Identification of Promising Drug Candidates against Prostate Cancer through Computationally-Driven Drug Repurposing
Abstract
1. Introduction
2. Results and Discussion
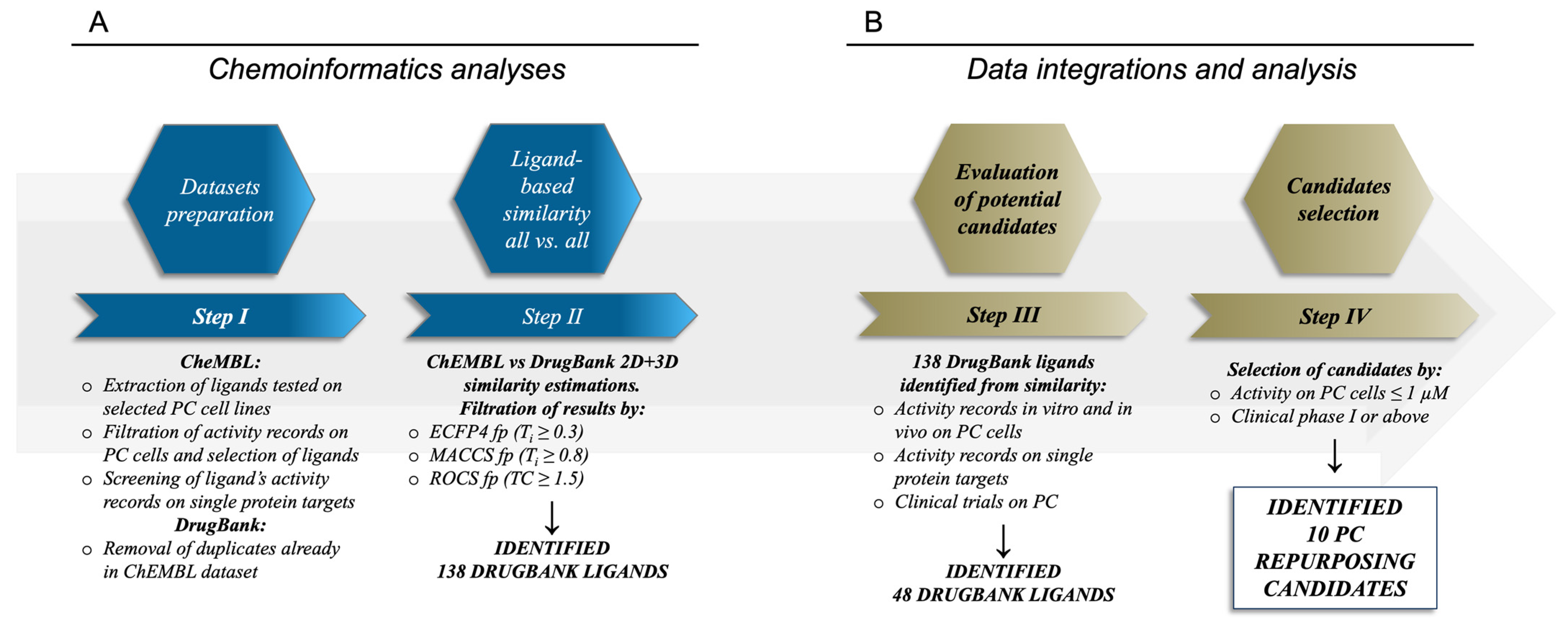
2.1. Analyses of the Compounds in the ChEMBL Dataset and of Their Target Activity Data
2.2. Similarity Estimations and Selection of the Candidates for Drug Repurposing
2.3. Molecules Already under Clinical Evaluation for PC
2.4. New Potential Candidates for Drug Repurposing
3. Materials and Methods
3.1. Curation of the Ligands and Targets Dataset
3.2. Ligand-Based Calculations
3.3. Data Integration
3.4. Analysis of Biological Target Activity Annotations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, M.C.S.; Goggins, W.B.; Wang, H.H.X.; Fung, F.D.H.; Leung, C.; Wong, S.Y.S.; Ng, C.F.; Sung, J.J.Y. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef]
- Howrey, B.T.; Kuo, Y.-F.; Lin, Y.-L.; Goodwin, J.S. The Impact of PSA Screening on Prostate Cancer Mortality and Overdiagnosis of Prostate Cancer in the United States. J. Gerontol. Ser. A 2013, 68, 56–61. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Sammon, J.D.; Abdollah, F.; D’Amico, A.; Gettman, M.; Haese, A.; Suardi, N.; Vickers, A.; Trinh, Q.-D. Predicting Life Expectancy in Men Diagnosed with Prostate Cancer. Eur. Urol. 2015, 68, 756–765. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Fang, D.; Zhou, L. Androgen Deprivation Therapy in Nonmetastatic Prostate Cancer Patients: Indications, Treatment Effects, and New Predictive Biomarkers. Asia-Pac. J. Clin. Oncol. 2019, 15, 108–120. [Google Scholar] [CrossRef]
- Banapour, P.; Schumacher, A.; Lin, J.C.; Finley, D.S. Radical Prostatectomy and Pelvic Lymph Node Dissection in Kaiser Permanente Southern California: 15-Year Experience. Perm. J. 2019, 23, 17–233. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Sayegh, N.; Swami, U.; Agarwal, N. Recent Advances in the Management of Metastatic Prostate Cancer. JCO Oncol. Pract. 2022, 18, 45–55. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate Cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Beatson, E.L.; Chau, C.H.; Price, D.K.; Figg, W.D. PARP Inhibitors on the Move in Prostate Cancer: Spotlight on Niraparib & Update on PARP Inhibitor Combination Trials. Am. J. Clin. Exp. Urol. 2022, 10, 252–257. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. The Treatment Landscape of Metastatic Prostate Cancer. Cancer Lett. 2021, 519, 20–29. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Kumar, R.; Harilal, S.; Gupta, S.V.; Jose, J.; Thomas Parambi, D.G.; Uddin, M.S.; Shah, M.A.; Mathew, B. Exploring the New Horizons of Drug Repurposing: A Vital Tool for Turning Hard Work into Smart Work. Eur. J. Med. Chem. 2019, 182, 111602. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Dickson, M.; Gagnon, J.P. Key Factors in the Rising Cost of New Drug Discovery and Development. Nat. Rev. Drug Discov. 2004, 3, 417–429. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Demus, T.; Moubarak, M.M.; Daher, D.; Alvarez Moreno, J.C.; Polit, F.; Lopez, O.; Merhe, A.; Abou-Kheir, W.; Nieder, A.M.; et al. Overcoming Drug Resistance in Advanced Prostate Cancer by Drug Repurposing. Med. Sci. 2022, 10, 15. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and Opportunities in Drug Discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Novac, N. Challenges and Opportunities of Drug Repositioning. Trends Pharmacol. Sci. 2013, 34, 267–272. [Google Scholar] [CrossRef]
- Rastelli, G.; Pellati, F.; Pinzi, L.; Gamberini, M.C. Repositioning Natural Products in Drug Discovery. Molecules 2020, 25, 1154. [Google Scholar] [CrossRef]
- March-Vila, E.; Pinzi, L.; Sturm, N.; Tinivella, A.; Engkvist, O.; Chen, H.; Rastelli, G. On the Integration of In Silico Drug Design Methods for Drug Repurposing. Front. Pharmacol. 2017, 8, 298. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E.; et al. The ChEMBL Database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic Target Database Update 2022: Facilitating Drug Discovery with Enriched Comparative Data of Targeted Agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef]
- Home-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 4 December 2022).
- Costa, F.F. Big data in biomedicine. Drug Discovery Today. 2014, 19, 433–440. [Google Scholar] [CrossRef]
- Pinzi, L.; Lherbet, C.; Baltas, M.; Pellati, F.; Rastelli, G. In Silico Repositioning of Cannabigerol as a Novel Inhibitor of the Enoyl Acyl Carrier Protein (ACP) Reductase (InhA). Molecules 2019, 24, E2567. [Google Scholar] [CrossRef]
- Carrella, D.; Manni, I.; Tumaini, B.; Dattilo, R.; Papaccio, F.; Mutarelli, M.; Sirci, F.; Amoreo, C.A.; Mottolese, M.; Iezzi, M.; et al. Computational Drugs Repositioning Identifies Inhibitors of Oncogenic PI3K/AKT/P70S6K-Dependent Pathways among FDA-Approved Compounds. Oncotarget 2016, 7, 58743–58758. [Google Scholar] [CrossRef]
- Maruca, A.; Rocca, R.; Catalano, R.; Mesiti, F.; Costa, G.; Lanzillotta, D.; Salatino, A.; Ortuso, F.; Trapasso, F.; Alcaro, S.; et al. Natural Products Extracted from Fungal Species as New Potential Anti-Cancer Drugs: A Structure-Based Drug Repurposing Approach Targeting HDAC7. Molecules 2020, 25, 5524. [Google Scholar] [CrossRef]
- Pinzi, L.; Tinivella, A.; Gagliardelli, L.; Beneventano, D.; Rastelli, G. LigAdvisor: A Versatile and User-Friendly Web-Platform for Drug Design. Nucleic Acids Res. 2021, 49, W326–W335. [Google Scholar] [CrossRef]
- Drugpro. Available online: https://drugrepo.org/ (accessed on 4 December 2022).
- Corsello, S.M.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M.; et al. The Drug Repurposing Hub: A next-Generation Drug Library and Information Resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef]
- Pinzi, L.; Tinivella, A.; Caporuscio, F.; Rastelli, G. Drug Repurposing and Polypharmacology to Fight SARS-CoV-2 Through Inhibition of the Main Protease. Front. Pharmacol. 2021, 12, 636989. [Google Scholar] [CrossRef]
- Masoudi-Sobhanzadeh, Y.; Omidi, Y.; Amanlou, M.; Masoudi-Nejad, A. Drug Databases and Their Contributions to Drug Repurposing. Genomics 2020, 112, 1087–1095. [Google Scholar] [CrossRef]
- Matsson, P.; Kihlberg, J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017, 60, 1662–1664. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Saranyutanon, S.; Deshmukh, S.K.; Dasgupta, S.; Pai, S.; Singh, S.; Singh, A.P. Cellular and Molecular Progression of Prostate Cancer: Models for Basic and Preclinical Research. Cancers 2020, 12, 2651. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and Characterization of a Human Prostatic Carcinoma Cell Line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a Human Prostate Carcinoma Cell Line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP Model of Human Prostatic Carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/MTOR Pathway in Castration-Resistant Prostate Cancer. Endocr. Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-MTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.S.; Kim, D.Y.; So, I.; Jeon, J.-H. PI3K Pathway in Prostate Cancer: All Resistant Roads Lead to PI3K. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2018, 1870, 198–206. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/MTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Jego, G.; Hazoumé, A.; Seigneuric, R.; Garrido, C. Targeting Heat Shock Proteins in Cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef]
- Taldone, T.; Gozman, A.; Maharaj, R.; Chiosis, G. Targeting Hsp90: Small-Molecule Inhibitors and Their Clinical Development. Curr. Opin. Pharmacol. 2008, 8, 370–374. [Google Scholar] [CrossRef]
- Gomez-Monterrey, I.; Sala, M.; Musella, S.; Campiglia, P. Heat Shock Protein 90 Inhibitors as Therapeutic Agents. Recent Pat. Anticancer Drug Discov. 2012, 7, 313–336. [Google Scholar] [CrossRef]
- Pinzi, L.; Benedetti, R.; Altucci, L.; Rastelli, G. Design of Dual Inhibitors of Histone Deacetylase 6 and Heat Shock Protein 90. ACS Omega 2020, 5, 11473–11480. [Google Scholar] [CrossRef]
- Anighoro, A.; Pinzi, L.; Marverti, G.; Bajorath, J.; Rastelli, G. Heat Shock Protein 90 and Serine/Threonine Kinase B-Raf Inhibitors Have Overlapping Chemical Space. RSC Adv. 2017, 7, 31069–31074. [Google Scholar] [CrossRef]
- Cairns, B.R. Emerging Roles for Chromatin Remodeling in Cancer Biology. Trends Cell Biol. 2001, 11, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Gupta, S. The Role of Histone Deacetylases in Prostate Cancer. Epigenetics 2008, 3, 300–309. [Google Scholar] [CrossRef]
- Li, G.; Tian, Y.; Zhu, W.-G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef] [PubMed]
- Willis-Martinez, D.; Richards, H.W.; Timchenko, N.A.; Medrano, E.E. Role of HDAC1 in senescence, aging, and cancer. Exp. Gerontol. 2010, 45, 279–285. [Google Scholar] [CrossRef]
- Weichert, W.; Röske, A.; Gekeler, V.; Beckers, T.; Stephan, C.; Jung, K.; Fritzsche, F.R.; Niesporek, S.; Denkert, C.; Dietel, M.; et al. Histone Deacetylases 1, 2 and 3 Are Highly Expressed in Prostate Cancer and HDAC2 Expression Is Associated with Shorter PSA Relapse Time after Radical Prostatectomy. Br. J. Cancer 2008, 98, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Halkidou, K.; Gaughan, L.; Cook, S.; Leung, H.Y.; Neal, D.E.; Robson, C.N. Upregulation and Nuclear Recruitment of HDAC1 in Hormone Refractory Prostate Cancer. Prostate 2004, 59, 177–189. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, S.-N.; Kim, Y.K. Involvement of HDAC1 in E-Cadherin Expression in Prostate Cancer Cells; Its Implication for Cell Motility and Invasion. Biochem. Biophys. Res. Commun. 2011, 404, 915–921. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Shen, K.-H.; Hung, S.-H.; Hsu, Y.-L. CXCL1/GROα Increases Cell Migration and Invasion of Prostate Cancer by Decreasing Fibulin-1 Expression through NF-ΚB/HDAC1 Epigenetic Regulation. Carcinogenesis 2012, 33, 2477–2487. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, G.; Zhao, T.C. HDAC4: Mechanism of Regulation and Biological Functions. Epigenomics 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Jin, K.; Zhao, W.; Xie, X.; Pan, Y.; Wang, K.; Zhang, H. MiR-520b Restrains Cell Growth by Targeting HDAC4 in Lung Cancer. Thorac. Cancer 2018, 9, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.-H.; Wang, C.-Y.; Zhang, W.-L.; Zhang, J.-T.; Yuan, C.-H.; Zhao, P.-W.; Lin, Y.-Y.; Hong, S.; Li, C.-Y.; Wang, L. Histone Deacetylase HDAC4 Promotes Gastric Cancer SGC-7901 Cells Progression via P21 Repression. PLoS ONE 2014, 9, e98894. [Google Scholar] [CrossRef] [PubMed]
- Mottet, D.; Pirotte, S.; Lamour, V.; Hagedorn, M.; Javerzat, S.; Bikfalvi, A.; Bellahcène, A.; Verdin, E.; Castronovo, V. HDAC4 Represses P21WAF1/Cip1 Expression in Human Cancer Cells through a Sp1-Dependent, P53-Independent Mechanism. Oncogene 2009, 28, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Byun, D.-S.; Nasser, S.; Murray, L.B.; Ayyanar, K.; Arango, D.; Figueroa, M.; Melnick, A.; Kao, G.D.; Augenlicht, L.H.; et al. HDAC4 Promotes Growth of Colon Cancer Cells via Repression of P21. Mol. Biol. Cell 2008, 19, 4062–4075. [Google Scholar] [CrossRef] [PubMed]
- Halkidou, K.; Cook, S.; Leung, H.Y.; Neal, D.E.; Robson, C.N. Nuclear Accumulation of Histone Deacetylase 4 (HDAC4) Coincides with the Loss of Androgen Sensitivity in Hormone Refractory Cancer of the Prostate. Eur. Urol. 2004, 45, 382–389. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, G.; Dong, Z.; Liu, Z.; Li, L.; Feng, Y.; Su, D.; Zhang, Y.; Huang, B.; Lu, J. Recruitment of HDAC4 by Transcription Factor YY1 Represses HOXB13 to Affect Cell Growth in AR-Negative Prostate Cancers. Int. J. Biochem. Cell Biol. 2009, 41, 1094–1101. [Google Scholar] [CrossRef]
- Wojcik, E.J.; Buckley, R.S.; Richard, J.; Liu, L.; Huckaba, T.M.; Kim, S. Kinesin-5: Cross-Bridging Mechanism to Targeted Clinical Therapy. Gene 2013, 531, 133–149. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, Q.; Tian, G.; Song, W.; Wang, T.; Wang, A.; Chen, X.; Qin, S. KIF11 Manipulates SREBP2-Dependent Mevalonate Cross Talk to Promote Tumor Progression in Pancreatic Ductal Adenocarcinoma. Cancer Med. 2022, 11, 3282–3295. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Wei, D.; Wang, B. KIF11 Is a Promising Therapeutic Target for Thyroid Cancer Treatment. Comput. Math. Methods Med. 2022, 2022, e6426800. [Google Scholar] [CrossRef]
- Ling, J.; Wang, Y.; Ma, L.; Zheng, Y.; Tang, H.; Meng, L.; Zhang, L. KIF11, a plus End-Directed Kinesin, as a Key Gene in Benzo(a)Pyrene-Induced Non-Small Cell Lung Cancer. Environ. Toxicol. Pharmacol. 2022, 89, 103775. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, G.; Cui, S.; Du, G. Upregulation of KIF11 in TP53 Mutant Glioma Promotes Tumor Stemness and Drug Resistance. Cell. Mol. Neurobiol. 2022, 42, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, Y.; Yi, L.; Gao, Z.; Lou, M.; Yuan, K. High KIF11 Expression Is Associated with Poor Outcome of NSCLC. Tumori J. 2022, 108, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-F.; Zeng, H.-J.; Shan, Z.; Ye, R.-Y.; Cheang, T.-Y.; Zhang, Y.-J.; Lu, S.-H.; Zhang, Q.; Shao, N.; Lin, Y. Overexpression of Kinesin Superfamily Members as Prognostic Biomarkers of Breast Cancer. Cancer Cell Int. 2020, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Liu, B.; Wei, S.; Wang, T.; Li, T.; Lin, J.; Ni, X. KIF11: A Potential Prognostic Biomarker for Predicting Bone Metastasis-free Survival of Prostate Cancer. Oncol. Lett. 2022, 24, 312. [Google Scholar] [CrossRef]
- Piao, X.-M.; Byun, Y.J.; Jeong, P.; Ha, Y.-S.; Yoo, E.S.; Yun, S.J.; Kim, W.-J. Kinesin Family Member 11 MRNA Expression Predicts Prostate Cancer Aggressiveness. Clin. Genitourin. Cancer 2017, 15, 450–454. [Google Scholar] [CrossRef]
- Xing, N.-D.; Ding, S.-T.; Saito, R.; Nishizawa, K.; Kobayashi, T.; Inoue, T.; Oishi, S.; Fujii, N.; Lv, J.-J.; Ogawa, O.; et al. A Potent Chemotherapeutic Strategy in Prostate Cancer: S-(Methoxytrityl)--Cysteine, a Novel Eg5 Inhibitor. Asian J. Androl. 2011, 13, 236–241. [Google Scholar] [CrossRef]
- Cochran, J.C.; Gatial, J.E.; Kapoor, T.M.; Gilbert, S.P. Monastrol Inhibition of the Mitotic Kinesin Eg5 *. J. Biol. Chem. 2005, 280, 12658–12667. [Google Scholar] [CrossRef]
- Lad, L.; Luo, L.; Carson, J.D.; Wood, K.W.; Hartman, J.J.; Copeland, R.A.; Sakowicz, R. Mechanism of Inhibition of Human KSP by Ispinesib. Biochemistry 2008, 47, 3576–3585. [Google Scholar] [CrossRef]
- Blagden, S.; Molife, L.R.; Seebaran, A.; Payne, M.; Reid, A.H.; Protheroe, A.S.; Vasist, L.S.; Williams, D.D.; Bowen, C.; Kathman, S.J.; et al. A phase I trial of ispinesib, a kinesin spindle protein inhibitor, with docetaxel in patients with advanced solid tumours. Br. J. Cancer 2008, 98, 894–899. [Google Scholar] [CrossRef]
- Beer, T.M.; Goldman, B.; Synold, T.W.; Ryan, C.W.; Vasist, L.S.; Van Veldhuizen, P.J.; Dakhil, S.R.; Lara, P.N.; Drelichman, A.; Hussain, M.H.A.; et al. Southwest Oncology Group Phase II Study of Ispinesib in Androgen-Independent Prostate Cancer Previously Treated with Taxanes. Clin. Genitourin. Cancer 2008, 6, 103–109. [Google Scholar] [CrossRef]
- Davis, D.A.; Sarkar, S.H.; Hussain, M.; Li, Y.; Sarkar, F.H. Increased Therapeutic Potential of an Experimental Anti-Mitotic Inhibitor SB715992 by Genistein in PC-3 Human Prostate Cancer Cell Line. BMC Cancer 2006, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Koller, E.; Fazli, L.; Gleave, M.E. Effects of Eg5 Knockdown on Human Prostate Cancer Xenograft Growth and Chemosensitivity. Prostate 2008, 68, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Panner, A.; Wurster, R.D. T-Type Calcium Channels and Tumor Proliferation. Cell Calcium 2006, 40, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Ho, C.; Ohe-Toyota, M.; Baylin, S.B.; Issa, J.-P.J. Inactivation of CACNA1G, a T-Type Calcium Channel Gene, by Aberrant Methylation of Its 5′ CpG Island in Human Tumors1. Cancer Res. 1999, 59, 4535–4541. [Google Scholar]
- Panner, A.; Cribbs, L.L.; Zainelli, G.M.; Origitano, T.C.; Singh, S.; Wurster, R.D. Variation of T-Type Calcium Channel Protein Expression Affects Cell Division of Cultured Tumor Cells. Cell Calcium 2005, 37, 105–119. [Google Scholar] [CrossRef]
- Barnes, S.; Haynes, L.W. Low-Voltage-Activated Calcium Channels in Human Retinoblastoma Cells. Brain Res. 1992, 598, 19–22. [Google Scholar] [CrossRef]
- Ohkubo, T.; Yamazaki, J. T-Type Voltage-Activated Calcium Channel Cav3.1, but Not Cav3.2, Is Involved in the Inhibition of Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells. Int. J. Oncol. 2012, 41, 267–275. [Google Scholar] [CrossRef]
- Banderali, U.; Jain, M.; Thakur, S.; Jayanthan, A.; Belke, D.D.; Giles, W.R.; Narendran, A. The T-Type Calcium Channel Cav3.1 in Y79 Retinoblastoma Cells Is Regulated by the Epidermal Growth Factor Receptor via the MAPK Signaling Pathway. Curr. Eye Res. 2022, 47, 426–435. [Google Scholar] [CrossRef]
- Suo, A.; Childers, A.; D’Silva, A.; Petersen, L.F.; Otsuka, S.; Dean, M.; Li, H.; Enwere, E.K.; Pohorelic, B.; Klimowicz, A.; et al. Cav3.1 Overexpression Is Associated with Negative Characteristics and Prognosis in Non-Small Cell Lung Cancer. Oncotarget 2018, 9, 8573–8583. [Google Scholar] [CrossRef]
- Haverstick, D.M.; Heady, T.N.; Macdonald, T.L.; Gray, L.S. Inhibition of Human Prostate Cancer Proliferation in Vitro and in a Mouse Model by a Compound Synthesized to Block Ca2+ Entry1. Cancer Res. 2000, 60, 1002–1008. [Google Scholar]
- Hu, S.; Li, L.; Huang, W.; Liu, J.; Lan, G.; Yu, S.; Peng, L.; Xie, X.; Yang, L.; Nian, Y.; et al. CAV3.1 Knockdown Suppresses Cell Proliferation, Migration and Invasion of Prostate Cancer Cells by Inhibiting AKT. Cancer Manag. Res. 2018, 10, 4603–4614. [Google Scholar] [CrossRef] [PubMed]
- Enserink, J.M.; Kolodner, R.D. An Overview of Cdk1-Controlled Targets and Processes. Cell Div. 2010, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Perfettini, J.-L.; Roumier, T.; Kroemer, G. Cyclin-Dependent Kinase-1: Linking Apoptosis to Cell Cycle and Mitotic Catastrophe. Cell Death Differ. 2002, 9, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Nikkhoo, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Mohammadi, H.; Yousefi, M.; Jadidi-Niaragh, F. CDK1 in Breast Cancer: Implications for Theranostic Potential. Anti-Cancer Agents Med. Chem. 2020, 20, 758–767. [Google Scholar] [CrossRef]
- Wijnen, R.; Pecoraro, C.; Carbone, D.; Fiuji, H.; Avan, A.; Peters, G.J.; Giovannetti, E.; Diana, P. Cyclin Dependent Kinase-1 (CDK-1) Inhibition as a Novel Therapeutic Strategy against Pancreatic Ductal Adenocarcinoma (PDAC). Cancers 2021, 13, 4389. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Xiao, D.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Proteasome-Mediated Degradation of Cell Division Cycle 25C and Cyclin-Dependent Kinase 1 in Phenethyl Isothiocyanate-Induced G2-M-Phase Cell Cycle Arrest in PC-3 Human Prostate Cancer Cells. Mol. Cancer Ther. 2004, 3, 567–576. [Google Scholar] [CrossRef]
- Agarwal, C.; Tyagi, A.; Agarwal, R. Gallic Acid Causes Inactivating Phosphorylation of Cdc25A/Cdc25C-Cdc2 via ATM-Chk2 Activation, Leading to Cell Cycle Arrest, and Induces Apoptosis in Human Prostate Carcinoma DU145 Cells. Mol. Cancer Ther. 2006, 5, 3294–3302. [Google Scholar] [CrossRef]
- Kan, S.-F.; Yu, C.-H.; Pu, H.-F.; Hsu, J.-M.; Chen, M.-J.; Wang, P.S. Anti-Proliferative Effects of Evodiamine on Human Prostate Cancer Cell Lines DU145 and PC3. J. Cell. Biochem. 2007, 101, 44–56. [Google Scholar] [CrossRef]
- Tsaur, I.; Makarević, J.; Hudak, L.; Juengel, E.; Kurosch, M.; Wiesner, C.; Bartsch, G.; Harder, S.; Haferkamp, A.; Blaheta, R.A. The Cdk1-Cyclin B Complex Is Involved in Everolimus Triggered Resistance in the PC3 Prostate Cancer Cell Line. Cancer Lett. 2011, 313, 84–90. [Google Scholar] [CrossRef]
- Tang, Z.; Pilié, P.G.; Geng, C.; Manyam, G.C.; Yang, G.; Park, S.; Wang, D.; Peng, S.; Wu, C.; Peng, G.; et al. ATR Inhibition Induces CDK1–SPOP Signaling and Enhances Anti–PD-L1 Cytotoxicity in Prostate Cancer. Clin. Cancer Res. 2021, 27, 4898–4909. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Yuan, X.; Bubley, G.J.; Balk, S.P. Androgen Receptor Phosphorylation and Stabilization in Prostate Cancer by Cyclin-Dependent Kinase 1. Proc. Natl. Acad. Sci. 2006, 103, 15969–15974. [Google Scholar] [CrossRef] [PubMed]
- Willder, J.M.; Heng, S.J.; McCall, P.; Adams, C.E.; Tannahill, C.; Fyffe, G.; Seywright, M.; Horgan, P.G.; Leung, H.Y.; Underwood, M.A.; et al. Androgen Receptor Phosphorylation at Serine 515 by Cdk1 Predicts Biochemical Relapse in Prostate Cancer Patients. Br. J. Cancer 2013, 108, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I Inhibitors: Camptothecins and Beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Hevener, K.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent Developments in Topoisomerase-Targeted Cancer Chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef]
- Malathi, K.; Paranjape, J.M.; Ganapathi, R.; Silverman, R.H. HPC1/RNASEL Mediates Apoptosis of Prostate Cancer Cells Treated with 2′,5′-Oligoadenylates, Topoisomerase I Inhibitors, and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. Cancer Res. 2004, 64, 9144–9151. [Google Scholar] [CrossRef]
- Yu, C.-C.; Pan, S.-L.; Chao, S.-W.; Liu, S.-P.; Hsu, J.-L.; Yang, Y.-C.; Li, T.-K.; Huang, W.-J.; Guh, J.-H. A Novel Small Molecule Hybrid of Vorinostat and DACA Displays Anticancer Activity against Human Hormone-Refractory Metastatic Prostate Cancer through Dual Inhibition of Histone Deacetylase and Topoisomerase I. Biochem. Pharmacol. 2014, 90, 320–330. [Google Scholar] [CrossRef]
- Roy, J.; Nguyen, T.X.; Kanduluru, A.K.; Venkatesh, C.; Lv, W.; Reddy, P.V.N.; Low, P.S.; Cushman, M. DUPA Conjugation of a Cytotoxic Indenoisoquinoline Topoisomerase I Inhibitor for Selective Prostate Cancer Cell Targeting. J. Med. Chem. 2015, 58, 3094–3103. [Google Scholar] [CrossRef]
- Tan, K.W.; Seng, H.L.; Lim, F.S.; Cheah, S.-C.; Ng, C.H.; Koo, K.S.; Mustafa, M.R.; Ng, S.W.; Maah, M.J. Towards a Selective Cytotoxic Agent for Prostate Cancer: Interaction of Zinc Complexes of Polyhydroxybenzaldehyde Thiosemicarbazones with Topoisomerase I. Polyhedron 2012, 38, 275–284. [Google Scholar] [CrossRef]
- Willems, L.; Tamburini, J.; Chapuis, N.; Lacombe, C.; Mayeux, P.; Bouscary, D. PI3K and MTOR Signaling Pathways in Cancer: New Data on Targeted Therapies. Curr. Oncol. Rep. 2012, 14, 129–138. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Thrasher, J.B.; Terranova, P. Glycogen Synthase Kinase-3: A Potential Preventive Target for Prostate Cancer Management. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.; et al. Stat3 Promotes Metastatic Progression of Prostate Cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.Y.; Frolov, A.; Li, R.; Ayala, G.; Greenberg, N.M. Targeting Aurora Kinases for the Treatment of Prostate Cancer. Cancer Res. 2006, 66, 4996–5002. [Google Scholar] [CrossRef]
- ROCS; 3.5.0.1. OpenEye Scientific Software: Santa Fe, NM, USA. Available online: https://www.eyesopen.com/news/2015/09/rocs-v3.2.1 (accessed on 19 November 2022).
- Gravina, G.L.; Marampon, F.; Sanità, P.; Mancini, A.; Colapietro, A.; Scarsella, L.; Jitariuc, A.; Biordi, L.; Ficorella, C.; Festuccia, C. Increased Expression and Activity of P75NTR Are Crucial Events in Azacitidine-Induced Cell Death in Prostate Cancer. Oncol. Rep. 2016, 36, 125–130. [Google Scholar] [CrossRef]
- Sonpavde, G.; Aparicio, A.M.; Zhan, F.; North, B.; DeLaune, R.; Garbo, L.E.; Rousey, S.R.; Weinstein, R.E.; Xiao, L.; Boehm, K.A.; et al. Azacitidine Favorably Modulates PSA Kinetics Correlating with Plasma DNA LINE-1 Hypomethylation in Men with Chemonaïve Castration-Resistant Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 682–689. [Google Scholar] [CrossRef]
- Witte, R.S.; Yeap, B.Y.; Trump, D.L. Trimetrexate in Advanced Hormone-Refractory Prostate Cancer. Investig. New Drugs 1994, 12, 255–258. [Google Scholar] [CrossRef]
- Dumble, M.; Crouthamel, M.-C.; Zhang, S.-Y.; Schaber, M.; Levy, D.; Robell, K.; Liu, Q.; Figueroa, D.J.; Minthorn, E.A.; Seefeld, M.A.; et al. Discovery of Novel AKT Inhibitors with Enhanced Anti-Tumor Effects in Combination with the MEK Inhibitor. PLoS ONE 2014, 9, e100880. [Google Scholar] [CrossRef]
- Lynch, J.T.; Polanska, U.M.; Hancox, U.; Delpuech, O.; Maynard, J.; Trigwell, C.; Eberlein, C.; Lenaghan, C.; Polanski, R.; Avivar-Valderas, A.; et al. Combined Inhibition of PI3Kβ and MTOR Inhibits Growth of PTEN-Null Tumors. Mol. Cancer Ther. 2018, 17, 2309–2319. [Google Scholar] [CrossRef]
- Beaufils, F.; Cmiljanovic, N.; Cmiljanovic, V.; Bohnacker, T.; Melone, A.; Marone, R.; Jackson, E.; Zhang, X.; Sele, A.; Borsari, C.; et al. 5-(4,6-Dimorpholino-1,3,5-Triazin-2-Yl)-4-(Trifluoromethyl)Pyridin-2-Amine (PQR309), a Potent, Brain-Penetrant, Orally Bioavailable, Pan-Class I PI3K/MTOR Inhibitor as Clinical Candidate in Oncology. J. Med. Chem. 2017, 60, 7524–7538. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Fultz, K.E.; Xu, S.; Xu, W.; Packard, G.; Khambatta, G.; Gamez, J.C.; Leisten, J.; Zhao, J.; Apuy, J.; et al. CC-223, a Potent and Selective Inhibitor of MTOR Kinase: In Vitro and In Vivo Characterization. Mol. Cancer Ther. 2015, 14, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Soh, C.K.; Goh, W.H.; Wang, H. Design, Synthesis, and Preclinical Evaluation of Fused Pyrimidine-Based Hydroxamates for the Treatment of Hepatocellular Carcinoma. J. Med. Chem. 2018, 61, 1552–1575. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.; Novotny-Diermayr, V.; Goh, K.C.; Williams, M.; Tan, Y.C.; Ong, L.C.; Cheong, A.; Ng, B.K.; Amalini, C.; Madan, B.; et al. VS-5584, a Novel and Highly Selective PI3K/MTOR Kinase Inhibitor for the Treatment of Cancer. Mol. Cancer Ther. 2013, 12, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Lin, M.-H.; Chao, M.-W.; Peng, S.-J.; Hsu, K.-C.; Eight Lin, T.; Chen, M.-C.; Lai, M.-J.; Pan, S.-L.; Liou, J.-P. Amide-Tethered Quinoline-Resorcinol Conjugates as a New Class of HSP90 Inhibitors Suppressing the Growth of Prostate Cancer Cells. Bioorganic Chem. 2019, 91, 103119. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K.; Zhang, H.; Brekken, J.; Huser, N.; Powell, R.E.; Timple, N.; Busch, D.J.; Neely, L.; Sensintaffar, J.L.; Yang, Y.; et al. BIIB021, an Orally Available, Fully Synthetic Small-Molecule Inhibitor of the Heat Shock Protein Hsp90. Mol. Cancer Ther. 2009, 8, 921–929. [Google Scholar] [CrossRef]
- Nong, H.; Zhang, Y.; Bai, Y.; Zhang, Q.; Liu, M.; Zhou, Q.; Shi, Z.; Zeng, G.; Zong, S.-H. Adapalene Inhibits Prostate Cancer Cell Proliferation In Vitro and In Vivo by Inducing DNA Damage, S-Phase Cell Cycle Arrest, and Apoptosis. Front. Pharmacol. 2022, 13, 801624. [Google Scholar] [CrossRef]
- Lu, X.P.; Fanjul, A.; Picard, N.; Shroot, B.; Pfahl, M. A Selective Retinoid with High Activity against an Androgen-resistant Prostate Cancer Cell Type. Int. J. Cancer. 1999, 80, 272–278. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Wang, G.; Lei, D.; Chen, X.; Lin, K.; Li, M.; Lin, H.; Li, D.; Zheng, Q. Picropodophyllin Inhibits the Proliferation of Human Prostate Cancer DU145 and LNCaP Cells via ROS Production and PI3K/AKT Pathway Inhibition. Biol. Pharm. Bull. 2022, 45, 1027–1035. [Google Scholar] [CrossRef]
- Scott, L.J. Azacitidine: A Review in Myelodysplastic Syndromes and Acute Myeloid Leukaemia. Drugs 2016, 76, 889–900. [Google Scholar] [CrossRef]
- Girnita, A.; Girnita, L.; del Prete, F.; Bartolazzi, A.; Larsson, O.; Axelson, M. Cyclolignans as Inhibitors of the Insulin-like Growth Factor-1 Receptor and Malignant Cell Growth. Cancer Res 2004, 64, 236–242. [Google Scholar] [CrossRef]
- Xie, H.; Lin, X.; Zhang, Y.; Tan, F.; Chi, B.; Peng, Z.; Dong, W.; An, D. Design, Synthesis and Biological Evaluation of Ring-Fused Pyrazoloamino Pyridine/Pyrimidine Derivatives as Potential FAK Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127459. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.C.; Lee, F.Y.F.; Fairchild, C.R.; Lynch, M.; Monticello, T.; Kramer, R.A.; Manne, V. Preclinical Antitumor Activity of BMS-214662, a Highly Apoptotic and Novel Farnesyltransferase Inhibitor. Cancer Res. 2001, 61, 7507–7517. [Google Scholar] [PubMed]
- Bailey, H.H.; Alberti, D.B.; Thomas, J.P.; Mulkerin, D.L.; Binger, K.A.; Gottardis, M.M.; Martell, R.E.; Wilding, G. Phase I Trial of Weekly Paclitaxel and BMS-214662 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zou, L.; Chen, W.; Yang, T.; Luo, J.; Wu, K.; Shu, F.; Tan, X.; Yang, Y.; Cen, S.; et al. Flubendazole, FDA-Approved Anthelmintic, Elicits Valid Antitumor Effects by Targeting P53 and Promoting Ferroptosis in Castration-Resistant Prostate Cancer. Pharmacol. Res. 2021, 164, 105305. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Shin, C.; Kim, C.-Y.; Ryu, B.; Kim, J.; Bang, J.; Park, J.-H. Albendazole Exerts Antiproliferative Effects on Prostate Cancer Cells by Inducing Reactive Oxygen Species Generation. Oncol. Lett. 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.Y.; Jung, B.K.; Hong, S.J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J Parasitol. 2021, 59(3), 189–225. [Google Scholar] [CrossRef]
- Weinberger, S.E.; Cockrill, B.A.; Mandel, J. 26-Pulmonary Complications in the Immunocompromised Host. In Principles of Pulmonary Medicine, 6th ed.; Weinberger, S.E., Cockrill, B.A., Mandel, J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2014; pp. 331–343. ISBN 978-1-4557-2532-8. [Google Scholar]
- Seefeld, M.A.; Rouse, M.B.; Heerding, D.A. Inhibitors of AKT Activity. Patent number US20110071182A1, 2011. [Google Scholar]
- Herberts, C.; Murtha, A.J.; Fu, S.; Wang, G.; Schönlau, E.; Xue, H.; Lin, D.; Gleave, A.; Yip, S.; Angeles, A.; et al. Activating AKT1 and PIK3CA Mutations in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2020, 78, 834–844. [Google Scholar] [CrossRef]
- Therapeutics, L. Laekna Therapeutics Reported Positive Results in Two Clinical Studies for the Treatment of Various Stages of Prostate Cancer at the ESMO Congress. Available online: https://www.prnewswire.com/news-releases/laekna-therapeutics-reported-positive-results-in-two-clinical-studies-for-the-treatment-of-various-stages-of-prostate-cancer-at-the-esmo-congress-301378428.html (accessed on 13 December 2022).
- Al-Ashmawy, A.A.K.; Ragab, F.A.; Elokely, K.M.; Anwar, M.M.; Perez-Leal, O.; Rico, M.C.; Gordon, J.; Bichenkov, E.; Mateo, G.; Kassem, E.M.M.; et al. Design, Synthesis and SAR of New-Di-Substituted Pyridopyrimidines as ATP-Competitive Dual PI3Kα/MTOR Inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3117–3122. [Google Scholar] [CrossRef]
- Basu, B.; Krebs, M.G.; Sundar, R.; Wilson, R.H.; Spicer, J.; Jones, R.; Brada, M.; Talbot, D.C.; Steele, N.; Ingles Garces, A.H.; et al. Vistusertib (Dual m-TORC1/2 Inhibitor) in Combination with Paclitaxel in Patients with High-Grade Serous Ovarian and Squamous Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 1918–1925. [Google Scholar] [CrossRef]
- Westin, S.N.; Litton, J.K.; Williams, R.A.; Shepherd, C.J.; Brugger, W.; Pease, E.J.; Soliman, P.T.; Frumovitz, M.M.; Levenback, C.F.; Sood, A.; et al. Phase I Trial of Olaparib (PARP Inhibitor) and Vistusertib (MTORC1/2 Inhibitor) in Recurrent Endometrial, Ovarian and Triple Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 5504. [Google Scholar] [CrossRef]
- Pancholi, S.; Leal, M.F.; Ribas, R.; Simigdala, N.; Schuster, E.; Chateau-Joubert, S.; Zabaglo, L.; Hills, M.; Dodson, A.; Gao, Q.; et al. Combination of MTORC1/2 Inhibitor Vistusertib plus Fulvestrant in Vitro and in Vivo Targets Oestrogen Receptor-Positive Endocrine-Resistant Breast Cancer. Breast Cancer Res. 2019, 21, 135. [Google Scholar] [CrossRef]
- Rageot, D.; Bohnacker, T.; Melone, A.; Langlois, J.-B.; Borsari, C.; Hillmann, P.; Sele, A.M.; Beaufils, F.; Zvelebil, M.; Hebeisen, P.; et al. Discovery and Preclinical Characterization of 5-[4,6-Bis({3-Oxa-8-Azabicyclo [3.2.1]Octan-8-Yl})-1,3,5-Triazin-2-Yl]-4-(Difluoromethyl)Pyridin-2-Amine (PQR620), a Highly Potent and Selective MTORC1/2 Inhibitor for Cancer and Neurological Disorders. J. Med. Chem. 2018, 61, 10084–10105. [Google Scholar] [CrossRef]
- Pacey, S.; Shah, N.; Davies, B.; Bratt, O.; Warren, A.; Baird, R.D.; Gnanapragasam, V.; Ingle, S.; Stearn, S.; Machin, A.; et al. A Pharmacodynamic Biomarker Study of Vistusertib (AZD2014), an MTORC1/2 Inhibitor, given Prior to Radical Prostatectomy (CANCAP02). J. Clin. Oncol. 2018, 36, 5081. [Google Scholar] [CrossRef]
- Hansen, A.R.; Shapiro, G.; Do, K.T.; Kumar, R.; Martin-Liberal, J.; Higano, C.S.; Wisinski, K.B.; Dean, E.J.; Heath, E.I.; Rathkopf, D.E.; et al. A First in Human Phase I Study of AZD8186, a Potent and Selective Inhibitor of PI3K in Patients with Advanced Solid Tumours as Monotherapy and in Combination with the Dual MTORC1/2 Inhibitor Vistusertib (AZD2014) or Abiraterone Acetate. J. Clin. Oncol. 2017, 35, 2570. [Google Scholar] [CrossRef]
- van Hattum, A.H.; Pinedo, H.M.; Schlüper, H.M.M.; Erkelens, C.A.M.; Tohgo, A.; Boven, E. The Activity Profile of the Hexacyclic Camptothecin Derivative DX-8951f in Experimental Human Colon Cancer and Ovarian Cancer. Biochem. Pharmacol. 2002, 64, 1267–1277. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Yu, F.; Sun, Y.; Huang, F.; Chen, Y.; Yang, Z.; Ding, G. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura Anjunae Oligopeptide (YVPGP) via PI3K/AKT/MTOR Signaling Pathway. Mar. Drugs 2018, 16, 325. [Google Scholar] [CrossRef]
- Facompre, N.D.; Sinha, I.; El-Bayoumy, K.; Pinto, J.T.; Sinha, R. Remarkable Inhibition of MTOR Signaling by the Combination of Rapamycin and 1,4-Phenylenebis(Methylene)Selenocyanate in Human Prostate Cancer Cells. Int. J. Cancer 2012, 131, 2134–2142. [Google Scholar] [CrossRef]
- Yang, K.; Tang, X.J.; Xu, F.F.; Liu, J.H.; Tan, Y.Q.; Gao, L.; Sun, Q.; Ding, X.; Liu, B.H.; Chen, Q.X. PI3K/mTORC1/2 inhibitor PQR309 inhibits proliferation and induces apoptosis in human glioblastoma cells. Oncol. Rep. 2020, 43, 773–782. [Google Scholar] [CrossRef]
- Mortensen, D.S.; Perrin-Ninkovic, S.M.; Shevlin, G.; Zhao, J.; Packard, G.; Bahmanyar, S.; Correa, M.; Elsner, J.; Harris, R.; Lee, B.G.S.; et al. Discovery of Mammalian Target of Rapamycin (MTOR) Kinase Inhibitor CC-223. J. Med. Chem. 2015, 58, 5323–5333. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, H.; Lundgren, K.; Wilson, L.; Burrows, F.; Shores, C.G. BIIB021, a Novel Hsp90 Inhibitor, Sensitizes Head and Neck Squamous Cell Carcinoma to Radiotherapy. Int. J. Cancer 2010, 126, 1216–1225. [Google Scholar] [CrossRef]
- Zhang, H.; Neely, L.; Lundgren, K.; Yang, Y.-C.; Lough, R.; Timple, N.; Burrows, F. BIIB021, a Synthetic Hsp90 Inhibitor, Has Broad Application against Tumors with Acquired Multidrug Resistance. Int. J. Cancer 2010, 126, 1226–1234. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, H.; Weng, M.; Zhang, H.; Wang, C.; Sun, L. CC-223, NSC781406, and BGT226 Exerts a Cytotoxic Effect Against Pancreatic Cancer Cells via MTOR Signaling. Front. Pharmacol. 2020, 11, 580407. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Zhang, X.; Li, X. CC-223 Inhibits Human Head and Neck Squamous Cell Carcinoma Cell Growth. Biochem. Biophys. Res. Commun. 2018, 496, 1191–1196. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, J.; Liu, M.; Chen, D.; Qiu, C.; Sun, K. CC-223 Blocks MTORC1/C2 Activation and Inhibits Human Hepatocellular Carcinoma Cells in Vitro and in Vivo. PLoS ONE 2017, 12, e0173252. [Google Scholar] [CrossRef]
- Goodyear, S.m.; Kheyfets, S.b.; Garcia, F.u.; Stearns, M.e. Role of the VEGFR3/VEGFD Receptor Axis in TGFβ1 Activation of Primary Prostate Cell Lines. Prostate 2009, 69, 982–990. [Google Scholar] [CrossRef]
- Bendell, J.C.; Kelley, R.K.; Shih, K.C.; Grabowsky, J.A.; Bergsland, E.; Jones, S.; Martin, T.; Infante, J.R.; Mischel, P.S.; Matsutani, T.; et al. A Phase I Dose-Escalation Study to Assess Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of the Dual MTORC1/MTORC2 Kinase Inhibitor CC-223 in Patients with Advanced Solid Tumors or Multiple Myeloma. Cancer 2015, 121, 3481–3490. [Google Scholar] [CrossRef]
- Shih, K.C.; Bendell, J.C.; Reinert, A.; Jones, S.; Kelley, R.K.; Infante, J.R.; Korn, M.; Hege, K.; Chopra, R.; Xu, S.; et al. Phase I Trial of an Oral TORC1/TORC2 Inhibitor (CC-223) in Advanced Solid and Hematologic Cancers. J. Clin. Oncol. 2012, 30, 3006. [Google Scholar] [CrossRef]
- Wolin, E.; Mita, A.; Mahipal, A.; Meyer, T.; Bendell, J.; Nemunaitis, J.; Munster, P.N.; Paz-Ares, L.; Filvaroff, E.H.; Li, S.; et al. A Phase 2 Study of an Oral MTORC1/MTORC2 Kinase Inhibitor (CC-223) for Non-Pancreatic Neuroendocrine Tumors with or without Carcinoid Symptoms. PLoS ONE 2019, 14, e0221994. [Google Scholar] [CrossRef]
- Böll, B.; Eltaib, F.; Reiners, K.S.; Von Tresckow, B.; Tawadros, S.; Simhadri, V.R.; Burrows, F.J.; Lundgren, K.; Hansen, H.P.; Engert, A.; et al. Heat Shock Protein 90 Inhibitor BIIB021 (CNF2024) Depletes NF-ΚB and Sensitizes Hodgkin’s Lymphoma Cells for Natural Killer Cell-Mediated Cytotoxicity. Clin. Cancer Res. 2009, 15, 5108–5116. [Google Scholar] [CrossRef]
- Shi, J.; Van de Water, R.; Hong, K.; Lamer, R.B.; Weichert, K.W.; Sandoval, C.M.; Kasibhatla, S.R.; Boehm, M.F.; Chao, J.; Lundgren, K.; et al. EC144 Is a Potent Inhibitor of the Heat Shock Protein 90. J. Med. Chem. 2012, 55, 7786–7795. [Google Scholar] [CrossRef] [PubMed]
- Taldone, T.; Patel, P.D.; Patel, M.; Patel, H.J.; Evans, C.E.; Rodina, A.; Ochiana, S.; Shah, S.K.; Uddin, M.; Gewirth, D.; et al. Experimental and Structural Testing Module to Analyze Paralogue-Specificity and Affinity in the Hsp90 Inhibitors Series. J. Med. Chem. 2013, 56, 6803–6818. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Plescia, J.; Goel, H.L.; Li, J.; Jiang, Z.; Cohen, R.J.; Languino, L.R.; Altieri, D.C. Cytoprotective Mitochondrial Chaperone TRAP-1 as a Novel Molecular Target in Localized and Metastatic Prostate Cancer. Am. J. Pathol. 2010, 176, 393–401. [Google Scholar] [CrossRef]
- Ernst, J.T.; Liu, M.; Zuccola, H.; Neubert, T.; Beaumont, K.; Turnbull, A.; Kallel, A.; Vought, B.; Stamos, D. Correlation between Chemotype-Dependent Binding Conformations of HSP90α/β and Isoform Selectivity—Implications for the Structure-Based Design of HSP90α/β Selective Inhibitors for Treating Neurodegenerative Diseases. Bioorg. Med. Chem. Lett. 2014, 24, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Piskin, S.; Uzunali, E. A Review of the Use of Adapalene for the Treatment of Acne Vulgaris. Ther. Clin. Risk Manag. 2007, 3, 621–624. [Google Scholar] [CrossRef]
- Charpentier, B.; Bernardon, J.-M.; Eustache, J.; Millois, C.; Martin, B.; Michel, S.; Shroot, B. Synthesis, Structure-Affinity Relationships, and Biological Activities of Ligands Binding to Retinoic Acid Receptor Subtypes. J. Med. Chem. 1995, 38, 4993–5006. [Google Scholar] [CrossRef]
- Pasquali, D.; Thaller, C.; Eichele, G. Abnormal Level of Retinoic Acid in Prostate Cancer Tissues. J. Clin. Endocrinol. Metab. 1996, 81, 2186–2191. [Google Scholar] [CrossRef]
- Hammond, L.A.; Brown, G.; Keedwell, R.G.; Durham, J.; Chandraratna, R.A. The Prospects of Retinoids in the Treatment of Prostate Cancer. Anti-Cancer Drugs 2002, 13, 781–790. [Google Scholar] [CrossRef]
- Yu, G.; Corn, P.G.; Shen, P.; Song, J.H.; Lee, Y.-C.; Lin, S.-C.; Pan, J.; Agarwal, S.K.; Panaretakis, T.; Pacifici, M.; et al. Retinoic Acid Receptor Activation Reduces Metastatic Prostate Cancer Bone Lesions by Blocking the Endothelial-to-Osteoblast Transition. Cancer Res. 2022, 82, 3158–3171. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, H.; Kanesaka, M.; Imamura, Y.; Sakamoto, S.; Ichikawa, T.; Kaneda, A. Epigenetic Modifications in Prostate Cancer. Int. J. Urol. 2021, 28, 140–149. [Google Scholar] [CrossRef]
- Pasquali, D.; Rossi, V.; Bellastella, G.; Bellastella, A.; Sinisi, A.A. Natural and Synthetic Retinoids in Prostate Cancer. Curr. Pharm. Des. 2006, 12, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.X.; Chung, E.P.; Bowser, R.; Sirianni, R.W. Lipid and Polymer Blended Polyester Nanoparticles Loaded with Adapalene for Activation of Retinoid Signaling in the CNS Following Intravenous Administration. J. Drug Deliv. Sci. Technol. 2019, 52, 927–933. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of Insulin-like Growth Factor 1 Receptor in Cancer Resistance to Chemotherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Bhattacharyya, B.; Wolff, J. Podophyllotoxin as a Probe for the Colchicine Binding Site of Tubulin. J. Biol. Chem. 1977, 252, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Patnaik, S.; Rup, A.R.; Gaurav, A. A Rare Case of Podophyllin Poisoning: Early Intervention Is Lifesaving. Indian J. Crit. Care Med. 2020, 24, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, M.; Lv, Q. Picropodophyllin Inhibits Type I Endometrial Cancer Cell Proliferation via Disruption of the PI3K/Akt Pathway. Acta Biochim. Et Biophys. Sin. 2019, 51, 753–760. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Mei, J.; Wang, X.; Zhu, X.; Zhang, Q.; Lv, J. Picropodophyllin Inhibits Epithelial Ovarian Cancer Cells in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2013, 435, 385–390. [Google Scholar] [CrossRef]
- Ohshima-Hosoyama, S.; Hosoyama, T.; Nelon, L.D.; Keller, C. IGF-1 Receptor Inhibition by Picropodophyllin in Medulloblastoma. Biochem. Biophys. Res. Commun 2010, 399, 727–732. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Sugiyama, T.; Yamamoto, S.; Sueishi, M.; Yoshida, T. Insulin-like Growth Factor-I Receptor in Proliferation and Motility of Pancreatic Cancer. World J. Gastroenterol. 2010, 16, 1854–1858. [Google Scholar] [CrossRef]
- Yin, S.; Girnita, A.; Strömberg, T.; Khan, Z.; Andersson, S.; Zheng, H.; Ericsson, C.; Axelson, M.; Nistér, M.; Larsson, O.; et al. Targeting the Insulin-like Growth Factor-1 Receptor by Picropodophyllin as a Treatment Option for Glioblastoma. Neuro Oncol. 2010, 12, 19–27. [Google Scholar] [CrossRef]
- Figel, S.; Gelman, I.H. Focal Adhesion Kinase Controls Prostate Cancer Progression Via Intrinsic Kinase and Scaffolding Functions. Anti-Cancer Agents Med. Chem. 2011, 11, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Pradines, A.; Favre, G. Perspectives on Farnesyl Transferase Inhibitors in Cancer Therapy. Cancer Lett. 2004, 206, 159–167. [Google Scholar] [CrossRef]
- Sivoňová, M.K.; Vilčková, M.; Kliment, J.; Mahmood, S.; Jurečeková, J.; Dušenková, S.; Waczulíková, I.; Slezák, P.; Dobrota, D. Association of P53 and P21 Polymorphisms with Prostate Cancer. Biomed. Rep. 2015, 3, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ding, Y.; Liu, H.; Sun, M.; Wang, H.; Wu, D. Flubendazole Plays an Important Anti-Tumor Role in Different Types of Cancers. Int. J. Mol. Sci. 2022, 23, 519. [Google Scholar] [CrossRef]
- Rushworth, L.K.; Hewit, K.; Munnings-Tomes, S.; Somani, S.; James, D.; Shanks, E.; Dufès, C.; Straube, A.; Patel, R.; Leung, H.Y. Repurposing Screen Identifies Mebendazole as a Clinical Candidate to Synergise with Docetaxel for Prostate Cancer Treatment. Br. J. Cancer 2020, 122, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Berthold, M.R.; Cebron, N.; Dill, F.; Gabriel, T.R.; Kötter, T.; Meinl, T.; Ohl, P.; Sieb, C.; Thiel, K.; Wiswedel, B. KNIME: The Konstanz Information Miner. In Proceedings of the Data Analysis, Machine Learning and Applications; Preisach; Preisach, C., Burkhardt, H., Schmidt-Thieme, L., Decker, R., Eds.; Springer: Berlin, Heidelberg, 2008; pp. 319–326. [Google Scholar] [CrossRef]
- OpenEye Toolkits; 2020.2.2. OpenEye Scientific Software: Santa Fe, NM, USA. Available online: https://www.eyesopen.com/ (accessed on 19 November 2022).
- OMEGA; 4.2.0.1. OpenEye Scientific Software: Santa Fe, NM, USA. Available online: https://www.eyesopen.com/omega (accessed on 19 November 2022).
- Pinzi, L.; Rastelli, G. Identification of Target Associations for Polypharmacology from Analysis of Crystallographic Ligands of the Protein Data Bank. J. Chem. Inf. Model. 2020, 60, 372–390. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
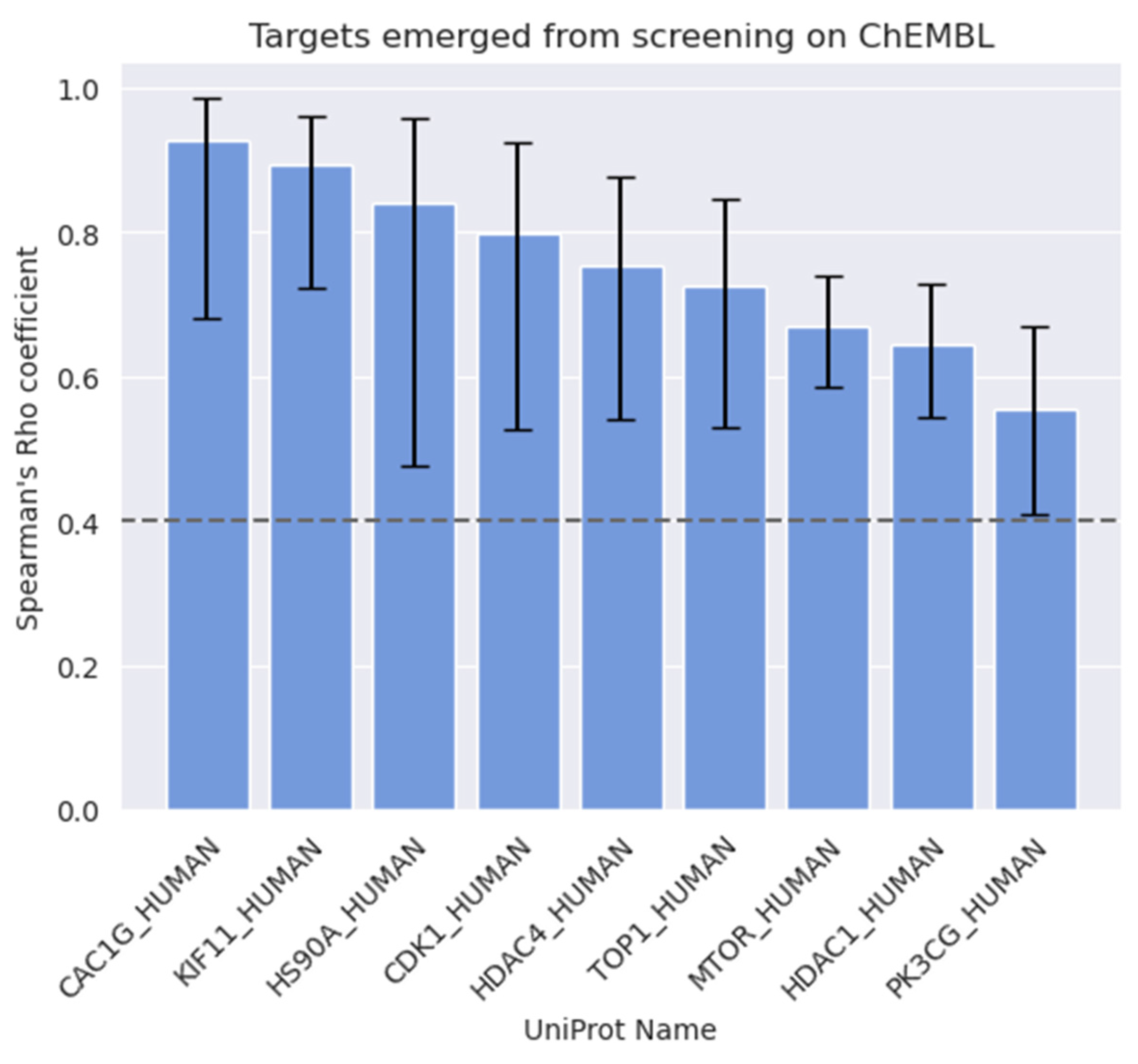
| Target Name | Protein Family | UniProt Name * | PC Cell |
|---|---|---|---|
| Kinesine-like protein KIF11 | Kinesin family, BimC subfamily | KIF11 | PC-3 |
| Heat shock protein HSP 90-alpha | Heat shock protein 90 family | HS90A | PC-3 |
| Histone deacetylase 4 | Histone deacetylase family, HD type 2 subfamily | HDAC4 | PC-3 |
| DNA Topoisomerase I | Type IB topoisomerase family | TOP1 | PC-3 |
| Serine/threonine-protein kinase mTOR | PI3/PI4-kinase family | MTOR | PC-3 |
| Histone deacetylase 1 | Histone deacetylase family, HD type 1 subfamily | HDAC1 | PC-3 |
| PI3-kinase p110-gamma subunit | PI3/PI4-kinase family | PK3CG | PC-3 |
| Voltage-gated T-type calcium channel alpha 1-G subunit | Calcium channel alpha-1 subunit family, CACNA1G subfamily | CAC1G | DU-145 |
| Cyclin-dependent kinase 1 | CMGC Ser/Thr protein kinase family, CDC2/CDKX subfamily | CDK1 | DU-145 |
| Molecule Name | 2D Structure | Activity In Vitro | Activity In Vivo | Max Phase | Clinical Trials | Disease | Primary Target * |
|---|---|---|---|---|---|---|---|
| Azacitidine | 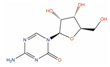 | Cell viability ~85% (0.5 µM, PC-3), ~80% (0.1 µM, 22Rv1) [118] | 4 | NCT00503984; Phase I [119] | Myelodysplastic syndrome (Approved) | RNA polymerase II [Inhibitor], RNA methyltransferase [Inhibitor] | |
| Trimetrexate |  | 4 | Phase II (Advanced hormone refractory-PC) [120] | Toxoplasmosis (Approved) | Dihydrofolate reductase (DYR) [Inhibitor] | ||
| Afuresertib | 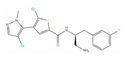 | IC50 = 104 nM (LNCaP) [121] | 2 | NCT04060394 | Leukemia (Phase 2); Solid tumor/cancer (Phase 2); Multiple myeloma (Phase 1) | RAC-alpha serine/threonine-protein kinase (AKT1) [Modulator] | |
| Vistusertib | 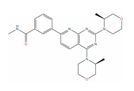 | Tumor reduction from 15 mg/kg (PC-3) [122] | 2 | NCT02064608; NCT01884285 | Solid tumor/cancer (Phase 2) | Serine/threonine-protein kinase mTOR (mTOR) [Inhibitor]; Mammalian target of rapamycin complex 1 (mTORC1) [Inhibitor]; Target of rapamycin complex 2 MAPKAP1 (MTORC2) [Inhibitor] | |
| Exatecan | 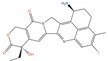 | 3 | NCT00004045 | Solid tumor/cancer (Phase 3) | DNA topoisomerase I (TOP1) [Inhibitor] | ||
| Bimiralisib | 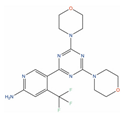 | T/C = 31–12% with 5–15 mg/kg (PC-3) [123] | 2 | Squamous head and neck cell carcinoma (Phase 2); Pain (Phase 1) | PI3-kinase beta (PIK3CB) [Inhibitor]; Serine/threonine-protein kinase mTOR (mTOR) [Inhibitor]; PI3-kinase gamma (PIK3CG) [Inhibitor] | ||
| Onatasertib(CC-223) | 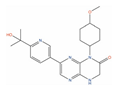 | IC50 = 0.114 µM (PC-3) | Tumor reduction from 5 mg/kg (PC-3) [124] | 2 | Solid tumor/cancer (Phase 1/2) | Serine/threonine-protein kinase mTOR (mTOR) [Modulator] | |
| VS-5584 | 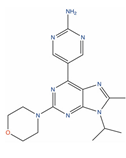 | IC50 = 0.18 µM (PC-3) [125] | TCI = 79% at 25 mg/kg (PC-3) [126] | 1 | Solid tumor/cancer (Phase 1); Malignant Mesothelioma (Phase 1) | Serine/threonine-protein kinase mTOR (mTOR) [Modulator]; PI3-kinase gamma (PIK3CG) [Modulator]; Mammalian target of rapamycin complex 1 (mTORC1) [Inhibitor]; Target of rapamycin complex 2 MAPKAP1 (MTORC2) [Inhibitor] | |
| BIIB021 | 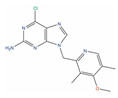 | GI50 = 0.28 µM (PC-3) [127] | Tumor growth inhibition 87% at 120 mg/kg (CWR22) [128] | 2 | Breast cancer (Phase 2) | Heat shock protein 90 alpha (HSP90A) [Inhibitor] | |
| Adapalene | 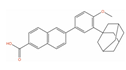 | IC50 = 8.23 µM at 24 h (RM-1); Cell viability (0.01 µM, 72 h)~65% (DU-145) [129,130] | Tumor reduction from 30 mg/kg (RM-1) [131] | 4 | Acne vulgaris (Approved) | Retinoic acid receptor gamma (RARG) [Agonist] | |
| Picropodophyllin | 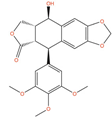 | IC50 = 0.802 µM (DU-145); IC50 = 0.899 µM (LNCaP) [132]; IC50 = 100 nM (PC-3) [133] | Tumor reduction from 20 mg/kg (PC-3) [133] | 2 | Solid tumor/cancer (Phase 2) | Insulin-like growth factor I receptor (IGF1R) [Inhibitor] | |
| VS-4718 | 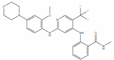 | IC50 = 0.62 µM (DU-145) [134] | 1 | Solid tumour/cancer (Phase 1) | Focal adhesion kinase 1 (FAK) [Inhibitor] | ||
| BMS-214662 | 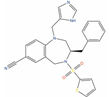 | IC50 = 0.16 µM (PC-3); IC50 = 0.14 µM (LNCaP) [135] | 1 | Phase I combination with placitaxel on 2 PC patients [136] | Non-small-cell lung cancer (Phase 1) | Farnesyl protein transferase (Ftase) [Modulator] | |
| Flubendazole |  | Cell viability (0.1 µM, 72 h) ~65% (PC-3), ~80% (DU-145) [137] | Tumor reduction from 10 mg/kg (PC-3). [137] | 4 | Worm infection (Approved) | ||
| Albendazole | 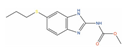 | Cell viability (48 h) ~70% (0.1 µM; PC-3, DU-145); ~90% (0.5 µM, LNCaP) [138] | 4 | Phase I 2 PC patients [139] | Worm infection (Approved) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, L.; Pinzi, L.; Rastelli, G. Identification of Promising Drug Candidates against Prostate Cancer through Computationally-Driven Drug Repurposing. Int. J. Mol. Sci. 2023, 24, 3135. https://doi.org/10.3390/ijms24043135
Bernal L, Pinzi L, Rastelli G. Identification of Promising Drug Candidates against Prostate Cancer through Computationally-Driven Drug Repurposing. International Journal of Molecular Sciences. 2023; 24(4):3135. https://doi.org/10.3390/ijms24043135
Chicago/Turabian StyleBernal, Leonardo, Luca Pinzi, and Giulio Rastelli. 2023. "Identification of Promising Drug Candidates against Prostate Cancer through Computationally-Driven Drug Repurposing" International Journal of Molecular Sciences 24, no. 4: 3135. https://doi.org/10.3390/ijms24043135
APA StyleBernal, L., Pinzi, L., & Rastelli, G. (2023). Identification of Promising Drug Candidates against Prostate Cancer through Computationally-Driven Drug Repurposing. International Journal of Molecular Sciences, 24(4), 3135. https://doi.org/10.3390/ijms24043135







