Hepatic Glucose Metabolism Disorder Induced by Adipose Tissue-Derived miR-548ag via DPP4 Upregulation
Abstract
:1. Introduction
2. Results
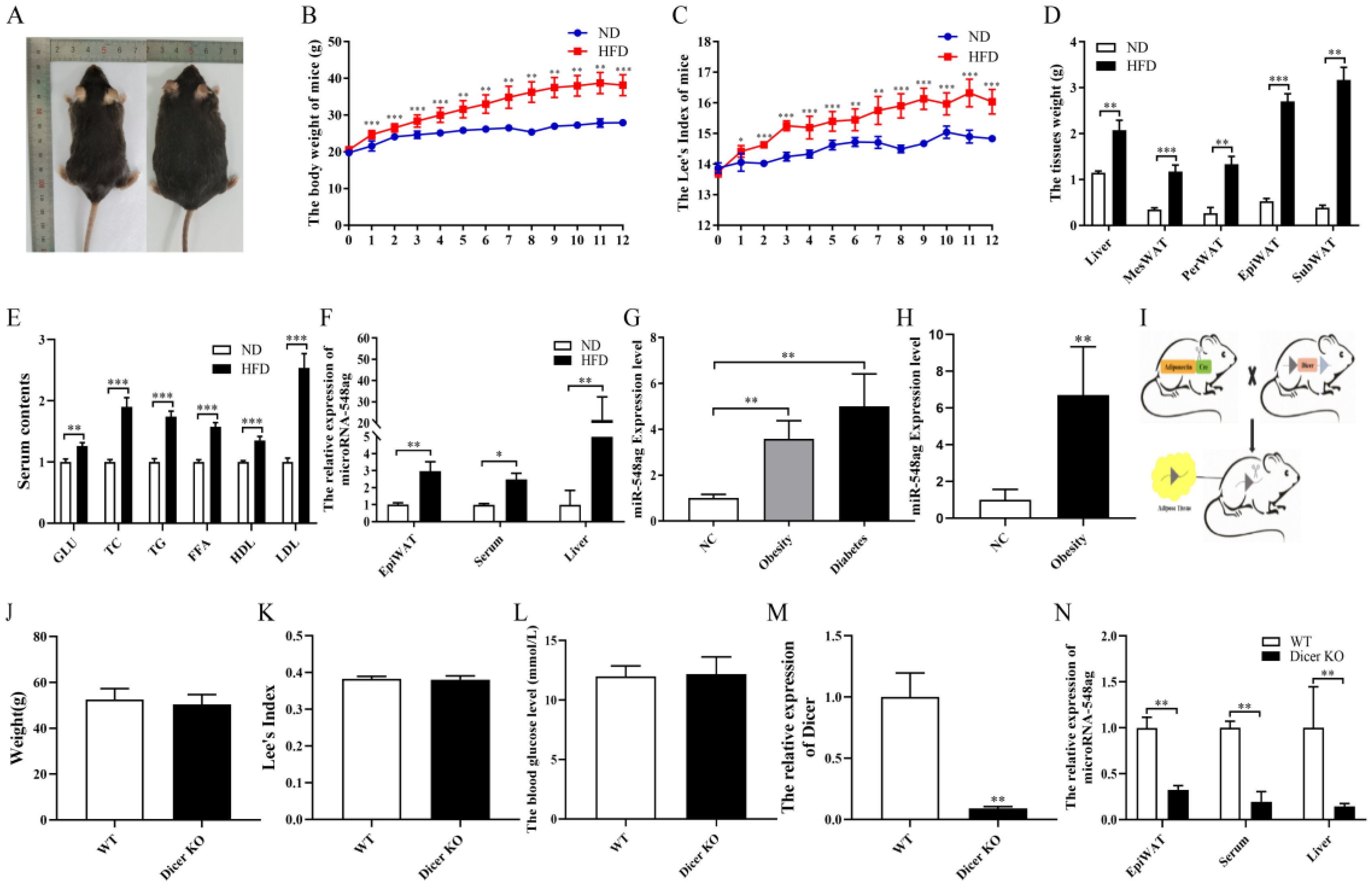
2.1. Adipose-Derived miR-548ag Levels Increased Conspicuously after the Onset of Obesity
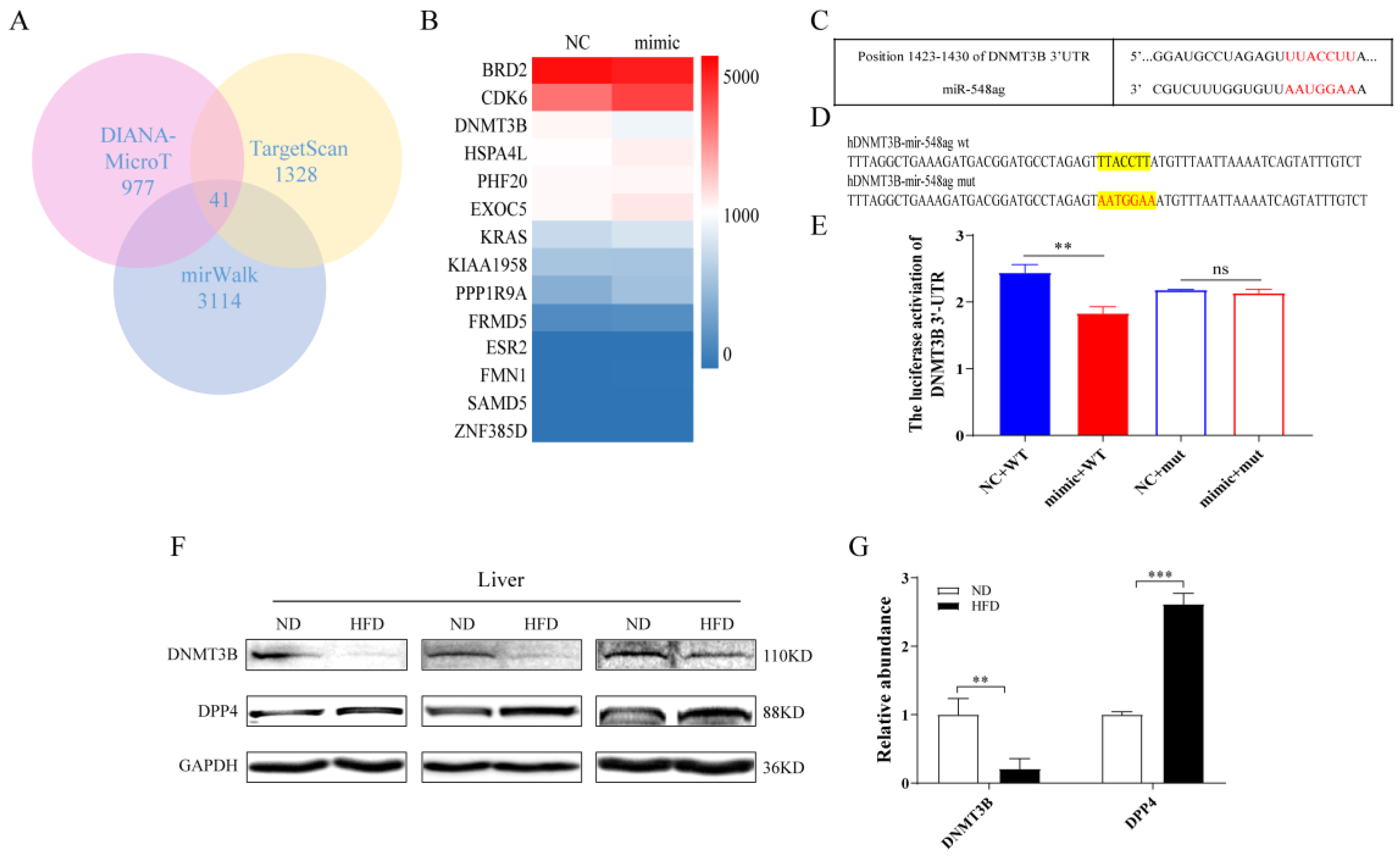
2.2. DNMT3B and DPP4 Could Be the Key Target Genes of miR-548ag
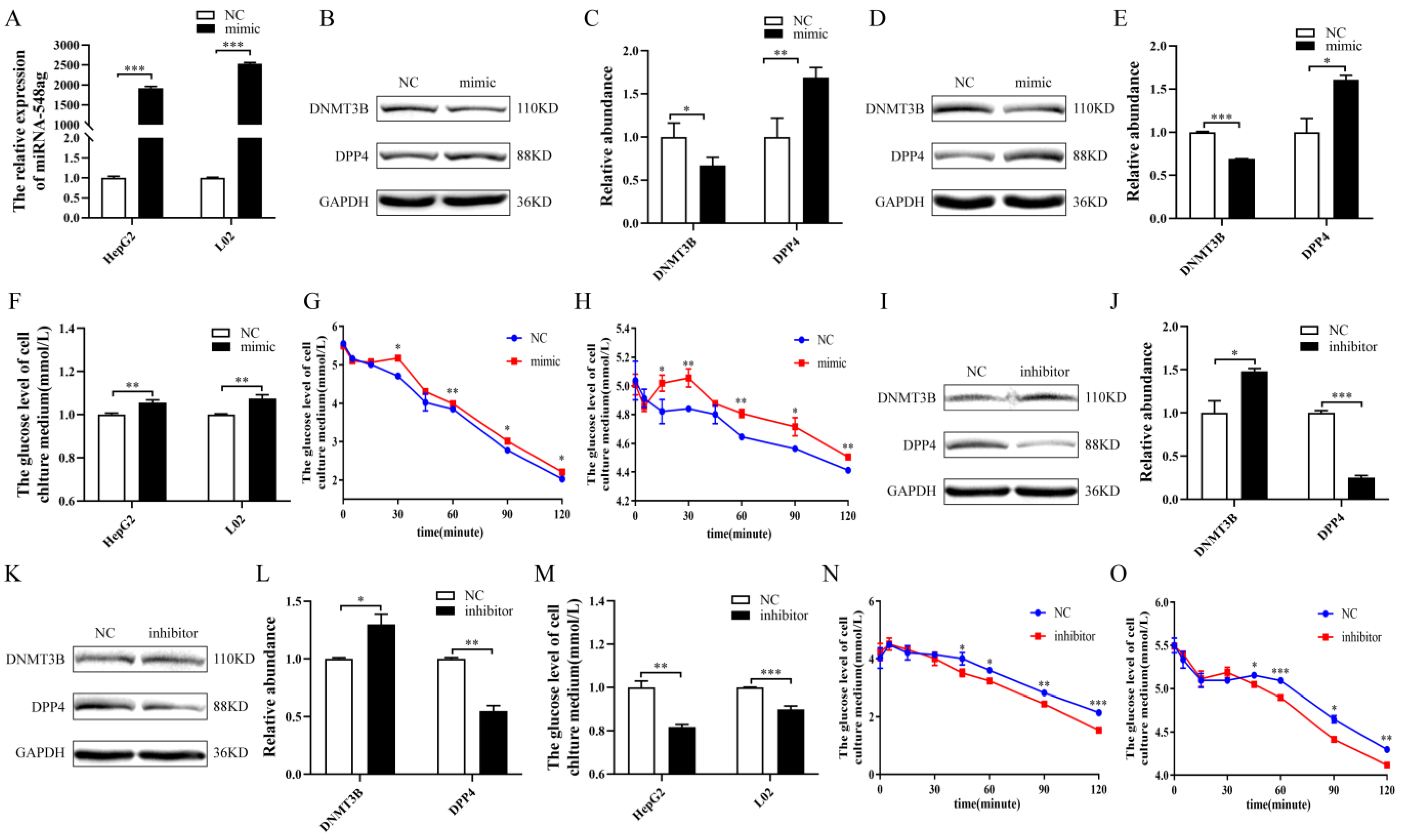
2.3. miR-548ag Impaired the Glucose Metabolism in Hepatocytes via DNMT3B Downregulation to Promote DPP4 Expression
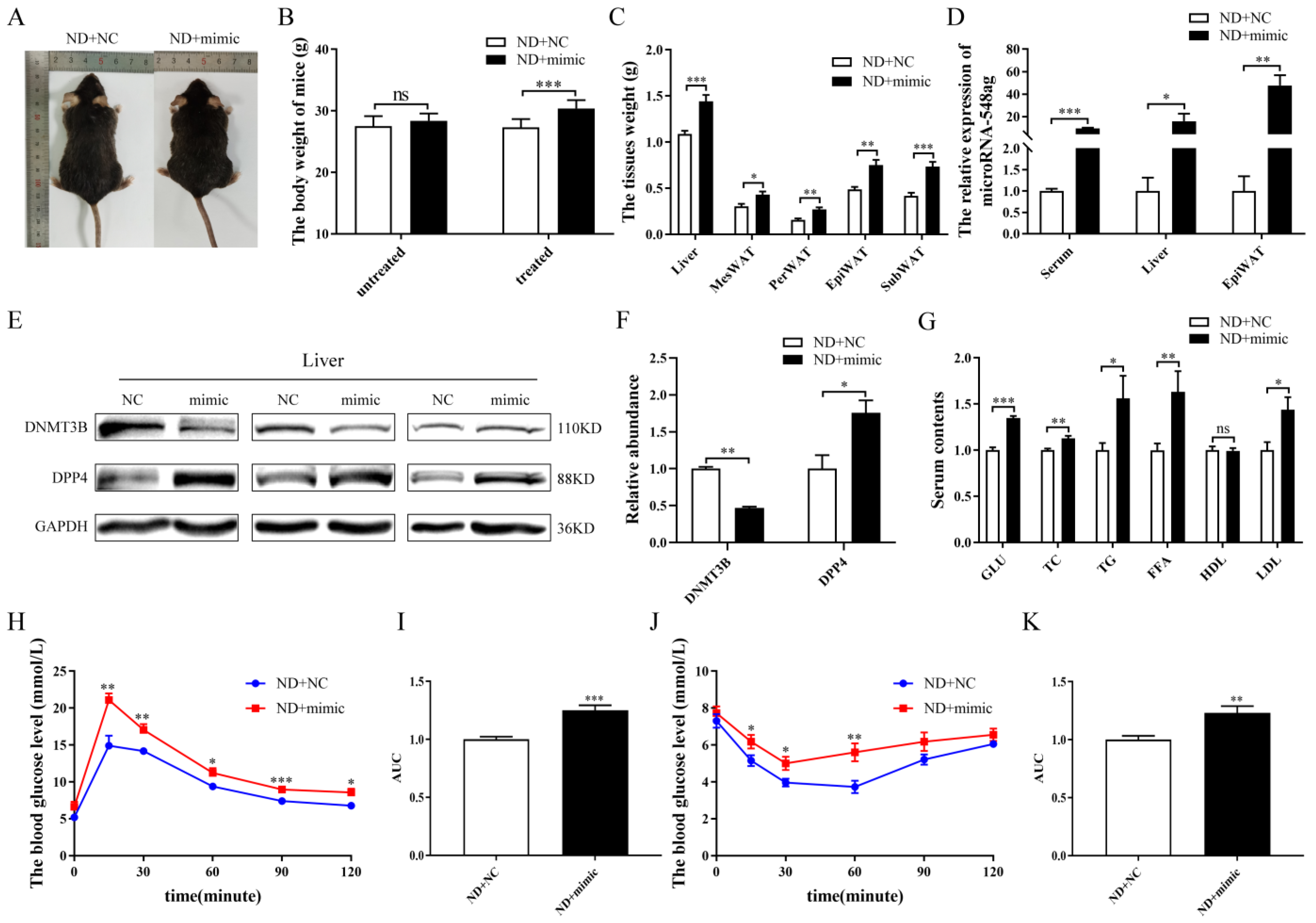
2.4. miR-548ag Impaired the Glucose Metabolism via DNMT3B Downregulation and DPP4 Upregulation In Vivo
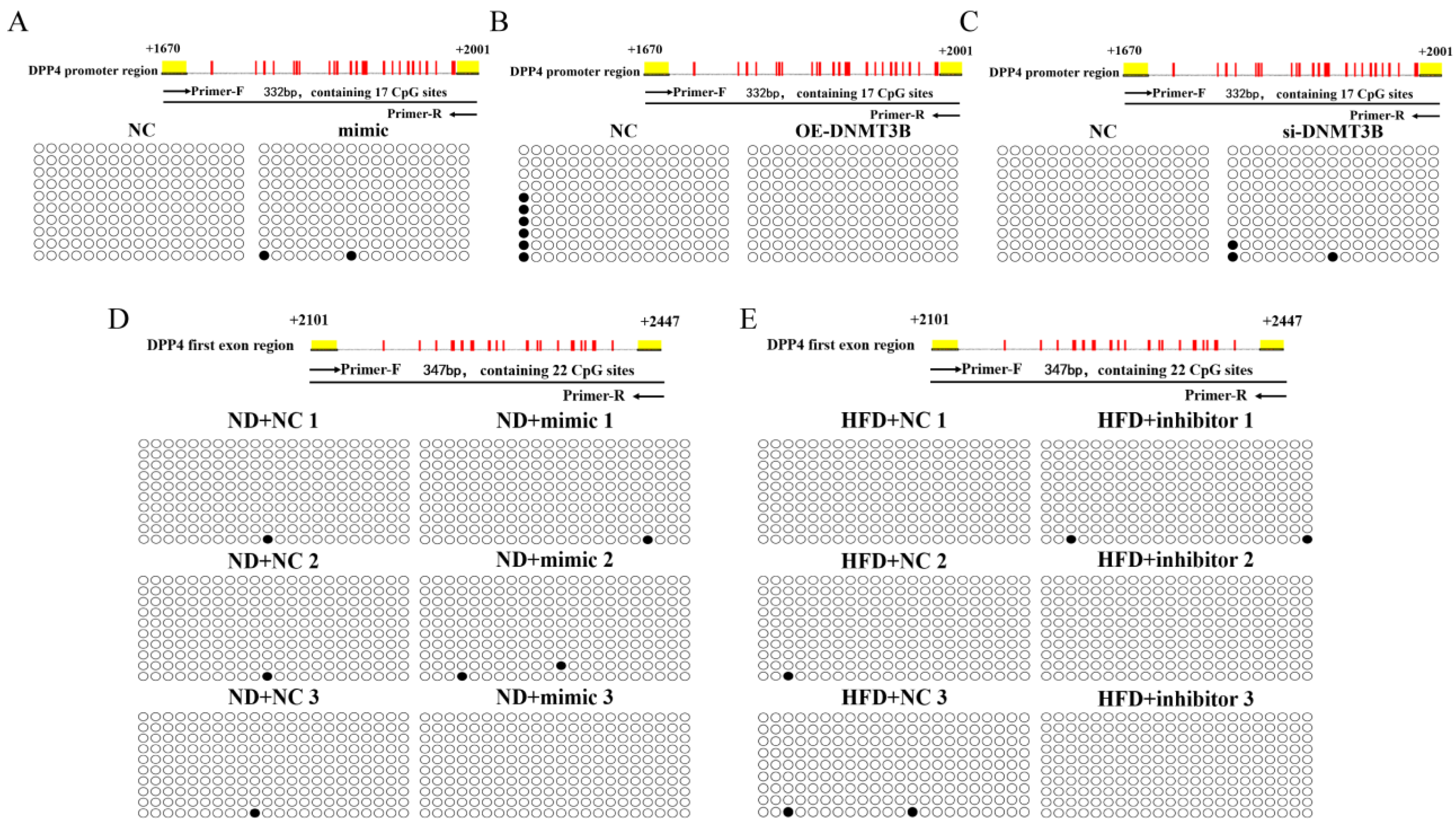
2.5. miR-548ag and DNMT3B Exerted no Significant Effect on the Methylation of the CpG Sites in the Promoter Region of DPP4
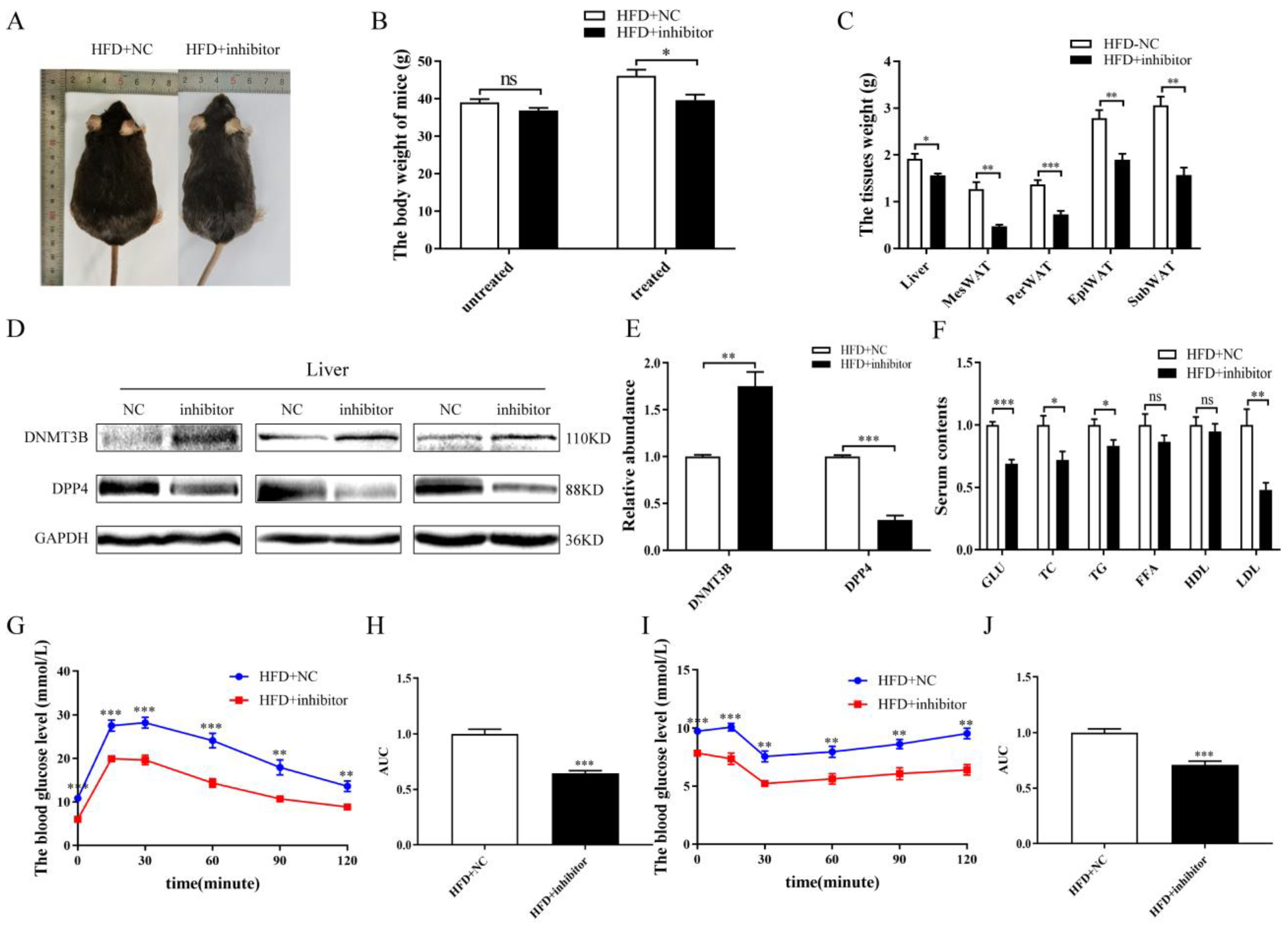
2.6. Inhibition of miR-548ag Ameliorated Glucose Tolerance and Insulin Sensitivity via DNMT3B Upregulation and DPP4 Downregulation in db/db Mice
3. Discussion
4. Material and Methods
4.1. Human Samples
4.2. Measurement of Biochemical Indices
4.3. Animal Care
4.4. Cell Culture
4.5. Adenovirus Amplification and Purification
4.6. Intraperitoneal Injection of the Adenovirus Vector in Mice
4.7. Cell Transfection
4.8. Cellular Glucose Consumption and Insulin Sensitivity Assay
4.9. Western Blotting and Antibodies
4.10. Real-Time PCR
4.11. Dual-Luciferase Reporter Assay
4.12. Plasmid Amplification and Extraction
4.13. Intraperitoneal Glucose Tolerance and Insulin Sensitivity Evaluations in Mice
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BSP | Bisulfite sequencing PCR |
| DNMT3B | DNA Methyltransferase 3B |
| DPP4 | Dipeptidyl peptidase-4 |
| EpiWAT | White adipose tissue of the epididymis |
| HDL | High-density lipoprotein |
| IPGTT | Intraperitoneal glucose tolerance trial |
| IR | Insulin resistance |
| ITT | Insulin tolerance test |
| LDL | Low-density lipoprotein |
| MesWAT | Mesenteric white adipose tissue |
| MiR-548ag | microRNA-548ag |
| PerWAT | Perirenal white adipose tissue |
| PCR | Polymerase chain reaction |
| qRT-PCR | Real-time quantitative polymerase chain reaction |
| SNP | Single nucleotide polymorphism |
| SubWAT | Subcutaneous white adipose tissue |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglyceride |
References
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The colors of adipose tissue. Los colores del tejido adiposo. Gac. Médica México 2020, 156, 142–149. [Google Scholar]
- Ou, X.H.; Zhu, C.C.; Sun, S.C. Effects of obesity and diabetes on the epigenetic modification of mammalian gametes. J. Cell Physiol. 2019, 234, 7847–7855. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipokines—Removing road blocks to obesity and diabetes therapy. Mol Metab. 2014, 3, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, F.; Gupte, M.; Putnam, K.; Cassis, L. Adipokines and blood pressure control. Curr. Opin. Nephrol. Hypertens. 2010, 19, 195–200. [Google Scholar] [CrossRef]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010, 393, 668–672. [Google Scholar] [CrossRef]
- Than, A.; He, H.L.; Chua, S.H.; Xu, D.; Sun, L.; Leow, M.K.; Chen, P. Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes. J. Biol. Chem. 2015, 290, 14679–14691. [Google Scholar] [CrossRef]
- Wattanachanya, L.; Lu, W.D.; Kundu, R.K.; Wang, L.; Abbott, M.J.; O’Carroll, D.; Quertermous, T.; Nissenson, R.A. Increased bone mass in mice lacking the adipokine apelin. Endocrinology 2013, 154, 2069–2080. [Google Scholar] [CrossRef]
- Arner, P.; Kulyté, A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 2015, 11, 276–288. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; Zeiher, A.M.; Dimmeler, S. Circulating microRNAs: Biomarkers or mediators of cardiovascular diseases? Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2383–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, J.T. MicroRNAs: Critical regulators of development, cellular physiology and malignancy. Cell Cycle 2005, 4, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Catalán, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gómez-Ambrosi, J.; Anglada, R.; Fernández-Formoso, J.A.; Ricart, W.; et al. Targeting the circulating microRNA signature of obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, J.; Mei, S.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell Mol. Med. 2017, 21, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Yu, M.; Tian, W. Diverse RNAs in adipose-derived extracellular vesicles and their therapeutic potential. Molecular therapy. Nucleic Acids 2021, 26, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Hussain, N.A.; Zhang, Q.; Chang, C.; Li, H.; Fu, Y.; Cao, L.; Han, W.; Stunkel, W.; Xu, F. miRNA-32 Drives Brown Fat Thermogenesis and Trans-activates Subcutaneous White Fat Browning in Mice. Cell Rep. 2017, 19, 1229–1246. [Google Scholar] [CrossRef]
- Fischer, C.; Seki, T.; Lim, S.; Nakamura, M.; Andersson, P.; Yang, Y.; Honek, J.; Wang, Y.; Gao, Y.; Chen, F.; et al. A miR-327-FGF10-FGFR2-mediated autocrine signaling mechanism controls white fat browning. Nat. Commun. 2017, 8, 2079. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.; Zhou, T.; Chen, C.; Cao, H.; Zhang, J.Z.; Ma, N.; Liu, C.; Song, M.; Shi, J.; et al. DUSP26 induces aortic valve calcification by antagonizing MDM2-mediated ubiquitination of DPP4 in human valvular interstitial cells. Eur. Heart J. 2021, 42, 2935–2951. [Google Scholar] [CrossRef]
- Nishina, S.; Hino, K. CD26/DPP4 as a Therapeutic Target in Nonalcoholic Steatohepatitis Associated Hepatocellular Carcinoma. Cancers 2022, 14, 454. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 642–653. [Google Scholar] [CrossRef]
- Sachinidis, A.; Nikolic, D.; Stoian, A.P.; Papanas, N.; Tarar, O.; Rizvi, A.A.; Rizzo, M. Cardiovascular outcomes trials with incretin-based medications: A critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism 2020, 111, 154343. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological treatment of hyperglycemia in type 2 diabetes. J. Clin. Investig. 2021, 131, e142243. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, K.M.; Montanari, V.; Raman, V.S.; Doyle, J.R.; Harwood, B.N.; Song, Y.C.; Fagan, M.P.; Rios, M.; Haines, D.R.; Kopin, A.S.; et al. A Non-Perturbative Molecular Grafting Strategy for Stable and Potent Therapeutic Peptide Ligands. ACS Cent. Sci. 2021, 7, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Dawwas, G.K.; Flory, J.H.; Hennessy, S.; Leonard, C.E.; Lewis, J.D. Comparative Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Dipeptidyl Peptidase 4 Inhibitors and Sulfonylureas on the Risk of Diabetic Ketoacidosis. Diabetes Care 2022, 45, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Mirna, M.; Paar, V.; Rezar, R.; Topf, A.; Eber, M.; Hoppe, U.C.; Lichtenauer, M.; Jung, C. MicroRNAs in Inflammatory Heart Diseases and Sepsis-Induced Cardiac Dysfunction: A Potential Scope for the Future? Cells 2019, 8, 1352. [Google Scholar] [CrossRef] [PubMed]
- Improta Caria, A.C.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; Souza, B.S.F. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Berindan-Neagoe, I.; del C Monroig, P.; Pasculli, B.; Calin, G.A. MicroRNAome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar]
- Miyazaki, M.; Kato, M.; Tanaka, K.; Tanaka, M.; Kohjima, M.; Nakamura, K.; Enjoji, M.; Nakamuta, M.; Kotoh, K.; Takayanagi, R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol. Med. Rep. 2012, 5, 729–733. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kang, Y.M.; Lee, S.E.; Lee, J.; Park, J.Y.; Lee, W.J.; Kim, Y.J.; Jung, C.H. Efficacy and safety of combination therapy with SGLT2 and DPP4 inhibitors in the treatment of type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2018, 44, 393–401. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Dahlet, T.; Argüeso Lleida, A.; Al Adhami, H.; Dumas, M.; Bender, A.; Ngondo, R.P.; Tanguy, M.; Vallet, J.; Auclair, G.; Bardet, A.F.; et al. Genome-wide analysis in the mouse embryo reveals the importance of DNA methylation for transcription integrity. Nat. Commun. 2020, 11, 3153. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Z.; Liu, X.; Wei, D.; Liu, P.; Nie, L.; Fan, K.; Kang, N.; Song, Y.; Xu, Q.; et al. Combined effects of progesterone and SOCS3 DNA methylation on T2DM: A case-control study. Clin. Epigenetics 2021, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.K.; Kumar, M.; Shanmugam, M.K.; Bhattacharya, A.; Rao, V.J.; Bhat, A.; Vasudevan, M.; Gopinath, K.S.; Mohiyuddin, A.; Chatterjee, A.; et al. Functional interplay between YY1 and CARM1 promotes oral carcinogenesis. Oncotarget 2019, 10, 3709–3724. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C.; Calkhoven, C.F. Emerging Role of C/EBPβ and Epigenetic DNA Methylation in Ageing. Trends Genet. 2020, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.; Harland, L.; Mustoe, T.; Williams, S.; Holdstock, J.; Yague, E.; Mulcahy, T.; Griffiths, M.; Edwards, S.; Ioannou, P.A.; et al. Transgenes encompassing dual-promoter CpG islands from the human TBP and HNRPA2B1 loci are resistant to heterochromatin-mediated silencing. Genomics 2003, 82, 269–279. [Google Scholar] [CrossRef]
- Mžik, M.; Chmelařová, M.; John, S.; Laco, J.; Slabý, O.; Kiss, I.; Bohovicová, L.; Palička, V.; Nekvindová, J. Aberrant methylation of tumour suppressor genes WT1, GATA5 and PAX5 in hepatocellular carcinoma. Clin. Chem. Lab Med. 2016, 54, 1971–1980. [Google Scholar] [CrossRef]
- Jin, L.; Ma, X.; Lei, X.; Tong, J.; Wang, R. Cyclophosphamide inhibits Pax5 methylation to regulate the growth of retinoblastoma via the Notch1 pathway. Hum. Exp. Toxicol. 2021, 40, S497–S508. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef]
- Öner, D.; Ghosh, M.; Bové, H.; Moisse, M.; Boeckx, B.; Duca, R.C.; Poels, K.; Luyts, K.; Putzeys, E.; Van Landuydt, K.; et al. Differences in MWCNT- and SWCNT-induced DNA methylation alterations in association with the nuclear deposition. Part Fibre Toxicol. 2018, 15, 11. [Google Scholar] [CrossRef]
- Chung, M.C.; Hung, P.H.; Hsiao, P.J.; Wu, L.Y.; Chang, C.H.; Wu, M.J.; Shieh, J.J.; Chung, C.J. Association of Sodium-Glucose Transport Protein 2 Inhibitor Use for Type 2 Diabetes and Incidence of Gout in Taiwan. JAMA Netw. Open 2021, 4, e2135353. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Lam, C.S.P.; Kim, D.J.; Cavender, M.A.; Norhammar, A.; Jørgensen, M.E.; Birkeland, K.I.; Holl, R.W.; Franch-Nadal, J.; Tangri, N.; et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: An analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020, 8, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, A.; Lair, A.; Arnaud, M.; Bégaud, B.; Raschi, E.; Pariente, A.; Salvo, F. DPP-4 inhibitors and venous thromboembolism: An analysis of the WHO spontaneous reporting database. Lancet Diabetes Endocrinol. 2020, 8, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, X.; Hou, Y.; Zhang, X.; Li, M.; Ma, D.; Tang, Y.; Yuan, C.; Sun, C.; Liang, M.; Liu, J.; et al. Hepatic Glucose Metabolism Disorder Induced by Adipose Tissue-Derived miR-548ag via DPP4 Upregulation. Int. J. Mol. Sci. 2023, 24, 2964. https://doi.org/10.3390/ijms24032964
Chu X, Hou Y, Zhang X, Li M, Ma D, Tang Y, Yuan C, Sun C, Liang M, Liu J, et al. Hepatic Glucose Metabolism Disorder Induced by Adipose Tissue-Derived miR-548ag via DPP4 Upregulation. International Journal of Molecular Sciences. 2023; 24(3):2964. https://doi.org/10.3390/ijms24032964
Chicago/Turabian StyleChu, Xiaolong, Yanting Hou, Xueting Zhang, Menghuan Li, Dingling Ma, Yihan Tang, Chenggang Yuan, Chaoyue Sun, Maodi Liang, Jie Liu, and et al. 2023. "Hepatic Glucose Metabolism Disorder Induced by Adipose Tissue-Derived miR-548ag via DPP4 Upregulation" International Journal of Molecular Sciences 24, no. 3: 2964. https://doi.org/10.3390/ijms24032964
APA StyleChu, X., Hou, Y., Zhang, X., Li, M., Ma, D., Tang, Y., Yuan, C., Sun, C., Liang, M., Liu, J., Wei, Q., Chang, Y., Wang, C., & Zhang, J. (2023). Hepatic Glucose Metabolism Disorder Induced by Adipose Tissue-Derived miR-548ag via DPP4 Upregulation. International Journal of Molecular Sciences, 24(3), 2964. https://doi.org/10.3390/ijms24032964





