Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons
Abstract
1. Introduction
2. Results
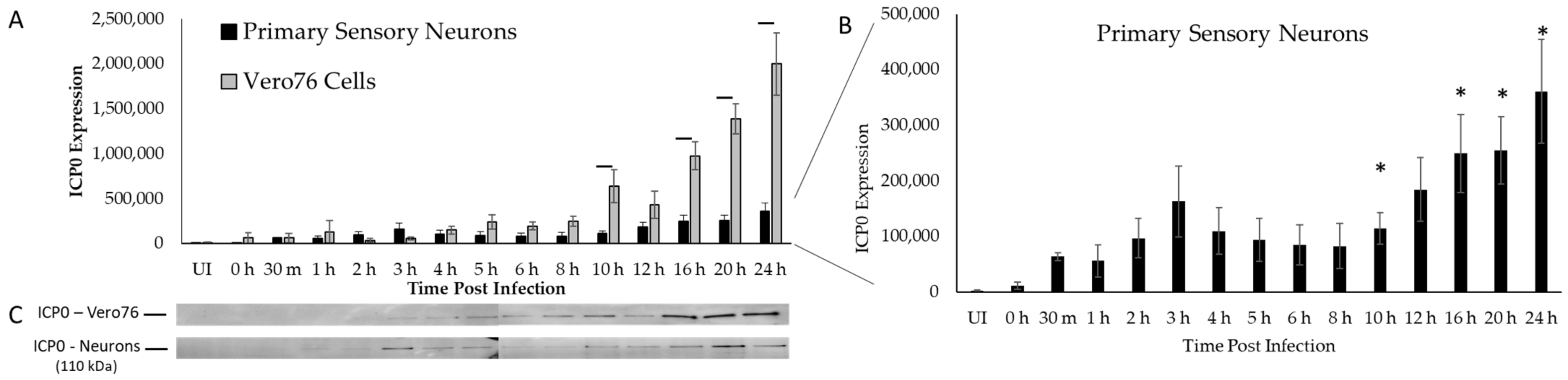
2.1. ICP0 Protein Profile Is Biphasic in Primary Adult Sensory Neurons
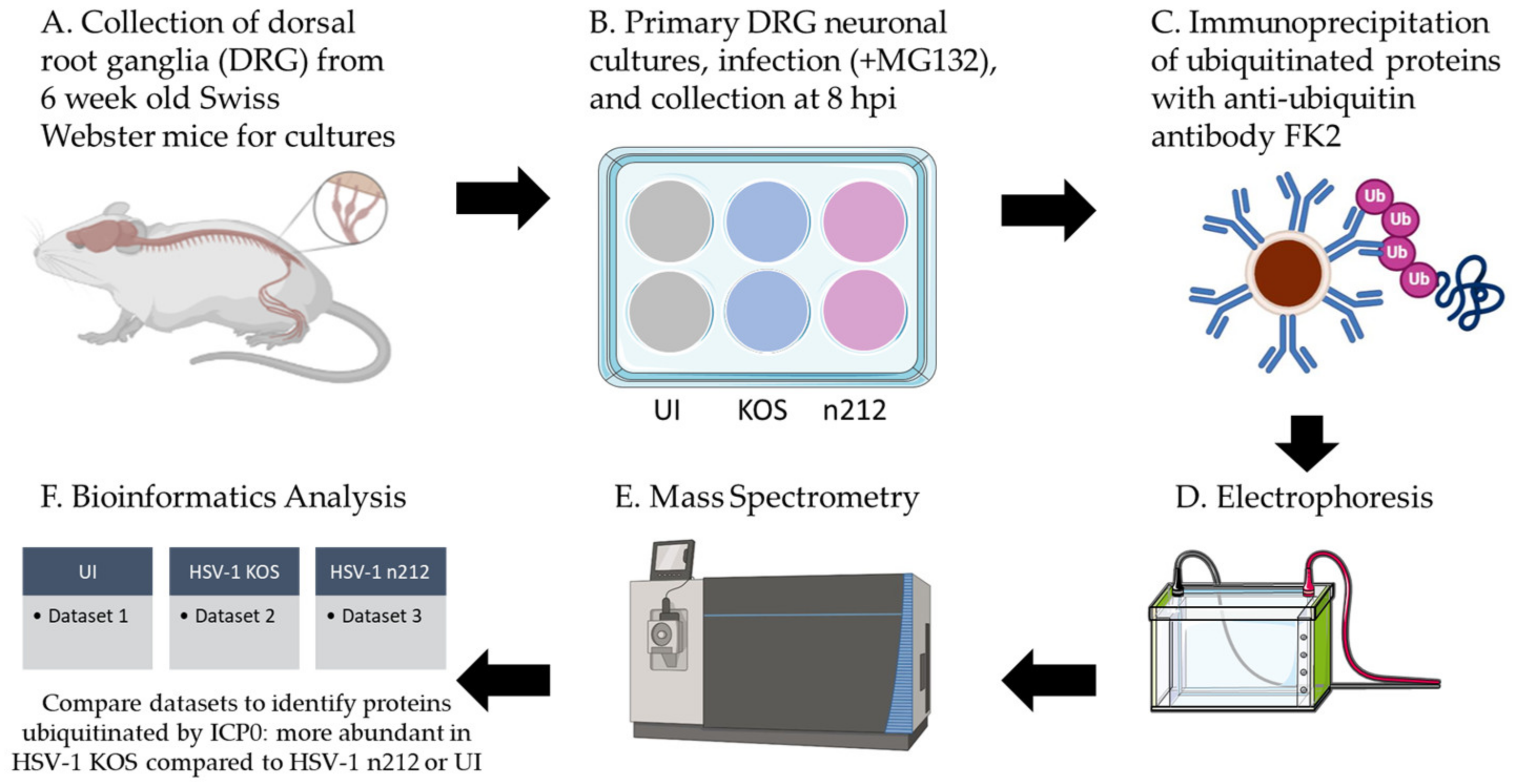
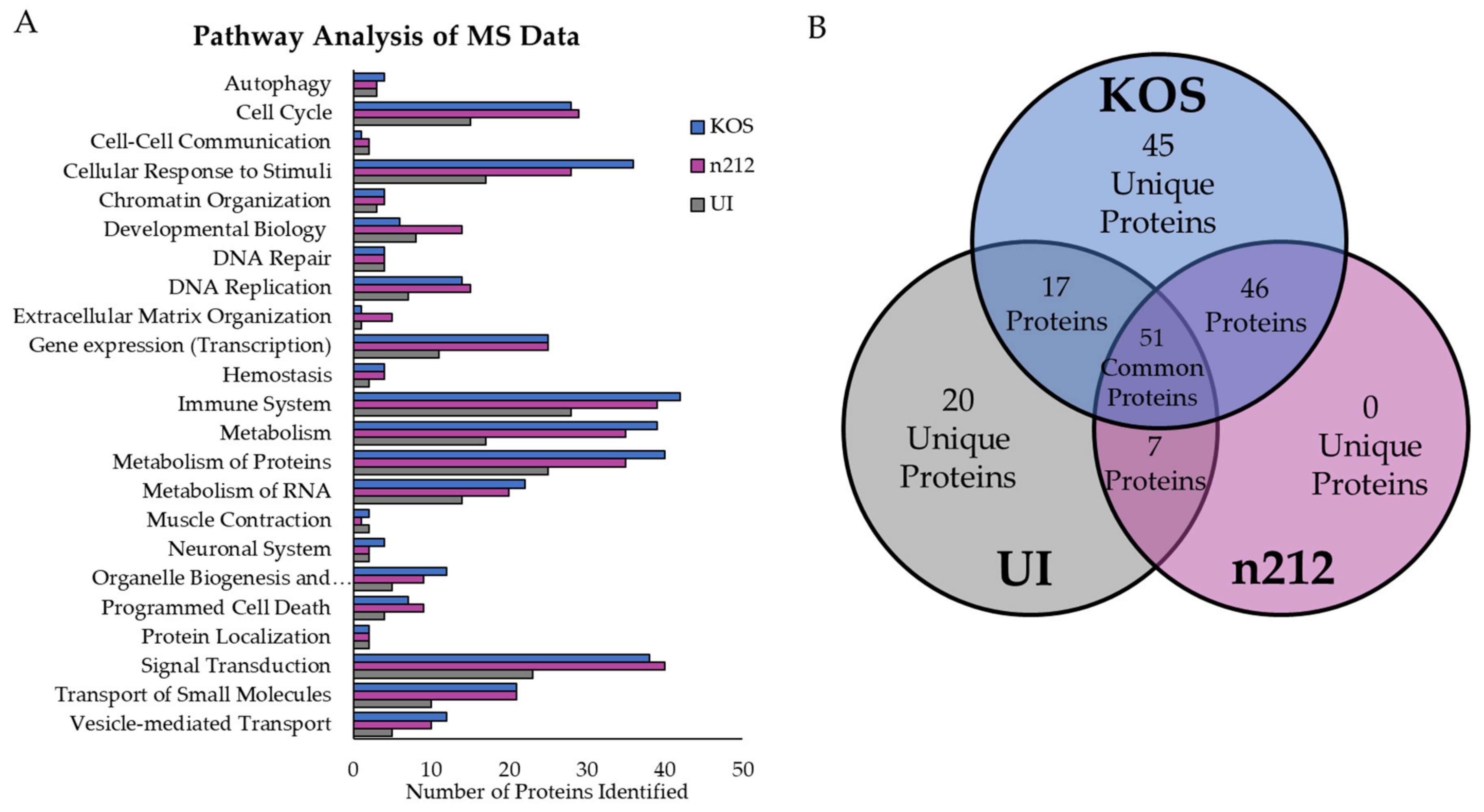
2.2. Mass Spectrometry Analysis of Proteins Ubiquitinated by ICP0
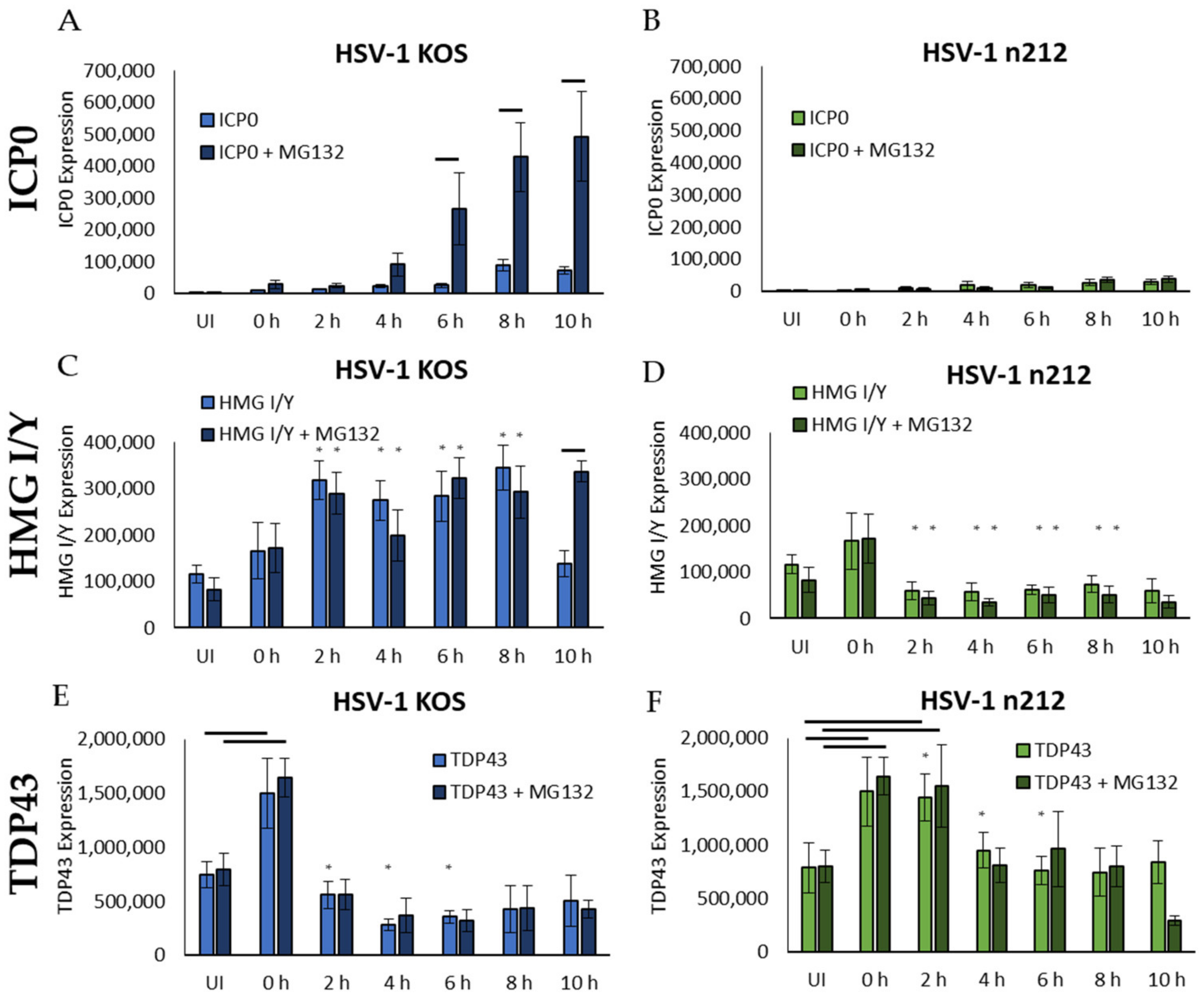
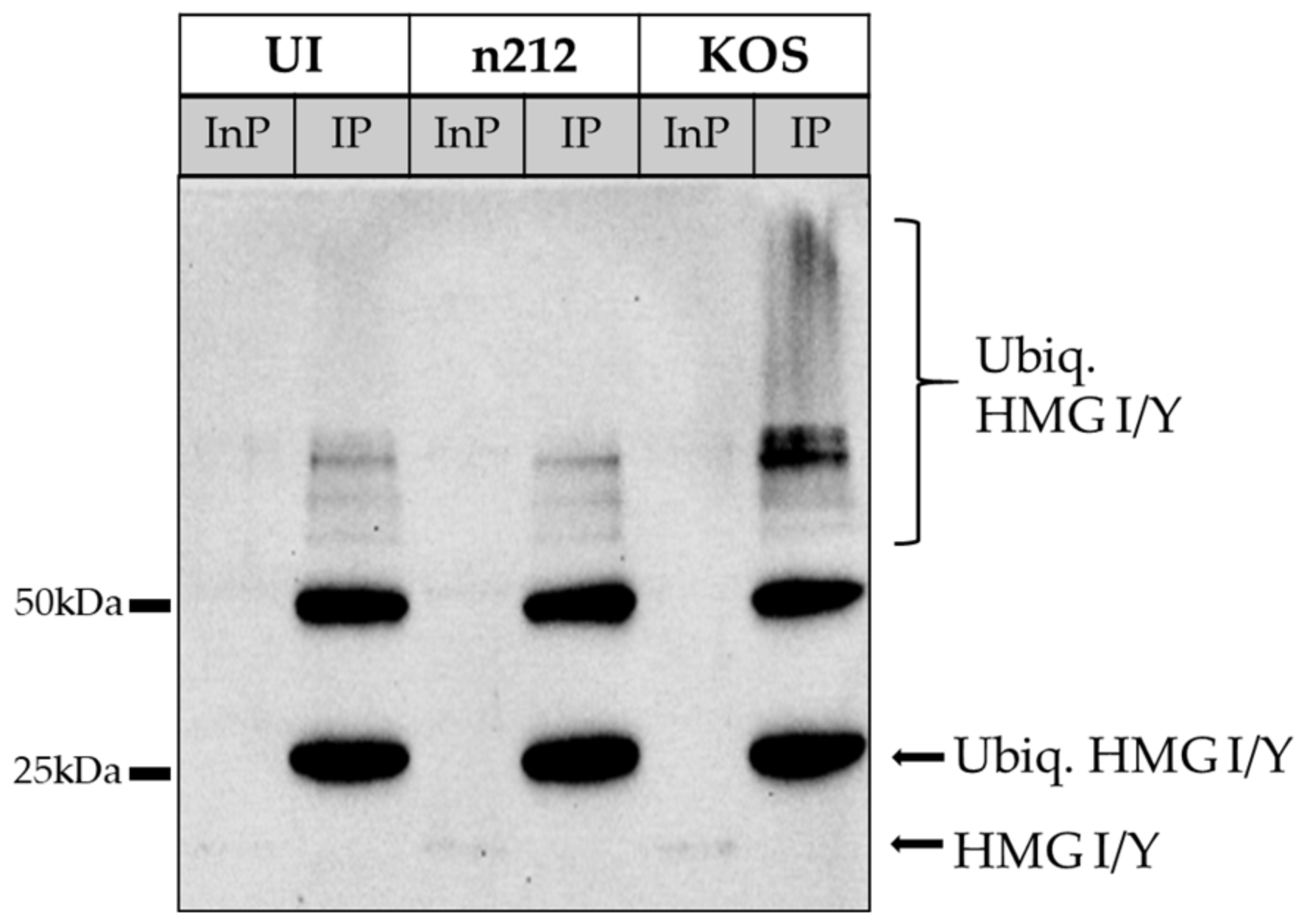
2.3. HMG I/Y and TDP43 Exhibit Increased Ubiquitination in the Presence of Functional ICP0
2.4. Productive HSV-1 Infection Alters HMG I/Y and TDP43 Proteins Profiles in Sensory Neurons
2.5. HMG I/Y Is Increasingly Ubiquitinated and Degraded by ICP0 between 8 and 10 hpi
3. Discussion
4. Conclusions and Future Studies
5. Materials and Methods
5.1. Cells and Viruses
5.2. Primary Adult Neuronal Cultures
5.3. Infection
5.4. Antibodies
5.5. LC-MS/MS
5.6. Data Processing
5.7. Statistical Analysis
5.8. Immunoblot
5.9. Immunoprecipitation
Supplementary Materials
Author Contributions
Funding
Institutional Animal Care and Use Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| HSV-1 | Herpes simplex virus 1 |
| KOS | HSV-1 wildtype strain KOS |
| n212 | HSV-1 strain n212, defective ICP0 protein |
| UI | Uninfected |
| hpi | Hours post inoculation |
| IE | Immediate early |
| ICP0 | Infected cell protein 0 |
| ICP22 | infected cell protein 22 |
| ICP27 | Infected cell protein 27 |
| ICP47 | Infected cell protein 47 |
| VP16 | HSV tegument viral protein 16, trans-inducing protein |
| TK | HSV thymidine kinase |
| DRG | Dorsal root ganglion |
| HMG I/Y | High mobility group protein I/Y |
| TDP43 | TAR-DNA binding protein 43 |
| USP7 | Ubiquitin-specific peptidase 7 |
| UbcH5a | Ubiquitin-conjugating enzyme H5a |
| UbcH6a | Ubiquitin-conjugating enzyme H6a |
| PML | Promyelocytic leukemia protein |
| LC-MS/MS | Nano liquid chromatography tandem mass spectrometry |
| PSM | Peptide spectrum matches |
| TCE | 2,2,2-Trichloroethanol |
| IP | Immunoprecipitation |
| InP | Input |
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence And Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Kolokotronis, A.; Doumas, S. Herpes Simplex Virus Infection, With Particular Reference To The Progression And Complications Of Primary Herpetic Gingivostomatitis. Clin. Microbiol. Infect. 2006, 12, 202–211. [Google Scholar] [CrossRef]
- Whitley, R.J.; Kimberlin, D.W.; Roizman, B. Herpes Simplex Viruses. Clin. Infect. Dis. 1998, 26, 541–553, Quiz 554–545. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.M.; St Leger, A.J.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes Keratitis. Prog. Retin Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Venkatesan, A. Herpes Simplex Virus-1 Encephalitis In Adults: Pathophysiology, Diagnosis, And Management. Neurotherapeutics 2016, 13, 493–508. [Google Scholar] [CrossRef]
- Carneiro, V.C.S.; Pereira, J.G.; De Paula, V.S. Family Herpesviridae And Neuroinfections: Current Status And Research In Progress. Mem. Inst. Oswaldo Cruz. 2022, 117, E220200. [Google Scholar] [CrossRef] [PubMed]
- Protto, V.; Marcocci, M.E.; Miteva, M.T.; Piacentini, R.; Li Puma, D.D.; Grassi, C.; Palamara, A.T.; De Chiara, G. Role Of Hsv-1 In Alzheimer’s Disease Pathogenesis: A Challenge For Novel Preventive/Therapeutic Strategies. Curr. Opin. Pharmacol. 2022, 63, 102200. [Google Scholar] [CrossRef]
- Kramer, T.; Enquist, L.W. Directional Spread Of Alphaherpesviruses In The Nervous System. Viruses 2013, 5, 678–707. [Google Scholar] [CrossRef]
- Bertke, A.S.; Swanson, S.M.; Chen, J.; Imai, Y.; Kinchington, P.R.; Margolis, T.P. A5-Positive Primary Sensory Neurons Are Nonpermissive For Productive Infection With Herpes Simplex Virus 1 In Vitro. J. Virol. 2011, 85, 6669–6677. [Google Scholar] [CrossRef]
- Bertke, A.S.; Ma, A.; Margolis, M.S.; Margolis, T.P. Different Mechanisms Regulate Productive Herpes Simplex Virus 1 (Hsv-1) And Hsv-2 Infections In Adult Trigeminal Neurons. J. Virol. 2013, 87, 6512–6516. [Google Scholar] [CrossRef]
- Yanez, A.A.; Harrell, T.; Sriranganathan, H.J.; Ives, A.M.; Bertke, A.S. Neurotrophic Factors Ngf, Gdnf And Ntn Selectively Modulate Hsv1 And Hsv2 Lytic Infection And Reactivation In Primary Adult Sensory And Autonomic Neurons. Pathogens 2017, 6, 5. [Google Scholar] [CrossRef]
- Lanfranca, M.P.; Mostafa, H.H.; Davido, D.J. Hsv-1 Icp0: An E3 Ubiquitin Ligase That Counteracts Host Intrinsic And Innate Immunity. Cells 2014, 3, 438–454. [Google Scholar] [CrossRef]
- Rice, S.A.; Davido, D.J. Hsv-1 Icp22: Hijacking Host Nuclear Functions To Enhance Viral Infection. Future Microbiol. 2013, 8, 311–321. [Google Scholar] [CrossRef]
- Goldsmith, K.; Chen, W.; Johnson, D.C.; Hendricks, R.L. Infected Cell Protein (Icp)47 Enhances Herpes Simplex Virus Neurovirulence By Blocking The Cd8+ T Cell Response. J. Exp. Med. 1998, 187, 341–348. [Google Scholar] [CrossRef]
- Orr, M.T.; Edelmann, K.H.; Vieira, J.; Corey, L.; Raulet, D.H.; Wilson, C.B. Inhibition Of Mhc Class I Is A Virulence Factor In Herpes Simplex Virus Infection Of Mice. PLoS Pathog. 2005, 1, E7. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.; Jensen, S.B.; Miettinen, J.J.; Luecke, S.; Prabakaran, T.; Reinert, L.S.; Mettenleiter, T.; Chen, Z.J.; Knipe, D.M.; Sandri-Goldin, R.M.; et al. Hsv-1 Icp27 Targets The Tbk1-Activated Sting Signalsome To Inhibit Virus-Induced Type I Ifn Expression. Embo J. 2016, 35, 1385–1399. [Google Scholar] [CrossRef]
- Long, M.C.; Leong, V.; Schaffer, P.A.; Spencer, C.A.; Rice, S.A. Icp22 And The Ul13 Protein Kinase Are Both Required For Herpes Simplex Virus-Induced Modification Of The Large Subunit Of Rna Polymerase Ii. J. Virol. 1999, 73, 5593–5604. [Google Scholar] [CrossRef]
- Grondin, B.; Deluca, N. Herpes Simplex Virus Type 1 Icp4 Promotes Transcription Preinitiation Complex Formation By Enhancing The Binding Of Tfiid To Dna. J. Virol. 2000, 74, 11504–11510. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Wang, S.; Zheng, C. Herpes Simplex Virus 1 E3 Ubiquitin Ligase Icp0 Protein Inhibits Tumor Necrosis Factor Alpha-Induced Nf-Kappab Activation By Interacting With P65/Rela And P50/Nf-Kappab1. J. Virol. 2013, 87, 12935–12948. [Google Scholar] [CrossRef]
- Van Lint, A.L.; Murawski, M.R.; Goodbody, R.E.; Severa, M.; Fitzgerald, K.A.; Finberg, R.W.; Knipe, D.M.; Kurt-Jones, E.A. Herpes Simplex Virus Immediate-Early Icp0 Protein Inhibits Toll-Like Receptor 2-Dependent Inflammatory Responses And Nf-Kappab Signaling. J. Virol. 2010, 84, 10802–10811. [Google Scholar] [CrossRef]
- Hobbs, W.E.; Deluca, N.A. Perturbation Of Cell Cycle Progression And Cellular Gene Expression As A Function Of Herpes Simplex Virus Icp0. J. Virol. 1999, 73, 8245–8255. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Schaffer, P.A. Herpes Simplex Virus Type 1 Icp0 Regulates Expression Of Immediate-Early, Early, And Late Genes In Productively Infected Cells. J. Virol. 1992, 66, 2904–2915. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Raja, P.; Knipe, D.M. Herpesviral Icp0 Protein Promotes Two Waves Of Heterochromatin Removal On An Early Viral Promoter During Lytic Infection. Mbio 2016, 7, E02007–E02015. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.T.; Dybas, J.M.; Kulej, K.; Reyes, E.D.; Price, A.M.; Akhtar, L.N.; Orr, A.; Garcia, B.A.; Boutell, C.; Weitzman, M.D. Comparative Proteomics Identifies Schlafen 5 (Slfn5) As A Herpes Simplex Virus Restriction Factor That Suppresses Viral Transcription. Nat. Microbiol. 2021, 6, 234–245. [Google Scholar] [CrossRef]
- Hagglund, R.; Roizman, B. Characterization Of The Novel E3 Ubiquitin Ligase Encoded In Exon 3 Of Herpes Simplex Virus-1-Infected Cell Protein 0. Proc. Natl. Acad. Sci. USA 2002, 99, 7889–7894. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Dybas, J.M.; Hughes, J.; Weitzman, M.D.; Boutell, C. The Hsv-1 Ubiquitin Ligase Icp0: Modifying The Cellular Proteome To Promote Infection. Virus Res. 2020, 285, 198015. [Google Scholar] [CrossRef]
- Daubeuf, S.; Singh, D.; Tan, Y.; Liu, H.; Federoff, H.J.; Bowers, W.J.; Tolba, K. Hsv Icp0 Recruits Usp7 To Modulate Tlr-Mediated Innate Response. Blood 2009, 113, 3264–3275. [Google Scholar] [CrossRef]
- Boutell, C.; Sadis, S.; Everett, R.D. Herpes Simplex Virus Type 1 Immediate-Early Protein Icp0 And Is Isolated Ring Finger Domain Act As Ubiquitin E3 Ligases In Vitro. J. Virol. 2002, 76, 841–850. [Google Scholar] [CrossRef]
- Boutell, C.; Everett, R.D. Regulation Of Alphaherpesvirus Infections By The Icp0 Family Of Proteins. J. Gen. Virol. 2013, 94, 465–481. [Google Scholar] [CrossRef]
- Boutell, C.; Everett, R.D. The Herpes Simplex Virus Type 1 (Hsv-1) Regulatory Protein Icp0 Interacts With And Ubiquitinates P53. J. Biol. Chem. 2003, 278, 36596–36602. [Google Scholar] [CrossRef]
- Lopez, P.; Van Sant, C.; Roizman, B. Requirements For The Nuclear-Cytoplasmic Translocation Of Infected-Cell Protein 0 Of Herpes Simplex Virus 1. J. Virol. 2001, 75, 3832–3840. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Van Sant, C.; Roizman, B. Herpes Simplex Virus 1 Alpha Regulatory Protein Icp0 Interacts With And Stabilizes The Cell Cycle Regulator Cyclin D3. J. Virol. 1997, 71, 7328–7336. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Deluca, N.A.; Schaffer, P.A. Overexpression Of The Herpes Simplex Virus Type 1 Immediate-Early Regulatory Protein, Icp27, Is Responsible For The Aberrant Localization Of Icp0 And Mutant Forms Of Icp4 In Icp4 Mutant Virus-Infected Cells. J. Virol. 1996, 70, 5346–5356. [Google Scholar] [CrossRef]
- Everett, R.D. Icp0 Induces The Accumulation Of Colocalizing Conjugated Ubiquitin. J. Virol. 2000, 74, 9994–10005. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, T.; Zhou, G.; Roizman, B. The Stability Of Herpes Simplex Virus 1 Icp0 Early After Infection Is Defined By The Ring Finger And The Ul13 Protein Kinase. J. Virol. 2014, 88, 5437–5443. [Google Scholar] [CrossRef]
- Gu, H.; Roizman, B. The Degradation Of Promyelocytic Leukemia And Sp100 Proteins By Herpes Simplex Virus 1 Is Mediated By The Ubiquitin-Conjugating Enzyme Ubch5a. Proc. Natl. Acad. Sci. USA 2003, 100, 8963–8968. [Google Scholar] [CrossRef]
- Boutell, C.; Canning, M.; Orr, A.; Everett, R.D. Reciprocal Activities Between Herpes Simplex Virus Type 1 Regulatory Protein Icp0, A Ubiquitin E3 Ligase, And Ubiquitin-Specific Protease Usp7. J. Virol. 2005, 79, 12342–12354. [Google Scholar] [CrossRef]
- Schaffer, W.Z.C.A.P.A. Herpes Simplex Virus Type 1 Icp0 Plays A Critical Role In The De Novo Synthesis Of Infectious Virus Following Transfection Of Viral Dna. J. Virol. 1989, 63, 4579–4589. [Google Scholar]
- Fujimuro, M.; Sawada, H.; Yokosawa, H. Production And Characterization Of Monoclonal Antibodies Specific To Multi-Ubiquitin Chains Of Polyubiquitinated Proteins. FEBS Lett. 1994, 349, 173–180. [Google Scholar] [CrossRef]
- Reeves, R. Structure And Function Of The Hmgi(Y) Family Of Architectural Transcription Factors. Environ. Health Perspect. 2000, 108 (Suppl. 5), 803–809. [Google Scholar] [CrossRef]
- Reeves, R. Nuclear Functions Of The Hmg Proteins. Biochim. Biophys. Acta 2010, 1799, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.T.; Pellacani, A.; Wang, H.; Lin, S.S.; Jain, M.K.; Perrella, M.A.; Lee, M.E. Enhancement Of Serum-Response Factor-Dependent Transcription And Dna Binding By The Architectural Transcription Factor Hmg-I(Y). J. Biol. Chem. 1998, 273, 9755–9760. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Xia, M.; Liu, C.; Zu, X.; Zhong, J. High Mobility Group A1 (Hmga1): Structure, Biological Function, And Therapeutic Potential. Int. J. Biol. Sci. 2022, 18, 4414–4431. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.; Nissen, M.S. The A.T-Dna-Binding Domain Of Mammalian High Mobility Group I Chromosomal Proteins. A Novel Peptide Motif For Recognizing Dna Structure. J. Biol. Chem. 1990, 265, 8573–8582. [Google Scholar] [CrossRef]
- Panagiotidis, C.A.; Silverstein, S.J. The Host-Cell Architectural Protein Hmg I(Y) Modulates Binding Of Herpes Simplex Virus Type 1 Icp4 To Its Cognate Promoter. Virology 1999, 256, 64–74. [Google Scholar] [CrossRef]
- Ou, S.H.; Wu, F.; Harrich, D.; Garcia-Martinez, L.F.; Gaynor, R.B. Cloning And Characterization Of A Novel Cellular Protein, Tdp-43, That Binds To Human Immunodeficiency Virus Type 1 Tar Dna Sequence Motifs. J. Virol. 1995, 69, 3584–3596. [Google Scholar] [CrossRef]
- Lalmansingh, A.S.; Urekar, C.J.; Reddi, P.P. Tdp-43 Is A Transcriptional Repressor: The Testis-Specific Mouse Acrv1 Gene Is A Tdp-43 Target In Vivo. J. Biol. Chem. 2011, 286, 10970–10982. [Google Scholar] [CrossRef]
- Polymenidou, M.; Lagier-Tourenne, C.; Hutt, K.R.; Huelga, S.C.; Moran, J.; Liang, T.Y.; Ling, S.C.; Sun, E.; Wancewicz, E.; Mazur, C.; et al. Long Pre-Mrna Depletion And Rna Missplicing Contribute To Neuronal Vulnerability From Loss Of Tdp-43. Nat. Neurosci. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Sadowski, M.; Sarcevic, B. Mechanisms Of Mono- And Poly-Ubiquitination: Ubiquitination Specificity Depends On Compatibility Between The E2 Catalytic Core And Amino Acid Residues Proximal To The Lysine. Cell Div. 2010, 5, 19. [Google Scholar] [CrossRef]
- Canning, M.; Boutell, C.; Parkinson, J.; Everett, R.D. A Ring Finger Ubiquitin Ligase Is Protected From Autocatalyzed Ubiquitination And Degradation By Binding To Ubiquitin-Specific Protease Usp7. J. Biol. Chem. 2004, 279, 38160–38168. [Google Scholar] [CrossRef]
- Xu, G.; Jaffrey, S.R. Proteomic Identification Of Protein Ubiquitination Events. Biotechnol. Genet. Eng. Rev. 2013, 29, 73–109. [Google Scholar] [CrossRef]
- Yao, F.; Courtney, R.J. Association Of Icp0 But Not Icp27 With Purified Virions Of Herpes Simplex Virus Type 1. J. Virol. 1992, 66, 2709–2716. [Google Scholar] [CrossRef]
- Margolis, T.P.; Dawson, C.R.; Lavail, J.H. Herpes Simplex Viral Infection Of The Mouse Trigeminal Ganglion. Immunohistochemical Analysis Of Cell Populations. Investig. Ophthalmol. Vis. Sci. 1992, 33, 259–267. [Google Scholar]
- Yang, L.; Voytek, C.C.; Margolis, T.P. Immunohistochemical Analysis Of Primary Sensory Neurons Latently Infected With Herpes Simplex Virus Type 1. J. Virol. 2000, 74, 209–217. [Google Scholar] [CrossRef]
- Margolis, T.P.; Imai, Y.; Yang, L.; Vallas, V.; Krause, P.R. Herpes Simplex Virus Type 2 (Hsv-2) Establishes Latent Infection In A Different Population Of Ganglionic Neurons Than Hsv-1: Role Of Latency-Associated Transcripts. J. Virol. 2007, 81, 1872–1878. [Google Scholar] [CrossRef]
- Bloom, D.C.; Giordani, N.V.; Kwiatkowski, D.L. Epigenetic Regulation Of Latent Hsv-1 Gene Expression. Biochim. Biophys. Acta 2010, 1799, 246–256. [Google Scholar] [CrossRef]
- Knipe, D.M.; Cliffe, A. Chromatin Control Of Herpes Simplex Virus Lytic And Latent Infection. Nat. Rev. Microbiol. 2008, 6, 211–221. [Google Scholar] [CrossRef]
- Nicoll, M.P.; Proenca, J.T.; Efstathiou, S. The Molecular Basis Of Herpes Simplex Virus Latency. Fems Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Kristie, T.M. Dynamic Modulation Of Hsv Chromatin Drives Initiation Of Infection And Provides Targets For Epigenetic Therapies. Virology 2015, 479-480, 555–561. [Google Scholar] [CrossRef]
- Lieberman, P.M. Epigenetics And Genetics Of Viral Latency. Cell Host Microb. 2016, 19, 619–628. [Google Scholar] [CrossRef]
- Lees-Miller, S.P.; Long, M.C.; Kilvert, M.A.; Lam, V.; Rice, S.A.; Spencer, C.A. Attenuation Of Dna-Dependent Protein Kinase Activity And Its Catalytic Subunit By The Herpes Simplex Virus Type 1 Transactivator Icp0. J. Virol. 1996, 70, 7471–7477. [Google Scholar] [CrossRef] [PubMed]
- Dembowski, J.A.; Deluca, N.A. Selective Recruitment Of Nuclear Factors To Productively Replicating Herpes Simplex Virus Genomes. PLoS Pathog. 2015, 11, E1004939. [Google Scholar] [CrossRef] [PubMed]
- Dembowski, J.A.; Deluca, N.A. Temporal Viral Genome-Protein Interactions Define Distinct Stages Of Productive Herpesviral Infection. Mbio 2018, 9, e01182-18. [Google Scholar] [CrossRef] [PubMed]
- Orzalli, M.H.; Broekema, N.M.; Knipe, D.M. Relative Contributions Of Herpes Simplex Virus 1 Icp0 And Vhs To Loss Of Cellular Ifi16 Vary In Different Human Cell Types. J. Virol. 2016, 90, 8351–8359. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.L.; Kennedy, M.A.; Hutton, J.E.; Liu, D.; Song, B.; Phelan, B.; Cristea, I.M. Systematic Profiling Of Protein Complex Dynamics Reveals Dna-Pk Phosphorylation Of Ifi16 En Route To Herpesvirus Immunity. Sci. Adv. 2021, 7, eabg6680. [Google Scholar] [CrossRef]
- Hicke, L. Protein Regulation By Monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001, 2, 195–201. [Google Scholar] [CrossRef]
- Boughton, A.J.; Krueger, S.; Fushman, D. Branching Via K11 And K48 Bestows Ubiquitin Chains With A Unique Interdomain Interface And Enhanced Affinity For Proteasomal Subunit Rpn1. Structure 2020, 28, 29–43.e26. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code In The Ubiquitin-Proteasome System And Autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Lange, S.M.; Kulathu, Y. Linking K29-Ub Chains To Biology. Nat. Chem. Biol. 2021, 17, 843–844. [Google Scholar] [CrossRef]
- Chen, A.; Kleiman, F.E.; Manley, J.L.; Ouchi, T.; Pan, Z.Q. Autoubiquitination Of The Brca1*Bard1 Ring Ubiquitin Ligase. J. Biol. Chem. 2002, 277, 22085–22092. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X. Regulation Of Apoptosis: The Ubiquitous Way. FAESB J. 2003, 17, 790–799. [Google Scholar] [CrossRef]
- Amemiya, Y.; Azmi, P.; Seth, A. Autoubiquitination Of Bca2 Ring E3 Ligase Regulates Its Own Stability And Affects Cell Migration. Mol. Cancer Res. 2008, 6, 1385–1396. [Google Scholar] [CrossRef]
- Gu, H.; Poon, A.P.; Roizman, B. During Its Nuclear Phase The Multifunctional Regulatory Protein Icp0 Undergoes Proteolytic Cleavage Characteristic Of Polyproteins. Proc. Natl. Acad. Sci. USA 2009, 106, 19132–19137. [Google Scholar] [CrossRef]
- Gu, H.; Zheng, Y.; Roizman, B. Interaction Of Herpes Simplex Virus Icp0 With Nd10 Bodies: A Sequential Process Of Adhesion, Fusion, And Retention. J. Virol. 2013, 87, 10244–10254. [Google Scholar] [CrossRef]
- Dremel, S.E.; Deluca, N.A. Herpes Simplex Viral Nucleoprotein Creates A Competitive Transcriptional Environment Facilitating Robust Viral Transcription And Host Shut Off. eLife 2019, 8, e51109. [Google Scholar] [CrossRef]
- Kops, A.D.B.; Knipe, D.M. Formation Of Dna Replication Structures In Herpes Virus-Infected Cells Requires A Viral Dna Binding Protein. Cell 1988, 55, 857–868. [Google Scholar] [CrossRef]
- Gao, M.; Knipe, D.M. Potential Role For Herpes Simplex Virus Icp8 Dna Replication Protein In Stimulation Of Late Gene Expression. J. Virol. 1991, 65, 2666–2675. [Google Scholar] [CrossRef]
- Field, H.J.; Wildy, P. The Pathogenicity Of Thymidine Kinase-Deficient Mutants Of Herpes Simplex Virus In Mice. J. Hyg. 1978, 81, 267–277. [Google Scholar] [CrossRef]
- Weller, S.K.; Coen, D.M. Herpes Simplex Viruses: Mechanisms Of Dna Replication. Cold Spring Harb. Perspect. Biol. 2012, 4, A013011. [Google Scholar] [CrossRef]
- Packard, J.E.; Dembowski, J.A. Hsv-1 Dna Replication-Coordinated Regulation By Viral And Cellular Factors. Viruses 2021, 13, 2015. [Google Scholar] [CrossRef]
- Arnoldo, L.; Sgarra, R.; Chiefari, E.; Iiritano, S.; Arcidiacono, B.; Pegoraro, S.; Pellarin, I.; Brunetti, A.; Manfioletti, G. A Novel Mechanism Of Post-Translational Modulation Of Hmga Functions By The Histone Chaperone Nucleophosmin. Sci. Rep. 2015, 5, 8552. [Google Scholar] [CrossRef] [PubMed]
- Cleynen, I.; Huysmans, C.; Sasazuki, T.; Shirasawa, S.; Van De Ven, W.; Peeters, K. Transcriptional Control Of The Human High Mobility Group A1 Gene: Basal And Oncogenic Ras-Regulated Expression. Cancer Res. 2007, 67, 4620–4629. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Iiritano, S.; Paonessa, F.; Le Pera, I.; Arcidiacono, B.; Filocamo, M.; Foti, D.; Liebhaber, S.A.; Brunetti, A. Pseudogene-Mediated Posttranscriptional Silencing Of Hmga1 Can Result In Insulin Resistance And Type 2 Diabetes. Nat. Commun. 2010, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Matta, M.K.; Panagiotidis, C.A. High-Mobility Group Protein A1 Binds Herpes Simplex Virus Gene Regulatory Sequences And Affects Their Expression. Arch. Virol. 2008, 153, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Mellone, M.; Rinaldi, C.; Massimi, I.; Petroni, M.; Veschi, V.; Talora, C.; Truffa, S.; Stabile, H.; Frati, L.; Screpanti, I.; et al. Human Papilloma Virus-Dependent Hmga1 Expression Is A Relevant Step In Cervical Carcinogenesis. Neoplasia 2008, 10, 773–781. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, J.; Gao, Z.; Zhang, S.; Chen, J.; He, J.; Guo, Y.; Deng, Q.; Xie, Y.; Liu, J.; et al. High Mobility Group At-Hook 1 (Hmga1) Is An Important Positive Regulator Of Hepatitis B Virus (Hbv) That Is Reciprocally Upregulated By Hbv X Protein. Nucleic Acids Res. 2022, 50, 2157–2171. [Google Scholar] [CrossRef]
- Yie, J.; Merika, M.; Munshi, N.; Chen, G.; Thanos, D. The Role Of Hmg I(Y) In The Assembly And Function Of The Ifn-Beta Enhanceosome. Embo J. 1999, 18, 3074–3089. [Google Scholar] [CrossRef]
- Bouallaga, I.; Massicard, S.; Yaniv, M.; Thierry, F. An Enhanceosome Containing The Jun B/Fra-2 Heterodimer And The Hmg-I(Y) Architectural Protein Controls Hpv 18 Transcription. Embo Rep. 2000, 1, 422–427. [Google Scholar] [CrossRef]
- Tollervey, J.R.; Curk, T.; Rogelj, B.; Briese, M.; Cereda, M.; Kayikci, M.; Konig, J.; Hortobagyi, T.; Nishimura, A.L.; Zupunski, V.; et al. Characterizing The Rna Targets And Position-Dependent Splicing Regulation By Tdp-43. Nat. Neurosci. 2011, 14, 452–458. [Google Scholar] [CrossRef]
- Reddi, P.P. Transcription And Splicing Factor Tdp-43: Role In Regulation Of Gene Expression In Testis. Semin. Reprod. Med. 2017, 35, 167–172. [Google Scholar] [CrossRef]
- Dewey, C.M.; Cenik, B.; Sephton, C.F.; Johnson, B.A.; Herz, J.; Yu, G. Tdp-43 Aggregation In Neurodegeneration: Are Stress Granules The Key? Brain Res. 2012, 1462, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sidibe, H.; Khalfallah, Y.; Xiao, S.; Gomez, N.B.; Fakim, H.; Tank, E.M.H.; Di Tomasso, G.; Bareke, E.; Aulas, A.; Mckeever, P.M.; et al. Tdp-43 Stabilizes G3bp1 Mrna: Relevance To Amyotrophic Lateral Sclerosis/Frontotemporal Dementia. Brain 2021, 144, 3461–3476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Cai, H.; Xue, W.; Wang, M.; Xia, T.; Li, W.J.; Xing, J.Q.; Zhao, M.; Huang, Y.J.; Chen, S.; et al. G3bp1 Promotes Dna Binding And Activation Of Cgas. Nat. Immunol. 2019, 20, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wiser, C.; Kim, B.; Ascano, M. G3bp1 Enhances Cytoplasmic Dna Pattern Recognition. Nat. Immunol. 2019, 20, 5–7. [Google Scholar] [CrossRef]
- Cai, W.; Astor, T.L.; Liptak, L.M.; Cho, C.; Coen, D.M.; Schaffer, P.A. The Herpes Simplex Virus Type 1 Regulatory Protein Icp0 Enhances Virus Replication During Acute Infection And Reactivation From Latency. J. Virol. 1993, 67, 7501–7512. [Google Scholar] [CrossRef]
- Samaniego, L.A.; Wu, N.; Deluca, N.A. The Herpes Simplex Virus Immediate-Early Protein Icp0 Affects Transcription From The Viral Genome And Infected-Cell Survival In The Absence Of Icp4 And Icp27. J. Virol. 1997, 71, 4614–4625. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The Pride Database Resources In 2022: A Hub For Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]


| Accession | Protein | Gene | UI | KOS | n212 | KOS/UI | KOS/n212 | KOS:UI (p Value) | KOS:n212 (p Value) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Viral Proteins | P03176 | Thymidine kinase | TK | 0.0 | 12.5 | 3.0 | NC | 4.17 | 0.0016 | 0.0136 |
| P08543 | Ribonucleoside-diphosphate reductase large subunit | RIR1 | 0.0 | 112.0 | 3.5 | NC | 32.00 | 0.0249 | 0.0266 | |
| P10221 | Inner tegument protein | UL37 | 0.0 | 58.5 | 0.0 | NC | NC | 0.0018 | 0.0018 | |
| P04296 | Major DNA-binding protein | DBP | 0.0 | 90.0 | 0.0 | NC | NC | 0.0202 | 0.0202 | |
| P04294 | Alkaline nuclease | UL12 | 0.0 | 41.0 | 0.0 | NC | NC | 0.0279 | 0.0279 | |
| P10211 | Envelope glycoprotein B | gB | 0.0 | 22.0 | 0.0 | NC | NC | 0.0670 | 0.0670 | |
| P04485 | Transcriptional regulator ICP22 | ICP22 | 0.0 | 21.0 | 0.0 | NC | NC | 0.0955 | 0.0955 | |
| P06492 | Tegument protein VP16 | UL48 | 0.0 | 21.0 | 0.0 | NC | NC | 0.1196 | 0.1196 | |
| P08392 | Major viral transcription factor ICP4 | ICP4 | 0.0 | 28.0 | 0.0 | NC | NC | 0.2222 | 0.2222 | |
| P06491 | Major capsid protein | MCP | 0.0 | 46.0 | 0.0 | NC | NC | 0.2421 | 0.2421 | |
| P08393 | E3 ubiquitin-protein ligase ICP0 | ICP0 | 0.0 | 36.0 | 0.0 | NC | NC | 0.2865 | 0.2865 | |
| P04488 | Envelope glycoprotein E | gE | 0.0 | 6.5 | 0.0 | NC | NC | 0.4226 | 0.4226 | |
| Host Proteins | Q60900 | ELAV-like protein 3 | Elavl3 | 0.0 | 21.0 | 0.0 | NC | NC | 0.0023 | 0.0023 |
| P61027 | Ras-related protein Rab-10 | Rab10 | 0.0 | 20.0 | 0.0 | NC | NC | 0.0025 | 0.0025 | |
| A0A1B0GS70 | Proteasome endopeptidase complex | Psma1 | 0.0 | 15.5 | 0.0 | NC | NC | 0.0250 | 0.0250 | |
| P54775 | 26S proteasome regulatory subunit 6B | Psmc4 | 0.0 | 21.5 | 0.0 | NC | NC | 0.0255 | 0.0255 | |
| Q9JKC6 | Cell cycle exit and neuronal differentiation protein 1 | Cend1 | 0.0 | 12.0 | 0.0 | NC | NC | 0.0572 | 0.0572 | |
| P49312 | Heterogeneous nuclear ribonucleoprotein A1 | Hnrnpa1 | 0.0 | 19.0 | 0.0 | NC | NC | 0.0628 | 0.0628 | |
| P17095 | High mobility group protein HMG-I/HMG-Y | Hmga1 | 0.0 | 12.5 | 0.0 | NC | NC | 0.4226 | 0.4226 | |
| P43274 | Histone H1.4 | H1-4 | 0.0 | 72.5 | 21.5 | NC | 3.37 | 0.2593 | 0.3874 | |
| P15864 | Histone H1.2 | H1-2 | 0.0 | 74.5 | 23.0 | NC | 3.24 | 0.3050 | 0.4444 | |
| Q91ZZ3 | Beta-synuclein | Sncb | 0.0 | 40.0 | 18.0 | NC | 2.22 | 0.0056 | 0.0258 | |
| Q8R0B4 | TAR DNA-binding protein 43 | Tardp | 0.0 | 29.0 | 15.5 | NC | 1.87 | 0.0105 | 0.0995 | |
| Q3THW5 | Histone H2A.V | H2az2 | 0.0 | 41.0 | 23.5 | NC | 1.74 | 0.0450 | 0.1917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrell, T.L.; Davido, D.J.; Bertke, A.S. Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons. Int. J. Mol. Sci. 2023, 24, 2931. https://doi.org/10.3390/ijms24032931
Harrell TL, Davido DJ, Bertke AS. Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons. International Journal of Molecular Sciences. 2023; 24(3):2931. https://doi.org/10.3390/ijms24032931
Chicago/Turabian StyleHarrell, Telvin L., David J. Davido, and Andrea S. Bertke. 2023. "Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons" International Journal of Molecular Sciences 24, no. 3: 2931. https://doi.org/10.3390/ijms24032931
APA StyleHarrell, T. L., Davido, D. J., & Bertke, A. S. (2023). Herpes Simplex Virus 1 (HSV-1) Infected Cell Protein 0 (ICP0) Targets of Ubiquitination during Productive Infection of Primary Adult Sensory Neurons. International Journal of Molecular Sciences, 24(3), 2931. https://doi.org/10.3390/ijms24032931






