V. Evolution, Functions, and Mysteries of Plant ARGONAUTE Proteins. Curr. Opin. Plant Biol. 2015, 27, 84–90. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Duan, X.; Liu, C.; Li, Z. Digital Quantitative Analysis of MicroRNA in Single Cell Based on Ligation-Depended Polymerase Colony (Polony). Biosens. Bioelectron. 2017, 95, 146–151. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of MiRNAs and SiRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhu, J.K. Micro RNAs and Short-Interfering RNAs in Plants. J. Integr. Plant Biol. 2007, 49, 817–826. [Google Scholar] [CrossRef]
- Kim, V.N. Small RNAs: Classification, Biogenesis, and Function. Mol. Cells 2005, 19, 1–15. [Google Scholar]
- Zilberman, D.; Cao, X.; Jacobsen, S.E. ARGONAUTE4 Control of Locus-Specific SiRNA Accumulation and DNA and Histone Methylation. Science 2003, 299, 716–719. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of Small RNAs in Animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Totoki, Y.; Sasaki, H.; Minami, N.; Imai, H. Analysis of Small RNA Profiles During Development. Methods Enzymol. 2007, 427, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A High Quality Draft Consensus Sequence of the Genome of a Heterozygous Grapevine Variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Xia, R.; Meyers, B.C. Phased, Secondary, Small Interfering RNAs in Posttranscriptional Regulatory Networks. Plant Cell 2013, 25, 2400–2415. [Google Scholar] [CrossRef] [PubMed]
- Creasey, K.M.; Zhai, J.; Borges, F.; Van Ex, F.; Regulski, M.; Meyers, B.C.; Martienssen, R.A. MiRNAs Trigger Widespread Epigenetically-Activated SiRNAs from Transposons in Arabidopsis. Nature 2014, 508, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Muhammad, S.; Cao, M.; Wu, L. Biogenesis and Regulatory Hierarchy of Phased Small Interfering RNAs in Plants. Plant Biotechnol. J. 2018, 16, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific Effects of MicroRNAs on the Plant Transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef]
- Axtell, M.J.; Meyers, B.C. Revisiting Criteria for Plant MicroRNA Annotation in the Era of Big Data. Plant Cell 2018, 30, 272–284. [Google Scholar] [CrossRef]
- Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Gilbert, K.B.; Montgomery, T.A.; Nguyen, T.; Cuperus, J.T.; Carrington, J.C. Functional Analysis of Three Arabidopsis Argonautes Using Slicer-Defective Mutants. Plant Cell 2012, 24, 3613–3629. [Google Scholar] [CrossRef]
- Köhler, A.; Hurt, E. Exporting RNA from the Nucleus to the Cytoplasm. Nat. Rev. Mol. Cell Biol. 2007, 8, 761–773. [Google Scholar] [CrossRef]
- Chen, X.; Sciences, P.; States, U. microRNA biogenesis and function in plants. FEBS Lett. 2005, 579, 5923–5931. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Small RNAs and Their Roles in Plant Development. Annu. Rev. Cell Dev. Biol. 2009, 35, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Hutva’gner, G.; Zamore, P.D. A MicroRNA in a Multiple- Turnover RNAi Enzyme Complex. Science 2002, 297, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.Y.; Sieburth, L.; Voinnet, O. Widespread Translational Inhibition by Plant MiRNAs and SiRNAs. Science 2008, 1185, 1185–1190. [Google Scholar] [CrossRef]
- Yang, L.; Wu, G.; Poethig, R.S. Mutations in the GW-Repeat Protein SUO Reveal a Developmental Function for MicroRNA-Mediated Translational Repression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 315–320. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Zhuang, X.; Yu, Y.; Liu, X.; Cui, X.; Ji, L.; Pan, Z.; Cao, X.; Mo, B.; et al. MicroRNAs Inhibit the Translation of Target MRNAs on the Endoplasmic Reticulum in Arabidopsis. Cell 2013, 153, 562–574. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of Flowering Time and Floral Organ Identity by a MicroRNA and Its APETALA2-like Target Genes The Plant Cell. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Gandikota, M.; Birkenbihl, R.P.; Höhmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The MiRNA156/157 Recognition Element in the 3′ UTR of the Arabidopsis SBP Box Gene SPL3 Prevents Early Flowering by Translational Inhibition in Seedlings. Plant J. 2007, 49, 683–693. [Google Scholar] [CrossRef]
- Chen, X. A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development. Science 2004, 303, 2022–2025. [Google Scholar] [CrossRef]
- Hou, C.Y.; Lee, W.C.; Chou, H.C.; Chen, A.P.; Chou, S.J.; Chen, H.M. Global Analysis of Truncated RNA Ends Reveals New Insights into Ribosome Stalling in Plants. Plant Cell 2016, 28, 2398–2416. [Google Scholar] [CrossRef]
- Li, S.; Le, B.; Ma, X.; Li, S.; You, C.; Yu, Y.; Zhang, B.; Liu, L.; Gao, L.; Shi, T.; et al. Biogenesis of Phased SiRNAs on Membrane-Bound Polysomes in Arabidopsis. Elife 2016, 5, e22750. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Willmann, M.R.; Anderson, S.J.; Gregory, B.D. Genome-Wide Mapping of Uncapped and Cleaved Transcripts Reveals a Role for the Nuclear Mrna Cap-Binding Complex in Cotranslational Rna Decay in Arabidopsis. Plant Cell 2016, 28, 2385–2397. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Small RNAs in Plants: Recent Development and Application for Crop Improvement. Front. Plant Sci. 2015, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Llave, C.; Xie, Z.; Kasschau, K.D.; Carrington, J.C. Cleavage of Scarecrow-like MRNA Targets Directed by a Class of Arabidopsis MiRNA. Science 2002, 297, 2053–2056. [Google Scholar] [CrossRef]
- German, M.A.; Pillay, M.; Jeong, D.H.; Hetawal, A.; Luo, S.; Janardhanan, P.; Kannan, V.; Rymarquis, L.A.; Nobuta, K.; German, R.; et al. Global Identification of MicroRNA-Target RNA Pairs by Parallel Analysis of RNA Ends. Nat. Biotechnol. 2008, 26, 941–946. [Google Scholar] [CrossRef]
- Mi, S.; Cai, T.; Hu, Y.; Chen, Y.; Hodges, E.; Ni, F.; Wu, L.; Li, S.; Zhou, H.; Long, C.; et al. Sorting of Small RNAs into Arabidopsis Argonaute Complexes Is Directed by the 5′ Terminal Nucleotide. Cell 2008, 133, 116–127. [Google Scholar] [CrossRef]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-MiR390 Interaction and Dual Functionality in TAS3 Trans-Acting SiRNA Formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef]
- Takeda, A.; Iwasaki, S.; Watanabe, T.; Utsumi, M.; Watanabe, Y. The Mechanism Selecting the Guide Strand from Small RNA Duplexes Is Different among Argonaute Proteins. Plant Cell Physiol. 2008, 49, 493–500. [Google Scholar] [CrossRef]
- Ji, L.; Liu, X.; Yan, J.; Wang, W.; Yumul, R.E.; Kim, Y.J.; Dinh, T.T.; Liu, J.; Cui, X.; Zheng, B.; et al. ARGONAUTE10 and ARGONAUTE1 Regulate the Termination of Floral Stem Cells through Two MicroRNAs in Arabidopsis. PLoS Genet. 2011, 7, e1001358. [Google Scholar] [CrossRef]
- Maunoury, N.; Vaucheret, H. AGO1 and AGO2 Act Redundantly in MiR408-Mediated Plantacyanin Regulation. PLoS ONE 2011, 6, e28729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, F.; Wang, R.; Zhou, X.; Sze, S.H.; Liou, L.W.; Barefoot, A.; Dickman, M.; Zhang, X. Arabidopsis Argonaute10 Specifically Sequesters MiR166/165 to Regulate Shoot Apical Meristem Development. Cell 2011, 145, 242–256. [Google Scholar] [CrossRef]
- Souret, F.F.; Kastenmayer, J.P.; Green, P.J. AtXRN4 Degrades MRNA in Arabidopsis and Its Substrates Include Selected MiRNA Targets. Mol. Cell 2004, 15, 173–183. [Google Scholar] [CrossRef]
- Ibrahim, F.; Rohr, J.; Jeong, W.J.; Hesson, J.; Cerutti, H. Untemplated Oligoadenylation Promotes Degradation of RISC-Cleaved Transcripts. Science 2006, 314, 1893. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xie, M.; Zhang, S.; Vinovskis, C.; Chen, X.; Yu, B. Methylation Protects MicroRNAs from an AGO1-Associated Activity That Uridylates 5′ RNA Fragments Generated by AGO1 Cleavage. Proc. Natl. Acad. Sci. USA 2014, 111, 6365–6370. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Dou, Y.; Zhang, C.; Chen, X.; Yu, B.; Ren, G. Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3′ Tailing of Small RNAs in Arabidopsis. PLoS Genet. 2015, 11, e1005091. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, F.; Sung, M.W.; Shu, C.; Castillo-González, C.; Koiwa, H.; Tang, G.; Dickman, M.; Li, P.; Zhang, X. RISC-Interacting Clearing 3′-5′ Exoribonucleases (RICES) Degrade Uridylated Cleavage Fragments to Maintain Functional RISC in Arabidopsis Thaliana. Elife 2017, 6, e24466. [Google Scholar] [CrossRef] [PubMed]
- Branscheid, A.; Marchais, A.; Schott, G.; Lange, H.; Gagliardi, D.; Andersen, S.U.; Voinnet, O.; Brodersen, P. SKI2 Mediates Degradation of RISC 5′-Cleavage Fragments and Prevents Secondary SiRNA Production from MiRNA Targets in Arabidopsis. Nucleic Acids Res. 2015, 43, 10975–10988. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Tomari, Y. Molecular Insights into MicroRNA-Mediated Translational Repression in Plants. Mol Cell 2013, 52, 591–601. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Chen, X. The ‘How’ and ‘Where’ of Plant MicroRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Z.; Gong, P.; Zhang, J.; Ziaf, K.; Li, H.; Xiao, F.; Ye, Z. Over-Expression of MicroRNA169 Confers Enhanced Drought Tolerance to Tomato. Biotechnol. Lett. 2011, 33, 403–409. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B. MicroRNAs in Control of Plant Development. J. Cell Physiol. 2016, 231, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Denyer, T. MiRNAs in Plant Development. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 689–712. ISBN 9781119312994. [Google Scholar]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant Noncoding RNAs: Hidden Players in Development and Stress Responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, P.; Liu, S.; Yang, Q.; Guo, H. The Control of Developmental Phase Transitions by MicroRNAs and Their Targets in Seed Plants. Int. J. Mol. Sci. 2020, 21, 1971. [Google Scholar] [CrossRef] [PubMed]
- Bhogireddy, S.; Mangrauthia, S.K.; Kumar, R.; Pandey, A.K.; Singh, S.; Jain, A.; Budak, H.; Varshney, R.K.; Kudapa, H. Regulatory Non-Coding RNAs: A New Frontier in Regulation of Plant Biology. Funct. Integr. Genom. 2021, 21, 313–330. [Google Scholar] [CrossRef]
- Liu, Y.; El-Kassaby, Y.A. Regulatory Crosstalk between MicroRNAs and Hormone Signalling Cascades Controls the Variation on Seed Dormancy Phenotype at Arabidopsis Thaliana Seed Set. Plant Cell Rep. 2017, 36, 705–717. [Google Scholar] [CrossRef]
- Das, S.S.; Karmakar, P.; Nandi, A.K.; Sanan-Mishra, N. Small RNA Mediated Regulation of Seed Germination. Front. Plant Sci. 2015, 6, 828. [Google Scholar] [CrossRef]
- Martin, R.C.; Asahina, M.; Liu, P.P.; Kristof, J.R.; Coppersmith, J.L.; Pluskota, W.E.; Bassel, G.W.; Goloviznina, N.A.; Nguyen, T.T.; Martínez-Andújar, C.; et al. The MicroRNA156 and MicroRNA172 Gene Regulation Cascades at Post-Germinative Stages in Arabidopsis. Seed Sci. Res. 2010, 20, 79–87. [Google Scholar] [CrossRef]
- Alptekin, B.; Langridge, P.; Budak, H. Abiotic Stress MiRNomes in the Triticeae. Funct. Integr. Genom. 2017, 17, 145–170. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Ferdous, J.; Sanchez-Ferrero, J.C.; Langridge, P.; Milne, L.; Chowdhury, J.; Brien, C.; Tricker, P.J. Differential Expression of MicroRNAs and Potential Targets under Drought Stress in Barley. Plant Cell Environ. 2017, 40, 11–24. [Google Scholar] [CrossRef]
- Li, W.X.; Oono, Y.; Zhu, J.; He, X.J.; Wu, J.M.; Iida, K.; Lu, X.Y.; Cui, X.; Jin, H.; Zhu, J.K. The Arabidopsis NFYA5 Transcription Factor Is Regulated Transcriptionally and Posttranscriptionally to Promote Drought Resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Lu, X.; Zeng, H.; Zhang, Y.; Zhu, J. Heat Stress Induction of MiR398 Triggers a Regulatory Loop That Is Critical for Thermotolerance in Arabidopsis. Plant J. 2013, 74, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Song, J.B.; Gao, S.; Wang, Y.; Li, B.W.; Zhang, Y.L.; Yang, Z.M. MiR394 and Its Target Gene LCR Are Involved in Cold Stress Response in Arabidopsis. Plant Gene 2016, 5, 56–64. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive Expression of a MiR319 Gene Alters Plant Development and Enhances Salt and Drought Tolerance in Transgenic Creeping Bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Guirao, R.; Baldrich, P.; Weigel, D.; Segundo, B.S.; Rubio-Somoza, I. The Microrna MiR773 Is Involved in the Arabidopsis Immune Response to Fungal Pathogens. Mol. Plant-Microbe Interact. 2018, 31, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, S.J.; Zhang, S.W.; Feng, T.; Zhang, Z.Y.; Luo, S.J.; Mao, H.Y.; Borkovich, K.A.; Ouyang, S.Q. SlymiR482e-3p Mediates Tomato Wilt Disease by Modulating Ethylene Response Pathway. Plant Biotechnol. J. 2021, 19, 17–19. [Google Scholar] [CrossRef]
- Canto-Pastor, A.; Santos, B.A.M.C.; Valli, A.A.; Summers, W.; Schornack, S.; Baulcombe, D.C. Enhanced Resistance to Bacterial and Oomycete Pathogens by Short Tandem Target Mimic RNAs in Tomato. Proc. Natl. Acad. Sci. USA 2019, 116, 2755–2760. [Google Scholar] [CrossRef]
- Hewezi, T.; Piya, S.; Qi, M.; Balasubramaniam, M.; Rice, J.H.; Baum, T.J. Arabidopsis MiR827 Mediates Post-Transcriptional Gene Silencing of Its Ubiquitin E3 Ligase Target Gene in the Syncytium of the Cyst Nematode Heterodera Schachtii to Enhance Susceptibility. Plant J. 2016, 88, 179–192. [Google Scholar] [CrossRef]
- Wamiq, G.; Khan, J.A. Overexpression of Ghr-MiR166b Generates Resistance against Bemisia Tabaci Infestation in Gossypium Hirsutum Plants. Planta 2018, 247, 1175–1189. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Wang, Y.; Li, H.; Li, S.; Zhao, H.; Zhang, H. Constitutive Expression of Mir408 Improves Biomass and Seed Yield in Arabidopsis. Front. Plant Sci. 2018, 8, 2114. [Google Scholar] [CrossRef]
- Gao, F.; Wang, K.; Liu, Y.; Chen, Y.; Chen, P.; Shi, Z.; Luo, J.; Jiang, D.; Fan, F.; Zhu, Y.; et al. Blocking MiR396 Increases Rice Yield by Shaping Inflorescence Architecture. Nat. Plants 2016, 2, 15196. [Google Scholar] [CrossRef] [PubMed]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of Jasmonate Biosynthesis and Senescence by MiR319 Targets. PLoS Biol. 2008, 6, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Ozseyhan, M.E.; Li, P.; Na, G.N.; Li, Z.; Wang, C.; Lu, C. Improved Fatty Acid Profiles in Seeds of Camelina Sativa by Artificial MicroRNA Mediated FATB Gene Suppression. Biochem. Biophys. Res. Commun. 2018, 503, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, H.; Hou, Z.; Xu, L.; Jin, W.; Liang, Z. Overexpression of Ath-MIR160b Increased the Biomass While Reduced the Content of Tanshinones in Salvia Miltiorrhiza Hairy Roots by Targeting ARFs Genes. Plant Cell Tissue Organ. Cult. 2020, 142, 327–338. [Google Scholar] [CrossRef]
- Jia, X.; Shen, J.; Liu, H.; Li, F.; Ding, N.; Gao, C.; Pattanaik, S.; Patra, B.; Li, R.; Yuan, L. Small Tandem Target Mimic-Mediated Blockage of MicroRNA858 Induces Anthocyanin Accumulation in Tomato. Planta 2015, 242, 283–293. [Google Scholar] [CrossRef]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Erratum: Posttranscriptional Induction of Two Cu/Zn Superoxide Dismutase Genes in Arabidopsis Is Mediated by Downregulation of MiR398 and Important for Oxidative Stress. Plant Cell 2006, 18, 2415. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, X.; Chen, X.; Shi, G.; Liu, Z.; Guo, C.; Xiao, K. Wheat MiRNA Taemir408 Acts as an Essential Mediator in Plant Tolerance to Pi Deprivation and Salt Stress via Modulating Stress-Associated Physiological Processes. Front. Plant Sci. 2018, 9, 499. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Hamera, S.; Chen, X.; Fang, R. MiR444a Has Multiple Functions in the Rice Nitrate-Signaling Pathway. Plant J. 2014, 78, 44–55. [Google Scholar] [CrossRef]
- Gao, S.; Guo, C.; Zhang, Y.; Zhang, F.; Du, X.; Gu, J.; Xiao, K. Wheat MicroRNA Member TaMIR444a Is Nitrogen Deprivation-Responsive and Involves Plant Adaptation to the Nitrogen-Starvation Stress. Plant Mol. Biol. Rep. 2016, 34, 931–946. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS Accumulation and Antiviral Defence Control by MicroRNA528 in Rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Kong, X.; Hamera, S.; Wu, Y.; Chen, X.; Fang, R.; Yan, Y. A Signaling Cascade from MiR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016, 170, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate Response Decay and Defense Metabolite Accumulation Contributes to Age-Regulated Dynamics of Plant Insect Resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef] [PubMed]
- Soto-Suárez, M.; Baldrich, P.; Weigel, D.; Rubio-Somoza, I.; San Segundo, B. The Arabidopsis MiR396 Mediates Pathogen-Associated Molecular Pattern-Triggered Immune Responses against Fungal Pathogens. Sci. Rep. 2017, 7, 44898. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396–OsGRF8–OsF3H-Flavonoid Pathway Mediates Resistance to the Brown Planthopper in Rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Zubair, M.; Khan, M.Z.; Rauf, I.; Raza, A.; Shah, A.H.; Hassan, I.; Amin, I.; Mansoor, S. Artificial Micro RNA (AmiRNA)-Mediated Resistance against Whitefly (Bemisia tabaci) Targeting Three Genes. Crop Prot. 2020, 137, 105308. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The Interplay between MiR156/SPL13 and DFR/WD40-1 Regulate Drought Tolerance in Alfalfa. BMC Plant Biol. 2019, 19, 434. [Google Scholar] [CrossRef]
- Visentin, I.; Pagliarani, C.; Deva, E.; Caracci, A.; Turečková, V.; Novák, O.; Lovisolo, C.; Schubert, A.; Cardinale, F. A Novel Strigolactone-MiR156 Module Controls Stomatal Behaviour during Drought Recovery. Plant Cell Environ. 2020, 43, 1613–1624. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwak, K.J.; Jung, H.J.; Lee, H.J.; Kang, H. MicroRNA402 Affects Seed Germination of Arabidopsis Thaliana under Stress Conditions via Targeting DEMETER-LIKE Protein3 MRNA. Plant Cell Physiol. 2010, 51, 1079–1083. [Google Scholar] [CrossRef]
- Lin, J.S.; Kuo, C.C.; Yang, I.C.; Tsai, W.A.; Shen, Y.H.; Lin, C.C.; Liang, Y.C.; Li, Y.C.; Kuo, Y.W.; King, Y.C.; et al. MicroRNA160 Modulates Plant Development and Heat Shock Protein Gene Expression to Mediate Heat Tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 68. [Google Scholar] [CrossRef]
- Giacomelli, J.I.; Weigel, D.; Chan, R.L.; Manavella, P.A. Role of Recently Evolved MiRNA Regulation of Sunflower HaWRKY6 in Response to Temperature Damage. New Phytol. 2012, 195, 766–773. [Google Scholar] [CrossRef]
- Ding, Y.; Gong, S.; Wang, Y.; Wang, F.; Bao, H.; Sun, J.; Cai, C.; Yi, K.; Chen, Z.; Zhu, C. MicroRNA166 Modulates Cadmium Tolerance and Accumulation in Rice. Plant Physiol. 2018, 177, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, K.; Liu, G.; Li, S.; Zhao, S.; Liu, X.; Yang, X.; Xiao, K. Global Identification and Characterization of MiRNA Family Members Responsive to Potassium Deprivation in Wheat (Triticum aestivum L.). Sci. Rep. 2020, 10, 15812. [Google Scholar] [CrossRef] [PubMed]
- Peragine, A.; Yoshikawa, M.; Wu, G.; Albrecht, H.L.; Poethig, R.S. SGS3 and SGS2/SDE1/RDR6 Are Required for Juvenile Development and the Production of Trans-Acting SiRNAs in Arabidopsis. Genes Dev. 2004, 18, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.; Vaucheret, H.; Rajagopalan, R.; Lepers, C.; Gasciolli, V.; Mallory, A.C.; Hilbert, J.L.; Bartel, D.P.; Crété, P. Endogenous Trans-Acting SiRNAs Regulate the Accumulation of Arabidopsis MRNAs. Mol. Cell 2004, 16, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. MicroRNA-Directed Phasing during Trans-Acting SiRNA Biogenesis in Plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef]
- Williams, L.; Carles, C.C.; Osmont, K.S.; Fletcher, J.C. A Database Analysis Method Identifies an Endogenous Trans-Acting Short-Interfering RNA That Targets the Arabidopsis ARF2, ARF3, and ARF4 Genes. Proc. Natl. Acad. Sci. USA 2005, 102, 9703–9708. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Vaucheret, H.; Trejo, J.; Bartel, D.P. A Diverse and Evolutionarily Fluid Set of MicroRNAs in Arabidopsis Thaliana. Genes Dev. 2006, 20, 3407–3425. [Google Scholar] [CrossRef]
- Arif, M.A.; Fattash, I.; Ma, Z.; Cho, S.H.; Beike, A.K.; Reski, R.; Axtell, M.J.; Frank, W. DICER-LIKE3 Activity in Physcomitrella Patens DICER-LIKE4 Mutants Causes Severe Developmental Dysfunction and Sterility. Mol. Plant 2012, 5, 1281–1294. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Wang, J.; Fang, J. Identification of Trans-Acting SiRNAs and Their Regulatory Cascades in Grapevine. Bioinformatics 2012, 28, 2561–2568. [Google Scholar] [CrossRef]
- Li, F.; Orban, R.; Baker, B. SoMART: A Web Server for Plant MiRNA, TasiRNA and Target Gene Analysis. Plant J. 2012, 70, 891–901. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, Q.; Han, C.; Ju, Z.; Cao, D.; Zhu, B.; Luo, Y.; Gao, L. SRNAome and Degradome Sequencing Analysis Reveals Specific Regulation of SRNA in Response to Chilling Injury in Tomato Fruit. Physiol. Plant 2017, 160, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mao, L.; Qi, Y. Roles of DICER-LIKE and ARGONAUTE Proteins in TAS-Derived Small Interfering RNA-Triggered DNA Methylation. Plant Physiol. 2012, 160, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.A.; Seong, J.Y.; Fahlgren, N.; Gilbert, S.D.; Howell, M.D.; Sullivan, C.M.; Alexander, A.; Nguyen, G.; Allen, E.; Ji, H.A.; et al. AGO1-MiR173 Complex Initiates Phased SiRNA Formation in Plants. Proc. Natl. Acad. Sci. USA 2008, 105, 20055–20062. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, J.T.; Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Burke, R.T.; Takeda, A.; Sullivan, C.M.; Gilbert, S.D.; Montgomery, T.A.; Carrington, J.C. Unique Functionality of 22-Nt MiRNAs in Triggering RDR6-Dependent SiRNA Biogenesis from Target Transcripts in Arabidopsis. Nat. Struct. Mol. Biol. 2010, 17, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Sanan-Mishra, N.; Abdul Kader Jailani, A.; Mandal, B.; Mukherjee, S.K. Secondary SiRNAs in Plants: Biosynthesis, Various Functions, and Applications in Virology. Front. Plant Sci. 2021, 12, 610283. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. HEAT-INDUCED TAS1 TARGET1 Mediates Thermotolerance via Heat Stress Transcription Factor A1a-Directed Pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780. [Google Scholar] [CrossRef]
- Kume, K.; Tsutsumi, K.I.; Saitoh, Y. TAS1 Trans-Acting SiRNA Targets Are Differentially Regulated at Low Temperature, and TAS1 Trans-Acting SiRNA Mediates Temperature-Controlled At1g51670 Expression. Biosci. Biotechnol. Biochem. 2010, 74, 1435–1440. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Nogueira, F.T.S.; Howell, M.D.; Montgomery, T.A.; Carrington, J.C.; Timmermans, M.C.P. Pattern Formation via Small RNA Mobility. Genes Dev. 2009, 23, 549–554. [Google Scholar] [CrossRef]
- Xia, J.; Wang, X.; Perroud, P.F.; He, Y.; Quatrano, R.; Zhang, W. Endogenous Small-Noncoding RNAs and Potential Functions in Desiccation Tolerance in Physcomitrella Patens. Sci. Rep. 2016, 6, 30118. [Google Scholar] [CrossRef]
- Shi, M.-Z.; Xie, D.-Y. Biosynthesis and Metabolic Engineering of Anthocyanins in Arabidopsis Thaliana. Recent Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Wu, J.; Ding, B.; Fei, Z. A Dynamic Evolutionary and Functional Landscape of Plant Phased Small Interfering RNAs. BMC Biol. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Khraiwesh, B.; Zhu, J.K.; Zhu, J. Role of MiRNAs and SiRNAs in Biotic and Abiotic Stress Responses of Plants. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Leng, J.; Ma, Y.; Fan, P.; Li, Y.; Yan, H.; Xu, Q. BrmiR828 Targets BrPAP1, BrMYB82, and BrTAS4 Involved in the Light Induced Anthocyanin Biosynthetic Pathway in Brassica Rapa. Int. J. Mol. Sci. 2020, 21, 4326. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Ji, N.; van Oudenaarden, A. Single Molecule Fluorescent in Situ Hybridization (SmFISH) of C. elegans Worms and Embryos. WormBook 2012, 1–16. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Chen, L.L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).