The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles
Abstract
1. Introduction

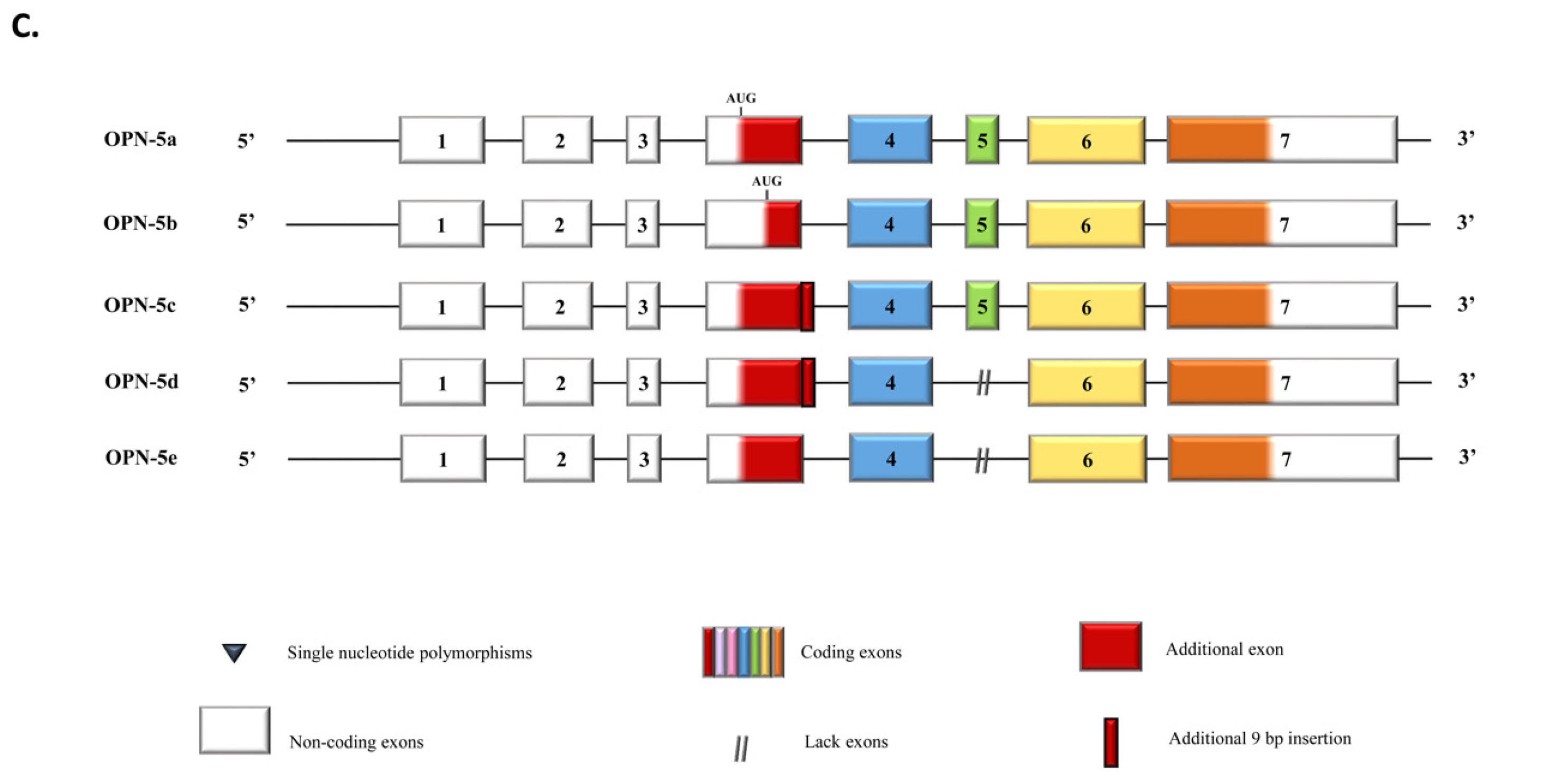

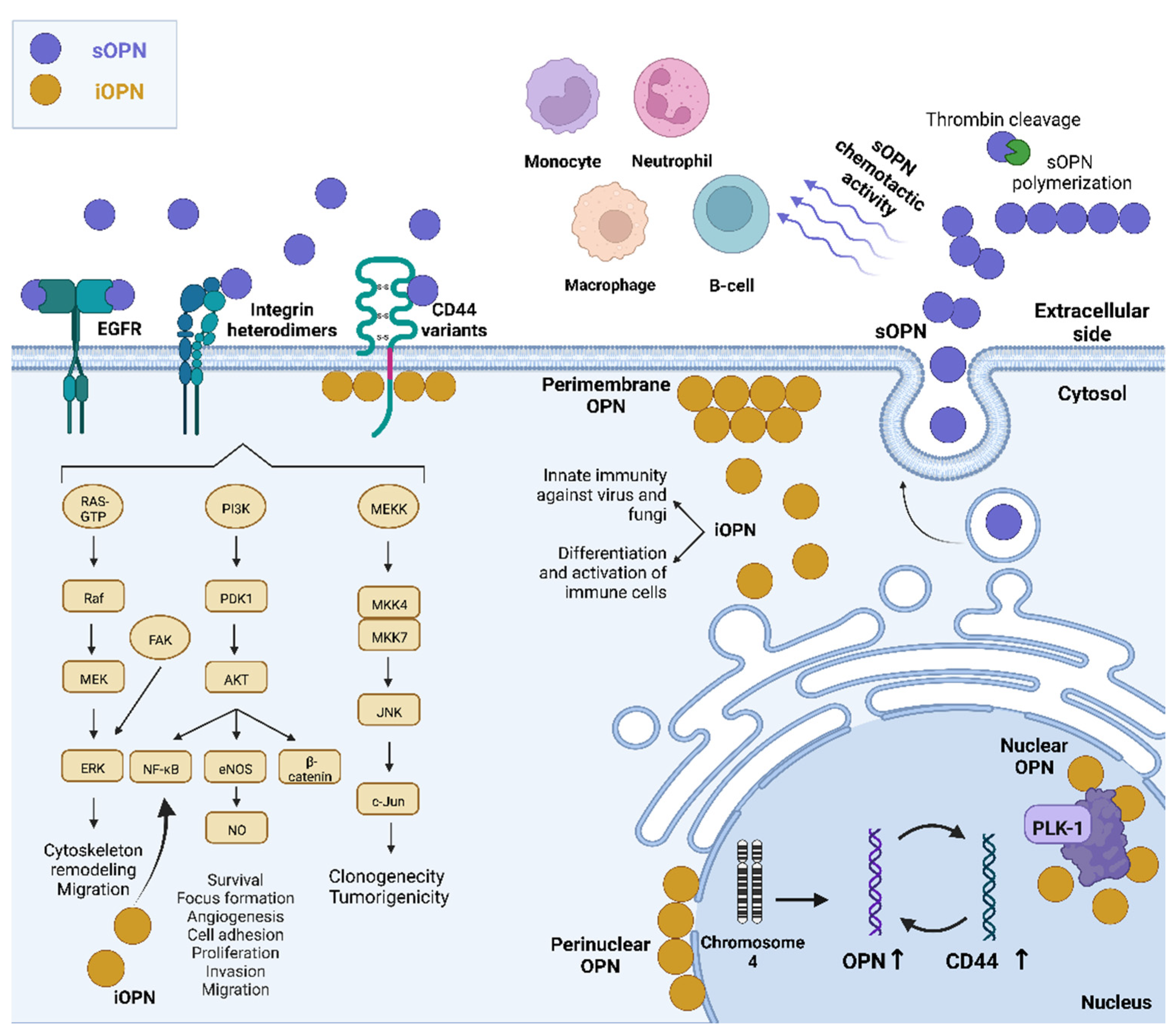
2. Secreted Roles of OPN
2.1. Secreted OPN Activities Mediated by the Integrin Receptors
2.2. Secreted OPN Activities Mediated by the CD44 Receptor
2.3. Secreted OPN Activities and Transactivation of EGFR
2.4. Secreted OPN Activities Mediated by More Than One Receptor
3. Intracellular OPN and Diseases
| iOPN Expression Pattern/Role | Main Findings | Cell Type | Approach/Methodology | Reference |
|---|---|---|---|---|
| OPN displayed a perinuclear and a perimembraneous distribution | iOPN was associated with stages of osteogenic cell differentiation | Fetal rat calvarial cells | Single-cell analysis, flow cytometry, and confocal microscopy | [62] |
| iOPN localizes in the cytoplasm but outside secretory vesicles | iOPN is translated from a non-AUG codon, accompanied by deletion of the N-terminal 16-aa signal sequence. | Dendritic and T cells | R’ RLM-RACE, in vitro translation, confocal microscopy, Ifna4 promoter reporter assay, isolation of secretory vesicles; in vivo assays, ELISA and intracellular cytokine flow cytometry, immunoblotting, RT-qPCR | [25,26] |
| Soluble OPN altered the actin cytoskeleton of tumor cells | iOPN induces rapid Tyr-418 dephosphorylation of c-Src, with decreases in actin stress fibers and increased binding to the vascular endothelium | Human melanoma and sarcoma cell lines; and patient-derived samples | Functional assays in vitro, in tumor cells, in mouse models, and ex vivo, using exogenous OPN or negative controls, including a site-directed mutant OPN | [72] |
| iOPN co-localizes with fungal PRRs in macrophages stimulated by Pneumocystis | iOPN is essential for generating antifungal innate immune responses in PRR recognition, signal transduction, phagocytosis, clearance of Pneumocystis, and cytokine production | Cells obtained from C57BL/6, Opn−/−, Rag2−/−, and Opn−/− Rag2−/− mice | RT-qPCR, confocal microscopy, immunoprecipitation, immunoblotting, ELISA, and analyses of phagocytosis, ROS production, and Pneumocystis clearance | [73] |
| iOPN expression is enhanced by VSV and SeV virus infection | iOPN acts as a positive regulator in innate antiviral immunity through the stabilization of TRAF3 | iOPN ectopic overexpression or OPN deficiency or knockdown; SPP1 knockout mice; Immunoprecipitation and immunoblotting; ELISA; RT-qPCR; ubiquitination assays | [74] |
4. Intracellular OPN and Immunity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Transl. Med. 2022, 2, 419–447. [Google Scholar] [CrossRef]
- Adu-Agyeiwaah, Y.; Grant, M.B.; Obukhov, A.G. The Potential Role of Osteopontin and Furin in Worsening Disease Outcomes in COVID-19 Patients with Pre-Existing Diabetes. Cells 2020, 9, 2528. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. The Multiple Functions and Mechanisms of Osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Nanda, R.; Mateos, B.; Hazan, S.; Matlahov, I.; Perelshtein, I.; Keinan-Adamsky, K.; Althoff-Ospelt, G.; Konrat, R.; Goobes, G. Osteopontin Regulates Biomimetic Calcium Phosphate Crystallization from Disordered Mineral Layers Covering Apatite Crystallites. Sci. Rep. 2020, 10, 15722. [Google Scholar] [CrossRef]
- Amar, A.; Afzal, A.; Hameed, A.; Ahmad, M.; Khan, A.R.; Najma, H.; Abid, A.; Khaliq, S. Osteopontin Promoter Polymorphisms and Risk of Urolithiasis: A Candidate Gene Association and Meta-Analysis Study. BMC Med. Genet. 2020, 21, 172. [Google Scholar] [CrossRef]
- Cao, D.-X.; Li, Z.-J.; Jiang, X.-O.; Lum, Y.L.; Khin, E.; Lee, N.P.; Wu, G.-H.; Luk, J.M. Osteopontin as Potential Biomarker and Therapeutic Target in Gastric and Liver Cancers. World J. Gastroenterol. 2012, 18, 3923–3930. [Google Scholar] [CrossRef]
- Peraramelli, S.; Zhou, Q.; Zhou, Q.; Wanko, B.; Zhao, L.; Nishimura, T.; Leung, T.H.; Mizuno, S.; Ito, M.; Myles, T.; et al. Thrombin Cleavage of Osteopontin Initiates Osteopontin’s Tumor-Promoting Activity. J. Thromb. Haemost. 2022, 20, 1256–1270. [Google Scholar] [CrossRef]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.-K. Osteopontin Splice Variants and Polymorphisms in Cancer Progression and Prognosis. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 93–108. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef]
- Chou, C.-F.; Huang, C.C.; Bin Dabil, N.; Chang, P.-L. Assessing SPP1/Osteopontin (OPN) Splice Variants and Their Association to Nonmelanoma Skin Cancer by Absolute Quantification: Identification of OPN-5 Subvariants and Their Protein Coding Potential. Cancer Investig. 2021, 39, 559–570. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in Immune Regulation and Stress Responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef]
- Kurzbach, D.; Platzer, G.; Schwarz, T.C.; Henen, M.A.; Konrat, R.; Hinderberger, D. Cooperative Unfolding of Compact Conformations of the Intrinsically Disordered Protein Osteopontin. Biochemistry 2013, 52, 5167–5175. [Google Scholar] [CrossRef]
- Lu, H.; Ng, D.Y.W.; Lieberwirth, I.; Weidner, T.; Bonn, M. Intrinsically Disordered Osteopontin Fragment Orders During Interfacial Calcium Oxalate Mineralization. Angew. Chem. Int. Ed. Engl. 2021, 60, 18577–18581. [Google Scholar] [CrossRef]
- Gimba, E.R.; Tilli, T.M. Human Osteopontin Splicing Isoforms: Known Roles, Potential Clinical Applications and Activated Signaling Pathways. Cancer Lett. 2013, 331, 11–17. [Google Scholar] [CrossRef]
- Silva, G.R.; Mattos, D.S.; Bastos, A.C.F.; Viana, B.P.P.B.; Brum, M.C.M.; Ferreira, L.B.; Gimba, E.R.P. Osteopontin-4 and Osteopontin-5 Splice Variants Are Expressed in Several Tumor Cell Lines. Mol. Biol. Rep. 2020, 47, 8339–8345. [Google Scholar] [CrossRef]
- Gimba, E.R.P.; Brum, M.C.M.; De Moraes, G.N. Full-Length Osteopontin and Its Splice Variants as Modulators of Chemoresistance and Radioresistance (Review). Int. J. Oncol. 2019, 54, 420–430. [Google Scholar] [CrossRef]
- Da Fonseca Bastos, A.C.S.; da Silva Rezende, A.C.; Ferreira, L.B.; Blunck, C.B.; Pombo-de-Oliveira, M.S.; Emerenciano, M.; Gimba, E.R.P. Osteopontin-c Is Overexpressed in KMT2A-AFF1 Positive Pediatric B-Cell Lymphoblastic Leukemia When Compared to Those with ETV6-RUNX1. Leuk. Res. 2020, 91, 106316. [Google Scholar] [CrossRef]
- Kamalabadi-Farahani, M.; Atashi, A.; Jabbarpour, Z.; Aghayan, S.S. Expression of Osteopontin-5 Splice Variant in the Mouse Primary and Metastatic Breast Cancer Cells. BMC Res. Notes 2022, 15, 286. [Google Scholar] [CrossRef]
- Grau, J.B.; Poggio, P.; Sainger, R.; Vernick, W.J.; Seefried, W.F.; Branchetti, E.; Field, B.C.; Bavaria, J.E.; Acker, M.A.; Ferrari, G. Analysis of Osteopontin Levels for the Identification of Asymptomatic Patients with Calcific Aortic Valve Disease. Ann. Thorac. Surg. 2012, 93, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Svezia, B.; Matteucci, M.; Botta, L.; Pucci, A.; Rinaldi, M.; Caselli, C.; Lionetti, V.; Del Ry, S. Myocardial Expression Analysis of Osteopontin and Its Splice Variants in Patients Affected by End-Stage Idiopathic or Ischemic Dilated Cardiomyopathy. PLoS ONE 2016, 11, e0160110. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Salazar, H.F.; Joseph, G.; Lok, Z.S.Y.; Caroti, C.M.; Weiss, D.; Taylor, W.R.; Lyle, A.N. Osteopontin Isoforms Differentially Promote Arteriogenesis in Response to Ischemia via Macrophage Accumulation and Survival. Lab. Investig. 2019, 99, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Many, G.M.; Yokosaki, Y.; Uaesoontrachoon, K.; Nghiem, P.P.; Bello, L.; Dadgar, S.; Yin, Y.; Damsker, J.M.; Cohen, H.B.; Kornegay, J.N.; et al. OPN-a Induces Muscle Inflammation by Increasing Recruitment and Activation of pro-Inflammatory Macrophages. Exp. Physiol. 2016, 101, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.L.; Kim, H.-J.; Kim, J.-H.; Garcia, V.A.; Cantor, H. Alternative Translation of Osteopontin Generates Intracellular and Secreted Isoforms That Mediate Distinct Biological Activities in Dendritic Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239. [Google Scholar] [CrossRef]
- Cantor, H.; Shinohara, M.L. Regulation of T-Helper-Cell Lineage Development by Osteopontin: The inside Story. Nat. Rev. Immunol. 2009, 9, 137–141. [Google Scholar] [CrossRef]
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human Osteopontin: Potential Clinical Applications in Cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef]
- Göthlin Eremo, A.; Lagergren, K.; Othman, L.; Montgomery, S.; Andersson, G.; Tina, E. Evaluation of SPP1/Osteopontin Expression as Predictor of Recurrence in Tamoxifen Treated Breast Cancer. Sci. Rep. 2020, 10, 1451. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Hiller, K.; Grote, A.; Scheer, M.; Münch, R.; Jahn, D. PrediSi: Prediction of Signal Peptides and Their Cleavage Positions. Nucleic Acids Res. 2004, 32, W375–W379. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of Protein Subcellular Localization Using Deep Learning. Bioinformatics 2017, 33, 4049. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Yao, Y.; Eubel, H.; Künzler, P.; Møller, I.M.; Xu, D. MULocDeep: A Deep-Learning Framework for Protein Subcellular and Suborganellar Localization Prediction with Residue-Level Interpretation. Comput. Struct. Biotechnol. J. 2021, 19, 4825–4839. [Google Scholar] [CrossRef]
- Brameier, M.; Krings, A.; MacCallum, R.M. NucPred—Predicting Nuclear Localization of Proteins. Bioinformatics 2007, 23, 1159–1160. [Google Scholar] [CrossRef]
- Chen, L.; Huan, X.; Xiao, G.-H.; Yu, W.-H.; Li, T.-F.; Gao, X.-D.; Zhang, Y.-C. Osteopontin and Its Downstream Carcinogenic Molecules: Regulatory Mechanisms and Prognostic Value in Cancer Progression. Neoplasma 2022, 69, 1253–1269. [Google Scholar] [CrossRef]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The Role of Osteopontin in Inflammatory Processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef]
- O’Regan, A.; Berman, J.S. Osteopontin: A Key Cytokine in Cell-Mediated and Granulomatous Inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef]
- Khongsti, K.; Das, K.B.; Das, B. MAPK Pathway and SIRT1 Are Involved in the Down-Regulation of Secreted Osteopontin Expression by Genistein in Metastatic Cancer Cells. Life Sci. 2021, 265, 118787. [Google Scholar] [CrossRef]
- Rangaswami, H.; Kundu, G.C. Osteopontin Stimulates Melanoma Growth and Lung Metastasis through NIK/MEKK1-Dependent MMP-9 Activation Pathways. Oncol. Rep. 2007, 18, 909–915. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Lu, X.; Qian, C.; Zhang, J.; Li, P.; Shi, L.; Zhao, P.; Fu, Z.; Pu, P.; et al. Overexpression of Osteopontin Induces Angiogenesis of Endothelial Progenitor Cells via the Avβ3/PI3K/AKT/ENOS/NO Signaling Pathway in Glioma Cells. Eur. J. Cell Biol. 2011, 90, 642–648. [Google Scholar] [CrossRef]
- Lund, S.A.; Wilson, C.L.; Raines, E.W.; Tang, J.; Giachelli, C.M.; Scatena, M. Osteopontin Mediates Macrophage Chemotaxis via A4 and A9 Integrins and Survival via the A4 Integrin. J. Cell. Biochem. 2013, 114, 1194–1202. [Google Scholar] [CrossRef]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-Driven Osteopontin Contributes to Breast Tumor Growth through Modulation of HIF1α-Mediated VEGF-Dependent Angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cui, Y.; Hu, M.U.; Zhi, X.; Zhang, L.; Li, W.; Wu, W.; Cheng, S.; Jiang, W.G. OPN-a Splicing Variant Expression in Non-Small Cell Lung Cancer and Its Effects on the Bone Metastatic Abilities of Lung Cancer Cells In Vitro. Anticancer Res. 2017, 37, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Myers, A.L.; Wang, Z.; Nancarrow, D.J.; Ferrer-Torres, D.; Handlogten, A.; Leverenz, K.; Bao, J.; Thomas, D.G.; Wang, T.D.; et al. Osteopontin (OPN/SPP1) Isoforms Collectively Enhance Tumor Cell Invasion and Dissemination in Esophageal Adenocarcinoma. Oncotarget 2015, 6, 22239–22257. [Google Scholar] [CrossRef] [PubMed]
- Jámbor, K.; Koroknai, V.; Kiss, T.; Szász, I.; Pikó, P.; Balázs, M. Gene Expression Patterns of Osteopontin Isoforms and Integrins in Malignant Melanoma. Pathol. Oncol. Res. 2022, 28, 1610608. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin Signaling Upregulates Cyclooxygenase-2 Expression in Tumor-Associated Macrophages Leading to Enhanced Angiogenesis and Melanoma Growth via A9β1 Integrin. Oncogene 2015, 34, 5408–5410. [Google Scholar] [CrossRef]
- Schack, L.; Stapulionis, R.; Christensen, B.; Kofod-Olsen, E.; Skov Sørensen, U.B.; Vorup-Jensen, T.; Sørensen, E.S.; Höllsberg, P. Osteopontin Enhances Phagocytosis through a Novel Osteopontin Receptor, the AlphaXbeta2 Integrin. J. Immunol. 2009, 182, 6943–6950. [Google Scholar] [CrossRef]
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin Promotes Mesenchymal Stem Cell Migration and Lessens Cell Stiffness via Integrin Β1, FAK, and ERK Pathways. Cell Biochem. Biophys. 2013, 65, 455–462. [Google Scholar] [CrossRef]
- Teramoto, H.; Castellone, M.D.; Malek, R.L.; Letwin, N.; Frank, B.; Gutkind, J.S.; Lee, N.H. Autocrine Activation of an Osteopontin-CD44-Rac Pathway Enhances Invasion and Transformation by H-RasV12. Oncogene 2005, 24, 489–501. [Google Scholar] [CrossRef]
- Castellone, M.D.; Celetti, A.; Guarino, V.; Cirafici, A.M.; Basolo, F.; Giannini, R.; Medico, E.; Kruhoffer, M.; Orntoft, T.F.; Curcio, F.; et al. Autocrine Stimulation by Osteopontin Plays a Pivotal Role in the Expression of the Mitogenic and Invasive Phenotype of RET/PTC-Transformed Thyroid Cells. Oncogene 2004, 23, 2188–2196. [Google Scholar] [CrossRef]
- Guarino, V.; Faviana, P.; Salvatore, G.; Castellone, M.D.; Cirafici, A.M.; De Falco, V.; Celetti, A.; Giannini, R.; Basolo, F.; Melillo, R.M.; et al. Osteopontin Is Overexpressed in Human Papillary Thyroid Carcinomas and Enhances Thyroid Carcinoma Cell Invasiveness. J. Clin. Endocrinol. Metab. 2005, 90, 5270–5278. [Google Scholar] [CrossRef][Green Version]
- Phillips, R.J.; Helbig, K.J.; Van der Hoek, K.H.; Seth, D.; Beard, M.R. Osteopontin Increases Hepatocellular Carcinoma Cell Growth in a CD44 Dependant Manner. World J. Gastroenterol. 2012, 18, 3389–3399. [Google Scholar] [CrossRef]
- Rao, G.; Wang, H.; Li, B.; Huang, L.; Xue, D.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y.; Lu, Y.; et al. Reciprocal Interactions between Tumor-Associated Macrophages and CD44-Positive Cancer Cells via Osteopontin/CD44 Promote Tumorigenicity in Colorectal Cancer. Clin. Cancer Res. 2013, 19, 785–797. [Google Scholar] [CrossRef]
- Cheng, Y.; Wen, G.; Sun, Y.; Shen, Y.; Zeng, Y.; Du, M.; Zhu, G.; Wang, G.; Meng, X. Osteopontin Promotes Colorectal Cancer Cell Invasion and the Stem Cell-Like Properties through the PI3K-AKT-GSK/3β-β/Catenin Pathway. Med. Sci. Monit. 2019, 25, 3014–3025. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S.E.; Jang, M.A.; Kim, C.D. Osteopontin Plays a Key Role in Vascular Smooth Muscle Cell Proliferation via EGFR-Mediated Activation of AP-1 and C/EBPβ Pathways. Pharmacol. Res. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Angelucci, A.; Festuccia, C.; Gravina, G.L.; Muzi, P.; Bonghi, L.; Vicentini, C.; Bologna, M. Osteopontin Enhances the Cell Proliferation Induced by the Epidermal Growth Factor in Human Prostate Cancer Cells. Prostate 2004, 59, 157–166. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Lee, H.-S.; Jin, J.-S.; Gao, H.-W.; Chao, T.-K.; Chen, A.; Nieh, S.; Chan, D.-C.; Chang, F.-N.; Lin, C.-K. Association between Osteopontin and EGFR Expression with Clinicopathological Parameters in Hepatocellular Carcinoma. Chin. J. Physiol. 2012, 55, 412–420. [Google Scholar] [CrossRef]
- Matušan-Ilijaš, K.; Damante, G.; Fabbro, D.; Dorđević, G.; Hadžisejdić, I.; Grahovac, M.; Avirović, M.; Grahovac, B.; Jonjić, N.; Lučin, K. EGFR Expression Is Linked to Osteopontin and Nf-ΚB Signaling in Clear Cell Renal Cell Carcinoma. Clin. Transl. Oncol. 2013, 15, 65–71. [Google Scholar] [CrossRef]
- Robertson, B.W.; Bonsal, L.; Chellaiah, M.A. Regulation of Erk1/2 Activation by Osteopontin in PC3 Human Prostate Cancer Cells. Mol. Cancer 2010, 9, 260. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, X.; Huang, Z.; Weber, G.F.; Zhang, G. Osteopontin Enhances the Expression and Activity of MMP-2 via the SDF-1/CXCR4 Axis in Hepatocellular Carcinoma Cell Lines. PLoS ONE 2011, 6, e23831. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Kizer, N.; Biswas, R.; Alvarez, U.; Strauss-Schoenberger, J.; Rifas, L.; Rittling, S.R.; Denhardt, D.T.; Hruska, K.A. Osteopontin Deficiency Produces Osteoclast Dysfunction Due to Reduced CD44 Surface Expression. Mol. Biol. Cell 2003, 14, 173–189. [Google Scholar] [CrossRef]
- Raineri, D.; Dianzani, C.; Cappellano, G.; Maione, F.; Baldanzi, G.; Iacobucci, I.; Clemente, N.; Baldone, G.; Boggio, E.; Gigliotti, C.L.; et al. Osteopontin Binds ICOSL Promoting Tumor Metastasis. Commun. Biol. 2020, 3, 615. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Lee, W.; Arora, P.; Cheifetz, S.; McCulloch, C.; Sodek, J. Single Cell Analysis of Intracellular Osteopontin in Osteogenic Cultures of Fetal Rat Calvarial Cells. J. Cell. Physiol. 1997, 170, 88–100. [Google Scholar] [CrossRef]
- Zohar, R.; Suzuki, N.; Suzuki, K.; Arora, P.; Glogauer, M.; McCulloch, C.A.; Sodek, J. Intracellular Osteopontin Is an Integral Component of the CD44-ERM Complex Involved in Cell Migration. J. Cell. Physiol. 2000, 184, 118–130. [Google Scholar] [CrossRef]
- Zhu, B.; Suzuki, K.; Goldberg, H.A.; Rittling, S.R.; Denhardt, D.T.; McCulloch, C.A.G.; Sodek, J. Osteopontin Modulates CD44-Dependent Chemotaxis of Peritoneal Macrophages through G-Protein-Coupled Receptors: Evidence of a Role for an Intracellular Form of Osteopontin. J. Cell. Physiol. 2004, 198, 155–167. [Google Scholar] [CrossRef]
- Ishizeki, K.; Shinagawa, T.; Nawa, T. Origin-Associated Features of Chondrocytes in Mouse Meckel’s Cartilage and Costal Cartilage: An In Vitro Study. Ann. Anat.—Anat. Anz. 2003, 185, 403–410. [Google Scholar] [CrossRef]
- Devoll, R.E.; Li, W.; Woods, K.V.; Pinero, G.J.; Butler, W.T.; Farach-Carson, M.C.; Happonen, R.-P. Osteopontin (OPN) Distribution in Premalignant and Malignant Lesions of Oral Epithelium and Expression in Cell Lines Derived from Squamous Cell Carcinoma of the Oral Cavity. J. Oral Pathol. Med. 1999, 28, 97–101. [Google Scholar] [CrossRef]
- Youssef, N.S.; Osman, W.M. Relationship between Osteopontin and β-Catenin Immunohistochemical Expression and Prognostic Parameters of Colorectal Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 1503–1514. [Google Scholar]
- Yushi, Q.; Li, Z.; Von Roemeling, C.A.; Doeppler, H.; Marlow, L.A.; Kim, B.Y.S.; Radisky, D.C.; Storz, P.; Copland, J.A.; Tun, H.W. Osteopontin Is a Multi-Faceted pro-Tumorigenic Driver for Central Nervous System Lymphoma. Oncotarget 2016, 7, 32156–32171. [Google Scholar] [CrossRef]
- Qin, X.; Yan, M.; Wang, X.; Xu, Q.; Wang, X.; Zhu, X.; Shi, J.; Li, Z.; Zhang, J.; Chen, W. Cancer-Associated Fibroblast-Derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-Kappa B Signaling Pathway. Theranostics 2018, 8, 921–940. [Google Scholar] [CrossRef]
- Iqbal, J.; Sarkar-Dutta, M.; McRae, S.; Ramachandran, A.; Kumar, B.; Waris, G. Osteopontin Regulates Hepatitis C Virus (HCV) Replication and Assembly by Interacting with HCV Proteins and Lipid Droplets and by Binding to Receptors AVβ3 and CD44. J. Virol. 2018, 92, e02116-17. [Google Scholar] [CrossRef]
- Fan, X.; He, C.; Jing, W.; Zhou, X.; Chen, R.; Cao, L.; Zhu, M.; Jia, R.; Wang, H.; Guo, Y.; et al. Intracellular Osteopontin Inhibits Toll-like Receptor Signaling and Impedes Liver Carcinogenesis. Cancer Res. 2015, 75, 86–97. [Google Scholar] [CrossRef]
- Mandelin, J.; Lin, E.C.K.; Hu, D.D.; Knowles, S.K.; Do, K.-A.; Wang, X.; Sage, E.H.; Smith, J.W.; Arap, W.; Pasqualini, R. Extracellular and Intracellular Mechanisms That Mediate the Metastatic Activity of Exogenous Osteopontin. Cancer 2009, 115, 1753–1764. [Google Scholar] [CrossRef]
- Inoue, M.; Moriwaki, Y.; Arikawa, T.; Chen, Y.-H.; Oh, Y.J.; Oliver, T.; Shinohara, M.L. Cutting Edge: Critical Role of Intracellular Osteopontin in Antifungal Innate Immune Responses. J. Immunol. 2011, 186, 19–23. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, M.; Zhang, L.; Wang, P.; Song, G.; Liu, B.; Wu, H.; Yin, Z.; Gao, C. Intracellular Osteopontin Stabilizes TRAF3 to Positively Regulate Innate Antiviral Response. Sci. Rep. 2016, 6, 23771. [Google Scholar] [CrossRef]
- Wung, J.K.; Perry, G.; Kowalski, A.; Harris, P.L.R.; Bishop, G.M.; Trivedi, M.A.; Johnson, S.C.; Smith, M.A.; Denhardt, D.T.; Atwood, C.S. Increased Expression of the Remodeling- and Tumorigenic-Associated Factor Osteopontin in Pyramidal Neurons of the Alzheimer’s Disease Brain. Curr. Alzheimer Res. 2007, 4, 67–72. [Google Scholar] [CrossRef]
- Gong, Q.; Chipitsyna, G.; Gray, C.F.; Anandanadesan, R.; Arafat, H.A. Expression and Regulation of Osteopontin in Type 1 Diabetes. Islets 2009, 1, 34–41. [Google Scholar] [CrossRef][Green Version]
- Junaid, A.; Moon, M.C.; Harding, G.E.J.; Zahradka, P. Osteopontin Localizes to the Nucleus of 293 Cells and Associates with Polo-like Kinase-1. Am. J. Physiol. Cell Physiol. 2007, 292, C919–C926. [Google Scholar] [CrossRef]
- Zduniak, K.; Ziolkowski, P.; Ahlin, C.; Agrawal, A.; Agrawal, S.; Blomqvist, C.; Fjällskog, M.-L.; Weber, G.F. Nuclear Osteopontin-c Is a Prognostic Breast Cancer Marker. Br. J. Cancer 2015, 112, 729–738. [Google Scholar] [CrossRef]
- Assidi, M.; Gomaa, W.; Jafri, M.; Hanbazazh, M.; Al-Ahwal, M.; Pushparaj, P.; Al-Harbi, A.; Al-Qahtani, M.; Buhmeida, A.; Al-Maghrabi, J. Prognostic Value of Osteopontin (SPP1) in Colorectal Carcinoma Requires a Personalized Molecular Approach. Tumour Biol. 2019, 41, 1010428319863627. [Google Scholar] [CrossRef]
- De Sousa, I.J.D.; Marques, D.S.; Príncipe, C.; Portugal, R.V.; Canberk, S.; Prazeres, H.; Lopes, J.M.; Gimba, E.R.P.; Lima, R.T.; Soares, P. Predictive Biomarkers and Patient Outcome in Platinum-Resistant (PLD-Treated) Ovarian Cancer. Diagnostics 2020, 10, 525. [Google Scholar] [CrossRef]
- Irion, C.I.; Dunkley, J.C.; John-Williams, K.; Condor Capcha, J.M.; Shehadeh, S.A.; Pinto, A.; Loebe, M.; Webster, K.A.; Brozzi, N.A.; Shehadeh, L.A. Nuclear Osteopontin Is a Marker of Advanced Heart Failure and Cardiac Allograft Vasculopathy: Evidence from Transplant and Retransplant Hearts. Front. Physiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.L.; Lu, L.; Bu, J.; Werneck, M.B.F.; Kobayashi, K.S.; Glimcher, L.H.; Cantor, H. Osteopontin Expression Is Essential for Interferon-Alpha Production by Plasmacytoid Dendritic Cells. Nat. Immunol. 2006, 7, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.; Cancila, V.; Sangaletti, S.; Botti, L.; Ratti, C.; Milani, M.; Dugo, M.; Bertoni, F.; Tripodo, C.; Chiodoni, C.; et al. Intracellular Osteopontin Protects from Autoimmunity-Driven Lymphoma Development Inhibiting TLR9-MYD88-STAT3 Signaling. Mol. Cancer 2022, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Leavenworth, J.W.; Verbinnen, B.; Wang, Q.; Shen, E.; Cantor, H. Intracellular Osteopontin Regulates Homeostasis and Function of Natural Killer Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 494–499. [Google Scholar] [CrossRef]
- Leavenworth, J.W.; Verbinnen, B.; Yin, J.; Huang, H.; Cantor, H. A P85α-Osteopontin Axis Couples the Receptor ICOS to Sustained Bcl-6 Expression by Follicular Helper and Regulatory T Cells. Nat. Immunol. 2015, 16, 96–106. [Google Scholar] [CrossRef]
- Kanayama, M.; Xu, S.; Danzaki, K.; Gibson, J.R.; Inoue, M.; Gregory, S.G.; Shinohara, M.L. Skewing of the Population Balance of Lymphoid and Myeloid Cells by Secreted and Intracellular Osteopontin. Nat. Immunol. 2017, 18, 973–984. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Intracellular Osteopontin (IOPN) and Immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef]
| OPN-SV | N-Terminal Sequence | Signal-6.0 SP Eukaryotic Consensus Sequence | Signal-6.0 Peptidase Cleavage Site 16/17 | PrediSi Probability of SP Prediction | DeepLoc Probability of Being Secreted | MULocDeep Probability of Subcellular Localization | NucPred Probability of Nuclear Localization |
|---|---|---|---|---|---|---|---|
| OPN-a | MRIAIVICFCLLGITCAI | yes | yes | 84.0% | >90% | 100% secreted | 61% |
| OPN-b | MRIAIVICFCLLGITCAI | yes | yes | 84.0% | >90% | 100% secreted | 60% |
| OPN-c | MRIAIVICFCLLGITCAI | yes | yes | 84.0% | >90% | 100% secreted | 54% |
| OPN-4 | MRIAIVICFCLLGITCAI | yes | yes | 84.0% | >90% | 100% secreted | 55% |
| OPN-5 | MGIVPRSLDKKAHRVQFQ | No | No | 0.0% | 81% | <10% secreted; ~30% mitochondrial; ~20% nuclear | 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, A.C.S.d.F.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942. https://doi.org/10.3390/ijms24032942
Bastos ACSdF, Gomes AVP, Silva GR, Emerenciano M, Ferreira LB, Gimba ERP. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. International Journal of Molecular Sciences. 2023; 24(3):2942. https://doi.org/10.3390/ijms24032942
Chicago/Turabian StyleBastos, Ana Clara Santos da Fonseca, Amanda Vitória Pampolha Gomes, Gabriela Ribeiro Silva, Mariana Emerenciano, Luciana Bueno Ferreira, and Etel Rodrigues Pereira Gimba. 2023. "The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles" International Journal of Molecular Sciences 24, no. 3: 2942. https://doi.org/10.3390/ijms24032942
APA StyleBastos, A. C. S. d. F., Gomes, A. V. P., Silva, G. R., Emerenciano, M., Ferreira, L. B., & Gimba, E. R. P. (2023). The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. International Journal of Molecular Sciences, 24(3), 2942. https://doi.org/10.3390/ijms24032942







