Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells
Abstract
1. Introduction
2. Cell Mediators: Helper T Cells Subpopulations
3. Importance of the Treg/Th17 Relationship
Regulation between Treg/Th17
4. Hypoxia
4.1. Hypoxia and Th17 Relationship
4.2. Hypoxia as Neuroglial Damage Mediator
4.3. Hypoxia and Blood Brain Barrier Dysfunction
4.4. Hypoxia and Neuroinflammation
4.5. Hypoxia and Autoimmune Diseases
5. Autoimmune Diseases and Neuroinflammation: Role of HIF-1 and Th17
5.1. Inflammatory Demyelinating Disease
5.2. Multiple Sclerosis
5.3. Alzheimer’s Disease
6. Potential Therapeutic Targets
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- López-Ornelas, A.; Jiménez, A.; Pérez-Sánchez, G.; Rodríguez-Pérez, C.E.; Corzo-Cruz, A.; Velasco, I.; Estudillo, E. The Impairment of Blood-Brain Barrier in Alzheimer’s Disease: Challenges and Opportunities with Stem Cells. Int. J. Mol. Sci. 2022, 23, 10136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, J. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Bautista, J.L.; Scott-Browne, J.; Mohan, J.F.; Hsieh, C.-S. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity 2012, 37, 475–486. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Klein, J.A.N.; Sato, A. The Immune System (First of Two Parts). N. Engl. J. Med. 2000, 343, 1132. [Google Scholar] [CrossRef]
- Takeuchi, A.; Saito, T. CD4 CTL, a cytotoxic subset of CD4+ T cells, their differentiation and function. Front. Immunol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.-Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.-R.; et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef]
- Ikejiri, A.; Nagai, S.; Goda, N.; Kurebayashi, Y.; Osada-Oka, M.; Takubo, K.; Suda, T.; Koyasu, S. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int. Immunol. 2012, 24, 137–146. [Google Scholar] [CrossRef]
- Uchiyama, R.; Yonehara, S.; Taniguchi, S.; Ishido, S.; Ishii, K.J.; Tsutsui, H. Inflammasome and Fas-Mediated IL-1β Contributes to Th17/Th1 Cell Induction in Pathogenic Bacterial Infection In Vivo. J. Immunol. 2017, 199, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Samany, P.G.; Thirumangalakudi, L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: Implications for disease pathogenesis. J. Alzheimer Dis. 2006, 9, 51–58. [Google Scholar] [CrossRef]
- El-Fawal, H.A.N. Neuroantibody biomarkers: Links and challenges in environmental neurodegeneration and autoimmunity. Autoimmune Dis. 2014, 2014, 340875. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Barbi, J.; Pardoll, D.; Pan, F. Metabolic control of the Treg/Th17 axis. Immunol. Rev. 2013, 252, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, C.; Xu, H.; Jia, J.; Wei, Z.; Guo, R.; Jia, Y.; Wu, Y.; Li, Y.; Qi, X.; et al. The immunoregulation of Th17 in host against intracellular bacterial infection. Mediat. Inflamm. 2018, 2018, 6587296. [Google Scholar] [CrossRef] [PubMed]
- Licona-Limón, P.; Arias-Rojas, A.; Olguín-Martínez, E. IL-9 and Th9 in parasite immunity. Semin. Immunopathol. 2017, 39, 29–38. [Google Scholar] [CrossRef]
- Lee, G.R. The balance of th17 versus treg cells in autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Hua, F.; Ji, L.; Zhan, Y.; Li, F.; Zou, S.; Chen, L.; Gao, S.; Li, Y.; Chen, H.; Cheng, Y. Aberrant frequency of IL-10-producing B cells and its association with Treg/Th17 in adult primary immune thrombocytopenia patients. Biomed Res. Int. 2014, 2014, 571302. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate immune system and preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Mohammadi, H.; Safarzadeh, E.; Hemmatzadeh, M.; Mahdian-Shakib, A.; Jadidi-Niaragh, F.; Azizi, G.; Baradaran, B. The paradox of Th17 cell functions in tumor immunity. Cell. Immunol. 2017, 322, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhao, E.; Kryczek, I.; Zou, W. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology 2012, 1, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Wacleche, V.S.; Landay, A.; Routy, J.-P.; Ancuta, P. The Th17 lineage: From barrier surfaces homeostasis to autoimmunity, cancer, and HIV-1 pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Cao, B.-B.; Qiu, Y.-H.; Peng, Y.-P. Treg Cells Attenuate Neuroinflammation and Protect Neurons in a Mouse Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2020, 15, 224–237. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, R.-T.; Du, T.; Yang, C.-L.; Liu, Y.-D.; Ge, M.-R.; Zhang, M.; Li, X.-L.; Li, H.; Dou, Y.-C.; et al. Exosomes derived from statin-modified bone marrow dendritic cells increase thymus-derived natural regulatory T cells in experimental autoimmune myasthenia gravis. J. Neuroinflammation 2019, 16, 202–212. [Google Scholar] [CrossRef]
- Koch, M.A.; Thomas, K.R.; Perdue, N.R.; Smigiel, K.S.; Srivastava, S.; Campbell, D.J. T-bet+ Treg Cells Undergo Abortive Th1 Cell Differentiation due to Impaired Expression of IL-12 Receptor β2. Immunity 2012, 37, 501–510. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the immune response by TGF-β: From conception to autoimmunity and infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshimi, R.; Yoshii, H.; Kim, D.; Dey, A.; Xiong, H.; Munasinghe, J.; Yazawa, I.; O’Donovan, M.; Maximova, O.A.; et al. The Transcription Factor IRF8 Activates Integrin-Mediated TGF-β Signaling and Promotes Neuroinflammation. Immunity 2014, 40, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Wang, Y.-L.; Fan, H.-C.; Lo, W.-T.; Wang, C.-C.; Sytwu, H.-K. Current status of the immunomodulation and immunomediated therapeutic strategies for multiple sclerosis. Clin. Dev. Immunol. 2012, 2012, 970789. [Google Scholar] [CrossRef]
- Ward-Hartstonge, K.A.; Kemp, R.A. Regulatory T-cell heterogeneity and the cancer immune response. Clin. Transl. Immunol. 2017, 6, e154. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sharma, S.; Edwards, J.; Feigenbaum, L.; Zhu, J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 2015, 16, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef]
- Krebs, C.F.; Paust, H.-J.; Krohn, S.; Koyro, T.; Brix, S.R.; Riedel, J.-H.; Bartsch, P.; Wiech, T.; Meyer-Schwesinger, C.; Huang, J.; et al. Autoimmune Renal Disease Is Exacerbated by S1P-Receptor-1-Dependent Intestinal Th17 Cell Migration to the Kidney. Immunity 2016, 45, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.; Dodd, W.; Motwani, K.; Hosaka, K.; Laurent, D.; Martinez, M.; Dugan, V.; Chalouhi, N.; Lucke-Wold, N.; Barpujari, A.; et al. Investigation and modulation of interleukin-6 following subarachnoid hemorrhage: Targeting inflammatory activation for cerebral vasospasm. J. Neuroinflammation 2022, 19, 1–16. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Fujimoto, M.; Kawakita, F.; Liu, L.; Nakano, F.; Nishikawa, H.; Okada, T.; Imanaka-Yoshida, K.; Yoshida, T.; Shiba, M. Toll-Like receptor 4 and tenascin-C signaling in cerebral vasospasm and brain injuries after subarachnoid hemorrhage. Acta Neurochir. Suppl. 2020, 127, 91–96. [Google Scholar]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Fasching, P.; Stradner, M.; Graninger, W.; Dejaco, C.; Fessler, J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules 2017, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Mole, D.R.; Ratcliffe, P. Cellular oxygen sensing in health and disease. Pediatr. Nephrol. 2008, 23, 681–694. [Google Scholar] [CrossRef]
- Klimova, T.; Chandel, N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008, 15, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Mohammed, R.; Ojha, U. What are the links between hypoxia and alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2019, 15, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Akanji, M.A.; Rotimi, D.; Adeyemi, O.S. Hypoxia-inducible factors as an alternative source of treatment strategy for cancer. Oxidative Med. Cell. Longev. 2019, 2019, 8547846. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-S.; Chiu, W.-T.; Hsu, P.-L.; Lin, S.-C.; Peng, I.-C.; Wang, C.-Y.; Tsai, S.-J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, M.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Vaupel, P. Hypoxia compromises anti-cancer immune responses. Adv. Exp. Med. Biol. 2020, 1232, 131–143. [Google Scholar] [PubMed]
- Nutsch, K.; Hsieh, C. When T cells run out of breath: The HIF-1α story. Cell 2011, 146, 673–674. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Physiological Effects of Chronic Hypoxia. N. Engl. J. Med. 2017, 376, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, J.-P.; Heikkilä, M.; Malinen, M.; Lee, H.-M.; Palvimo, J.J.; Wei, G.-H.; Myllyharju, J. A long hypoxia-inducible factor 3 isoform 2 is a transcription activator that regulates erythropoietin. Cell. Mol. Life Sci. 2020, 77, 3627–3642. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Patkar, S.; Ogunshola, O. Cell-specific blood-brain barrier regulation in health and disease: A focus on hypoxia. Br. J. Pharmacol. 2014, 171, 1210–1230. [Google Scholar] [CrossRef] [PubMed]
- Lisy, K.; Peet, D. Turn me on: Regulating HIF transcriptional activity. Cell Death Differ. 2008, 15, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hassan, H. Hypoxia in Alzheimer’s disease: Effects of hypoxia inducible factors. Neural Regen. Res. 2021, 16, 310–311. [Google Scholar] [CrossRef]
- Mennerich, D.; Kubaichuk, K.; Raza, G.S.; Fuhrmann, D.C.; Herzig, K.-H.; Brüne, B.; Kietzmann, T. ER-stress promotes VHL-independent degradation of hypoxia-inducible factors via FBXW1A/βTrCP. Redox Biol. 2022, 50, 102243. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Darnay, B.G.; Powis, G. Hypoxia-Associated Factor, a Novel E3-Ubiquitin Ligase, Binds and Ubiquitinates Hypoxia-Inducible Factor 1α, Leading to Its Oxygen-Independent Degradation. Mol. Cell. Biol. 2008, 28, 7081–7095. [Google Scholar] [CrossRef]
- Montagner, M.; Enzo, E.; Forcato, M.; Zanconato, F.; Parenti, A.; Rampazzo, E.; Basso, G.; Leo, G.; Rosato, A.; Bicciato, S.; et al. SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature 2012, 487, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Ni, A. Histone deacetylase inhibitor induced pVHL-independent degradation of HIF-1α and hierarchical quality control of pVHL via chaperone system. PLoS ONE 2021, 16, e0248019. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Zhao, Y.; Yue, X.; Wu, H.; Huang, S.; Chen, J.; Tomsky, K.; Xie, H.; Khella, C.A.; et al. Parkin targets HIF-1α for ubiquitination and degradation to inhibit breast tumor progression. Nat. Commun. 2017, 8, 1823. [Google Scholar] [CrossRef] [PubMed]
- Harnanik, T.; Soeroso, J.; Suryokusumo, M.G.; Juliandhy, T. Effects of hyperbaric oxygen on t helper 17/regulatory t polarization in antigen and collagen-induced arthritis: Hypoxia-inducible factor-1α as a target. Oman Med. J. 2020, 35, e90. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Duan, J.; Liu, G.; Tan, B.; Yang, G.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. mTORC1 signaling and IL-17 expression: Defining pathways and possible therapeutic targets. Eur. J. Immunol. 2016, 46, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, K.S.; Park, H.S.; Park, S.J.; Min, K.H.; Moon, H.; Puri, K.D.; Lee, Y.C. HIF-1α inhibition ameliorates an allergic airway disease via VEGF suppression in bronchial epithelium. Eur. J. Immunol. 2010, 40, 2858–2869. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, L.; Wang, S.; Liang, G. Dexmedetomidine hydrochloride up-regulates expression of hypoxia inducible factor-1α to alleviate renal ischemiareperfusion injury in diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 944–949. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Gołas, A.; Krzeptowski, W.; Kajta, M. Neuroprotective action of raloxifene against hypoxia-induced damage in mouse hippocampal cells depends on ERα but not ERβ or GPR30 signalling. J. Steroid Biochem. Mol. Biol. 2015, 146, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, L.; Liu, D.; Wang, J.; Wang, S.; Zhang, Q.; Gong, Y.; Liu, H.; Hao, A.; Wang, Z. Hydrogen sulfide attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Pharmacol. Res. 2014, 84, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hafeez, A.; Noorulla, F.; Geng, X.; Shao, G.; Ren, C.; Lu, G.; Zhao, H.; Ding, Y.; Ji, X. Preconditioning in neuroprotection: From hypoxia to ischemia. Prog. Neurobiol. 2017, 157, 79–91. [Google Scholar] [CrossRef]
- Benderro, G.F.; LaManna, J.C. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011, 1389, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.S.; Metang, P.; Egge, N.; Liu, Y.; Zuurbier, K.R.; Sivaprakasam, K.; Shirazi, S.; Chuah, A.; Arneaud, S.L.; Konopka, G.; et al. Glycolytic preconditioning in astrocytes mitigates trauma-induced neurodegeneration. eLife 2021, 10, e69438. [Google Scholar] [CrossRef] [PubMed]
- Traxler, L.; Herdy, J.R.; Stefanoni, D.; Eichhorner, S.; Pelucchi, S.; Szücs, A.; Santagostino, A.; Kim, Y.; Agarwal, R.K.; Schlachetzki, J.C.; et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 2022, 34, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.P.; Stys, P.K. Mechanisms of axonal injury: Internodal nanocomplexes and calcium deregulation. Trends Mol. Med. 2010, 16, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Correia, S.; dos Santos, R.X.C.; Cardoso, S.; Moreira, P.; Clark, T.A.; Zhu, X.; Smith, M.A.; Perry, G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J. Bioenerg. Biomembr. 2009, 41, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, B.; Jensen, A.; Roth-Härer, A.; Dermietzel, R.; Meier, C. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012, 1487, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Pu, H.; Hu, X.; Wei, Z.; Hong, D.; Zhang, W.; Gao, Y.; Chen, J.; Shi, Y. A Post-stroke Therapeutic Regimen with Omega-3 Polyunsaturated Fatty Acids that Promotes White Matter Integrity and Beneficial Microglial Responses after Cerebral Ischemia. Transl. Stroke Res. 2016, 7, 548–561. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Gunzer, M.; Gutzeit, H.O.; Ullrich, O.; Reymann, K.G.; Dinkel, K. Microglia provide neuroprotection after ischemia. FASEB J. 2006, 20, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.; Teschemacher, A.G.; Kasparov, S.; Gourine, A.V. Glia, sympathetic activity and cardiovascular disease. Exp. Physiol. 2016, 101, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.C.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R.S. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Chun, Y.; Kim, J. Ampk–mtor signaling and cellular adaptations in hypoxia. Int. J. Mol. Sci. 2021, 22, 9765. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Schenk, G.J.; de Vries, H.E. Altered blood–brain barrier transport in neuro-inflammatory disorders. Drug Discov. Today Technol. 2016, 20, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Candia, A.; Rogers, N.K.; Castillo, R.L. Blood-Brain Barrier Dysfunction in the Detrimental Brain Function. In Connectivity and Functional Specialization in the Brain; IntechOpen Limited: London, UK, 2021. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wei, T.; Shen, Z.; Bei, Y.; Lin, H.; Dai, H. Classical HDACs in the regulation of neuroinflammation. Neurochem. Int. 2021, 150, 105182. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain–Immune Interactions and Neuroinflammation after Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Adzemovic, M.Z.; Zeitelhofer, M.; Eriksson, U.; Olsson, T.; Nilsson, I. Imatinib Ameliorates Neuroinflammation in a Rat Model of Multiple Sclerosis by Enhancing Blood-Brain Barrier Integrity and by Modulating the Peripheral Immune Response. PLoS ONE 2013, 8, e56586. [Google Scholar] [CrossRef]
- Wojkowska, D.; Szpakowski, P.; Ksiazek-Winiarek, D.; Leszczynski, M.; Glabinski, A. Interactions between neutrophils, Th17 Cells, and chemokines during the initiation of experimental model of multiple sclerosis. Mediat. Inflamm. 2014, 2014, 590409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, X.-Y.; Zhu, J. Th1/Th2/Th17/Treg cytokines in Guillain-Barré syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev. 2013, 24, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Krauthausen, M.; Hofer, M.J.; Heneka, M.T.; Campbell, I.L.; Müller, M. CNS-Targeted Production of IL-17A Induces Glial Activation, Microvascular Pathology and Enhances the Neuroinflammatory Response to Systemic Endotoxemia. PLoS ONE 2013, 8, e57307. [Google Scholar] [CrossRef]
- Luo, B.; Jiang, M.; Yang, X.; Zhang, Z.; Xiong, J.; Schluesener, H.J.; Zhang, Z.; Wu, Y. Erythropoietin is a hypoxia inducible factor-induced protective molecule in experimental autoimmune neuritis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1260–1270. [Google Scholar] [CrossRef]
- Watts, E.R.; Walmsley, S.R. Inflammation and Hypoxia: HIF and PHD Isoform Selectivity. Trends Mol. Med. 2019, 25, 33–46. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, P.; Schwab, J.; Mirakaj, V.; Masekowsky, E.; Mager, A.; Morote-Garcia, J.C.; Unertl, K.E.; Eltzschig, H.K. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009, 10, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.S.; Keith, B.; Simon, M.C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 2016, 16, 663–673. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; White, E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019, 33, 610–619. [Google Scholar] [CrossRef]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010, 41, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.C.; Pennypacker, K.R. Neuroinflammation and MMPs: Potential therapeutic targets in neonatal hypoxic-ischemic injury. J. Neuroinflammation 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, C.; Tüzün, E.; Kürtüncü, M.; Türkoğlu, R.; Akman-Demir, G.; Eraksoy, M. Comparison of the cytokine profiles of patients with neuronal-antibody- associated central nervous system disorders. Int. J. Neurosci. 2012, 122, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 2013, 208093. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R.; Suarez-Fueyo, A.; Meidan, E.; Li, H.; Mizui, M.; Tsokos, G.C. Pathogenesis of Human Systemic Lupus Erythematosus: A Cellular Perspective. Trends Mol. Med. 2017, 23, 615–635. [Google Scholar] [CrossRef]
- Sundrud, M.S.; Trivigno, C. Identity crisis of Th17 cells: Many forms, many functions, many questions. Semin. Immunol. 2013, 25, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Whitson, H.E.; Colton, C.; El Khoury, J.; Gate, D.; Goate, A.; Heneka, M.T.; Kaddurah-Daouk, R.; Klein, R.S.; Shinohara, M.L.; Sisodia, S.; et al. Infection and inflammation: New perspectives on Alzheimer’s disease. Brain Behav. Immun. Health 2022, 22, 100462. [Google Scholar] [CrossRef] [PubMed]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; De Los Santos, M.B.; et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zheng, H. Peripheral immune system in aging and Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, E.; Kwon, S.-H.; Yu, M.-Y.; Cha, R.-H.; Lee, H.; Kim, D.K.; Lee, J.P.; Ye, S.-K.; Yoo, J.-Y.; et al. Transcriptional modulation of the T helper 17/interleukin 17 axis ameliorates renal ischemia-reperfusion injury. Nephrol. Dial. Transplant. 2019, 34, 1481–1498. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Desai, R.A.; Bloomfield, P.S.; McIntosh, P.R.; Chapple, K.J.; Linington, C.; Fairless, R.; Diem, R.; Kasti, M.; Murphy, M.P.; et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 2013, 74, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kito, H.; Tanaka, R.; Kajikuri, J.; Tanaka, S.; Elboray, E.E.; Suzuki, T.; Ohya, S. Possible contribution of inflammation-associated hypoxia to increased k2p5.1 k+ channel expression in CD4+ t cells of the mouse model for inflammatory bowel disease. Int. J. Mol. Sci. 2020, 21, 38. [Google Scholar] [CrossRef]
- Wan, C.; Bi, W.; Lin, P.; Zhang, Y.; Tian, J.; Fang, S.; Li, Z.; Wang, X.; Qu, Y.; Mu, L.; et al. MicroRNA 182 promotes T helper 1 cell by repressing hypoxia induced factor 1 alpha in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2019, 49, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Shobatake, R.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; Takasawa, S. The Impact of Intermittent Hypoxia on Metabolism and Cognition. Int. J. Mol. Sci. 2022, 23, 12957. [Google Scholar] [CrossRef]
- Farias, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological models and approaches for pathophysiological conditions associated with hypoxia and oxidative stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, S.; Zhao, D.; Zeng, Z.; Mo, H.; Hu, K. Orexin A improves the cognitive impairment induced by chronic intermittent hypoxia in mice. Brain Res. Bull. 2021, 173, 203–210. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B.; et al. Intermittent hypoxia-hyperoxia training improves cognitive function and decreases circulating biomarkers of Alzheimer’s disease in patients with mild cognitive impairment: A pilot study. Int. J. Mol. Sci. 2019, 20, 5405. [Google Scholar] [CrossRef]
- Guglani, L.; A Khader, S. Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS 2010, 5, 120–127. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Sommer, A.; Marxreiter, F.; Krach, F.; Fadler, T.; Grosch, J.; Maroni, M.; Graef, D.; Eberhardt, E.; Riemenschneider, M.J.; Yeo, G.W.; et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell 2018, 23, 123–131. [Google Scholar] [CrossRef]
- Yao, K.; Graham, J.; Akahata, Y.; Oh, U.; Jacobson, S. Mechanism of neuroinflammation: Enhanced cytotoxicity and IL-17 production via CD46 binding. J. Neuroimmune Pharmacol. 2010, 5, 469–478. [Google Scholar] [CrossRef]
- Kim, C.F.; Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 2011, 12, 370–383. [Google Scholar] [CrossRef]
- Liu, Y.; Holdbrooks, A.T.; De Sarno, P.; Rowse, A.L.; Yanagisawa, L.L.; McFarland, B.C.; Harrington, L.E.; Raman, C.; Sabbaj, S.; Benveniste, E.N.; et al. Therapeutic Efficacy of Suppressing the JAK/STAT Pathway in Multiple Models of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2014, 192, 59–72. [Google Scholar] [CrossRef]
- Shu, L.; Xu, C.-Q.; Yan, Z.-Y.; Yan, Y.; Jiang, S.-Z.; Wang, Y.-R. Post-Stroke Microglia Induce Sirtuin2 Expression to Suppress the Anti-inflammatory Function of Infiltrating Regulatory T Cells. Inflammation 2019, 42, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients with Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef] [PubMed]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, V.; Anrather, J.; Orzi, F.; Iadecola, C. Th17 and Cognitive Impairment: Possible Mechanisms of Action. Front. Neuroanat. 2019, 13, 95. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Moon, C.; Kim, S. Erythropoietin and autoimmune neuroinflammation: Lessons from experimental autoimmune encephalomyelitis and experimental autoimmune neuritis. Anat. Cell Biol. 2012, 45, 215–220. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.-Y.; Ma, C.; Wu, J.; Adem, A.; Zhu, J.; Zhang, H.-L. Mitigated tregs and augmented Th17 cells and cytokines are associated with severity of experimental autoimmune neuritis. Scand. J. Immunol. 2014, 80, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.M.; Shriver, L.P.; Bodiga, V.L.; Ray, A.; Basu, S.; Ahuja, R.; Jana, A.; Pahan, K.; Dittel, B.N. Lymphocytes with cytotoxic activity induce rapid microtubule axonal destabilization independently and before signs of neuronal death. ASN Neuro 2013, 5, AN20120087. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009, 11, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Cavani, A.; Schmidt-Weber, C.B. IL-17 and IL-22: Siblings, not twins. Trends Immunol. 2010, 31, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gomez, S.; Binder, J.; Heneka, M.T. Neuroinflammation as motor of Alzheimer’s disease. Nervenarzt 2019, 90, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R. Molecular basis of etiological implications in alzheimer’s disease: Focus on neuroinflammation. Mol. Neurobiol. 2013, 48, 412–428. [Google Scholar] [CrossRef]
- Sun, L.; Ju, T.; Wang, T.; Zhang, L.; Ding, F.; Zhang, Y.; An, R.; Sun, Y.; Li, Y.; Lu, Y.; et al. Decreased netrin-1 and correlated Th17/Tregs balance disorder in Aβ1-42 induced Alzheimer’s disease model rats. Front. Aging Neurosci. 2019, 11, 124. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, K.-F.; Liu, Z.; Qiu, Y.-H.; Peng, Y.-P. Th17 Cell-Mediated Neuroinflammation Is Involved in Neurodegeneration of Aβ1-42-Induced Alzheimer’s Disease Model Rats. PLoS ONE 2013, 8, e75786. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 943–972. [Google Scholar] [CrossRef]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Guan, S.; Yan, H.; Huang, X.; Liang, M.; Zhang, R.; Luo, T. Cyclin-dependent kinase 5 inhibitor attenuates lipopolysaccharide-induced neuroinflammation through metabolic reprogramming. Eur. J. Pharmacol. 2022, 929, 175118. [Google Scholar] [CrossRef]
- Kong, L.; Ma, Y.; Wang, Z.; Liu, N.; Ma, G.; Liu, C.; Shi, R.; Du, G. Inhibition of hypoxia inducible factor 1 by YC-1 attenuates tissue plasminogen activator induced hemorrhagic transformation by suppressing HMGB1/TLR4/NF-κB mediated neutrophil infiltration in thromboembolic stroke rats. Int. Immunopharmacol. 2021, 94, 107507. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calle, R.; Vicente-Rodríguez, M.; Gramage, E.; Pita, J.; Pérez-García, C.; Ferrer-Alcón, M.; Uribarri, M.; Ramos, M.P.; Herradón, G. Pleiotrophin regulates microglia-mediated neuroinflammation. J. Neuroinflammation 2017, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Yilmaz, Ö.H. Molecular pathways: Dietary regulation of stemness and tumor initiation by the PPAR-d pathway. Clin. Cancer Res. 2016, 22, 5636–5641. [Google Scholar] [CrossRef]
- Strosznajder, A.K.; Wójtowicz, S.; Jeżyna, M.J.; Sun, G.Y. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. NeuroMolecular Med. 2021, 23, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Brigas, H.C.; Ribeiro, M.; Coelho, J.E.; Gomes, R.; Gomez-Murcia, V.; Carvalho, K.; Faivre, E.; Costa-Pereira, S.; Darrigues, J.; de Almeida, A.A.; et al. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 2021, 36, 257–270. [Google Scholar] [CrossRef]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
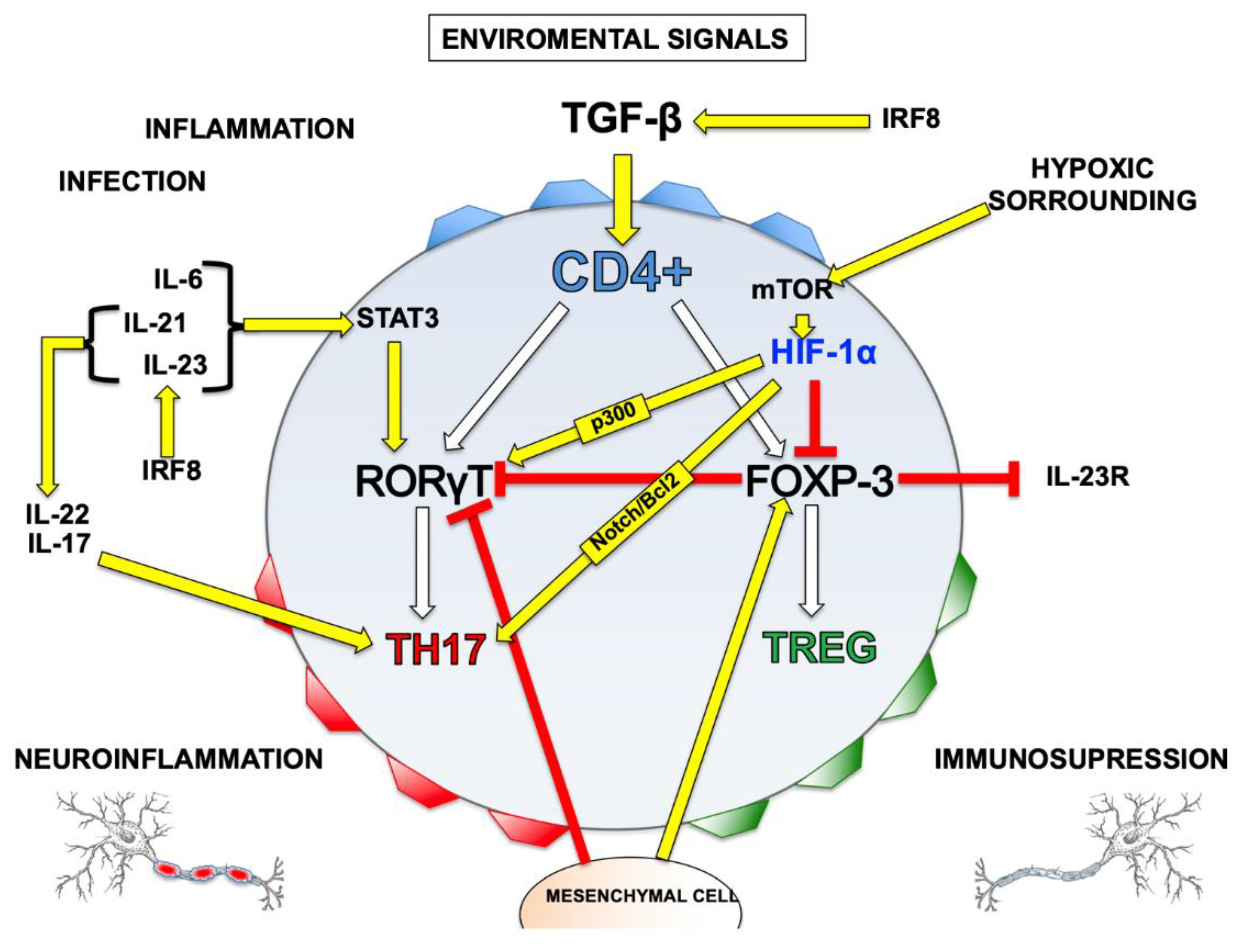
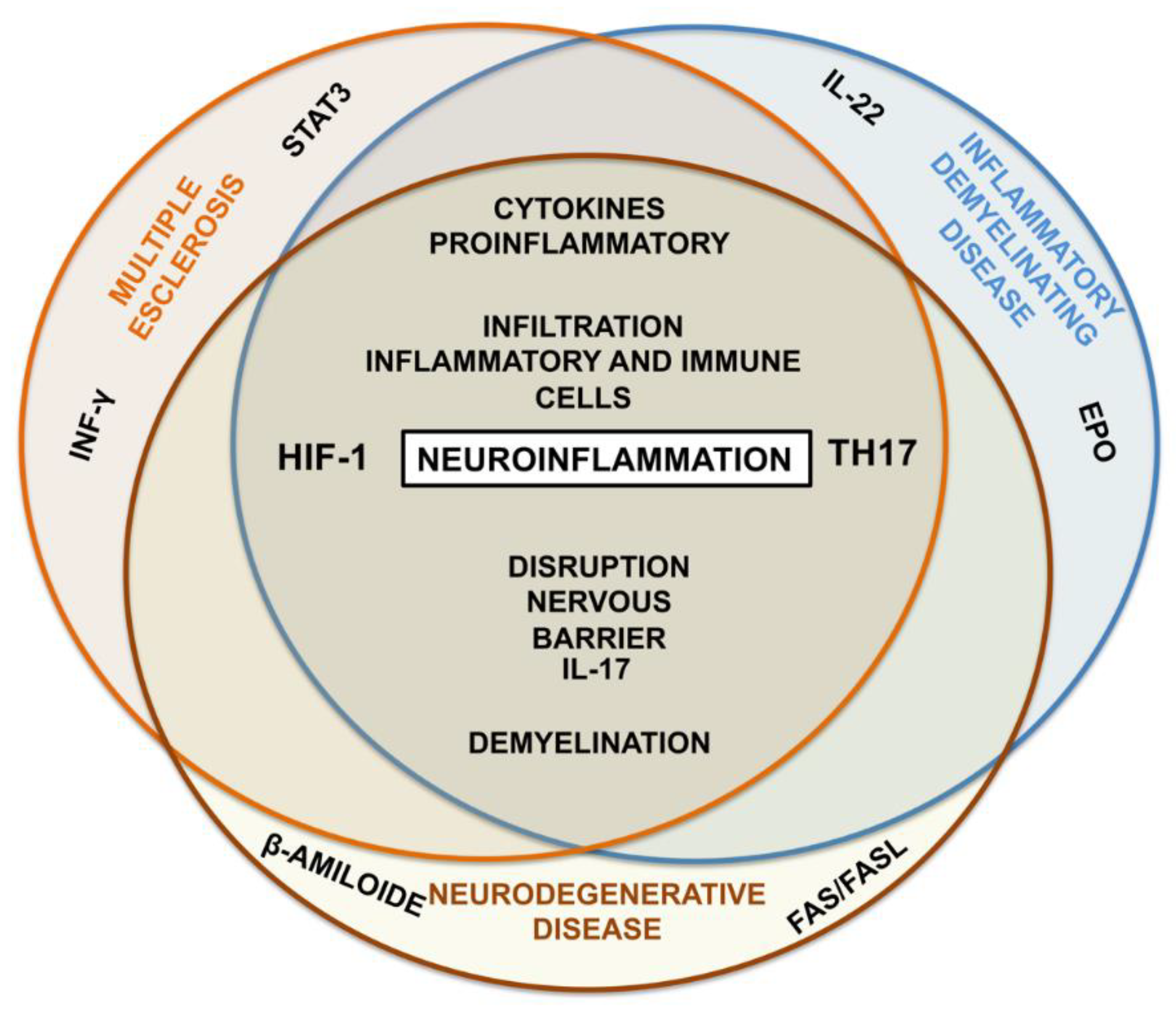
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, C.; Sepúlveda, P.; Castillo, R.L.; Salazar, L.A. Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells. Int. J. Mol. Sci. 2023, 24, 3073. https://doi.org/10.3390/ijms24043073
Arias C, Sepúlveda P, Castillo RL, Salazar LA. Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells. International Journal of Molecular Sciences. 2023; 24(4):3073. https://doi.org/10.3390/ijms24043073
Chicago/Turabian StyleArias, Consuelo, Paulina Sepúlveda, Rodrigo L. Castillo, and Luis A. Salazar. 2023. "Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells" International Journal of Molecular Sciences 24, no. 4: 3073. https://doi.org/10.3390/ijms24043073
APA StyleArias, C., Sepúlveda, P., Castillo, R. L., & Salazar, L. A. (2023). Relationship between Hypoxic and Immune Pathways Activation in the Progression of Neuroinflammation: Role of HIF-1α and Th17 Cells. International Journal of Molecular Sciences, 24(4), 3073. https://doi.org/10.3390/ijms24043073






