Abstract
Acute liver injury (ALI) is a globally important public health issue that, when severe, rapidly progresses to acute liver failure, seriously compromising the life safety of patients. The pathogenesis of ALI is defined by massive cell death in the liver, which triggers a cascade of immune responses. Studies have shown that the aberrant activation of the nod-like receptor protein 3 (NLRP3) inflammasome plays an important role in various types of ALI and that the activation of the NLRP3 inflammasome causes various types of programmed cell death (PCD), and these cell death effectors can in turn regulate NLRP3 inflammasome activation. This indicates that NLRP3 inflammasome activation is inextricably linked to PCD. In this review, we summarize the role of NLRP3 inflammasome activation and PCD in various types of ALI (APAP, liver ischemia reperfusion, CCl4, alcohol, Con A, and LPS/D-GalN induced ALI) and analyze the underlying mechanisms to provide references for future relevant studies.
1. Introduction
The liver is the largest parenchymal organ in the human body and is the key hub of many physiological processes, including nutrient metabolism, blood volume regulation, immune regulation, and detoxification [1,2]. As such, the normal physiological structure and function of the liver are essential for maintaining human health [3]. Liver failure, due to various factors, is a global health problem. Acute liver injury (ALI) is a common disease that seriously threatens the health of patients, and the massive death of hepatocytes in patients within a short period of time leads to a dramatic decline in their liver function [4]. The etiology of ALI can generally be defined as either drug-induced liver injury, ischemia-reperfusion liver injury, autoimmune liver injury, or viral liver injury [5,6]; viral causes predominate in developing countries while drug causes predominate in developed countries such as the United States and Western Europe [7]. ALI progresses rapidly, and once acute liver failure has occurred, liver transplantation is the only effective treatment [8]; however, the scarcity of liver sources, the cost, and high mortality rates after urgent transplantation limit its application [9]. Various causes of innate immunity drive a local sterile inflammatory response and are the most direct cause of liver injury and failure [10], so it is particularly important to better understand the immune response in ALI and inhibit its progression.
The nod-like receptor protein 3 (NLRP3) inflammasome is the most widely studied of the inflammasomes. It is composed of a sensor (NLRP3), an adapter (apoptosis-associated spotted protein, ASC), and an effector (cysteine aspartate protease 1, caspase-1) [11,12]. The level of NLRP3 expression under steady-state conditions is insufficient for the activation of the NLRP3 inflammasome [13]. Thus, its activation requires two steps [14,15,16]. Signal 1 (pathogen-associated molecular patterns (PAMPs) and cell factors) stimulates toll-like receptor 4 (TLR4), activating nuclear factor kappa-B (NF-ĸB) and promoting its translocation to the nucleus [17,18]; this then upregulates the expression of NLRP3, pro-interleukin-1β (IL-1β), and pro-interleukin-18 (IL-18) [19]. Signal 2 (damage-associated molecular patterns (DAMPs) and PAMPs) then induces NLRP3 protein oligomerization, recruits the ASC and pro-caspase-1 molecules [20], and then interacts with them to assemble the NLRP3 inflammasome, activating caspase-1 [15,21]. On the one hand, activated caspase-1 promotes the maturation of proinflammatory cytokines, such as IL-1β and IL-18 [22]. On the other hand, activated caspase-1 also cleaves gasdermin D (GSDMD) and releases its N-terminal domain, which translocates to the cell membrane and forms pores, leading to the release of cellular contents such as IL-1β and IL-18, inducing pyroptosis (Figure 1) [13,15,23,24]. Studies have shown that the NLRP3 inflammasome plays an important role in various diseases throughout the body, including chronic liver injury (viral hepatitis, non-alcoholic fatty liver disease, etc.) and ALI [25,26,27,28,29,30,31,32].
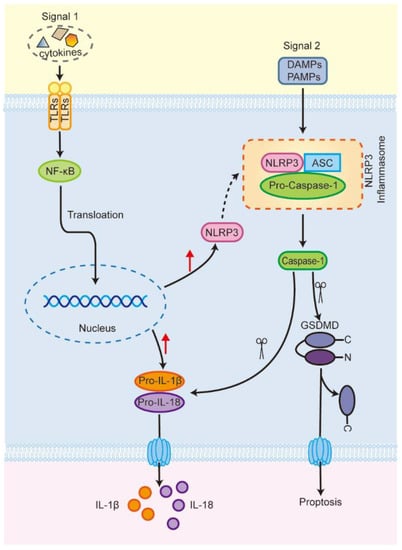
Figure 1.
The activation process of NLRP3 inflammasome (Scissors represent clipping; red arrows represent rising). TLRs, toll-like receptors; NF-κB, nuclear factor kappa-B; NLRP3, nod-like receptor protein 3; Pro-IL-1β, pro-interleukin-1β; Pro-IL-18, pro-interleukin-18; ASC, apoptosis-associated spotted protein; Pro-caspase-1, pro-cysteine aspartate protease 1; GSDMD, gasdermin D.
Biologists have long regarded cell death as an inevitable, passive outcome of life. However, as research has developed over the past few decades, studies have shown that organisms can actively clear damaged, harmful, and superfluous cells through certain specific cell death programs; this process is essential for maintaining the homeostasis of the human body environment [33,34]. Depending on whether the cell death process can be regulated, it can be classified as either accidental cell death (ACD) or regulated cell death (RCD) [35]. ACD is uncontrollable, mainly because the cells are subjected to extreme physical, chemical, or mechanical stimuli (e.g., high temperature, high pressure, pH changes, and shear forces). However, RCD is induced by precise molecular mechanisms that intervene in regulated cell death at the biological level [36]. RCD that occurs under physiological conditions that lack any exogenous environmental disturbance is also known as programmed cell death (PCD); this category includes apoptosis, necroptosis, pyroptosis, ferroptosis, and autophagy, among other forms [37,38,39,40]. In recent years, intensive research into the molecular mechanisms at play during cell death has shown that multiple effectors of PCD can regulate the activation of the NLRP3 inflammasome and that the activation of the NLRP3 inflammasome can also lead to different forms of PCD, indicating that PCD is inseparable from the activation of NLRP3 inflammasome [41,42,43]. In addition, with the rapid development of single-cell sequencing technology, several results of liver transcriptomic analysis have further demonstrated that NLRP3 inflammasome is closely related to various regulatory and effector factors of PCD [44,45,46,47]. In this review, we focused on current studies regarding the NLRP3 inflammasome and PCD in ALI and analyzed the underlying mechanisms to provide references for future research and treatment of ALI.
2. NLRP3 Inflammasome and Programmed Cell Death
PCD is an active, orderly mode of cell death that is encoded in specific genes. Different types of PCD differ in their morphological features, signaling both the pathways and their resulting outcomes; here, we will mainly discuss the characteristics of the following types of PCD and their mutual regulation with the NLRP3 inflammasome: apoptosis, necroptosis, pyroptosis, and autophagy.
2.1. Apoptosis
As the first to be defined in the literature, apoptosis is the most classic form of PCD [48,49,50]. Apoptosis was first observed in 1842 by Karl Vogt during cell growth and development in toads and was then formally described by Kerr in 1972 [51,52]. The main morphological characteristics of apoptosis are cell deformation, loss of cell membrane integrity, DNA degradation, and the formation of apoptotic bodies (Figure 2) [53]. According to their inducing factors, cases of apoptosis can be divided into cell mitochondria/caspase-9 mediated endogenous apoptosis and death receptor/caspase-8 mediated exogenous apoptosis [54,55,56]. Although the initiation signaling pathways vary, all cases share common downstream pathways that culminate in the execution of apoptosis through a family of caspases (Caspase-3, -6, -7) [41,57,58]. Studies have shown that the family of caspases, which are initiators and executors of apoptosis, can promote the activation of the NLRP3 inflammasome by inducing potassium ion efflux [59,60]. In addition, several different effectors of apoptosis, such as the Bcl-2-associated X protein and Bcl-2 homologous antagonist killer, can regulate mitochondrial homeostasis and thereby induce NLRP3 inflammasome activation [61]. However, there are also some effectors of apoptosis that have diametrically opposite effects; for example, cytochrome c released from the inner mitochondrial membrane upon apoptosis can competitively bind to the LRR domain of NLRP3, thereby inhibiting its activation [62,63]. The intrinsic link between apoptosis and NLRP3 inflammasome activation is complex, and so a careful distinction between the two is required when judging the relationship between apoptosis and NLRP3 inflammasome in disease.
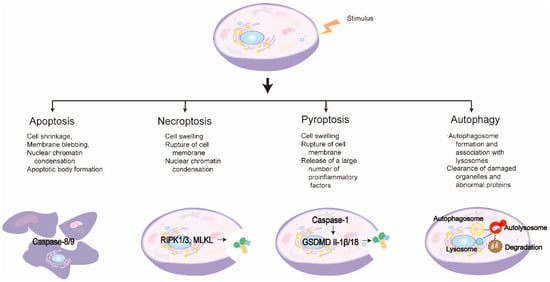
Figure 2.
Characteristics of different programmed cell death pathways.
2.2. Necroptosis
Necroptosis is another type of PCD that occurs when the normal apoptotic pathway of cells is inhibited [64,65]. In 1998, Vercammen et al. found that fibrosarcoma cells induced a controlled form of necrosis in the absence of caspase [66]. In contrast to apoptosis, necroptosis does not affect karyopyknosis, nor produce nucleosome DNA fragments, which are mainly manifested through mitochondrial dysfunction, organelle disruption, and leakage of cell contents [67,68]. According to various inducing factors, necroptosis is divided into tumor necrosis factor-α(TNF-α)-induced external necroptosis, intrinsic necroptosis induced by reactive oxygen species (ROS), and endogenous necroptosis induced by ischemia [69]. Of these, TNF-α-mediated necroptosis is the most prevalent form in the literature and is widely studied [64,70]. The activation of receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like (MLKL) is key in the necroptosis pathway, and studies have shown that they can promote NLRP3 inflammasome activation through multiple pathways [71,72,73,74]. In addition, Z-DNA binding protein 1 (ZBP1), a recently discovered effector of necroptosis, has also been shown to play an important role in NLRP3 inflammasome activation [75,76].
2.3. Pyroptosis
Pyroptosis is a type of programmed necrosis that is dependent on the gasdermin family [37]. In 1992, Zychlinsky et al. discovered pyroptosis in Shigella flexneri-induced macrophages, but incorrectly classified it as a specific category of apoptosis [77]. It was not until 2001 that Cookson formally proposed the concept of pyroptosis, defined as caspase-1-dependent inflammatory programmed cell death [78]. It is mainly characterized by cell swelling, pyknosis of the nucleus and DNA fragmentation, and the rupture of the cell membrane to form pores that then release a large number of inflammatory factors [79,80]. The occurrence of pyroptosis is mainly divided into the caspase-1-dependent canonical pathway and the caspase-4, -5, -11-dependent non-canonical pathway [79,81,82,83]. Pyroptosis is the most closely related form of PCD to the NLRP3 inflammasome, as the canonical pyroptosis pathway can be initiated by caspase-1 and the inflammasome initiates caspase-1 activation, of which the NLRP3 inflammasome is currently the most extensively studied [84].
2.4. Autophagy
Autophagy is a lysosome-dependent phagocytic phenomenon that is genetically regulated and is also considered a type II PCD [85]. Autophagy plays a role in the energy cycle and in maintaining cellular homeostasis by degrading proteins and damaged organelles inside the cell [86,87,88,89,90]. The primary morphological feature of autophagy is the formation and accumulation of either crescent-like or cup-shaped phagocytic vacuoles [91,92]. Autophagy can be divided into non-selective autophagy, such as macroautophagy and microautophagy, and selective autophagy, which includes mitophagy, peroxisomal autophagy, endoplasmic reticulum autophagy, as well as ribosomal autophagy, among others [87,93]. Macroautophagy is currently the most widely studied form of autophagy; in general, the word autophagy is used to refer to macroautophagy. Autophagy is reciprocally regulated with the NLRP3 inflammasome and can either reduce DAMPs release by removing damaged organelles [94], thereby inhibiting NLRP3 inflammasome activation [95,96,97], or inhibit its activation by engulfing and degrading NLRP3 inflammasome components [98,99,100,101]. The NLRP3 inflammasome can also inhibit autophagy activation through autophagy protein interactions [102,103,104].
3. NLRP3 Inflammasome and Programmed Cell Death in ALI
The NLRP3 inflammasome, as well as various forms of PCD, have been shown to play important roles in the pathogenesis of ALI, and their in-depth study has contributed to the development of new therapeutic strategies for ALI. Here, we summarize both the pathogenesis of the NLRP3 inflammasome and PCD in various types of ALI and the latest therapeutic strategies targeting them.
3.1. NLRP3 Inflammasome and Programmed Cell Death in Acetaminophen (APAP)-Induced Acute Liver Injury
Drug-induced liver injury (DILI) is the main form of acute liver injury in developed countries [105,106]. APAP is a widely used antipyretic and analgesic drug that is safe to use at therapeutic doses [107,108]. However, when it is over-used it can produce dose-dependent hepatotoxicity and even liver failure [109]. APAP-induced acute liver injury is the leading cause of drug-induced liver injury in western countries; in both the United States and the United Kingdom, the greatest predisposition for cases of acute liver failure is the excessive use of APAP [110,111,112]. N-acetylcysteine (NAC) is the only clinically approved drug for the treatment of APAP-induced liver injury. However, the NAC therapeutic window is quite narrow and can have adverse effects [113,114,115]. At a normal dose, APAP is converted in the liver, primarily by UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT), into a nontoxic compound and excreted with urine; only a small amount is metabolized to n-acetyl-p-benzoquinoneimine (NAPQI) by cytochrome P450 enzymes (CYP) [116]. NAPQI is a highly reactive intermediate compound that is cytotoxic and can be rapidly detoxified in combination with glutathione (GSH) in the liver [117,118]. However, once GSH is depleted, NAPQI covalently binds to protein sulfhydryl groups, especially in the mitochondria, leading to mitochondrial oxidative stress, and thereby inducing hepatocytes to undergo both apoptosis and necrosis [119,120,121]. Mice are preferred for studying APAP overdose because the doses of APAP that cause toxicity are similar in mice and humans, and the mechanisms of toxicity are similar in mice and humans. However, the time course of liver injury differs slightly between humans and mice, with hepatotoxicity developing slightly faster in mice, peaking at around 12–24 h compared with 24–72 h in humans [122].
In recent years, increasing evidence has demonstrated that apoptosis plays an important role in APAP-induced liver injury; tune staining has also demonstrated that hepatocytes underwent massive apoptosis in an APAP-induced acute liver injury model in mice [123,124]. Kaempferol (KA), a flavonoid compound extracted from Penthorum chinense, has powerful anti-inflammatory and antioxidant activities. Du et al. found that KA inhibited the high mobility group protein B1 (HMGB1)/TLR4/NF-κB signaling pathway and the activation of the NLRP3 inflammasome, thereby protecting the liver from APAP-induced inflammatory responses and apoptosis [125]. Elshal et al. found that diacerein reduced NLRP3/caspase-1/IL-1β by downregulating the IL-4/Monocyte chemoattractant protein-1 (MCP-1) and TNF-α/ NF-κB inflammatory signaling pathways and mediating oxidative stress, mitochondrial dysfunction, necrosis, sterile inflammation, and apoptosis, thereby preventing and reversing APAP hepatotoxicity in mice [126]. In addition, recent studies have shown that pyroptosis and necroptosis also play a role in APAP-induced acute liver injury. Wang et al. found that targeting mitochondrial ROS (via peroxidase 3) inhibited NLRP3 inflammasome activation and prevented APAP-induced pyroptosis, thereby exerting hepatoprotective effects [127]. Receptor-interacting serine-threonine kinase (RIPK1) and RIPK3 play important roles in necroptosis, and Deutsch et al. found that in APAP mediated ALI, RIPK3 deletion or specifically blocking RIPK1 using Necrostatin-1 (Nec-1), an inhibitor of RIPK1, was protective against liver injury and associated with inhibition of NLRP3 inflammasome activation [128]. Another study suggested that Nec-1 attenuates APAP-induced liver injury by inhibiting the interaction between necroptosis and the NLRP3 inflammasome [129].
3.2. NLRP3 Inflammasome and Programmed Cell Death in Liver Ischemia-Reperfusion Injury
Liver ischemia-reperfusion injury (LIRI), an inevitable pathophysiological process that occurs during several clinical procedures, such as liver transplantation and liver resection, is a common type of acute liver injury [130]. Severe LIRI causes liver dysfunction and failure, which leads to surgical failure [131,132]. To date, no effective method for the prevention and treatment of LIRI has been found. Therefore, in order to provide a clinical solution to this problem, it is important to deeply investigate the pathogenesis of LIRI. LIRI can be divided into two phases, ischemia and reperfusion, which are characterized by hypoxia-induced cell injury in the ischemic phase and immunoinflammation after the restoration of blood flow [133]. During ischemia, ROS production as well as organelle damage, are induced by glycogen depletion, inadequate oxygen supply, and ATP depletion, which can lead to hepatocyte injury and death [134,135]. Subsequent reperfusion not only causes cellular metabolic disorders but also triggers a series of inflammatory cascades, exacerbating hepatocyte injury [10]. LIRI can be divided into warm ischemia and cold ischemia. Except for liver transplantation, partial hepatectomy, trauma as well as hemorrhagic shock were warm ischemia. The warm rodent model (orthotopic) LIRI model is the most widely used because of its high feasibility, few ethical concerns, and low expense [136].
There are many different pathways of PCD, all of which can initiate inflammatory immune cascades during LIRI [10]. The NLRP3 inflammasome has been shown to play an important role in LIRI pathogenesis, regulating hepatocyte injury, immune cell activation, and hepatic inflammatory responses [137,138]. Inoue et al. found that NLRP3 expression was elevated in a murine partial (70%) hepatic warm ischemia-reperfusion model, but that NLRP3-/- mice showed reduced levels of inflammation and apoptosis; the authors suggested that NLRP3 regulated neutrophil function by influencing chemokine-mediated signaling [139]. γ- Oryzanol (ORY), an important bioactive ingredient isolated from rice bran oil, has anti-inflammation and anti-oxidation properties and can exert protective effects in a variety of liver disease models [140,141]. Du et al. found that ORY protected the liver from I/R-induced inflammasome activation and apoptosis by inhibiting the HMGB1/NLRP3/IL-1β signaling pathway by establishing a hepatic 70% warm ischemia-reperfusion model after mice were fed ORY for seven days [142]. Studies have also shown that dietary restrictions exert protective effects in IRI in multiple organs, including the liver [143,144]. Miyauchi et al. found that twelve hours of fasting enhanced β-Hydroxybutyric acid expression, promoting the up-regulated expression of acetylated histone-3 and the activation of forkhead box protein O1 (FOXO1) and heme oxygenase 1 (HO-1) and enabling NF-κB and NLRP3 inactivation, thereby playing a protective role against LIRI-caused hepatocyte apoptosis and necrosis [145]. El-Sisi et al. found that octreotide could also play a protective role in LIRI by disrupting TLR4-mediated NLRP3 inflammasome activation and pyroptosis, and then inhibiting pyroptosis-triggered apoptosis [146].
An increasing number of studies have focused on the interaction between autophagy and the NLRP3 inflammasome in LIRI. Cao et al. found that pretreatment with 25 hydroxycholesterol (25HC)-activated mitophagy inhibited NLRP3 inflammasome activity in a rat LIRI model; the protective effects on the liver and the inhibitory effect of the NLRP3 inflammasome were attenuated after the inhibition of mitophagy, suggesting that the effect of 25HC pretreatment on LIRI may depend on upregulating mitophagy and inhibiting NLRP3 inflammasome activation [147]. Xue et al. demonstrated that lycopene promotes nuclear factor erythroid 2-related factor 2 (Nrf2)/ HO-1 pathway activation and further inhibits the NLRP3 inflammasome by enhancing pooled autophagy, thereby alleviating LIRI [148]. Wang et al. found that Eva1a inhibited NLRP3 activation, thereby alleviating LIRI by inducing Kupffer cell autophagy [149]. Zhang et al. found that transient receptor potential melastatin 2 knockdown attenuated hepatocyte oxygen-glucose deprivation/reoxygenation (OGD/R)-induced cell injury by enhancing autophagy and negatively regulated the NLRP3 inflammasome pathway. In addition, the use of INF39, an inhibitor of NLRP3, could increase cell viability and reduce cell apoptosis caused by OGD/R [150].
3.3. NLRP3 Inflammasome and Programmed Cell Death in CCl4-Induced Acute Liver Injury
Carbon tetrachloride (CCl4), an industrial solvent that causes the necrosis and apoptosis of hepatocytes in the centrilobular region, is a typical hepatotoxicant [151,152]. CCl4 is metabolized in the liver into trichloromethyl radical and peroxyl radical by cytochrome P450 enzyme (CYP2E1). The trichloromethyl radical can cause a peroxidation chain reaction to produce more toxic free radicals (such as superoxide and hydroxide anions), leading to free radical-mediated lipid peroxidation (membrane rupture, loss of membrane integrity) and thus liver cell damage [153,154]. Acute liver injury induced by CCl4 in animals is extremely similar to acute chemical liver injury in humans and is therefore widely used to investigate potential hepatoprotective strategies [155].
Salidroside (Sal), the active component of the plant salidroside, has many effects, including the inhibition of inflammatory responses. Zhang et al. found that Sal exerts protective effects against CCl4-induced ALI by reducing hepatocyte apoptosis, inhibiting oxidative stress, and reducing inflammatory responses by downregulating CYP2E1 expression and inhibiting NLRP3 inflammasome activation [156]. Zhao et al. found that Ginsenoside Rg1 ameliorated acute liver injury via autophagy and may be associated with the NF-κB/NLRP3 inflammasome signaling pathway [157].
3.4. NLRP3 Inflammasome and Programmed Cell Death in Alcohol-Induced Acute Liver Injury
Alcoholic liver injury is the most common cause of liver injury worldwide [158,159]. Drinking a lot in a short period of time can cause acute liver damage, even liver failure, and the mortality rate is as high as 44% [160]. Excessive drinking will activate the CYP2E1 in the liver, resulting in the excessive production and accumulation of ROS, and enhancing lipid peroxidation, leading to the swelling and disintegration of liver cells [161,162,163]. To date, only a few corticosteroid drugs are clinically available to treat alcohol-induced acute liver injury, and their efficacy remains unsatisfactory [164].
Liu et al. found that quercetin can reduce ROS production by inducing the expression of HO-1 and then downregulating the activation of NLRP3 inflammatory bodies and the secretion of proinflammatory cytokines to protect the liver from acute alcohol attack [164]. However, in the above studies, there was no further examination of whether NLRP3 inflammatory bodies could also regulate the PCD process.
3.5. NLRP3 Inflammasome and Programmed Cell Death in Con A-Induced Autoimmune Hepatitis
Autoimmune hepatitis (AIH), a complex hepatic inflammatory disease mediated by autoimmune reactions, can present in both acute and chronic forms [165]. Its clinical features are characterized by elevated serum transaminases, high γ- Hypogammaglobulinemia, autoantibody positivity, and interface hepatitis, with a predominance of lymphocytic and plasma cell infiltrates [166]. It occurs worldwide, with a relatively high incidence in European and American countries, and severe cases can rapidly progress to acute liver failure. Concanavalin A (Con A), a lectin extracted from concanavalin, causes liver injury via the tail vein injection of Con A and is a model of acute hepatitis because the damage it causes usually lasts only 48 h [6]. Most of its pathogenic features are similar to those of patients with clinical acute autoimmune hepatitis (AIH); as such, it has become one of the most commonly used research models for AIH [167,168].
Studies have shown that, in a Con A-induced ALI model in mice, the signal pathway involved in pyroptosis was significantly enhanced, which proved that pyroptosis was a key cell death event in AIH [169]. Phenethyl isothiocyanate (PEITC) is a widely distributed natural compound derived from the secondary metabolites of Brassicaceae plants; it has antimicrobial, anti-inflammatory, and antioxidant properties [170,171]. Wang et al., by constructing a Con A-induced acute immune liver injury model in mice, found that PEITC could reduce NLRP3 production and casp1 and GSDMD cleavage in the liver of ALI-suffering mice and directly interact with the cysteine at position 191 of GSDMD to inhibit hepatocyte pyroptosis, thereby exerting a significant hepatoprotective effect [172]. The work of Shi et al. showed that dimethyl fumarate promoted NLRP3 phosphorylation on Ser/Thr residues at specific sites of protein kinase A (PKA) and enhanced PKA signaling to inhibit NLRP3 inflammasome activation, pyroptosis, and IL-1 β Secretion, thereby treating Con A-induced AIH [173]. Deutsch et al. found that RIPK3 deletion was protective in Con A-induced AIH, whereas RIPK1 inhibition using Nec-1 exacerbated liver injury, and this effect was associated with increased hepatocyte apoptosis [128].
3.6. NLRP3 Inflammasome and Programmed Cell Death in LPS/D-GalN-Induced Acute Liver Injury
Lipopolysaccharide (LPS)/D-galactosamine (D-GalN) is a common hepatotoxic substance [174]. LPS/D-GalN combined with intraperitoneal injection can cause the diffuse necrosis of liver cells; this process is similar to the changes in liver pathology that occur after clinical acute viral hepatitis. Therefore, the animal model of ALI induced by LPS/D-GalN is widely used to explore the mechanism of clinical fulminant liver failure and test potential therapeutic drugs [175,176,177]. ALI induced by LPS/D-GalN overproduces TNF-α, IL-1β, IL-6, and other inflammatory cytokines, which together lead to the necrosis of liver cells and liver failure [178,179].
NLRP3-Inflammed Bodies and Autophagy Seem to Play an Important Role in LPS/D-GalN-Induced ALI. Several research groups have shown that natural products, such as Biochanin A, daphnetin, Licochalcone A, Mangiferin, and Salvia miltiorrhiza can reduce LPS/D-GalN-induced ALI by inducing autophagy to inhibit the activation of NLRP3 inflammasome [180,181,182,183,184]. Furthermore, Yang et al. found that maresin 1 could inhibit mitogen-activated protein kinase/NF-κB signaling and NLRP3 inflammasome-induced pyroptosis to ameliorate inflammation during LPS/D-GalN-induced ALI [185]. IL-1 exerts its effects through the cell surface interleukin-1 receptor type 1 (IL-1R1). Gehrke et al. found that LPS/D-GalN-induced acute liver injury was significantly attenuated in IL-1R1Hep-/- mice, the expression of NLRP3 inflammasome and cell apoptosis were significantly inhibited, and the use of anakinra, an IL-1R antagonist, had the same effect [186]. S100 calcium-binding protein A9 (S100A9) is an important novel DAMP molecule that plays an important role in necroptosis. Bai et al. found that Paquinimod (an inhibitor of S100A9) significantly inhibited necroptosis and NLRP3 inflammasome activation in D-GalN/LPS-induced acute liver injury, thereby attenuating ALI [187].
4. Conclusions
The incidence of liver disease is increasing year by year and is the leading cause of death worldwide, and the immune response is central to almost all acute and chronic liver diseases. The NLRP3 inflammasome plays a critical role in host immune responses; However, its aberrant activation promotes the development of multiple chronic liver diseases (including viral hepatitis, alcoholic and nonalcoholic steatohepatitis, and liver cancer, among others) and ALI. ALI seriously threatens human health and quality of life and understanding how cell damage in the liver participates in the pathological process of ALI, no matter the cause, is the key to finding hepatoprotective measures. The role of the NLRP3 inflammasome in ALI has been widely recognized, and research on NLRP3 inflammasome activation has been intensive. A large number of studies have shown that NLRP3 inflammasome activation is inextricably linked to various types of PCD.
In this review, we summarize the pathogenesis of different types of ALI and discuss the role of the NLRP3 inflammasome and PCD therein (Table 1). The pathogeneses of various types of ALI are different, but a ROS signaling imbalance and an oxidative stress response seem to play a key role in all types. Several natural compounds ameliorate NLRP3 inflammasome activation and PCD in ALI by regulating oxidative stress and inhibiting the production of ROS. However, the specific targets of these compounds are not clear, and previous studies have been mostly based on animal models and cellular levels. The research is still lacking in vivo experimental validation, which limits the clinical use of these compounds in the treatment of ALI. The role of various targeted small molecule inhibitors in ALI has also been validated, suggesting that it may become a potential new drug in the clinic. In recent years, ferroptosis as one of the PCD forms is a research hotspot and was shown to play an important role in several diseases. However, this article mainly summarizes the interaction of NLRP3 inflammasome with apoptosis, necroptosis, pyroptosis, as well as autophagy, in ALI (Figure 3) and does not involve iron death in ALI, whether NLRP3 inflammasome can interact with ferroptosis and thereby participate in the pathogenesis of ALI needs to be further clarified and studied.

Table 1.
Summary of the roles of NLRP3 inflammasome and programmed cell death in ALI animal models.
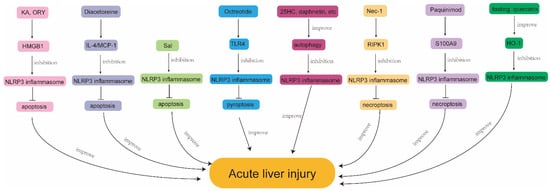
Figure 3.
Mechanisms exerting protective effects by regulating NLRP3 inflammasome and PCD in ALI. KA, kaempferol; ORY, γ-Oryzanol; HMGB1, high mobility group protein B1; NLRP3, nod-like receptor protein 3; MCP-1, monocyte chemoattractant protein-1; Sal, salidroside; TLR4, toll-like receptor 4; 25HC, 25 hydroxycholesterol; Nec-1, Necrostatin-1; RIPK1, receptor interacting serine-threonine kinase 1; S100A9, S100 calcium binding protein A9; HO-1, heme oxygenase 1.
In conclusion, NLRP3 inflammasome and PCD are involved in the pathophysiology of ALI, and the rapid development of single-cell gene sequencing technology may help to further elucidate how PCD is coordinated with the activation of NLRP3 inflammasome, and how NLRP3 inflammasome regulates PCD. A full understanding of the link between them may not only improve our understanding of their pathogenesis but also facilitate the investigation of new targeted inhibitors, thereby providing a new strategy for the clinical treatment of ALI.
Author Contributions
Conceptualization, C.Y.; writing—original draft preparation, C.Y. and P.C.; writing—review and editing, C.Y. and G.D.; making the table, L.M.; drawing the figures, C.Y. and P.C.; funding acquisition, G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China Grants (2018YFA0109800) and the Shandong Provincial Natural Science Foundation grant (ZR2022MH183).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| ACD | accidental cell death |
| AIH | autoimmune hepatitis |
| ALI | acute liver injury |
| APAP | acetaminophen |
| ASC | apoptosis-associated spotted protein |
| Caspase-1 | cysteine aspartate protease 1 |
| Con A | concanavalin A |
| CYP | cytochrome P450 enzymes |
| CYP2E1 | cytochrome P450 2E1 |
| DAMPs | damage-associated molecular patterns |
| D-GalN | D-galactosamine |
| DILI | drug-induced liver injury |
| FOXO1 | forkhead box protein O1 |
| GSDMD | gasdermin D |
| GSH | glutathione |
| HO-1 | heme oxygenase 1 |
| HMGB1 | high mobility group protein B1 |
| IL-1β | interleukin-1β |
| IL-1R1 | interleukin-1 receptor type 1 |
| IL-18 | interleukin-18 |
| KA | kaempferol |
| LPS | lipopolysaccharide |
| MCP-1 | monocyte chemoattractant protein-1 |
| MLKL | mixed lineage kinase domain-like |
| NAC | N-acetylcysteine |
| NAPQI | n-acetyl-p-benzoquinoneimine |
| Nec-1 | Necrostatin-1 |
| NLRP3 | nod-like receptor protein 3 |
| NF-ĸB | nuclear factor kappa-B |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OGD/R | oxygen-glucose deprivation/reoxygenation |
| ORY | γ- Oryzanol |
| PAMPs | pathogen-associated molecular patterns |
| PCD | programmed cell death |
| PEITC | phenethyl isothiocyanate |
| PKA | protein kinase A |
| RCD | regulated cell death |
| ROS | reactive oxygen species |
| RIPK1 | receptor-interacting serine-threonine kinase 1 |
| RIPK3 | receptor-interacting serine-threonine kinase 3 |
| Sal | salidroside |
| SULT | sulfotransferase |
| S100A9 | S100 calcium-binding protein A9 |
| TNF-α | tumor necrosis factor-α |
| TLR4 | toll-like receptor 4 |
| UGT | UDP-glucuronosyltransferase |
| ZBP1 | Z-DNA binding protein 1 |
| 25HC | 25 hydroxycholesterol |
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Matsubara, Y.; Kiyohara, H.; Teratani, T.; Mikami, Y.; Kanai, T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology 2022, 205, 108915. [Google Scholar] [CrossRef]
- Harrell, C.R.; Pavlovic, D.; Djonov, V.; Volarevic, V. Therapeutic potential of mesenchymal stem cells in the treatment of acute liver failure. World J. Gastroenterol. 2022, 28, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Tujios, S.; Stravitz, R.T.; Lee, W.M. Management of Acute Liver Failure: Update 2022. Semin. Liver Dis. 2022, 42, 362–378. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, R.; Chen, P. Gut Microbiota and Chemical-Induced Acute Liver Injury. Front. Physiol. 2021, 12, 688780. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.; Chauhan, A. The role of platelet mediated thromboinflammation in acute liver injury. Front. Immunol. 2022, 13, 1037645. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Z.; Wang, Q. Pluripotent Stem Cell-derived Strategies to Treat Acute Liver Failure: Current Status and Future Directions. J. Clin. Transl. Hepatol. 2022, 10, 692–699. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Hirao, H.; Nakamura, K.; Kupiec-Weglinski, J.W. Liver ischaemia-reperfusion injury: A new understanding of the role of innate immunity. Nat. Reviews. Gastroenterol. Hepatol. 2022, 19, 239–256. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, X.; Zhang, H.; Li, S.; Yang, P.; Tan, Q. Relevance of NLRP3 Inflammasome-Related Pathways in the Pathology of Diabetic Wound Healing and Possible Therapeutic Targets. Oxidative Med. Cell. Longev. 2022, 2022, 9687925. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Chatterjee, A.; Gupta, S.; Nagar, A. Activation and Regulation of NLRP3 by Sterile and Infectious Insults. Front. Immunol. 2022, 13, 896353. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Puhach, A.; Frieser, D.; Arunkumar, M.; Lehner, L.; Seeholzer, T.; Garcia-Lopez, A.; van der Wal, M.; Fibi-Smetana, S.; Dietschmann, A.; et al. Human T(H)17 cells engage gasdermin E pores to release IL-1α on NLRP3 inflammasome activation. Nat. Immunol. 2023, 24, 295–308. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, W.; Wu, Q.; Liu, T.; Wei, Y.; Ding, J.; Zhou, C.; Xu, H.; Yang, S. NLRP3 Inflammasome Activation: A Therapeutic Target for Cerebral Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2022, 15, 847440. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2022; in press. [Google Scholar]
- Chen, C.; Xu, P. Activation and Pharmacological Regulation of Inflammasomes. Biomolecules 2022, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Ribeiro, M.; Szabo, G. Role of the Inflammasome in Liver Disease. Annu. Rev. Pathol. 2022, 17, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Gu, Y.; Li, H.; Liang, B.; Han, C.; Zhang, Y.; Liu, Q.; Wei, W.; Ma, Y. NLRP3 inflammasome activation mechanism and its role in autoimmune liver disease. Acta Biochim. Biophys. Sin. 2022, 54, 1577–1586. [Google Scholar] [CrossRef]
- Torres, S.; Segalés, P.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria and the NLRP3 Inflammasome in Alcoholic and Nonalcoholic Steatohepatitis. Cells 2022, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hong, W.; Lu, S.; Li, Y.; Guan, Y.; Weng, X.; Feng, Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front. Pharmacol. 2022, 13, 780496. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, H.; Fu, X.; Chen, C.; Liu, H.; Wang, H.; Wu, D. The Role of Endoplasmic Reticulum Stress and NLRP3 Inflammasome in Liver Disorders. Int. J. Mol. Sci. 2022, 23, 3528. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.L.; Wang, L.; Gardner, C.L.; Corkum, C.P.; Grant, M.D.; Hirasawa, K.; Russell, R.S. Crosstalk Between Pyroptosis and Apoptosis in Hepatitis C Virus-induced Cell Death. Front. Immunol. 2022, 13, 788138. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef]
- Zuo, R.M.; Jiao, J.Y.; Chen, N.; Jiang, X.L.; Wu, Y.L.; Nan, J.X.; Lian, L.H. Carnosic acid suppressed the formation of NETs in alcoholic hepatosteatosis based on P2X7R-NLRP3 axis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 110, 154599. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Cell Death in Development. Cold Spring Harb. Perspect. Biol. 2022, 14, a041095. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Tweedell, R.E.; Kanneganti, T.D. Programming inflammatory cell death for therapy. Pharmacol. Ther. 2022, 232, 108010. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Cockell, S.; Tiniakos, D.; Queen, R.; Younes, R.; Vacca, M.; Alexander, L.; Ravaioli, F.; Palmer, J.; Petta, S.; et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020, 12, eaba4448. [Google Scholar] [CrossRef] [PubMed]
- Chapin, C.A.; Taylor, S.A.; Malladi, P.; Neighbors, K.; Melin-Aldana, H.; Kreiger, P.A.; Bowsher, N.; Schipma, M.J.; Loomes, K.M.; Behrens, E.M.; et al. Transcriptional Analysis of Liver Tissue Identifies Distinct Phenotypes of Indeterminate Pediatric Acute Liver Failure. Hepatol. Commun. 2021, 5, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Nakib, D.; Perciani, C.T.; MacParland, S.A. The immune niche of the liver. Clin. Sci. 2021, 135, 2445–2466. [Google Scholar] [CrossRef]
- Steensels, S.; Qiao, J.; Ersoy, B.A. Transcriptional Regulation in Non-Alcoholic Fatty Liver Disease. Metabolites 2020, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Galle, P.R.; Schuchmann, M. Apoptosis in liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2006, 26, 904–911. [Google Scholar] [CrossRef]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–249. [Google Scholar] [CrossRef]
- Hockenbery, D. Defining apoptosis. Am. J. Pathol. 1995, 146, 16–19. [Google Scholar]
- O’Reilly, L.A.; Strasser, A. Apoptosis and autoimmune disease. Inflamm. Res. 1999, 48, 5–21. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Delhalle, S.; Duvoix, A.; Schnekenburger, M.; Morceau, F.; Dicato, M.; Diederich, M. An introduction to the molecular mechanisms of apoptosis. Ann. NY Acad. Sci. 2003, 1010, 1–8. [Google Scholar] [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2022, 29, 272–284. [Google Scholar] [CrossRef]
- Hague, A.; Paraskeva, C. Apoptosis and disease: A matter of cell fate. Cell Death Differ. 2004, 11, 1366–1372. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.; Sasaki, T.; Elia, A.J.; Cheng, H.Y.; Ravagnan, L.; et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001, 410, 549–554. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [CrossRef]
- Khoury, M.K.; Gupta, K.; Franco, S.R.; Liu, B. Necroptosis in the Pathophysiology of Disease. Am. J. Pathol. 2020, 190, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhong, C.Q.; Zhang, D.W. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat. Immunol. 2011, 12, 1143–1149. [Google Scholar] [CrossRef]
- Vercammen, D.; Beyaert, R.; Denecker, G.; Goossens, V.; Van Loo, G.; Declercq, W.; Grooten, J.; Fiers, W.; Vandenabeele, P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1998, 187, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflammation 2018, 15, 199. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef]
- Speir, M.; Lawlor, K.E. RIP-roaring inflammation: RIPK1 and RIPK3 driven NLRP3 inflammasome activation and autoinflammatory disease. Semin. Cell Dev. Biol. 2021, 109, 114–124. [Google Scholar] [CrossRef]
- Conos, S.A.; Chen, K.W.; De Nardo, D.; Hara, H.; Whitehead, L.; Núñez, G.; Masters, S.L.; Murphy, J.M.; Schroder, K.; Vaux, D.L.; et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA 2017, 114, E961–E969. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Fu, R.; Zhou, M.; Wang, S.; Huang, Y.; Hu, H.; Zhao, J.; Gaskin, F.; Yang, N.; Fu, S.M. Pathogenesis of lupus nephritis: RIP3 dependent necroptosis and NLRP3 inflammasome activation. J. Autoimmun. 2019, 103, 102286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Inuzuka, H.; Wei, W. Necroptosis pathways in tumorigenesis. Semin. Cancer Biol. 2022, 86, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Y.T.; Wang, L.Q.; Li, L.Y.; Bao, Q.; Tian, S.; Chen, M.X.; Chen, H.X.; Cui, J.; Li, C.W. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J. Allergy Clin. Immunol. 2019, 144, 777–787.e9. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef] [PubMed]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Live to die another way: Modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 329–344. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Cui, Y.; Zhang, H.; Zhang, D. Autophagy is associated with cell fate in the process of macrophage-derived foam cells formation and progress. J. Biomed. Sci. 2016, 23, 57. [Google Scholar] [CrossRef]
- Kanayama, M.; Shinohara, M.L. Roles of Autophagy and Autophagy-Related Proteins in Antifungal Immunity. Front. Immunol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef]
- Kovács, A.L.; Pálfia, Z.; Réz, G.; Vellai, T.; Kovács, J. Sequestration revisited: Integrating traditional electron microscopy, de novo assembly and new results. Autophagy 2007, 3, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, H.; Yang, Y.; Wang, H. The Role of Autophagy and Pyroptosis in Liver Disorders. Int. J. Mol. Sci. 2022, 23, 6208. [Google Scholar] [CrossRef] [PubMed]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay Between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Bae, S.H.; Ryu, J.C.; Kwon, Y.; Oh, J.H.; Kwon, J.; Moon, J.S.; Kim, K.; Miyawaki, A.; Lee, M.G.; et al. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 2016, 12, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Jain, A.; Choi, S.W.; Mandell, M.A.; Johansen, T.; Deretic, V. TRIM-directed selective autophagy regulates immune activation. Autophagy 2017, 13, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Spalinger, M.R.; Lang, S.; Gottier, C.; Dai, X.; Rawlings, D.J.; Chan, A.C.; Rogler, G.; Scharl, M. PTPN22 regulates NLRP3-mediated IL1B secretion in an autophagy-dependent manner. Autophagy 2017, 13, 1590–1601. [Google Scholar] [CrossRef]
- Allaeys, I.; Marceau, F.; Poubelle, P.E. NLRP3 promotes autophagy of urate crystals phagocytized by human osteoblasts. Arthritis Res. Ther. 2013, 15, R176. [Google Scholar] [CrossRef]
- Lai, M.; Yao, H.; Shah, S.Z.A.; Wu, W.; Wang, D.; Zhao, Y.; Wang, L.; Zhou, X.; Zhao, D.; Yang, L. The NLRP3-Caspase 1 Inflammasome Negatively Regulates Autophagy via TLR4-TRIF in Prion Peptide-Infected Microglia. Front. Aging Neurosci. 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Nagasu, H.; Murakami, T.; Hoang, H.; Broderick, L.; Hoffman, H.M.; Horng, T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 15514–15519. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- McAtee, C. Drug-Induced Liver Injury. Crit. Care Nurs. Clin. N. Am. 2022, 34, 267–275. [Google Scholar] [CrossRef]
- Jaeschke, H.; Adelusi, O.B.; Akakpo, J.Y.; Nguyen, N.T.; Sanchez-Guerrero, G.; Umbaugh, D.S.; Ding, W.X.; Ramachandran, A. Recommendations for the use of the acetaminophen hepatotoxicity model for mechanistic studies and how to avoid common pitfalls. Acta Pharm. Sin. B 2021, 11, 3740–3755. [Google Scholar] [CrossRef]
- Luo, G.; Huang, L.; Zhang, Z. The molecular mechanisms of acetaminophen-induced hepatotoxicity and its potential therapeutic targets. Exp. Biol. Med. 2023, 15353702221147563. [Google Scholar] [CrossRef]
- Jaeschke, H. Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients. Dig. Dis. 2015, 33, 464–471. [Google Scholar] [CrossRef]
- Bernal, W.; Williams, R. Acute Liver Failure. Clin. Liver Dis. 2020, 16, 45–55. [Google Scholar] [CrossRef]
- González-Recio, I.; Simón, J.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Mercado-Gómez, M.; Rodríguez-Agudo, R.; Lachiondo-Ortega, S.; Gil-Pitarch, C.; Fernández-Rodríguez, C.; Castellana, D.; et al. Restoring cellular magnesium balance through Cyclin M4 protects against acetaminophen-induced liver damage. Nat. Commun. 2022, 13, 6816. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Q.; Ni, J.; Liu, X.; Zhu, J.; Zhou, T.; Huang, H.; OuYang, K.; Wu, Y.; Yang, Z. Gut Microbiota Mediates the Therapeutic Effect of Monoclonal Anti-TLR4 Antibody on Acetaminophen-Induced Acute Liver Injury in Mice. Microbiol. Spectr. 2022, 10, e0064722. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hua, S.; Deng, J.; Du, Z.; Zhang, D.; Liu, Z.; Khan, N.U.; Zhou, M.; Chen, Z. Astaxanthin Activated the Nrf2/HO-1 Pathway to Enhance Autophagy and Inhibit Ferroptosis, Ameliorating Acetaminophen-Induced Liver Injury. ACS Appl. Mater. Interfaces 2022, 14, 42887–42903. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cui, Y.; Wang, J.; Liu, D.; Tian, Y.; Liu, K.; Wang, X.; Liu, L.; He, Y.; Pei, Y.; et al. Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res. Ther. 2022, 13, 94. [Google Scholar] [CrossRef]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef]
- Larson, A.M.; Polson, J.; Fontana, R.J.; Davern, T.J.; Lalani, E.; Hynan, L.S.; Reisch, J.S.; Schiødt, F.V.; Ostapowicz, G.; Shakil, A.O.; et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef]
- Laine, J.E.; Auriola, S.; Pasanen, M.; Juvonen, R.O. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica Fate Foreign Compd. Biol. Syst. 2009, 39, 11–21. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Thorgeirsson, S.S.; Potter, W.Z.; Jollow, D.J.; Keiser, H. Acetaminophen-induced hepatic injury: Protective role of glutathione in man and rationale for therapy. Clin. Pharmacol. Ther. 1974, 16, 676–684. [Google Scholar] [CrossRef]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Animal models of drug-induced liver injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, Q.; Sun, L.; Wu, M.; Li, S.; Hua, H.; Sun, Y.; Ni, T.; Zhou, C.; Huang, S.; et al. Acetaminophen-induced reduction of NIMA-related kinase 7 expression exacerbates acute liver injury. JHEP Rep. Innov. Hepatol. 2022, 4, 100545. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Duan, L.; Akakpo, J.Y.; Farhood, A.; Ramachandran, A. The role of apoptosis in acetaminophen hepatotoxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 709–718. [Google Scholar] [CrossRef]
- Du, Y.C.; Lai, L.; Zhang, H.; Zhong, F.R.; Cheng, H.L.; Qian, B.L.; Tan, P.; Xia, X.M.; Fu, W.G. Kaempferol from Penthorum chinense Pursh suppresses HMGB1/TLR4/NF-κB signaling and NLRP3 inflammasome activation in acetaminophen-induced hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef] [PubMed]
- Elshal, M.; Abdelmageed, M.E. Diacerein counteracts acetaminophen-induced hepatotoxicity in mice via targeting NLRP3/caspase-1/IL-1β and IL-4/MCP-1 signaling pathways. Arch. Pharmacal Res. 2022, 45, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Wang, Z.; Sun, R.; Zou, B.; Li, R.; Liu, D.; Lin, M.; Zhou, J.; Ning, S.; et al. Peroxiredoxin 3 Inhibits Acetaminophen-Induced Liver Pyroptosis Through the Regulation of Mitochondrial ROS. Front. Immunol. 2021, 12, 652782. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.; Graffeo, C.S.; Rokosh, R.; Pansari, M.; Ochi, A.; Levie, E.M.; Van Heerden, E.; Tippens, D.M.; Greco, S.; Barilla, R.; et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 2015, 6, e1759. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shan, S.; Kang, K.; Zhang, C.; Kou, R.; Song, F. The cross-talk of NLRP3 inflammasome activation and necroptotic hepatocyte death in acetaminophen-induced mice acute liver injury. Hum. Exp. Toxicol. 2021, 40, 673–684. [Google Scholar] [CrossRef]
- Huang, J.; Xian, S.; Liu, Y.; Chen, X.; Pu, K.; Wang, H. A Renally Clearable Activatable Polymeric Nanoprobe for Early Detection of Hepatic Ischemia-Reperfusion Injury. Adv. Mater. 2022, 34, e2201357. [Google Scholar] [CrossRef]
- Tang, S.P.; Mao, X.L.; Chen, Y.H.; Yan, L.L.; Ye, L.P.; Li, S.W. Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death. Front. Immunol. 2022, 13, 870239. [Google Scholar] [CrossRef]
- Peng, Y.; Yin, Q.; Yuan, M.; Chen, L.; Shen, X.; Xie, W.; Liu, J. Role of hepatic stellate cells in liver ischemia-reperfusion injury. Front. Immunol. 2022, 13, 891868. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, H.; Han, B.; Xia, Y.; Kong, X.; Gu, J. Natural Killer Cells in Hepatic Ischemia-Reperfusion Injury. Front. Immunol. 2022, 13, 870038. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; He, S.; Mao, X.; Zhang, Y.; Cai, Y.; Li, S. Effect of Hepatic Macrophage Polarization and Apoptosis on Liver Ischemia and Reperfusion Injury During Liver Transplantation. Front. Immunol. 2020, 11, 1193. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Braz, M.; Elias-Miró, M.; Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Ramalho, F.S.; Peralta, C. The current state of knowledge of hepatic ischemia-reperfusion injury based on its study in experimental models. J. Biomed. Biotechnol. 2012, 2012, 298657. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, V.; Thygesen, S.J.; Sester, D.P.; Idris, A.; Cridland, J.A.; Vajjhala, P.R.; Roberts, T.L.; Schroder, K.; Vince, J.E.; Hill, J.M.; et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013, 20, 1149–1160. [Google Scholar] [CrossRef]
- Inoue, Y.; Shirasuna, K.; Kimura, H.; Usui, F.; Kawashima, A.; Karasawa, T.; Tago, K.; Dezaki, K.; Nishimura, S.; Sagara, J.; et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J. Immunol. 2014, 192, 4342–4351. [Google Scholar] [CrossRef]
- Shu, G.; Qiu, Y.; Hao, J.; Fu, Q.; Deng, X. γ-Oryzanol alleviates acetaminophen-induced liver injury: Roles of modulating AMPK/GSK3β/Nrf2 and NF-κB signaling pathways. Food Funct. 2019, 10, 6858–6872. [Google Scholar] [CrossRef]
- Guo, X.X.; Zeng, Z.; Qian, Y.Z.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.P.; Zhou, F. Wheat Flour, Enriched with γ-Oryzanol, Phytosterol, and Ferulic Acid, Alleviates Lipid and Glucose Metabolism in High-Fat-Fructose-Fed Rats. Nutrients 2019, 11, 1697. [Google Scholar] [CrossRef]
- Du, Y.; Zhong, F.; Cheng, H.; Li, T.; Chen, Y.; Tan, P.; Huang, M.; Liang, T.; Liu, Y.; Xia, X.; et al. The Dietary Supplement γ-Oryzanol Attenuates Hepatic Ischemia Reperfusion Injury via Inhibiting Endoplasmic Reticulum Stress and HMGB1/NLRP3 Inflammasome. Oxidative Med. Cell. Longev. 2021, 2021, 4628050. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Rickenbacher, A.; Jang, J.H.; Limani, P.; Ungethüm, U.; Lehmann, K.; Oberkofler, C.E.; Weber, A.; Graf, R.; Humar, B.; Clavien, P.A. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. J. Hepatol. 2014, 61, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Uchida, Y.; Kadono, K.; Hirao, H.; Kawasoe, J.; Watanabe, T.; Ueda, S.; Okajima, H.; Terajima, H.; Uemoto, S. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc. Natl. Acad. Sci. USA 2019, 116, 13533–13542. [Google Scholar] [CrossRef]
- El-Sisi, A.E.E.; Sokar, S.S.; Shebl, A.M.; Mohamed, D.Z.; Abu-Risha, S.E. Octreotide and melatonin alleviate inflammasome-induced pyroptosis through inhibition of TLR4-NF-κB-NLRP3 pathway in hepatic ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2021, 410, 115340. [Google Scholar] [CrossRef]
- Cao, Q.; Luo, J.; Xiong, Y.; Liu, Z.; Ye, Q. 25-Hydroxycholesterol mitigates hepatic ischemia reperfusion injury via mediating mitophagy. Int. Immunopharmacol. 2021, 96, 107643. [Google Scholar] [CrossRef]
- Xue, R.; Qiu, J.; Wei, S.; Liu, M.; Wang, Q.; Wang, P.; Sha, B.; Wang, H.; Shi, Y.; Zhou, J.; et al. Lycopene alleviates hepatic ischemia reperfusion injury via the Nrf2/HO-1 pathway mediated NLRP3 inflammasome inhibition in Kupffer cells. Ann. Transl. Med. 2021, 9, 631. [Google Scholar] [CrossRef]
- Wang, Z.; Han, S.; Chen, X.; Li, X.; Xia, N.; Pu, L. Eva1a inhibits NLRP3 activation to reduce liver ischemia-reperfusion injury via inducing autophagy in kupffer cells. Mol. Immunol. 2021, 132, 82–92. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, W.; Ma, Y. Down-regulation of TRPM2 attenuates hepatic ischemia/reperfusion injury through activation of autophagy and inhibition of NLRP3 inflammasome pathway. Int. Immunopharmacol. 2022, 104, 108443. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, F.; Wu, C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Kamel, R.; El Morsy, E.M. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch. Pharmacal Res. 2013, 36, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wen, X.; Liu, J.; Kan, J.; Qian, C.; Wu, C.; Jin, C. Protective effect of an arabinogalactan from black soybean against carbon tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2018, 117, 659–664. [Google Scholar] [CrossRef]
- Chen, M.; Huang, W.; Wang, C.; Nie, H.; Li, G.; Sun, T.; Yang, F.; Zhang, Y.; Shu, K.; Wang, C.; et al. High-mobility group box 1 exacerbates CCl₄-induced acute liver injury in mice. Clin. Immunol. 2014, 153, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, J.; Xu, S.; Li, J.; Liu, J.; Lu, Y.; Shi, J.; Zhou, S.; Wu, Q. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 117, 109073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuang, G.; Wan, J.; Jiang, R.; Ma, L.; Gong, X.; Liu, X. Salidroside protects mice against CCl4-induced acute liver injury via down-regulating CYP2E1 expression and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 85, 106662. [Google Scholar] [CrossRef]
- Zhao, J.; He, B.; Zhang, S.; Huang, W.; Li, X. Ginsenoside Rg1 alleviates acute liver injury through the induction of autophagy and suppressing NF-κB/NLRP3 inflammasome signaling pathway. Int. J. Med. Sci. 2021, 18, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, H.; Xu, L.; Zhang, W.; Lv, J.; Chen, Y. The amelioration of alcohol-induced liver and intestinal barrier injury by Lactobacillus rhamnosus Gorbach-Goldin (LGG) is dependent on Interleukin 22 (IL-22) expression. Bioengineered 2022, 13, 12650–12660. [Google Scholar] [CrossRef]
- Hughes, E.; Hopkins, L.J.; Parker, R. Survival from alcoholic hepatitis has not improved over time. PLoS ONE 2018, 13, e0192393. [Google Scholar]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 245–254. [Google Scholar]
- Yang, P.; Wang, Z.; Zhan, Y.; Wang, T.; Zhou, M.; Xia, L.; Yang, X.; Zhang, J. Endogenous A1 adenosine receptor protects mice from acute ethanol-induced hepatotoxicity. Toxicology 2013, 309, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Samakidou, A.; Lyberopoulou, A.; Banakou, E.; Gatselis, N.K. Recent advances in the diagnosis and management of autoimmune hepatitis. Pol. Arch. Intern. Med. 2022, 132, 16334. [Google Scholar] [CrossRef]
- Liberal, R.; de Boer, Y.S.; Heneghan, M.A. Established and novel therapeutic options for autoimmune hepatitis. Lancet Gastroenterol. Hepatol. 2021, 6, 315–326. [Google Scholar] [CrossRef]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef]
- Lee, W.Y.; Salmi, M.; Kelly, M.M.; Jalkanen, S.; Kubes, P. Therapeutic advantage of anti-VAP-1 over anti-α4 integrin antibody in concanavalin a-induced hepatitis. Hepatology 2013, 58, 1413–1423. [Google Scholar] [CrossRef]
- Luan, J.; Zhang, X.; Wang, S.; Li, Y.; Fan, J.; Chen, W.; Zai, W.; Wang, S.; Wang, Y.; Chen, M.; et al. NOD-Like Receptor Protein 3 Inflammasome-Dependent IL-1β Accelerated ConA-Induced Hepatitis. Front. Immunol. 2018, 9, 758. [Google Scholar] [CrossRef]
- Aggarwal, M.; Saxena, R.; Sinclair, E.; Fu, Y.; Jacobs, A.; Dyba, M.; Wang, X.; Cruz, I.; Berry, D.; Kallakury, B.; et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016, 23, 1615–1627. [Google Scholar] [CrossRef]
- Ioannides, C.; Konsue, N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab. Rev. 2015, 47, 356–373. [Google Scholar] [CrossRef]
- Wang, J.; Shi, K.; An, N.; Li, S.; Bai, M.; Wu, X.; Shen, Y.; Du, R.; Cheng, J.; Wu, X.; et al. Direct Inhibition of GSDMD by PEITC Reduces Hepatocyte Pyroptosis and Alleviates Acute Liver Injury in Mice. Front. Immunol. 2022, 13, 825428. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.L.; Ni, S.T.; Luo, S.Q.; Hu, B.; Xu, R.; Liu, S.Y.; Huang, X.D.; Zeng, B.; Liang, Q.Q.; Chen, S.Y.; et al. Dimethyl fumarate ameliorates autoimmune hepatitis in mice by blocking NLRP3 inflammasome activation. Int. Immunopharmacol. 2022, 108, 108867. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, H.I.; Kim, S.J.; Park, J.H.; Kim, J.S.; Kim, Y.H.; Lee, S.K.; Kwak, J.H.; Lee, S.M. Protective effect of linarin against D-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur. J. Pharmacol. 2014, 738, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Pervin, M.; Kuramochi, M.; Karim, M.R.; Izawa, T.; Kuwamura, M.; Yamate, J. M1/M2-macrophage Polarization-based Hepatotoxicity in d-galactosamine-induced Acute Liver Injury in Rats. Toxicol. Pathol. 2018, 46, 764–776. [Google Scholar] [CrossRef]
- Gehrke, N.; Wörns, M.A.; Mann, A.; Hövelmeyer, N.; Waisman, A.; Straub, B.K.; Galle, P.R.; Schattenberg, J.M. Hepatocyte Bcl-3 protects from death-receptor mediated apoptosis and subsequent acute liver failure. Cell Death Dis. 2022, 13, 510. [Google Scholar] [CrossRef]
- Tao, Y.C.; Wang, Y.H.; Wang, M.L.; Jiang, W.; Wu, D.B.; Chen, E.Q.; Tang, H. Upregulation of microRNA-125b-5p alleviates acute liver failure by regulating the Keap1/Nrf2/HO-1 pathway. Front. Immunol. 2022, 13, 988668. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Wan, R.Z.; Zhang, P.; Zhang, B. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure in mice. Int. Immunopharmacol. 2017, 46, 124–132. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Xie, S.; Lai, Y.; Mo, C.; Zeng, T.; Kuang, S.; Deng, G.; Zhou, C.; Chen, Y.; et al. Hepatic TGFβr1 Deficiency Attenuates Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure Through Inhibiting GSK3β-Nrf2-Mediated Hepatocyte Apoptosis and Ferroptosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1649–1672. [Google Scholar] [CrossRef]
- Lv, H.; Yang, H.; Wang, Z.; Feng, H.; Deng, X.; Cheng, G.; Ci, X. Nrf2 signaling and autophagy are complementary in protecting lipopolysaccharide/d-galactosamine-induced acute liver injury by licochalcone A. Cell Death Dis. 2019, 10, 313. [Google Scholar] [CrossRef]
- Lv, H.; Fan, X.; Wang, L.; Feng, H.; Ci, X. Daphnetin alleviates lipopolysaccharide/d-galactosamine-induced acute liver failure via the inhibition of NLRP3, MAPK and NF-κB, and the induction of autophagy. Int. J. Biol. Macromol. 2018, 119, 240–248. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Liu, X.; Cai, L.; Qi, J.; Zhang, P.; Li, Y. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2016, 38, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.W.; Pan, Z.Z.; Hu, J.J.; Chen, W.L.; Zhou, G.Y.; Lin, W.; Jin, L.X.; Xu, C.L. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur. J. Pharmacol. 2016, 770, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Huang, J.; Wang, K.; Yu, Q.; Zhu, C.; Ren, H. Pterostilbene Protects Against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Upregulating the Nrf2 Pathway and Inhibiting NF-κB, MAPK, and NLRP3 Inflammasome Activation. J. Med. Food 2020, 23, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, K.; Zhang, P.; Chen, X.; Sun, X.; Li, R. Maresin 1 protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting macrophage pyroptosis and inflammatory response. Biochem. Pharmacol. 2022, 195, 114863. [Google Scholar] [CrossRef]
- Gehrke, N.; Hövelmeyer, N.; Waisman, A.; Straub, B.K.; Weinmann-Menke, J.; Wörns, M.A.; Galle, P.R.; Schattenberg, J.M. Hepatocyte-specific deletion of IL1-RI attenuates liver injury by blocking IL-1 driven autoinflammation. J. Hepatol. 2018, 68, 986–995. [Google Scholar] [CrossRef]
- Bai, L.; Kong, M.; Duan, Z.; Liu, S.; Zheng, S.; Chen, Y. M2-like macrophages exert hepatoprotection in acute-on-chronic liver failure through inhibiting necroptosis-S100A9-necroinflammation axis. Cell Death Dis. 2021, 12, 93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).