Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis
Abstract
1. Introduction
2. Results
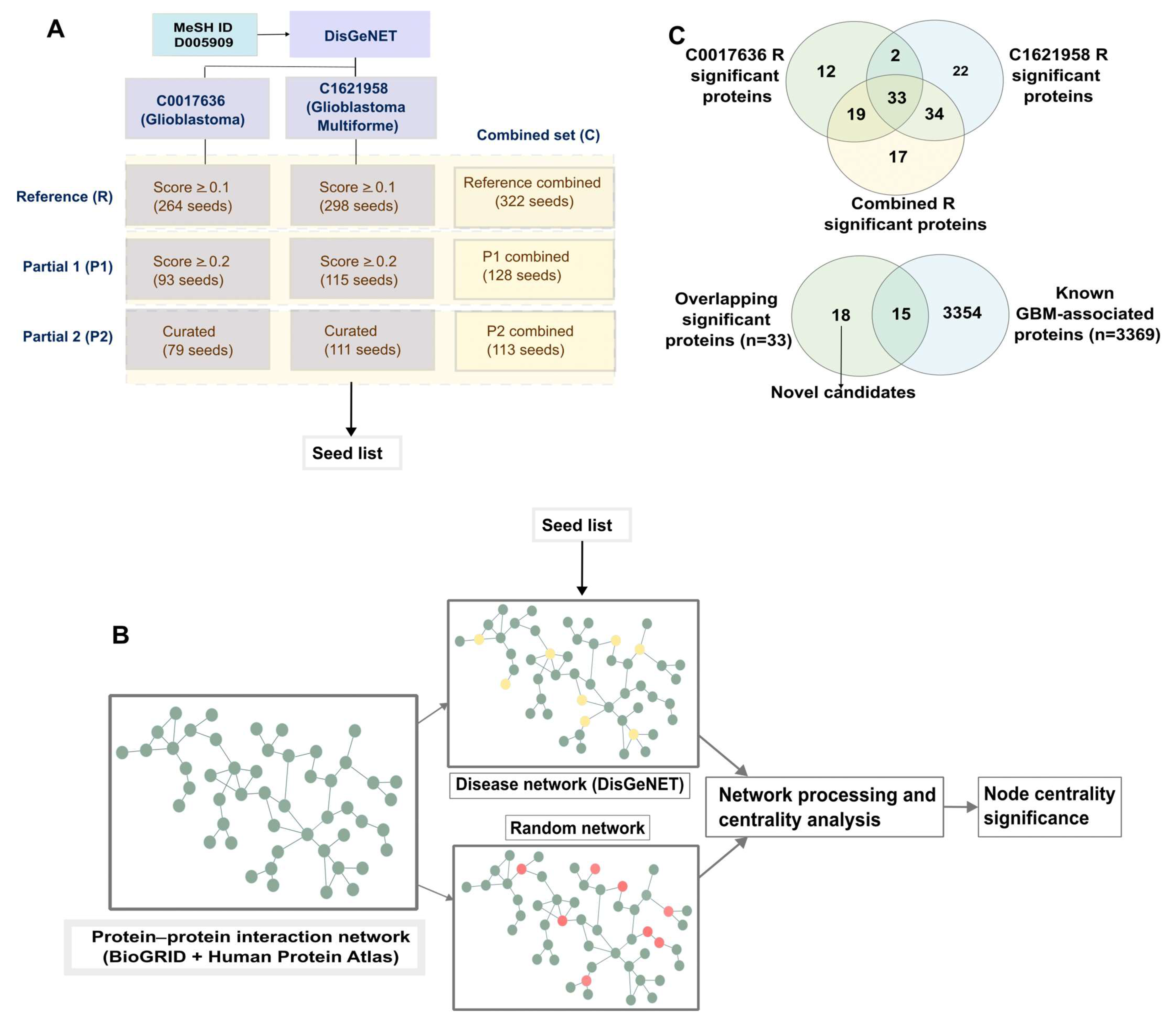
2.1. Overlapping Top-Ranking Nodes on Applying Background-Corrected Centrality Analysis across Topologically Varying Glioblastoma Networks Yields Robust Putative Glioblastoma Candidates
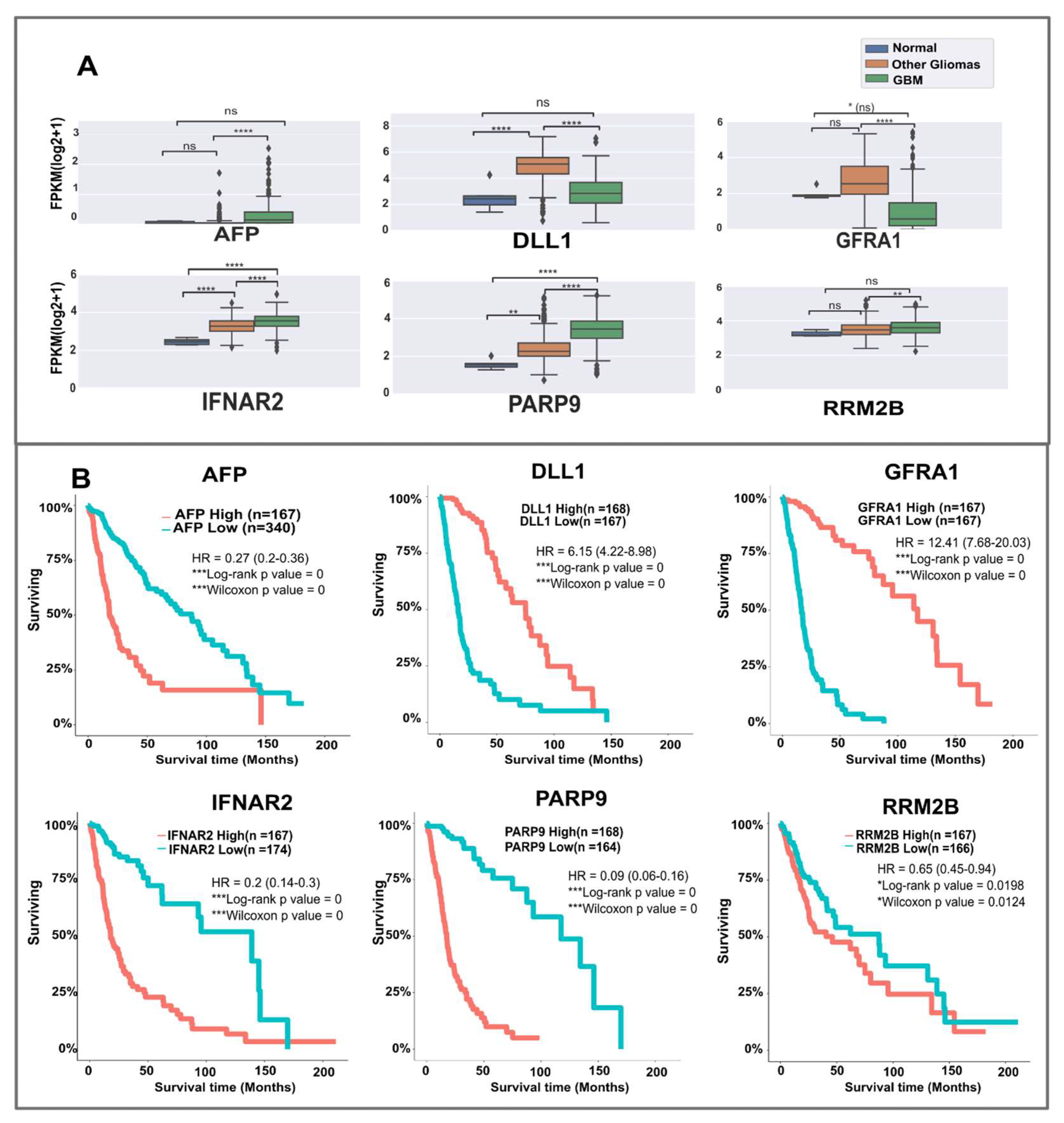
2.2. Eighteen Novel Candidates Identified in the Study Show Links to GBM/Glioma Based on Mutations, Literature Evidence, Expression, and Survival Analysis
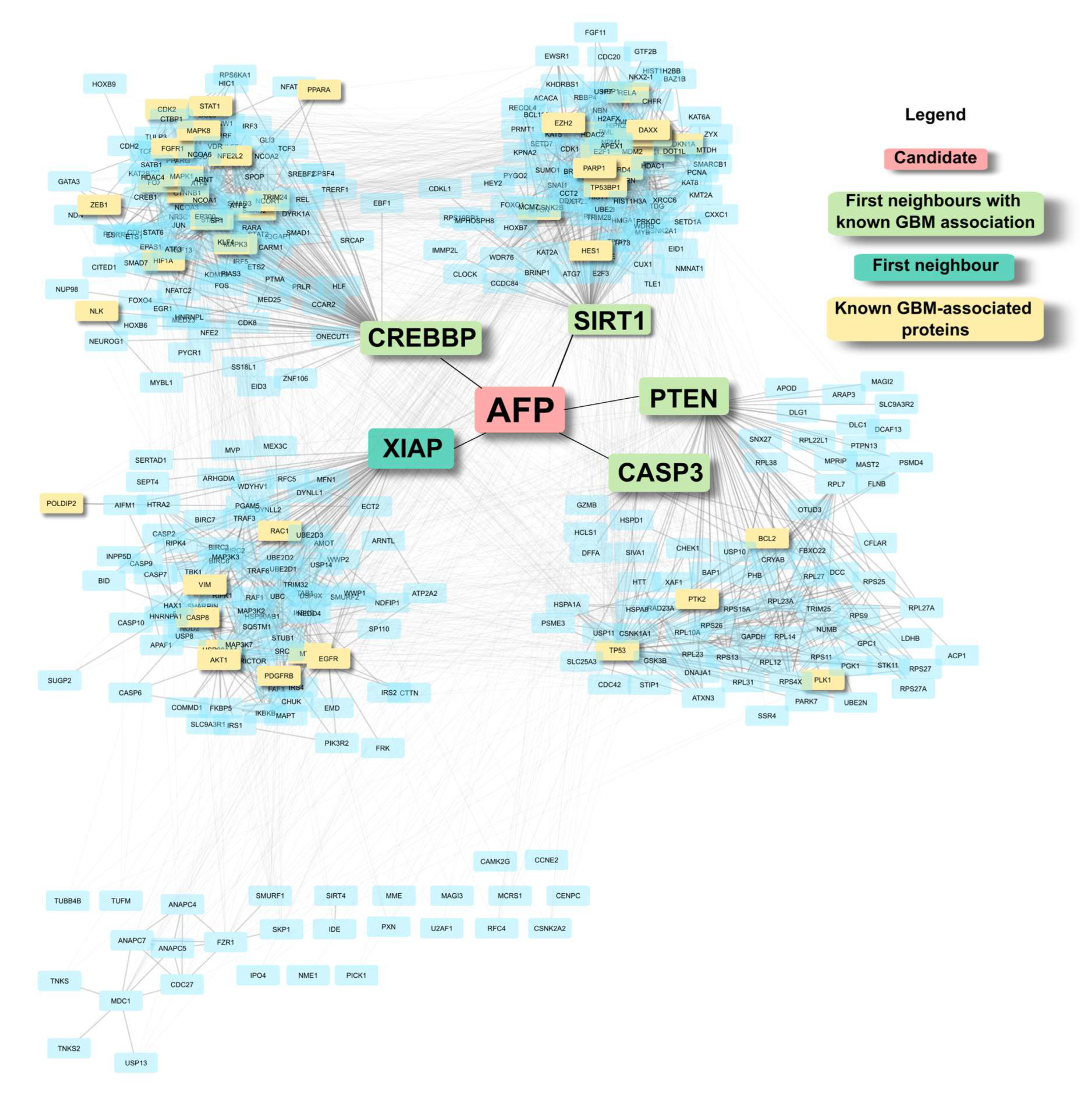
2.3. Background Correction Highlights Low-Degree Structurally Critical Proteins That Connect Several Known GBM Proteins
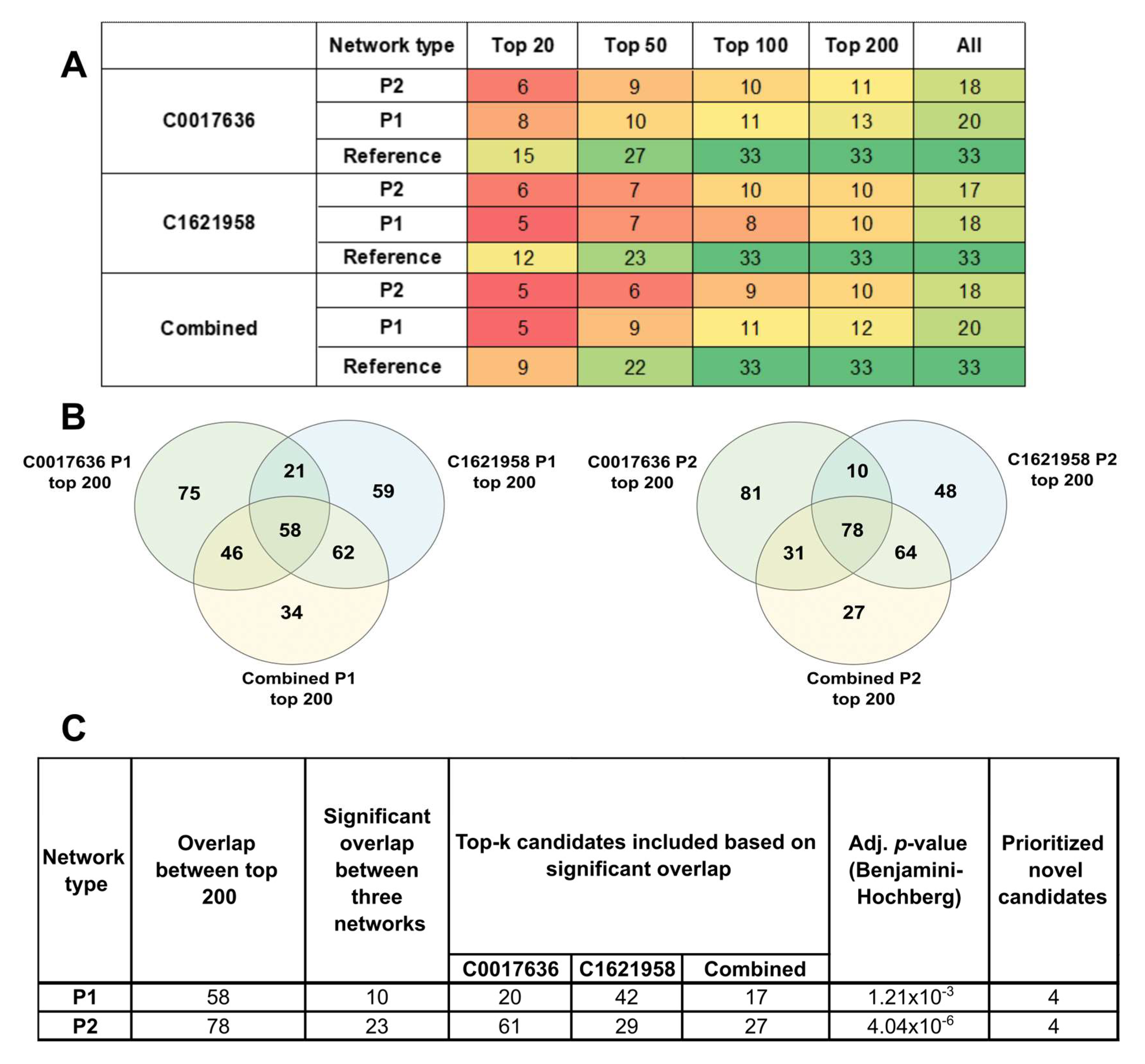
2.4. Partial Networks Also Return Top-Ranked Nodes as the Top Hits, Thus Indicating the Robustness of the Method to Recover Topologically Critical Nodes
3. Discussion
4. Methods and Materials
4.1. Obtaining Seed Lists
4.2. Mapping to PPI
4.3. Computational Pipeline and Resources
4.4. Overlap Significance
4.5. Validation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreatsoulas, D.; Bolyard, C.; Wu, B.X.; Cam, H.; Giglio, P.; Li, Z. Translational landscape of glioblastoma immunotherapy for physicians: Guiding clinical practice with basic scientific evidence. J. Hematol. Oncol. 2022, 15, 80. [Google Scholar] [CrossRef]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef]
- Liu, A.; Hou, C.; Chen, H.; Zong, X.; Zong, P. Genetics and Epigenetics of Glioblastoma: Applications and Overall Incidence of IDH1 Mutation. Front. Oncol. 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- A Yabo, Y.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro-Oncology 2021, 24, 669–682. [Google Scholar] [CrossRef]
- Badkas, A.; De Landtsheer, S.; Sauter, T. Topological network measures for drug repositioning. Briefings Bioinform. 2020, 22, bbaa357. [Google Scholar] [CrossRef]
- Choobdar, S.; The DREAM Module Identification Challenge Consortium; Ahsen, M.E.; Crawford, J.; Tomasoni, M.; Fang, T.; Lamparter, D.; Lin, J.; Hescott, B.; Hu, X.; et al. Assessment of network module identification across complex diseases. Nat. Methods 2019, 16, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Martins, E.; Vinga, S.; Costa, B. The Role of Network Science in Glioblastoma. Cancers 2021, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Chalise, P.; Ni, Y.; Fridley, B.L. Network-based integrative clustering of multiple types of genomic data using non-negative matrix factorization. Comput. Biol. Med. 2020, 118, 103625. [Google Scholar] [CrossRef]
- Uthamacumaran, A.; Craig, M. Algorithmic reconstruction of glioblastoma network complexity. Iscience 2022, 25, 104179. [Google Scholar] [CrossRef]
- Park, J.H.; Feroze, A.H.; Emerson, S.N.; Mihalas, A.B.; Keene, C.D.; Cimino, P.J.; de Lomana, A.L.G.; Kannan, K.; Wu, W.-J.; Turkarslan, S.; et al. A single-cell based precision medicine approach using glioblastoma patient-specific models. NPJ Precis. Oncol. 2022, 6, 55. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2016, 45, D833–D839. [Google Scholar] [CrossRef]
- Badkas, A.; De Landtsheer, S.; Sauter, T. Construction and contextualization approaches for protein-protein interaction networks. Comput. Struct. Biotechnol. J. 2022, 20, 3280–3290. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zeng, A.; Fan, Y.; Di, Z. Robustness of centrality measures against network manipulation. Phys. A Stat. Mech. Appl. 2015, 438, 124–131. [Google Scholar] [CrossRef]
- Frantz, T.L.; Cataldo, M.; Carley, K.M. Robustness of centrality measures under uncertainty: Examining the role of network topology. Comput. Math. Organ. Theory 2009, 15, 303. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Carley, K.M.; Krackhardt, D. On the robustness of centrality measures under conditions of imperfect data. Soc. Networks 2006, 28, 124–136. [Google Scholar] [CrossRef]
- Iyer, S.; Killingback, T.; Sundaram, B.; Wang, Z. Attack Robustness and Centrality of Complex Networks. PLoS One 2013, 8, e59613. [Google Scholar] [CrossRef]
- Martin, C.; Niemeyer, P. Influence of measurement errors on networks: Estimating the robustness of centrality measures. Netw. Sci. 2019, 7, 180–195. [Google Scholar] [CrossRef]
- Durón, C.; Pan, Y.; Gutmann, D.H.; Hardin, J.; Radunskaya, A. Variability of Betweenness Centrality and Its Effect on Identifying Essential Genes. Bull. Math. Biol. 2019, 81, 3655–3673. [Google Scholar] [CrossRef]
- Badkas, A.; Nguyen, T.-P.; Caberlotto, L.; Schneider, J.; De Landtsheer, S.; Sauter, T. Degree Adjusted Large-Scale Network Analysis Reveals Novel Putative Metabolic Disease Genes. Biology 2021, 10, 107. [Google Scholar] [CrossRef]
- Rahman, M.; Jackson, L.K.; Johnson, W.; Li, D.Y.; Bild, A.H.; Piccolo, S.R. Alternative preprocessing of RNA-Sequencing data in The Cancer Genome Atlas leads to improved analysis results. Bioinformatics 2015, 31, 3666–3672. [Google Scholar] [CrossRef]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.W.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro. Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef]
- Shurin, M.R.; Lu, L.; Kalinski, P.; Stewart-Akers, A.M.; Lotze, M.T. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin. Immunopathol. 1999, 21, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.H.; Apeltsin, L.; Newman, A.M.; Baumbach, J.; Wittkop, T.; Su, G.; Bader, G.D.; Ferrin, T.E. Clustermaker: A multi-algorithm clustering plugin for Cytoscape. BMC Bioinform. 2011, 12, 436. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: A novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics 1998, 14, 656–664. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Hariri, H.; St-Arnaud, R. Expression and Role of Ubiquitin-Specific Peptidases in Osteoblasts. Int. J. Mol. Sci. 2021, 22, 7746. [Google Scholar] [CrossRef]
- Jalili, M.; Salehzadeh-Yazdi, A.; Asgari, Y.; Arab, S.S.; Yaghmaie, M.; Ghavamzadeh, A.; Alimoghaddam, K. CentiServer: A Comprehensive Resource, Web-Based Application and R Package for Centrality Analysis. PLoS ONE 2015, 10, e0143111. [Google Scholar] [CrossRef]
- Rogers, F.B. Medical subject headings. Bull. Med. Libr. Assoc. 1963, 51, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Stark, C.; Breitkreutz, B.J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A general repository for interaction datasets. Nucleic Acids Res. 2006, 34, D535–D539. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Varrette, S.; Bouvry, P.; Cartiaux, H.; Georgatos, F. Management of an academic HPC cluster: The UL experience. In Proceedings of the 2014 International Conference on High Performance Computing & Simulation (HPCS), Bologna, Italy, 21–25 July 2014; pp. 959–967. [Google Scholar] [CrossRef]
- Amand, J.; Fehlmann, T.; Backes, C.; Keller, A. DynaVenn: Web-based computation of the most significant overlap between ordered sets. BMC Bioinform. 2019, 20, 743. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]

| Diseases/Pathology | Function | p-Value | Candidates | Number of Proteins (/18) |
|---|---|---|---|---|
| Cell Death and Survival | Apoptosis | 0.000115 | STAT4, DLL1, TLN1, IFNAR2, GFRA1, IL6R, CDC37, SH3RF1, AFP, RRM2B, USP53 | 11 |
| Cell Death and Survival, Organismal Injury and Abnormalities | Necrosis | 0.000173 | STAT4, DLL1, TLN1, IFNAR2, GFRA1, IL6R, CDC37, SH3RF1, AFP, RRM2B, USP53 | 11 |
| Cell-To-Cell Signalling and Interaction | Activation of lymphatic system cells | 0.000192 | DLL1, STAT4, TLN1, IL6R, AFP | 5 |
| Tissue Morphology | Quantity of cells | 0.000288 | STAT4, DLL1, TLN1, IFNAR2, GFRA1, IL6R, SHC2, AFP, KALRN | 9 |
| Cell Death and Survival, Organismal Injury and Abnormalities | Cell death of tumor cell lines | 0.000662 | DLL1, TLN1, IFNAR2, IL6R, CDC37, SH3RF1, AFP, RRM2B | 8 |
| Cell Death and Survival, Organismal Injury and Abnormalities | Cell death of immune cells | 0.000773 | DLL1, STAT4, TLN1, IL6R, AFP | 5 |
| Cellular Movement | Migration of cells | 0.0038 | DLL1, TLN1, GFRA1, IL6R, PARP9, SH3RF1, EPB41L5, KALRN | 8 |
| Nervous System Development and Function | Morphology of nervous system | 0.00591 | GFRA1, IL6R, SHC2, RRM2B, KALRN | 5 |
| Cell Morphology, Nervous System Development and Function, Tissue Morphology | Morphology of neurons | 0.00604 | GFRA1, IL6R, SHC2, KALRN | 4 |
| Neurological Disease, Organismal Injury and Abnormalities | Progressive neurological disorder | 0.0116 | DLL1, IFNAR2, IL6R, RRM2B, USP53 | 5 |
| Cancer, Organismal Injury and Abnormalities | Carcinoma | 0.0142 | STAT4, TLN1, IFNAR2, GFRA1, IL6R, SHC2, PARP9, EIF1AD, CDC37, SH3RF1, AFP, RRM2B, DLL1, EPB41L5, PSKH1, KALRN, GRB14, USP53 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badkas, A.; De Landtsheer, S.; Sauter, T. Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis. Int. J. Mol. Sci. 2023, 24, 3075. https://doi.org/10.3390/ijms24043075
Badkas A, De Landtsheer S, Sauter T. Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis. International Journal of Molecular Sciences. 2023; 24(4):3075. https://doi.org/10.3390/ijms24043075
Chicago/Turabian StyleBadkas, Apurva, Sébastien De Landtsheer, and Thomas Sauter. 2023. "Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis" International Journal of Molecular Sciences 24, no. 4: 3075. https://doi.org/10.3390/ijms24043075
APA StyleBadkas, A., De Landtsheer, S., & Sauter, T. (2023). Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis. International Journal of Molecular Sciences, 24(4), 3075. https://doi.org/10.3390/ijms24043075






