Epstein-Barr Virus and Multiple Sclerosis: A Convoluted Interaction and the Opportunity to Unravel Predictive Biomarkers
Abstract
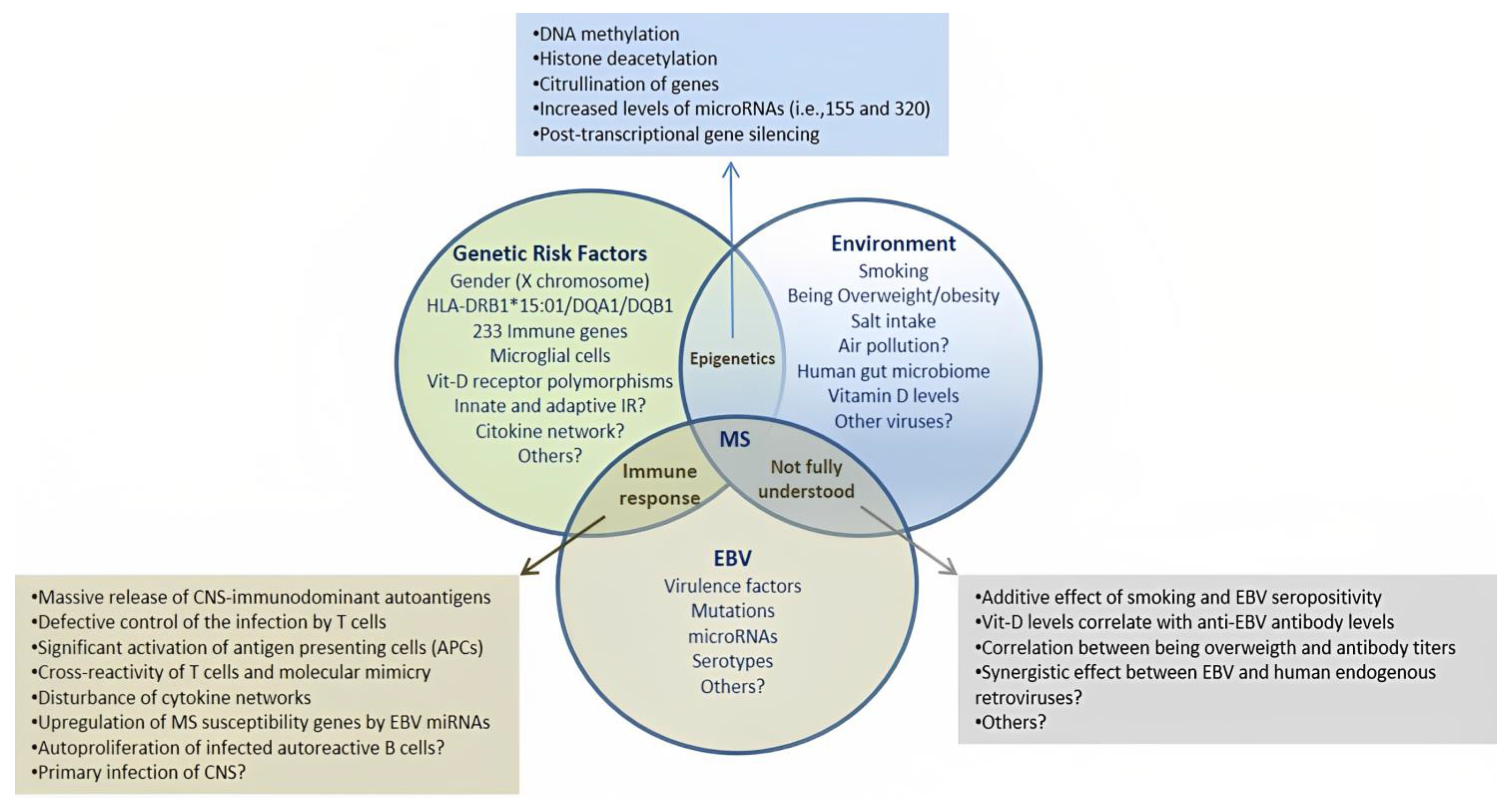
1. Introduction
2. Overview of the Pathophysiology of EBV Infection
The Infectious Cycle of EBV
3. EBV and the Risk for MS: Evidence from Genomic Studies
3.1. Regulation of MS Susceptibility Genes by EBV-Transcriptional Factors
3.2. Genomic Control of EBNA2 in Other Chronic Inflammatory Diseases
3.3. EBV-microRNAs in the Pathogenesis of MS and Cancer
4. EBV Decides the Fate of Infected B Cells and Likely of Autoreactive B Cells
4.1. The Role of Other EBV-Encoding Products in the Pathogenesis of MS
4.2. EBV and Autoreactive B Cells in MS
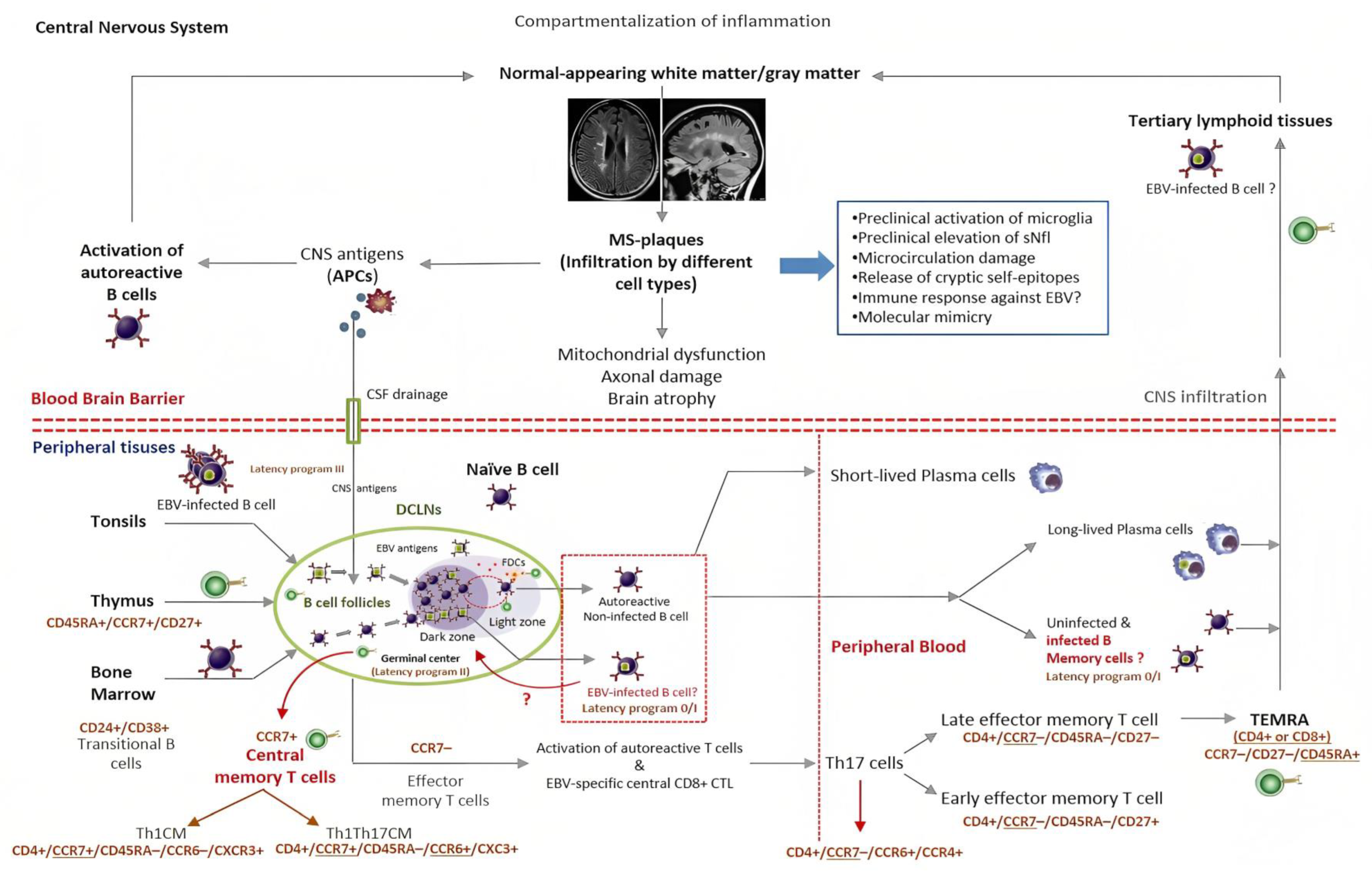
5. Where Does EBV Actually Reside in Patients with MS?
6. EBV Infection and the Chance of Discovering Predictive Biomarkers for MS
6.1. EBV and Neurodegeneration in MS
6.2. EBV Vaccination and Anti-CD20 Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIM | Acute infectious mononucleosis. |
| APCs | Antigen-presenting cells. |
| BART microRNAs | Short non-coding single-stranded RNA molecules implicated in the post-transcriptional regulation of genes via either translation repression or RNA degradation. EBV encodes 25 microRNAs precursors, which contain 49 mature microRNAs. EBV-microRNAs are all overexpressed during latency. |
| BCR | B cell receptor. |
| BL | Burkitt’s lymphoma. |
| CD21 | Complement receptor 2 transmembrane protein expressed on B cells and serving as the receptor for EBV entry. |
| CD40L | A protein transiently expressed by T lymphocytes during the immunological synapse with APCs (engagement with CD40 receptors), that up-regulates the initiation and further development of cellular and humoral adaptive immune response. |
| CNS | Central Nervous System. |
| CTL | Cytotoxic T lymphocytes. |
| DCLNs | Deep cervical lymph nodes. |
| DLBCL | Diffuse large B-cell lymphoma. |
| DMTs | Disease-modifying therapies. |
| EBERs | Non-coding RNAs expressed abundantly in latently EBV-infected cells. They are responsible for malignant phenotypes of BL cells including resistance to apoptosis. |
| EBNA1 | EBNA1 attaches viral episomes to the host-DNA, easing the replication of the viral-genome. EBNA1 suppresses HLA-I restricted antigen presentation. It also works as a transcriptional activator of host and viral genes. |
| EBNA2 | One of the first proteins expressed upon acute infection of B cells. EBNA2 likely interacts with enhancer elements in the host cell and EBV-latent genes, blocking cellular differentiation and driving uncontrolled proliferation. |
| EBNA3A and 3C | These proteins can displace EBNA2 from the Cp promoter and recruit repressor proteins that lead to epigenetic silencing of Cp and the suppression of EBNA2 transcription. Cessation of EBNA2 synthesis can cause growth arrest and allow infected B cells to assume a germinal center-phenotype and express the default program (II). |
| EBNALP | EBNALP is thought to contribute to the immortalization of infected-B cells by enhancing EBNA2-mediated transcriptional activation of the LMP1 gene. |
| Episome | Circular protective shape adopted by the genome of herpes viruses that can be present inside the host cell’s nucleus during the latent phase of infection closely associated with the DNA but which does not integrate into the host DNA. |
| GC | The germinal centers are immune organs located in the secondary lymphoid organs. These structures are anatomically specialized in supporting the proliferation and maturation of B cells. |
| gp350 | EBV-encoded glycoprotein expressed on the virion’s envelope, carrying the virus attachment moiety for the CD21 receptor in B lymphocytes. |
| gp42 | EBV glycoprotein that belongs to the C-type lectin superfamily with homology to the natural killer cell’s receptors and which binds HLA-II molecules expressed on B cells. |
| HL | Hodgkin lymphoma. |
| HLA-II | Major histocompatibility complex class II. These molecules are normally expressed only by professional antigen-presenting cells and activated T cells. |
| LMP1 and -2 | Viral ligand-independent BCR signal membrane proteins expressed on the membrane of EBV-infected B cells in the default program (II). They mimic two essential signals (T helper cell and BCR) required for rescuing the GC cells in the memory pool. |
| NFC | Nasopharyngeal carcinoma. |
| SHM | Somatic hypermutation. |
| sNFL | Serum neurofilament light chain protein. |
| TRAF-3 | Tumor necrosis factor receptor-associated factor 3. An intracellular protein involved in the signal transduction of CD40. TRAF-3 induces the activation of NF-kappa β transcription factor, which eventually suppresses apoptosis and triggers the proliferation of immune cells. |
References
- Thacker, E.L.; Mirzaei, F.; Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: A meta-analysis. Ann. Neurol. 2006, 59, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hiyoshi, A.; Smith, K.A.; Piehl, F.; Olsson, T.; Fall, K.; Montgomery, S. Association of Infectious Mononucleosis in Childhood and Adolescence With Risk for a Subsequent Multiple Sclerosis Diagnosis Among Siblings. JAMA Netw. Open 2021, 4, e2124932. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Anti-Epstein-Barr virus antibodies as serological markers of multiple sclerosis: A prospective study among United States military personnel. Mult. Scler. 2011, 17, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.I. Temporal Relationship Between Elevation of Epstein-Barr Virus Antibody Titers and Initial Onset of Neurological Symptoms in Multiple Sclerosis. JAMA 2005, 293, 2496. [Google Scholar] [CrossRef]
- Almohmeed, Y.H.; Avenell, A.; Aucott, L.; Vickers, M.A. Systematic Review and Meta-Analysis of the Sero-Epidemiological Association between Epstein Barr Virus and Multiple Sclerosis. PLoS ONE 2013, 8, e61110. [Google Scholar] [CrossRef]
- Hedström, A.K.; Huang, J.; Michel, A.; Butt, J.; Brenner, N.; Hillert, J.; Waterboer, T.; Kockum, I.; Olsson, T.; Alfredsson, L. High Levels of Epstein–Barr Virus Nuclear Antigen-1-Specific Antibodies and Infectious Mononucleosis Act Both Independently and Synergistically to Increase Multiple Sclerosis Risk. Front. Neurol. 2020, 10, 1368. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Khan, G.; Fitzmaurice, C.; Naghavi, M.; Ahmed, L.A. Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990–2017. BMJ 2020, 10, e037505. [Google Scholar] [CrossRef]
- Handel, A.E.; Williamson, A.J.; Disanto, G.; Handunnetthi, L.; Giovannoni, G.; Ramagopalan, S.V. An Updated Meta-Analysis of Risk of Multiple Sclerosis following Infectious Mononucleosis. PLoS ONE 2010, 5, e12496. [Google Scholar] [CrossRef]
- Jacobs, B.M.; Giovannoni, G.; Cuzick, J.; Dobson, R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Mult. Scler. 2020, 26, 1281–1297. [Google Scholar] [CrossRef]
- Rolf, L.; Muris, A.H.; Mathias, A.; Du Pasquier, R.; Koneczny, I.; Disanto, G.; Kuhle, J.; Ramagopalan, S.; Damoiseaux, J.; Smolders, J.; et al. Exploring the effect of vitamin D3 supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult. Scler. 2018, 24, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Brenner, N.; Butt, J.; Hillert, J.; Waterboer, T.; Olsson, T.; Alfredsson, L. Overweight/obesity in young adulthood interacts with aspects of EBV infection in MS etiology. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, e912. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L.; Lennette, E.T.; Spiegelman, D.; Hernán, M.A.; Olek, M.J.; Hankinson, S.E.; Hunter, D.J. Epstein-Barr Virus Antibodies and Risk of Multiple Sclerosis. JAMA 2001, 286, 3083. [Google Scholar] [CrossRef] [PubMed]
- Sumaya, C.V.; Myers, L.W.; Ellison, G.W.; Ench, Y. Increased prevalence and titer of Epstein-Barr virus antibodies in patients with multiple sclerosis. Ann. Neurol. 1985, 17, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.D.; Bloomer, L.C.; Bray, P.F. Epstein-Barr nuclear antigen and viral capsid antigen antibody titers in multiple sclerosis. Neurology 1985, 35, 435–438. [Google Scholar] [CrossRef]
- Guan, Y.; Jakimovski, D.; Ramanathan, M.; Weinstock-Guttman, B.; Zivadinov, R. The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural. Regen. Res. 2019, 14, 373. [Google Scholar]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [Google Scholar] [CrossRef]
- Willis, S.N.; Stadelmann, C.; Rodig, S.J.; Caron, T.; Gattenloehner, S.; Mallozzi, S.S.; Roughan, J.E.; Almendinger, S.E.; Blewett, M.M.; Brück, W.; et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 2009, 132, 3318–3328. [Google Scholar] [CrossRef]
- Sargsyan, S.A.; Shearer, A.J.; Ritchie, A.M.; Burgoon, M.P.; Anderson, S.; Hemmer, B.; Stadelmann, C.; Gattenlohner, S.; Owens, G.P.; Gilden, D.; et al. Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 2010, 74, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Niedobitek, G.; Aloisi, F.; Middeldorp, J.M. Epstein-Barr virus in the multiple sclerosis brain: A controversial issue--report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 2011, 134, 2772–2786. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Or-Geva, N.; Aftab, B.T.; Khanna, R.; Croze, E.; Steinman, L.; Han, M.H. Molecular signature of Epstein-Barr virus infection in MS brain lesions. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e466. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Veroni, C.; Mazzola, G.A.; Aloisi, F. Epstein-Barr Virus-Specific CD8 T Cells Selectively Infiltrate the Brain in Multiple Sclerosis and Interact Locally with Virus-Infected Cells: Clue for a Virus-Driven Immunopathological Mechanism. J. Virol. 2019, 93, e00980-19. [Google Scholar] [CrossRef]
- Wandinger, K.P.; Jabs, W.; Siekhaus, A.; Bubel, S.; Trillenberg, P.; Wagner, H.J.; Wessel, K.; Kirchner, H.; Hennig, H. Association between clinical disease activity and Epstein–Barr virus reactivation in MS. Neurology 2000, 55, 178–184. [Google Scholar] [CrossRef]
- Torkildsen, Ø.; Nyland, H.; Myrmel, H.; Myhr, K.M. Epstein–Barr virus reactivation and multiple sclerosis. Eur. J. Neurol. 2008, 15, 106–108. [Google Scholar] [CrossRef]
- Farrell, R.A.; Antony, D.; Wall, G.R.; Clark, D.A.; Fisniku, L.; Swanton, J.; Khaleeli, Z.; Schmierer, K.; Miller, D.H.; Giovannoni, G. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 2009, 73, 32–38. [Google Scholar] [CrossRef]
- Ingram, G.; Bugert, J.J.; Loveless, S.; Robertson, N.P. Anti-EBNA-1 IgG is not a reliable marker of multiple sclerosis clinical disease activity. Eur. J. Neurol. 2010, 17, 1386–1389. [Google Scholar] [CrossRef]
- Veroni, C.; Serafini, B.; Rosicarelli, B.; Fagnani, C.; Aloisi, F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflamm. 2018, 15, 18. [Google Scholar] [CrossRef]
- Ramesh, A.; Schubert, R.D.; Greenfield, A.L.; Dandekar, R.; Loudermilk, R.; Sabatino, J.J.; Koelzer, M.T.; Tran, E.B.; Koshal, K.; Kim, K.; et al. A pathogenic and clonally expanded B cell transcriptome in active multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 22932–22943. [Google Scholar] [CrossRef]
- Tyler, K.L. The enigmatic links between Epstein-Barr virus infection and multiple sclerosis. J. Clin. Investig. 2022, 132, e160468. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Zabriskie, J.B.; Plank, C.; Ablashi, D.; Whitman, J.; Shahan, B.; Edgell, R.; Shieh, M.; Rapalino, O.; Zimmerman, R.; et al. A randomized clinical trial of valacyclovir in multiple sclerosis. Mult. Scler. 2005, 11, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.; Marta, M.; Meier, U.C.; Christensen, T.; Miller, D.; Altmann, D.; Holden, D.; Bianchi, L.; Adiutori, R.; MacManus, D.; et al. A phase II baseline versus treatment study to determine the efficacy of raltegravir (Isentress) in preventing progression of relapsing remitting multiple sclerosis as determined by gadolinium-enhanced MRI: The INSPIRE study. Mult. Scler. Relat. Disord. 2018, 24, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Csurhes, P.A.; Smith, C.; Douglas, N.L.; Neller, M.A.; Matthews, K.K.; Beagley, L.; Rehan, S.; Crooks, P.; Hopkins, T.J.; et al. Epstein-Barr virus–specific T cell therapy for progressive multiple sclerosis. JCI. Insight 2018, 3, e12471. [Google Scholar] [CrossRef]
- Nantes University Hospital. Trial to Assess the Safety and Feasibility of Adoptive Cell Therapy with Autologous EBV-Specific Cytotoxic T Lymphocytes (CTL) in Patients with a First Clinical Episode Highly Suggestive of Multiple Sclerosis (MS and EBV-CTL). In ClinicalTrials.gov; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT02912897 (accessed on 14 January 2023).
- Queen Mary University of London. A Phase 2 Open Label Clinical Trial to Determine the Effect of Famciclovir on Epstein-Barr Virus Activity as Measured by EBV Shedding in Saliva of Patients with Multiple Sclerosis. In ClinicalTrials.gov; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05283551 (accessed on 14 January 2023).
- Thorley-Lawson, D.A. EBV Persistence—Introducing the Virus. Curr. Top. Microbiol. Immunol. 2015, 390, 151–209. [Google Scholar]
- Stanfield, B.A.; Luftig, M.A. Recent advances in understanding Epstein-Barr virus. F1000Research 2017, 6, 386. [Google Scholar] [CrossRef]
- Tengvall, K.; Huang, J.; Hellström, C.; Kammer, P.; Biström, M.; Ayoglu, B.; Lima Bomfim, I.; Stridh, P.; Butt, J.; Brenner, N.; et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. USA 2019, 116, 16955–16960. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein-Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front. Oncol. 2018, 8, 211. [Google Scholar] [CrossRef]
- Münz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700. [Google Scholar] [CrossRef]
- Hammerschmidt, W.; Sugden, B. Replication of Epstein-Barr Viral DNA. Cold. Spring. Harb. Perspect. Biol. 2013, 5, a013029. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Noda, T.; Ohba, Y. Epstein–Barr Virus Acquires Its Final Envelope on Intracellular Compartments With Golgi Markers. Front. Microbiol. 2018, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Croft, N.P.; Shannon-Lowe, C.; Bell, A.I.; Horst, D.; Kremmer, E.; Ressing, M.E.; Wiertz, E.J.H.J.; Middeldorp, J.; Rowe, M.; Rickinson, A.B.; et al. Stage-Specific Inhibition of MHC Class I Presentation by the Epstein-Barr Virus BNLF2a Protein during Virus Lytic Cycle. PLoS Pathog. 2009, 5, e1000490. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kieff, E. Epstein–Barr virus latent genes. Exp. Mol. Med. 2015, 47, e131. [Google Scholar] [CrossRef]
- Yin, H.; Qu, J.; Peng, Q.; Gan, R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med. Microbiol. Immunol. 2019, 208, 573–583. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Parnell, G.P.; Fewings, N.; Schibeci, S.D.; Basuki, M.A.; Chandramohan, R.; Zhou, Y.; Taylor, B.; Brown, D.A.; Swaminathan, S.; et al. Evidence from genome wide association studies implicates reduced control of Epstein-Barr virus infection in multiple sclerosis susceptibility. Genome Med. 2019, 11, 26. [Google Scholar] [CrossRef]
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef]
- Holden, D.W.; Gold, J.; Hawkes, C.H.; Giovannoni, G.; Saxton, J.M.; Carter, A.; Sharrack, B. Epstein Barr virus shedding in multiple sclerosis: Similar frequencies of EBV in saliva across separate patient cohorts. Mult. Scler. Relat. Disord. 2018, 25, 197–199. [Google Scholar] [CrossRef]
- Singavi, A.K.; Harrington, A.M.; Fenske, T.S. Post-transplant lymphoproliferative disorders. Cancer. Treat. Res. 2015, 165, 305–327. [Google Scholar]
- Germini, D.; Sall, F.B.; Shmakova, A.; Wiels, J.; Dokudovskaya, S.; Drouet, E.; Vassetzky, Y. Oncogenic Properties of the EBV ZEBRA Protein. Cancers 2020, 12, 1479. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Parnell, G.P.; Swaminathan, S.; Stewart, G.J.; Booth, D.R. The interaction of Multiple Sclerosis risk loci with Epstein-Barr virus phenotypes implicates the virus in pathogenesis. Sci. Rep. 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Parameswaran, S.; Donmez, O.A.; Miller, D.; Forney, C.; Lape, M.; Ribeiro, M.S.J.; Liang, J.; Edsall, L.E.; Magnusen, A.F.; et al. Epstein-Barr virus nuclear antigen 2 extensively rewires the human chromatin landscape at autoimmune risk loci. Genome Res. 2021, 31, 2185–2198. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.T.; Afrasiabi, A.; Schibeci, S.D.; Swaminathan, S.; Parnell, G.P.; Booth, D.R. The interaction of Epstein-Barr virus encoded transcription factor EBNA2 with multiple sclerosis risk loci is dependent on the risk genotype. EBioMedicine 2021, 71, 103572. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef] [PubMed]
- Kempkes, B.; Ling, P.D. EBNA2 and Its Coactivator EBNA-LP. Curr. Top. Microbiol. Immunol. 2015, 391, 35–59. [Google Scholar]
- Harley, J.B.; Chen, X.; Pujato, M.; Miller, D.; Maddox, A.; Forney, C.; Magnusen, A.F.; Lynch, A.; Chetal, K.; Yukawa, M.; et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat. Genet. 2018, 50, 699–707. [Google Scholar] [CrossRef]
- Hanlon, P.; Avenell, A.; Aucott, L.; Vickers, M.A. Systematic review and meta-analysis of the sero-epidemiological association between Epstein-Barr virus and systemic lupus erythematosus. Arthritis. Res. Ther. 2014, 16, R3. [Google Scholar] [CrossRef]
- Houen, G.; Trier, N.H. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front. Immunol. 2021, 11, 587380. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Wu, W.; Wang, Y.; Ding, H.; Qian, L. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int. J. Biol. Sci. 2018, 14, 565–576. [Google Scholar] [CrossRef]
- Hassani, A.; Khan, G. Epstein-Barr Virus and miRNAs: Partners in Crime in the Pathogenesis of Multiple Sclerosis? Front. Immunol. 2019, 10, 695. [Google Scholar] [CrossRef]
- Domínguez-Andrés, J.; Netea, M.G. Impact of Historic Migrations and Evolutionary Processes on Human Immunity. Trends Immunol. 2019, 40, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Van Langelaar, J.; Wierenga-Wolf, A.F.; Samijn, J.P.A.; Luijks, C.J.M.; Siepman, T.A.; van Doorn, P.A.; Bell, A.; van Zelm, M.C.; Smolders, J.; van Luijn, M.M. The association of Epstein-Barr virus infection with CXCR3+ B-cell development in multiple sclerosis: Impact of immunotherapies. Eur. J. Immunol. 2021, 51, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Csurhes, P.A.; Burrows, J.M.; Burrows, S.R. Defective T-cell control of Epstein–Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 2017, 6, e126. [Google Scholar] [CrossRef]
- Pender, M.P.; Csurhes, P.A.; Pfluger, C.M.; Burrows, S.R. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult. Scler. 2014, 20, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.F.; Serafini, B.; Piras, E.; Severa, M.; Coccia, E.M.; Rosicarelli, B.; Ruggieri, S.; Gasperini, C.; Buttari, F.; Centonze, D.; et al. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013, 9, e1003220. [Google Scholar] [CrossRef] [PubMed]
- Rang, X.; Liu, Y.; Wang, J.; Wang, Y.; Xu, C.; Fu, J. Identification of multiple sclerosis-related genes regulated by EBV-encoded microRNAs in B cells. Mult. Scler. Relat. Disord. 2022, 59, 103563. [Google Scholar] [CrossRef]
- Hochberg, D.; Souza, T.; Catalina, M.; Sullivan, J.L.; Luzuriaga, K.; Thorley-Lawson, D.A. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 2004, 78, 5194–5204. [Google Scholar] [CrossRef]
- Mrozek-Gorska, P.; Buschle, A.; Pich, D.; Schwarzmayr, T.; Fechtner, R.; Scialdone, A.; Hammerschmidt, W. Epstein-Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc. Natl. Acad. Sci. USA 2019, 116, 16046–16055. [Google Scholar] [CrossRef]
- Salahuddin, S.; Fath, E.K.; Biel, N.; Ray, A.; Moss, C.R.; Patel, A.; Patel, S.; Hilding, L.; Varn, M.; Ross, T.; et al. Epstein-Barr Virus Latent Membrane Protein-1 Induces the Expression of SUMO-1 and SUMO-2/3 in LMP1-positive Lymphomas and Cells. Sci. Rep. 2019, 9, 208. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Fewings, N.L.; Schibeci, S.D.; Keane, J.T.; Booth, D.R.; Parnell, G.P.; Swaminathan, S. The Interaction of Human and Epstein–Barr Virus miRNAs with Multiple Sclerosis Risk Loci. Int. J. Mol. Sci. 2021, 22, 2927. [Google Scholar] [CrossRef]
- Pender, M.P. The Essential Role of Epstein-Barr Virus in the Pathogenesis of Multiple Sclerosis. Neuroscientist 2011, 17, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P. Does Epstein-Barr virus infection in the brain drive the development of multiple sclerosis? Brain 2009, 132, 3196–3198. [Google Scholar] [CrossRef] [PubMed]
- Van Zwam, M.; Huizinga, R.; Melief, M.J.; Wierenga-Wolf, A.F.; Van Meurs, M.; Voerman, J.S.; Biber, K.P.H.; Boddeke, H.W.G.M.; Höpken, U.E.; Meisel, C.; et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J. Mol. Med. 2009, 87, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.N.H.; Yaari, G.; vander Heiden, J.A.; Church, G.; Donahue, W.F.; Hintzen, R.Q.; Huttner, A.J.; Laman, J.D.; Nagra, R.M.; Nylander, A.; et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci. Transl. Med. 2014, 6, 248ra107. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Paroni, M.; Pagani, M.; Galimberti, D.; De Francesco, R.; Scarpini, E.; Abrignani, S. The Enigmatic Role of Viruses in Multiple Sclerosis: Molecular Mimicry or Disturbed Immune Surveillance? Trends Immunol. 2017, 38, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Niller, H.H.; Wolf, H.; Minarovits, J. Regulation and dysregulation of Epstein-Barr virus latency: Implications for the development of autoimmune diseases. Autoimmunity 2008, 41, 298–328. [Google Scholar] [CrossRef] [PubMed]
- Lindhout, E.; Lakeman, A.; Mevissen, M.L.; De Groot, C. Functionally active Epstein-Barr virus-transformed follicular dendritic cell-like cell lines. J. Exp. Med. 1994, 179, 1173–1184. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Styles, C.T.; Bazot, Q.; Parker, G.A.; White, R.E.; Paschos, K.; Allday, M.J. EBV epigenetically suppresses the B cell-to-plasma cell differentiation pathway while establishing long-term latency. PLoS Biol. 2017, 15, e2001992. [Google Scholar] [CrossRef]
- Bonnan, M. Intrathecal Immunoglobulin Synthesis in MS—A Complete Reappraisal. In Trending Topics in Multiple Sclerosis; Gonzalez-Quevedo, A., Ed.; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/chapters/50603 (accessed on 14 January 2023).
- Nissimov, N.; Hajiyeva, Z.; Torke, S.; Grondey, K.; Brück, W.; Häusser-Kinzel, S.; Weber, M.S. B cells reappear less mature and more activated after their anti-CD20–mediated depletion in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 25690–25699. [Google Scholar] [CrossRef]
- Barnett, M.H.; Prineas, J.W. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann. Neurol. 2004, 55, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017, 133, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Aloisi, F.; Stigliano, E. Epstein-barr virus in the central nervous system and cervical lymph node of a patient with primary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2014, 73, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Stuhlmann-Laeisz, C.; Oschlies, I.; Klapper, W. Detection of EBV in reactive and neoplastic lymphoproliferations in adults—When and how? J. Hematop. 2014, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kuerten, S.; Schickel, A.; Kerkloh, C.; Recks, M.S.; Addicks, K.; Ruddle, N.H.; Lehmann, P.V. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012, 124, 861–873. [Google Scholar] [CrossRef]
- Hassani, A.; Corboy, J.R.; Al-Salam, S.; Khan, G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS ONE 2018, 13, e0192109. [Google Scholar] [CrossRef]
- Aloisi, F.; Serafini, B.; Magliozzi, R.; Howell, O.W.; Reynolds, R. Detection of Epstein-Barr virus and B-cell follicles in the multiple sclerosis brain: What you find depends on how and where you look. Brain 2010, 133, e157. [Google Scholar] [CrossRef]
- Willekens, B.; Presas-Rodríguez, S.; Mansilla, M.J.; Derdelinckx, J.; Lee, W.P.; Nijs, G.; De Laere, M.; Wens, I.; Cras, P.; Parizel, P.; et al. Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): A harmonised study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open 2019, 9, e030309. [Google Scholar] [CrossRef]
- Laman, J.D.; Weller, R.O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J. Neuroimmune Pharmacol. 2013, 8, 840–856. [Google Scholar] [CrossRef]
- Albayram, M.S.; Smith, G.; Tufan, F.; Tuna, I.S.; Bostancıklıoğlu, M.; Zile, M.; Albayram, O. Non-invasive MR imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat. Commun. 2022, 13, 203. [Google Scholar] [CrossRef]
- Jacob, L.; De Brito Neto, J.; Lenck, S.; Corcy, C.; Benbelkacem, F.; Geraldo, L.H.; Xu, Y.; Thomas, J.-M.; El Kamouh, M.-R.; Spajer, M.; et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med. 2022, 219, e20220035. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Marrie, R.A. The multiple sclerosis prodrome: Emerging evidence, challenges, and opportunities. Mult. Scler. 2021, 27, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Munger, K.L.; Makhani, N. The Multiple Sclerosis Prodrome: Evidence to Action. Front. Neurol. 2021, 12, 761408. [Google Scholar] [CrossRef] [PubMed]
- Dobrow, M.J.; Hagens, V.; Chafe, R.; Sullivan, T.; Rabeneck, L. Consolidated principles for screening based on a systematic review and consensus process. Can. Med. Assoc. J. 2018, 190, E422–E429. [Google Scholar] [CrossRef] [PubMed]
- Louapre, C.; Lubetzki, C. Neurodegeneration in multiple sclerosis is a process separate from inflammation: Yes. Mult. Scler. 2015, 21, 1626–1628. [Google Scholar] [CrossRef]
- Hutchinson, M. Neurodegeneration in multiple sclerosis is a process separate from inflammation: No. Mult. Scler. 2015, 21, 1628–1631. [Google Scholar] [CrossRef]
- Witte, M.E.; Mahad, D.J.; Lassmann, H.; Van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014, 20, 179–187. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X.; et al. Targeting the signaling in Epstein–Barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal. Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef]
- Vernon, S.D.; Whistler, T.; Cameron, B.; Hickie, I.B.; Reeves, W.C.; Lloyd, A. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr Virus. BMC. Infect. Dis. 2006, 6, 15. [Google Scholar] [CrossRef]
- Bjornevik, K.; Munger, K.L.; Cortese, M.; Barro, C.; Healy, B.C.; Niebuhr, D.W.; Scher, A.I.; Kuhle, J.; Ascherio, A. Serum Neurofilament Light Chain Levels in Patients With Presymptomatic Multiple Sclerosis. JAMA. Neurol. 2020, 77, 58. [Google Scholar] [CrossRef]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Varhaug, K.N.; Barro, C.; Bjørnevik, K.; Myhr, K.M.; Torkildsen, Ø.; Wergeland, S.; Bindoff, L.A.; Kuhle, J.; Vedeler, C. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e422. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Tur, C.; Eshaghi, A.; Doshi, A.; Chan, D.; Binks, S.; Wellington, H.; Heslegrave, A.; Zetterberg, H.; Chataway, J. Serum neurofilament light and MRI predictors of cognitive decline in patients with secondary progressive multiple sclerosis: Analysis from the MS-STAT randomised controlled trial. Mult. Scler. 2022, 28, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases (NIAID). Safety and Immunogenicity of an Epstein-Barr Virus (EBV) gp350-Ferritin Nanoparticle Vaccine in Healthy Adults with or without EBV Infection. In ClinicalTrials.gov; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04645147 (accessed on 14 January 2023).
- Moderna, TX Inc. A Study of an Epstein-Barr Virus (EBV) Candidate Vaccine, mRNA-1189, in 18- to 30-Year-Old Healthy Adults. In ClinicalTrials.gov; U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05164094 (accessed on 14 January 2023).
- Berger, J.R.; Kakara, M. The elimination of circulating Epstein-Barr virus infected B cells underlies anti-CD20 monoclonal antibody activity in multiple sclerosis: A hypothesis. Mult. Scler. Relat. Disord. 2022, 59, 103678. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
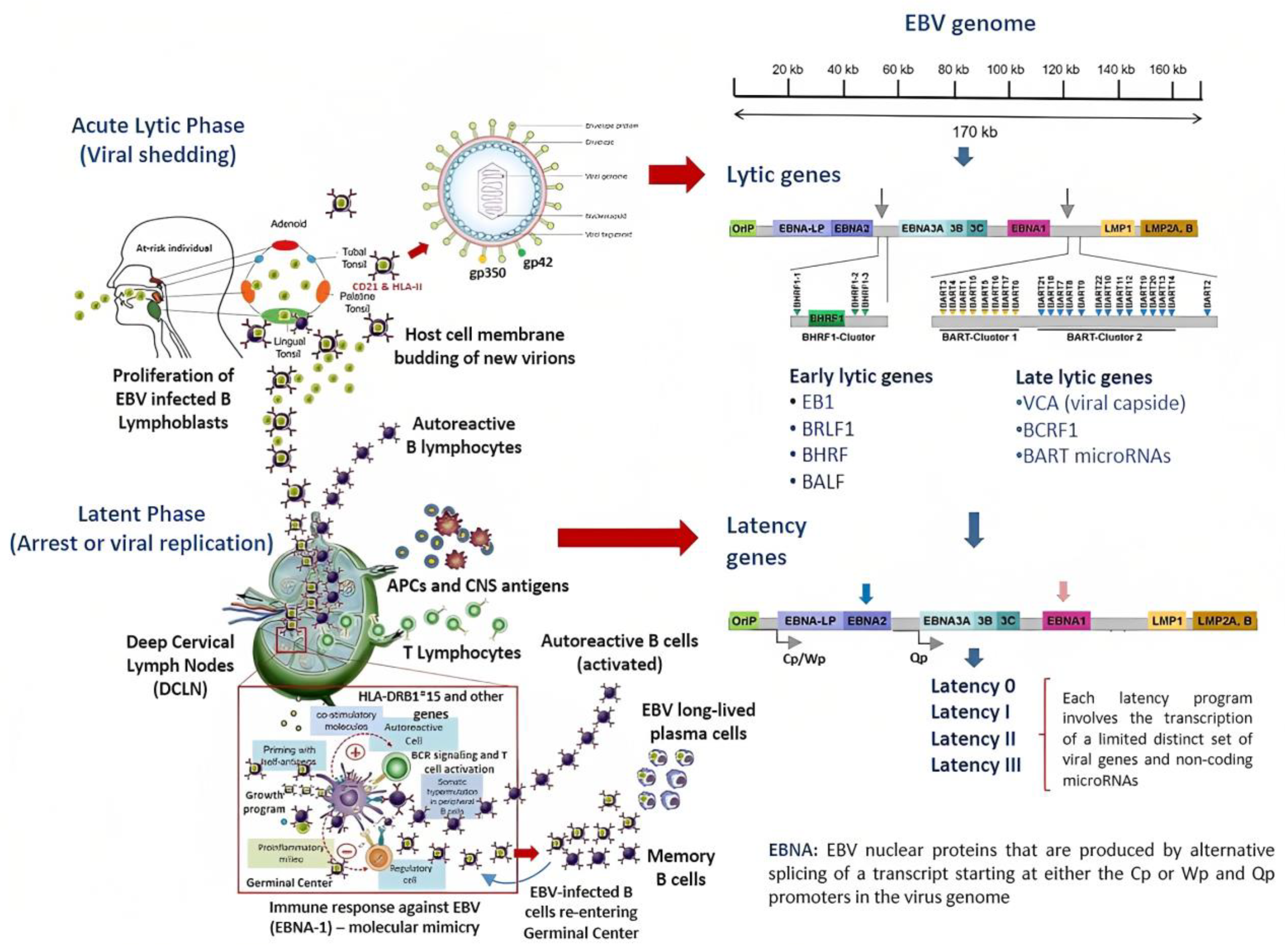
| Latency Program | Alternative Name | Gene Products 1 | Site In Vivo | Stage of Normal B-Cell Development | Events | Biomarkers | Associated Disease 1,2 | Ref |
|---|---|---|---|---|---|---|---|---|
| 0 | Latency program 0 | EBERs BART microRNAs (non-coding genes) | Peripheral circulation | Latently infected memory B cells | Downregulation of most viral protein-encoding genes suppressing the production of virions | None | None | [37] |
| I | Latency program I | EBNA1 3 EBER1/2 RNA BART microRNAs | Peripheral circulation | The homeostatic proliferation of memory B cells is not driven by the virus | Replication of EBV genome harnessing the mitotic cycle of memory B cells | Anti-EBNA1 IgG Anti-VCA IgG | BL | [42] |
| II | Default program | EBNA1 3 EBER1/2 RNA BART microRNAs LMP1 LMP2A/B | Tonsil germinal center (GC) and lymph nodes | Naïve B cells infected by EBV gain access into the GC for the normal process of differentiation | EBV set a limited transcriptional program that rescues EBV-DNA into the memory B cell compartment where viral DNA persists as the episome | Anti-EBNA1 IgG Anti-VCA IgG | HL T/NK-cell malignancies Epithelial malignancies NFC | [41,51,52] |
| III | Growth program | EBNA1 3 EBNA2 EBNA3A, 3B, 3C EBER1/2 RNA EBNALP BART microRNAs LMP1 LMP2A/B | Tonsil GC/Lymph nodes | Activation of naïve B cells infected by EBV In this lytic phase, B cells become proliferating B-blasts before entering the GC | Full expression of EBV proteins CTL trigger a strong immune response to suppress EBV-infected B cells Selective silencing of EBV genes upon unknown individual and environmental conditions | Anti-EA IgM Anti-EBNA1 &-2 IgM Anti-VCA IgM/IgG CTL | DLBCL Post-transplant lymphoproliferative disorders CNS lymphoma MS? | [41,42,47,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Hernandez, O.-D.; Martínez-Cáceres, E.M.; Presas-Rodríguez, S.; Ramo-Tello, C. Epstein-Barr Virus and Multiple Sclerosis: A Convoluted Interaction and the Opportunity to Unravel Predictive Biomarkers. Int. J. Mol. Sci. 2023, 24, 7407. https://doi.org/10.3390/ijms24087407
Ortega-Hernandez O-D, Martínez-Cáceres EM, Presas-Rodríguez S, Ramo-Tello C. Epstein-Barr Virus and Multiple Sclerosis: A Convoluted Interaction and the Opportunity to Unravel Predictive Biomarkers. International Journal of Molecular Sciences. 2023; 24(8):7407. https://doi.org/10.3390/ijms24087407
Chicago/Turabian StyleOrtega-Hernandez, Oscar-Danilo, Eva M. Martínez-Cáceres, Silvia Presas-Rodríguez, and Cristina Ramo-Tello. 2023. "Epstein-Barr Virus and Multiple Sclerosis: A Convoluted Interaction and the Opportunity to Unravel Predictive Biomarkers" International Journal of Molecular Sciences 24, no. 8: 7407. https://doi.org/10.3390/ijms24087407
APA StyleOrtega-Hernandez, O.-D., Martínez-Cáceres, E. M., Presas-Rodríguez, S., & Ramo-Tello, C. (2023). Epstein-Barr Virus and Multiple Sclerosis: A Convoluted Interaction and the Opportunity to Unravel Predictive Biomarkers. International Journal of Molecular Sciences, 24(8), 7407. https://doi.org/10.3390/ijms24087407







