Monoglyceride Lipase Deficiency Is Associated with Altered Thrombogenesis in Mice
Abstract
1. Introduction
2. Results
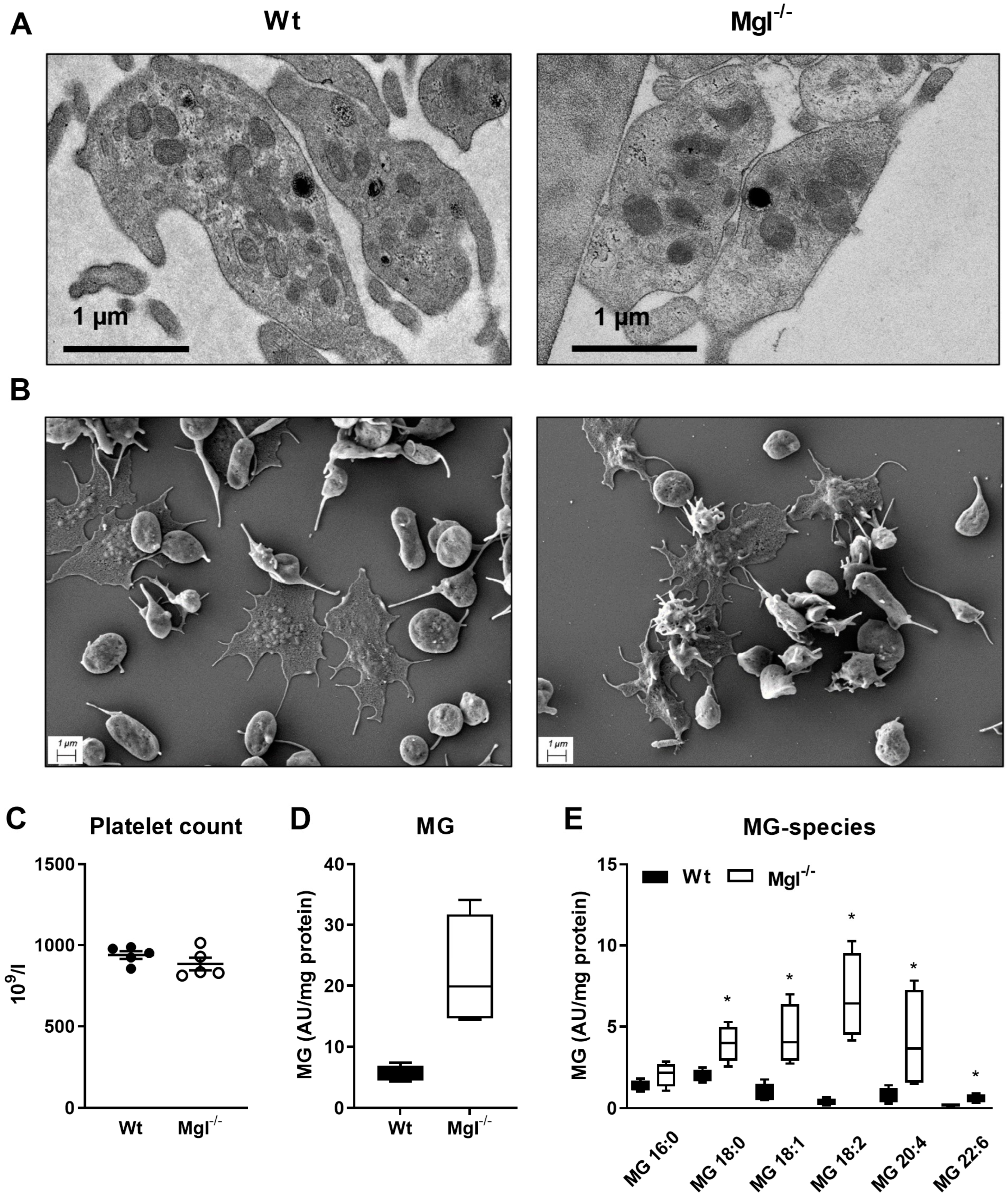
2.1. Unaltered Morphology and Spreading in Mgl−/− Platelets
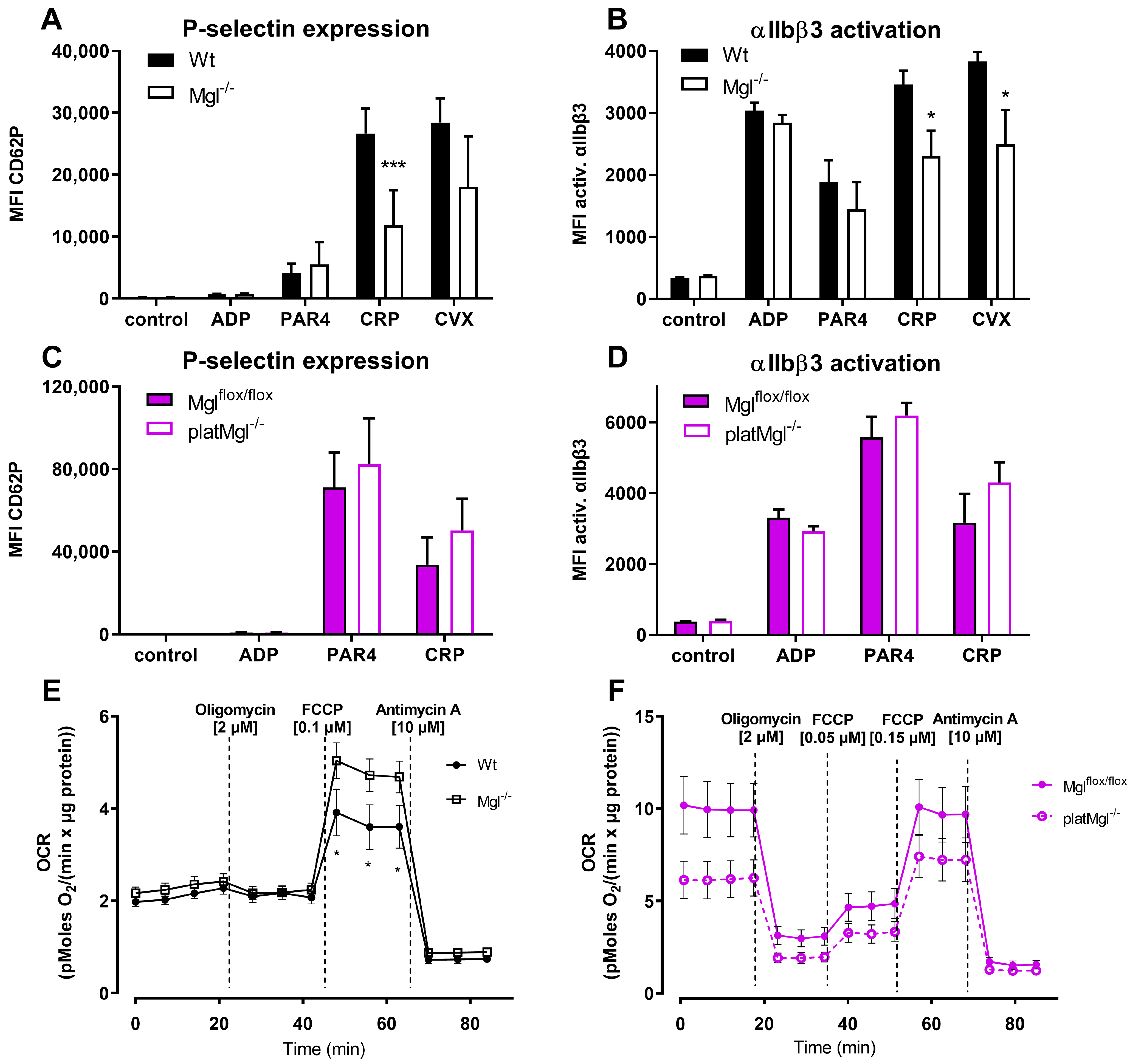
2.2. Increased Mitochondrial Respiration in Mgl−/− Platelets
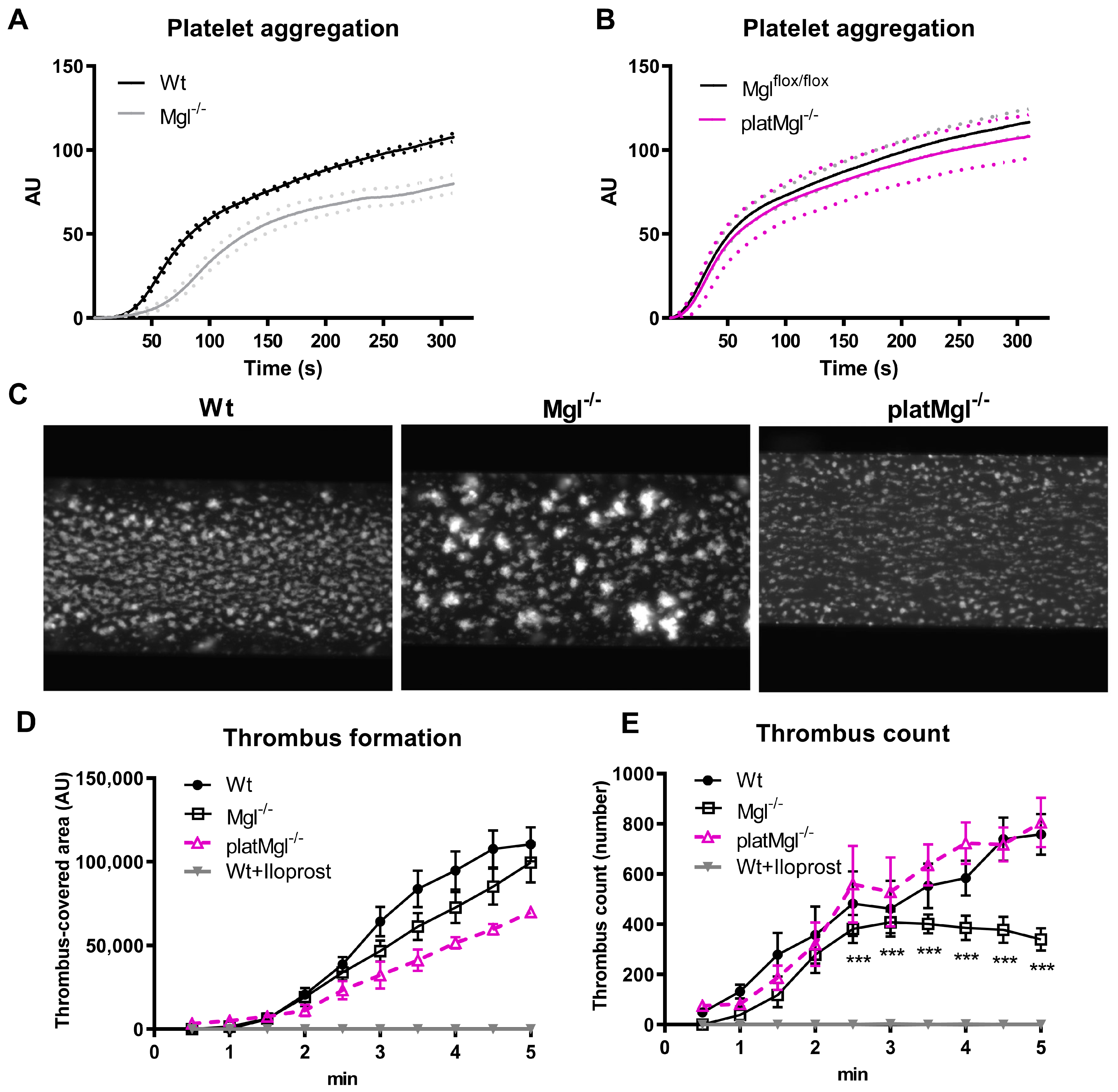
2.3. Loss of MGL Affects Thrombus Formation In Vitro
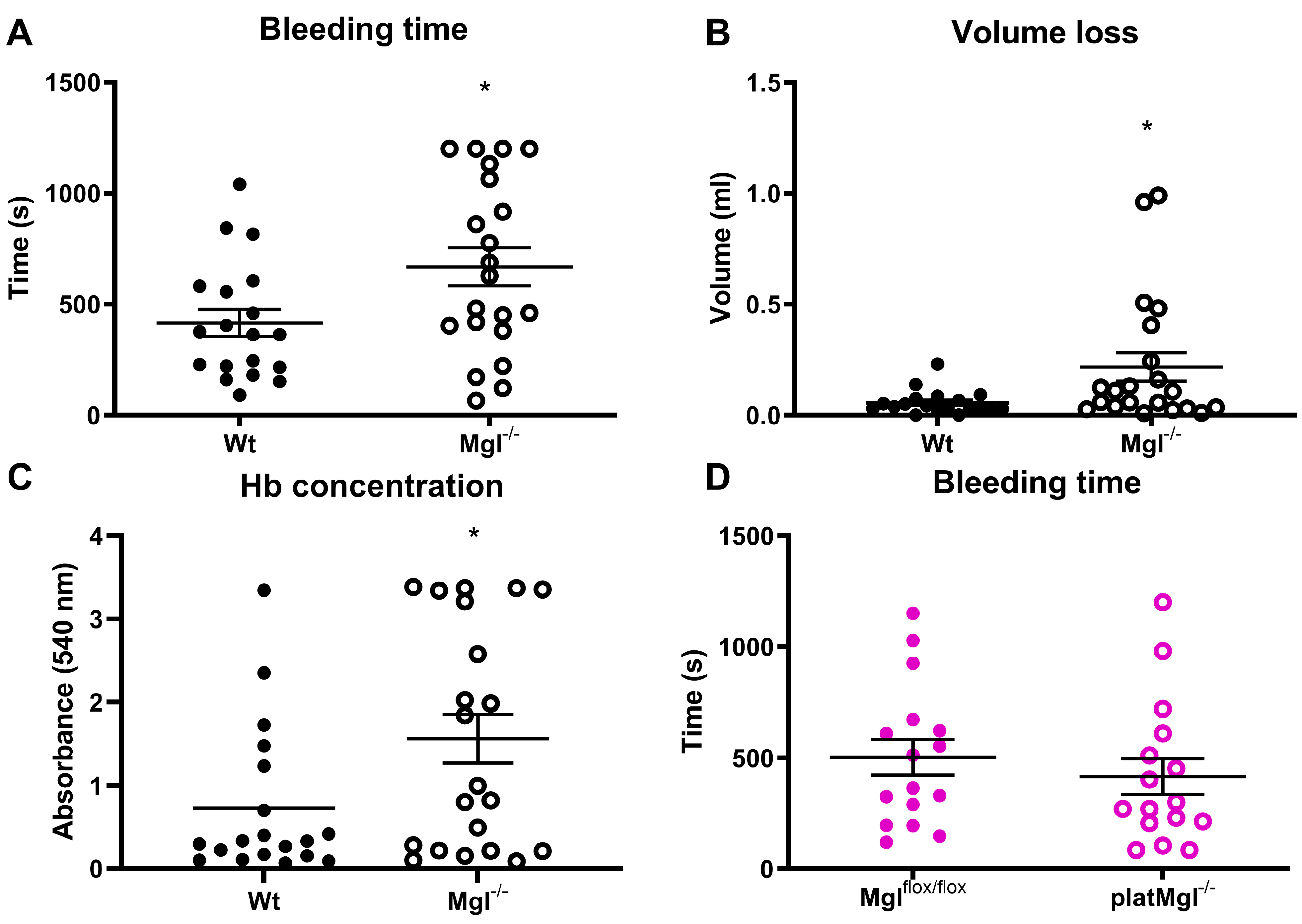
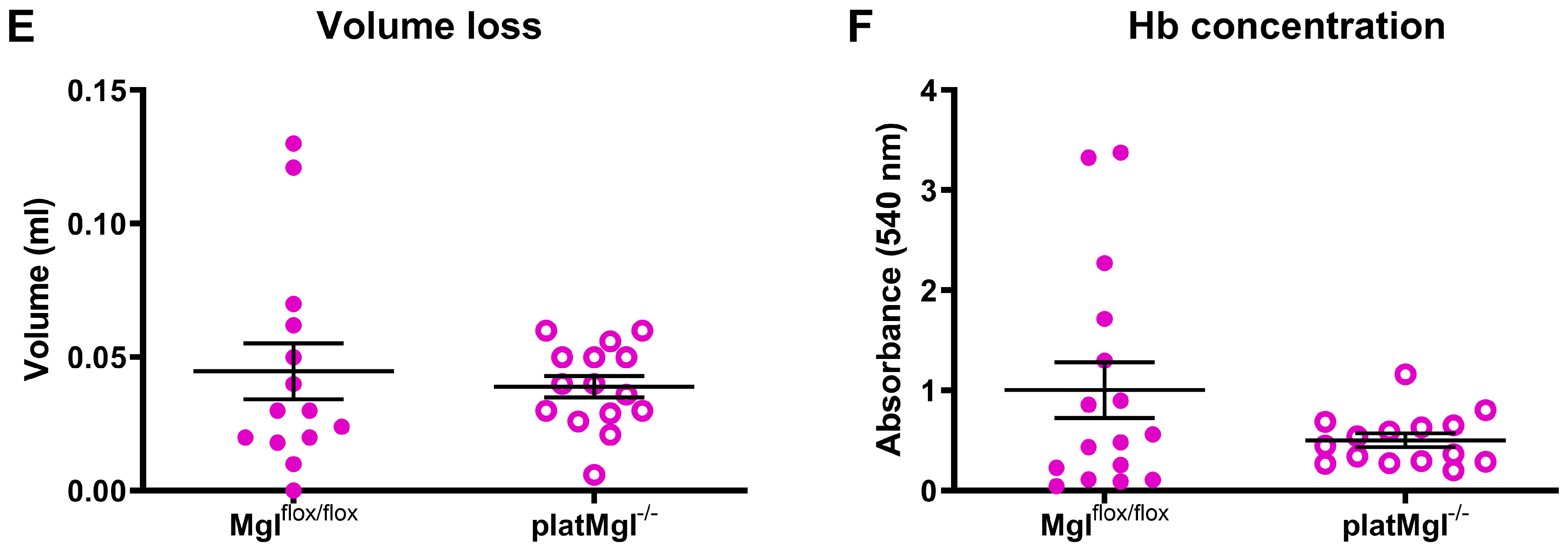
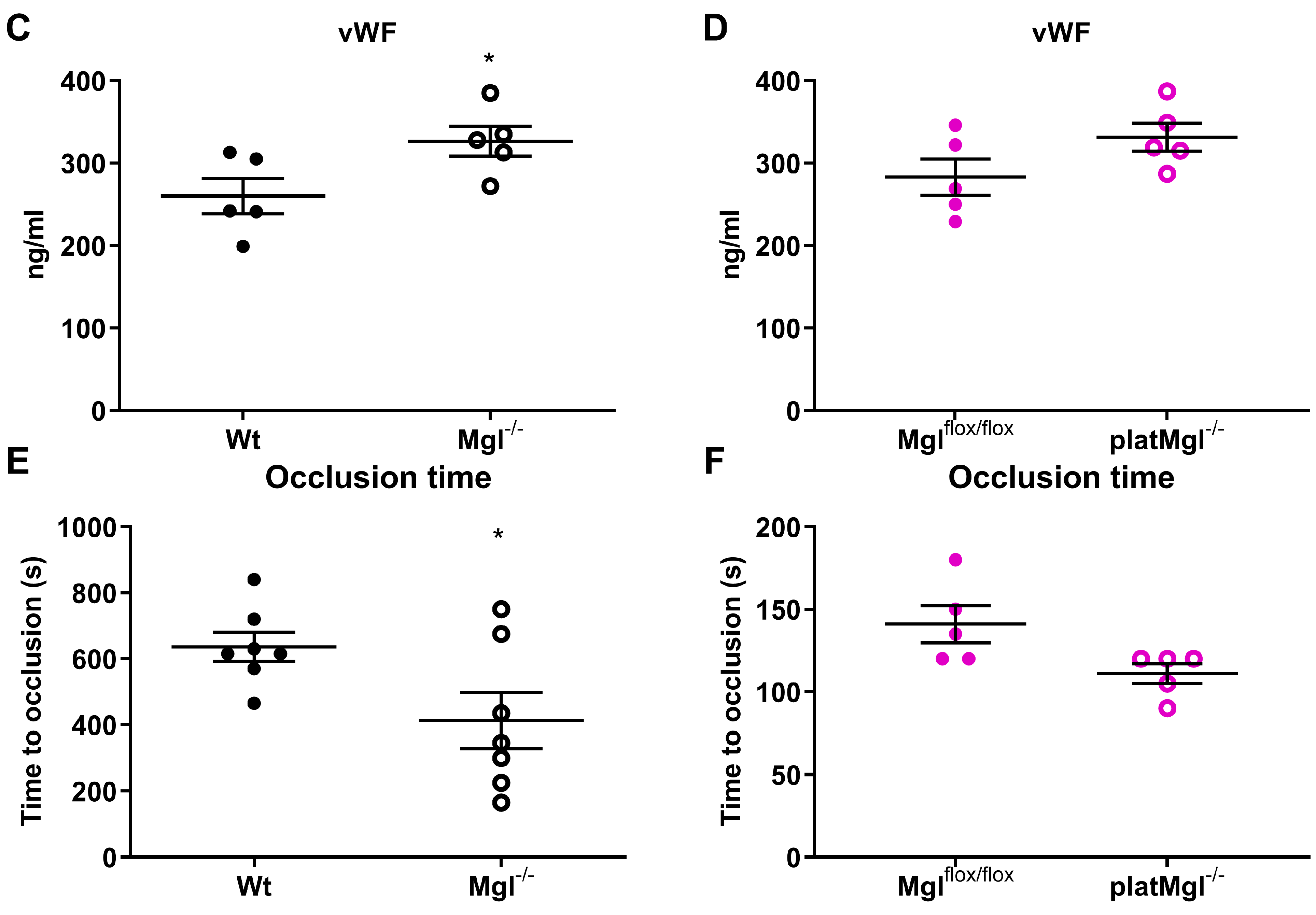
2.4. Reduced Hemostatic Function in Mgl−/− but Not in platMgl−/− Mice
2.5. Loss of MGL Affects Thrombus Formation In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Platelet Isolation and Purification
4.3. Transmission and Scanning Electron Microscopy
4.4. Targeted Lipidomic Analysis
4.5. Flow Cytometric Analyses of P-Selectin and αIIbβ3 Activation
4.6. Platelet Aggregation, Tail Bleeding, and Hemoglobin Assays
4.7. Mitochondrial Respiration Measurement
4.8. Thrombus Formation In Vitro
4.9. Thrombus Formation In Vivo
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C.; Colloquium, P.P. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B.; Murphy, R.C.; Watson, S.P. Platelet lipidomics: Modern day perspective on lipid discovery and characterization in platelets. Circ. Res. 2014, 114, 1185–1203. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb. Haemost. 2011, 106, 827–838. [Google Scholar]
- Vaughan, M.; Berger, J.E.; Steinberg, D. Hormone-sensitive Lipase and Monoglyceride Lipase Activities in Adipose Tissue. J. Biol. Chem. 1964, 239, 401–409. [Google Scholar] [CrossRef]
- Prescott, S.M.; Majerus, P.W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J. Biol. Chem. 1983, 258, 764–769. [Google Scholar] [CrossRef]
- Savinainen, J.R.; Järvinen, T.; Laine, K.; Laitinen, J.T. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001, 134, 664–672. [Google Scholar] [CrossRef]
- Gonsiorek, W.; Lunn, C.; Fan, X.; Narula, S.; Lundell, D.; Hipkin, R.W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol. Pharmacol. 2000, 57, 1045–1050. [Google Scholar]
- De Angelis, V.; Koekman, A.C.; Weeterings, C.; Roest, M.; de Groot, P.G.; Herczenik, E.; Maas, C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS ONE 2014, 9, e108282. [Google Scholar] [CrossRef]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Varga, K.; Wagner, J.A.; Bridgen, D.T.; Kunos, G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacol 2018, 43, 155–172. [Google Scholar] [CrossRef]
- Brantl, S.A.; Khandoga, A.L.; Siess, W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets 2014, 25, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.V.; Gasperi, V.; Catanzaro, G.; Baldassarri, S.; Bertoni, A.; Sinigaglia, F.; Avigliano, L.; Maccarrone, M. Human Platelets Express Authentic CB1 and CB2 Receptors. Curr. Neurovasc. Res. 2010, 7, 311–318. [Google Scholar] [CrossRef]
- Malorni, W.; Bari, M.; Straface, E.; Battista, N.; Matarrese, P.; Finazzi-Agrò, A.; Del Principe, D.; Maccarrone, M. Morphological evidence that 2-arachidonoylglycerol is a true agonist of human platelets. Thromb. Haemost. 2004, 92, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, V.; Avigliano, L.; Evangelista, D.; Oddi, S.; Chiurchiù, V.; Lanuti, M.; Maccarrone, M.; Catani, M.V. 2-Arachidonoylglycerol enhances platelet formation from human megakaryoblasts. Cell Cycle 2014, 13, 3938–3947. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Menichelli, A.; Giuliani, E.; Del Principe, D.; Finazzi-Agrò, A. Human platelets bind and degrade 2-arachidonoylglycerol, which activates these cells through a cannabinoid receptor. Eur. J. Biochem. 2001, 268, 819–825. [Google Scholar] [CrossRef]
- Baldassarri, S.; Bertoni, A.; Bagarotti, A.; Sarasso, C.; Zanfa, M.; Catani, M.V.; Avigliano, L.; Maccarrone, M.; Torti, M.; Sinigaglia, F. The endocannabinoid 2-arachidonoylglycerol activates human platelets through non-CB1/CB2 receptors. J. Thromb. Haemost. 2008, 6, 1772–1779. [Google Scholar] [CrossRef]
- Taschler, U.; Radner, F.P.W.; Heier, C.; Schreiber, R.; Schweiger, M.; Schoiswohl, G.; Preiss-Landl, K.; Jaeger, D.; Reiter, B.; Koefeler, H.C.; et al. Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-induced Insulin Resistance. J. Biol. Chem. 2011, 286, 17467–17477. [Google Scholar] [CrossRef]
- Vujic, N.; Schlager, S.; Eichmann, T.O.; Madreiter-Sokolowski, C.T.; Goeritzer, M.; Rainer, S.; Schauer, S.; Rosenberger, A.; Woelfler, A.; Doddapattar, P.; et al. Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice. Atherosclerosis 2016, 244, 9–21. [Google Scholar] [CrossRef]
- Rowley, J.W.; Oler, A.J.; Tolley, N.D.; Hunter, B.N.; Low, E.N.; Nix, D.A.; Yost, C.C.; Zimmerman, G.A.; Weyrich, A.S. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011, 118, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Goeritzer, M.; Schlager, S.; Kuentzel, K.B.; Vujić, N.; Korbelius, M.; Rainer, S.; Kolb, D.; Mussbacher, M.; Salzmann, M.; Schrottmaier, W.C.; et al. Adipose Triglyceride Lipase Deficiency Attenuates In Vitro Thrombus Formation without Affecting Platelet Activation and Bleeding In Vivo. Cells 2022, 11, 850. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Jandrot-Perrus, M.; Lagrue, A.-H.; Okuma, M.; Bon, C. Adhesion and Activation of Human Platelets Induced by Convulxin Involve Glycoprotein VI and Integrin α2β1. J. Biol. Chem. 1997, 272, 27035–27041. [Google Scholar] [CrossRef] [PubMed]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Schlager, S.; Goeritzer, M.; Jandl, K.; Frei, R.; Vujic, N.; Kolb, D.; Strohmaier, H.; Dorow, J.; Eichmann, T.O.; Rosenberger, A.; et al. Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis. J. Leukoc. Biol. 2015, 98, 837–850. [Google Scholar] [CrossRef]
- Eckly, A.; Hechler, B.; Freund, M.; Zerr, M.; Cazenave, J.P.; Lanza, F.; Mangin, P.H.; Gachet, C. Mechanisms underlying FeCl3-induced arterial thrombosis. J. Thromb. Haemost. 2011, 9, 779–789. [Google Scholar] [CrossRef]
- Bender, M.; Hagedorn, I.; Nieswandt, B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl3-induced thrombosis. J. Thromb. Haemost. 2011, 9, 1423–1426. [Google Scholar] [CrossRef]
- Anajirih, N.; O’Sullivan, S.E.; Alexander, S.P.H. Endocannabinoid hydrolases differentially distribute in platelets and red blood cells and are differentially released by thrombin. Prostaglandins Other Lipid Mediat. 2023, 164, 106692. [Google Scholar] [CrossRef]
- Maccarrone, M.; Dainese, E.; Oddi, S. Intracellular trafficking of anandamide: New concepts for signaling. Trends Biochem. Sci. 2010, 35, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Tsuboi, K.; Uyama, T. Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways. Febs. J. 2013, 280, 1874–1894. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A Comprehensive Profile of Brain Enzymes that Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Bergmeier, W.; Hynes, R.O. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb. Perspect. Biol. 2012, 4, a005132. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Z.S.; Jackson, S.P. The role of platelets in atherothrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 51–61. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Moliterno, D.J. The role of the platelet in the pathogenesis of atherothrombosis. Am. J. Cardiovasc. Drugs 2005, 5, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.L.; Hultin, M.B.; James, A.H.; Manco-Johnson, M.J.; Montgomery, R.R.; Ortel, T.L.; Rick, M.E.; Sadler, J.E.; Weinstein, M.; Yawn, B.P. von Willebrand disease (VWD): Evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008, 14, 171–232. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., 3rd; Lu, Y.; Whinna, H.C.; Gachet, C.; Fay, W.P.; Mackman, N. Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J. Thromb. Haemost. 2011, 9, 1862–1863. [Google Scholar] [CrossRef]
- Engelmann, B.; Kögl, C.; Kulschar, R.; Schaipp, B. Transfer of phosphatidylcholine, phosphatidylethanolamine and sphingomyelin from low- and high-density lipoprotein to human platelets. Biochem. J. 1996, 315, 781–789. [Google Scholar] [CrossRef]
- Paszek, E.; Zajdel, W.; Rajs, T.; Żmudka, K.; Legutko, J.; Kleczyński, P. Profilin 1 and Mitochondria—Partners in the Pathogenesis of Coronary Artery Disease? Int. J. Mol. Sci. 2021, 22, 1100. [Google Scholar] [CrossRef] [PubMed]
- Iritani, N.; Ikeda, Y.; Kajitani, H. Selectivities of 1-acylglycerophosphorylcholine acyltransferase and acyl-CoA synthetase for n-3 polyunsaturated fatty acids in platelets and liver microsomes. Biochim. Biophys. Acta 1984, 793, 416–422. [Google Scholar] [PubMed]
- McKean, M.L.; Smith, J.B.; Silver, M.J. Phospholipid biosynthesis in human platelets. Formation of phosphatidylcholine from 1-acyl lysophosphatidylcholine by acyl-CoA:1-acyl-sn-glycero-3-phosphocholine acyltransferase. J. Biol. Chem. 1982, 257, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Bevers, E.M.; Comfurius, P.; Zwaal, R.F. Changes in membrane phospholipid distribution during platelet activation. Biochim. Biophys. Acta 1983, 736, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Münzer, P.; Mittelstädt, S.; Geue, S.; Manke, M.-C.; Walker-Allgaier, B.; Lang, F.; Gawaz, M.; Borst, O. Ceramidase critically affects GPVI-dependent platelet activation and thrombus formation. Biochem. Biophys. Res. Commun. 2018, 496, 792–798. [Google Scholar] [CrossRef]
- Huo, Y.; Schober, A.; Forlow, S.B.; Smith, D.F.; Hyman, M.C.; Jung, S.; Littman, D.R.; Weber, C.; Ley, K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003, 9, 61–67. [Google Scholar] [CrossRef]
- Grabner, G.F.; Eichmann, T.O.; Wagner, B.; Gao, Y.; Farzi, A.; Taschler, U.; Radner, F.P.W.; Schweiger, M.; Lass, A.; Holzer, P.; et al. Deletion of Monoglyceride Lipase in Astrocytes Attenuates Lipopolysaccharide-induced Neuroinflammation. J. Biol. Chem. 2016, 291, 913–923. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Weberhofer, B.P.; Eichmann, T.O.; Kohlwein, S.D.; Rechberger, G.N. A versatile ultra-high performance LC-MS method for lipid profiling. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 951–952, 119–128. [Google Scholar] [CrossRef]
- Fernandez-Acosta, M.; Romero, J.I.; Bernabó, G.; Velázquez-Campos, G.M.; Gonzalez, N.; Mares, M.L.; Werbajh, S.; Avendaño-Vázquez, L.A.; Rechberger, G.N.; Kühnlein, R.P.; et al. orsai, the Drosophila homolog of human ETFRF1, links lipid catabolism to growth control. BMC Biol. 2022, 20, 233. [Google Scholar] [CrossRef]
- Liu, Y.; Jennings, N.L.; Dart, A.M.; Du, X.J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J. Exp. Med. 2012, 2, 30–36. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goeritzer, M.; Kuentzel, K.B.; Beck, S.; Korbelius, M.; Rainer, S.; Bradić, I.; Kolb, D.; Mussbacher, M.; Schrottmaier, W.C.; Assinger, A.; et al. Monoglyceride Lipase Deficiency Is Associated with Altered Thrombogenesis in Mice. Int. J. Mol. Sci. 2023, 24, 3116. https://doi.org/10.3390/ijms24043116
Goeritzer M, Kuentzel KB, Beck S, Korbelius M, Rainer S, Bradić I, Kolb D, Mussbacher M, Schrottmaier WC, Assinger A, et al. Monoglyceride Lipase Deficiency Is Associated with Altered Thrombogenesis in Mice. International Journal of Molecular Sciences. 2023; 24(4):3116. https://doi.org/10.3390/ijms24043116
Chicago/Turabian StyleGoeritzer, Madeleine, Katharina B. Kuentzel, Sarah Beck, Melanie Korbelius, Silvia Rainer, Ivan Bradić, Dagmar Kolb, Marion Mussbacher, Waltraud C. Schrottmaier, Alice Assinger, and et al. 2023. "Monoglyceride Lipase Deficiency Is Associated with Altered Thrombogenesis in Mice" International Journal of Molecular Sciences 24, no. 4: 3116. https://doi.org/10.3390/ijms24043116
APA StyleGoeritzer, M., Kuentzel, K. B., Beck, S., Korbelius, M., Rainer, S., Bradić, I., Kolb, D., Mussbacher, M., Schrottmaier, W. C., Assinger, A., Schlagenhauf, A., Rost, R., Gottschalk, B., Eichmann, T. O., Züllig, T., Graier, W. F., Vujić, N., & Kratky, D. (2023). Monoglyceride Lipase Deficiency Is Associated with Altered Thrombogenesis in Mice. International Journal of Molecular Sciences, 24(4), 3116. https://doi.org/10.3390/ijms24043116










