The Expression Pattern of Insulin-Like Growth Factor Subtype 3 (igf3) in the Orange-Spotted Grouper Epinephelus coioides and Its Function on Ovary Maturation
Abstract
1. Introduction
2. Results
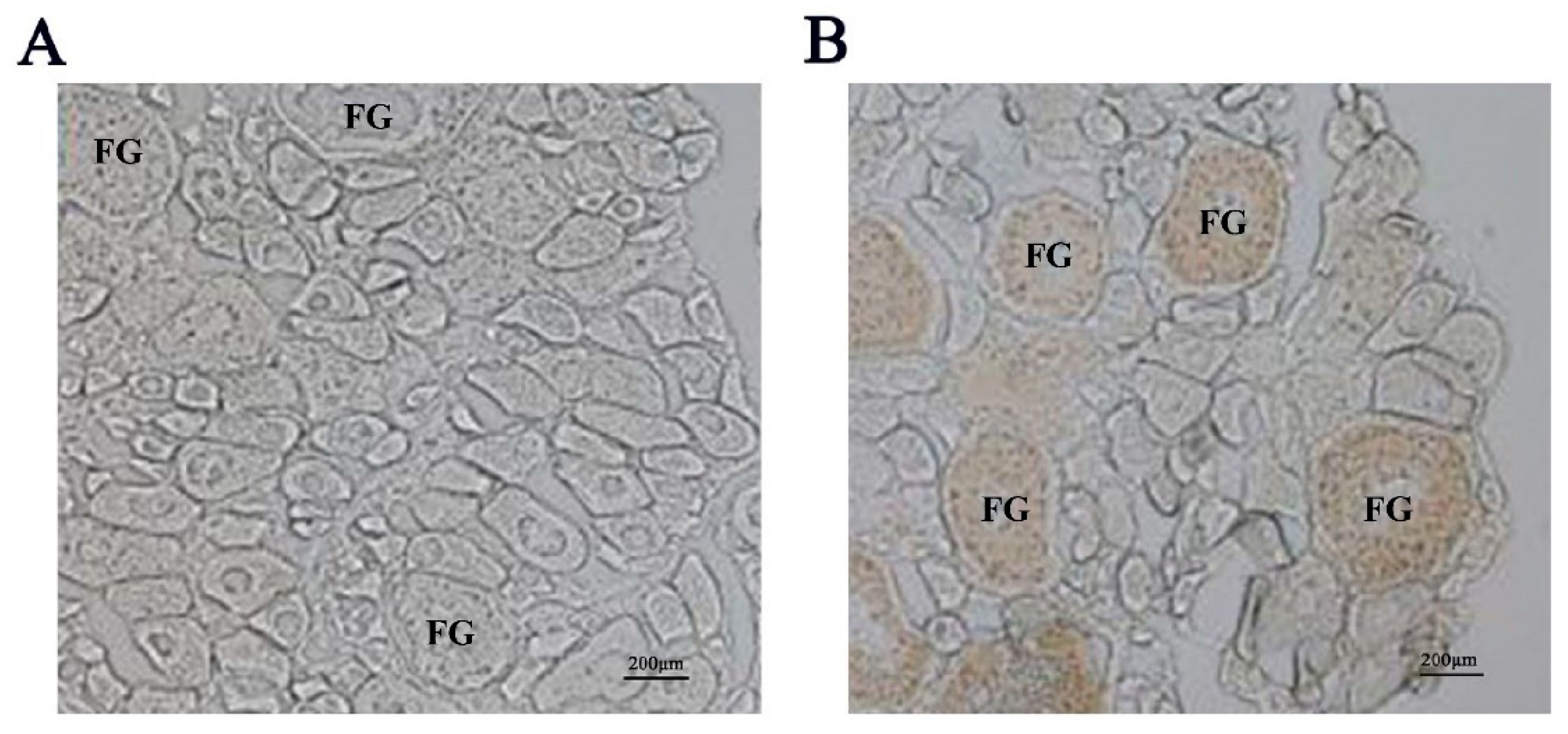
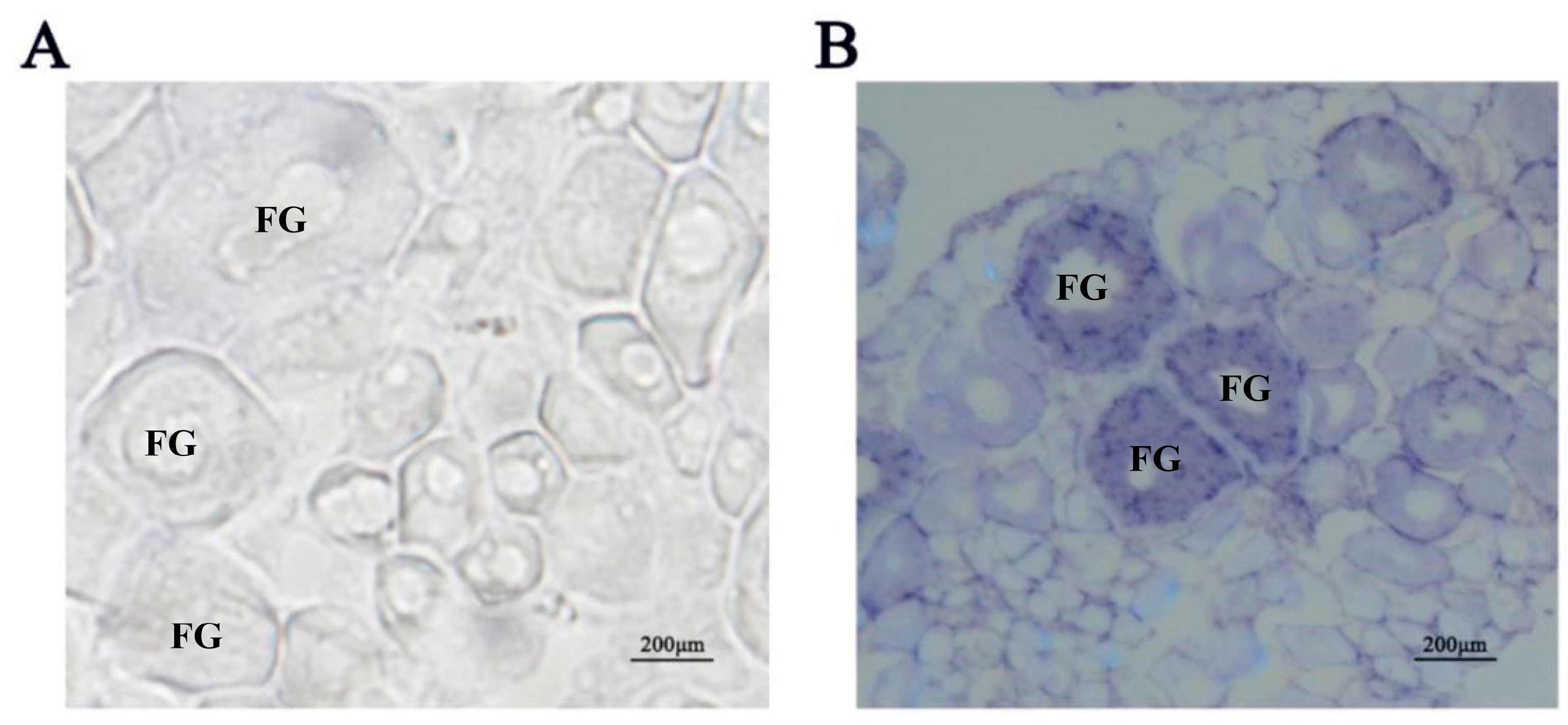
2.1. Distribution of igf3 mRNA in the Developing Ovary
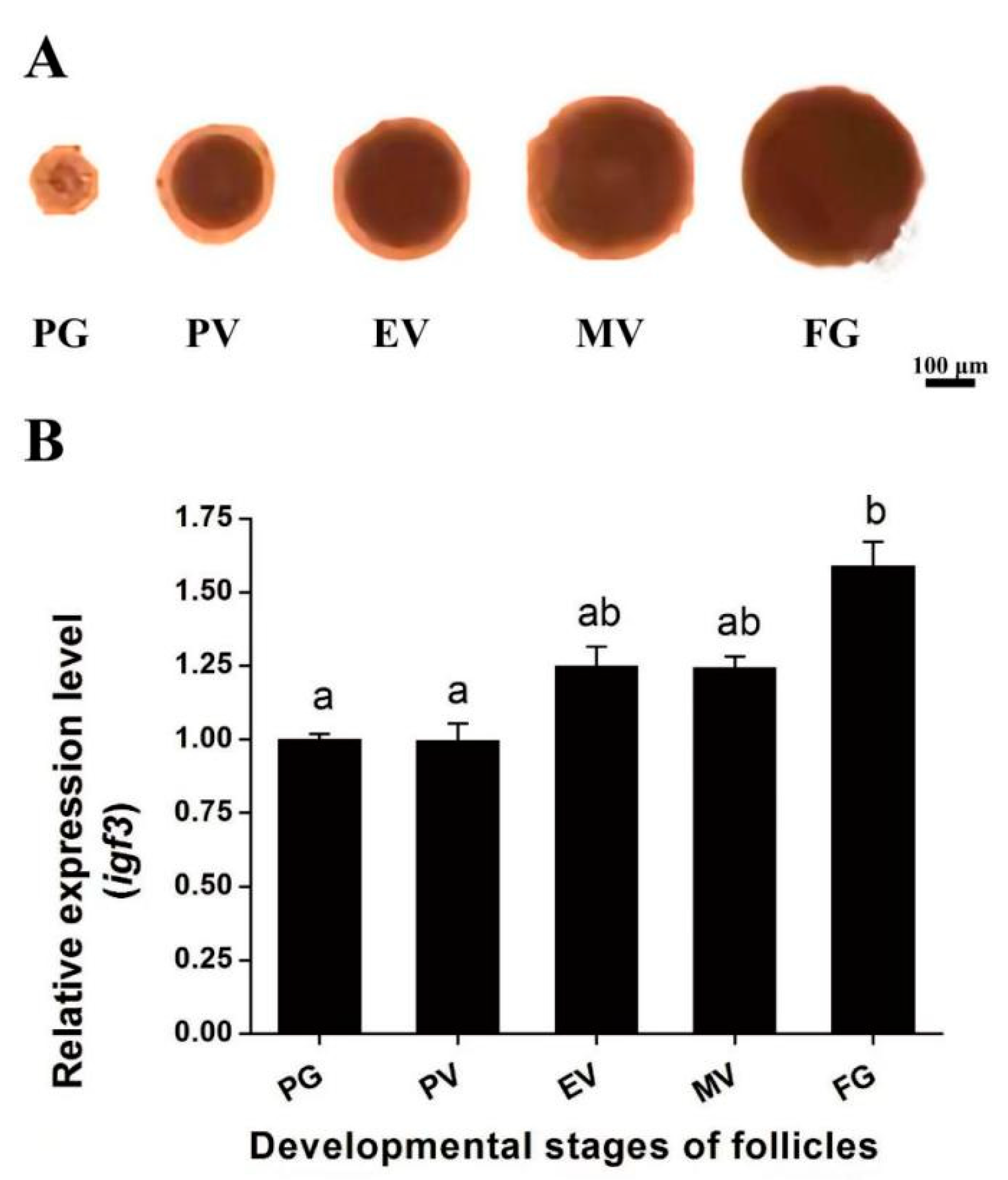
2.2. Expression of igf3 mRNA in Different Stages of Ovarian Follicular
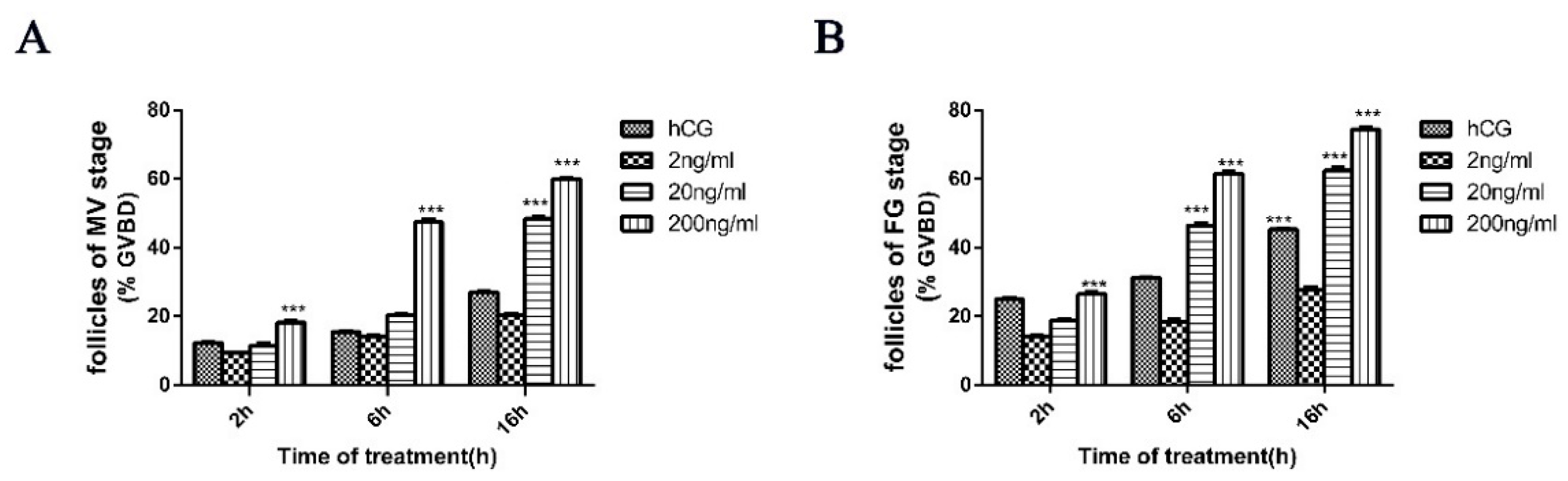
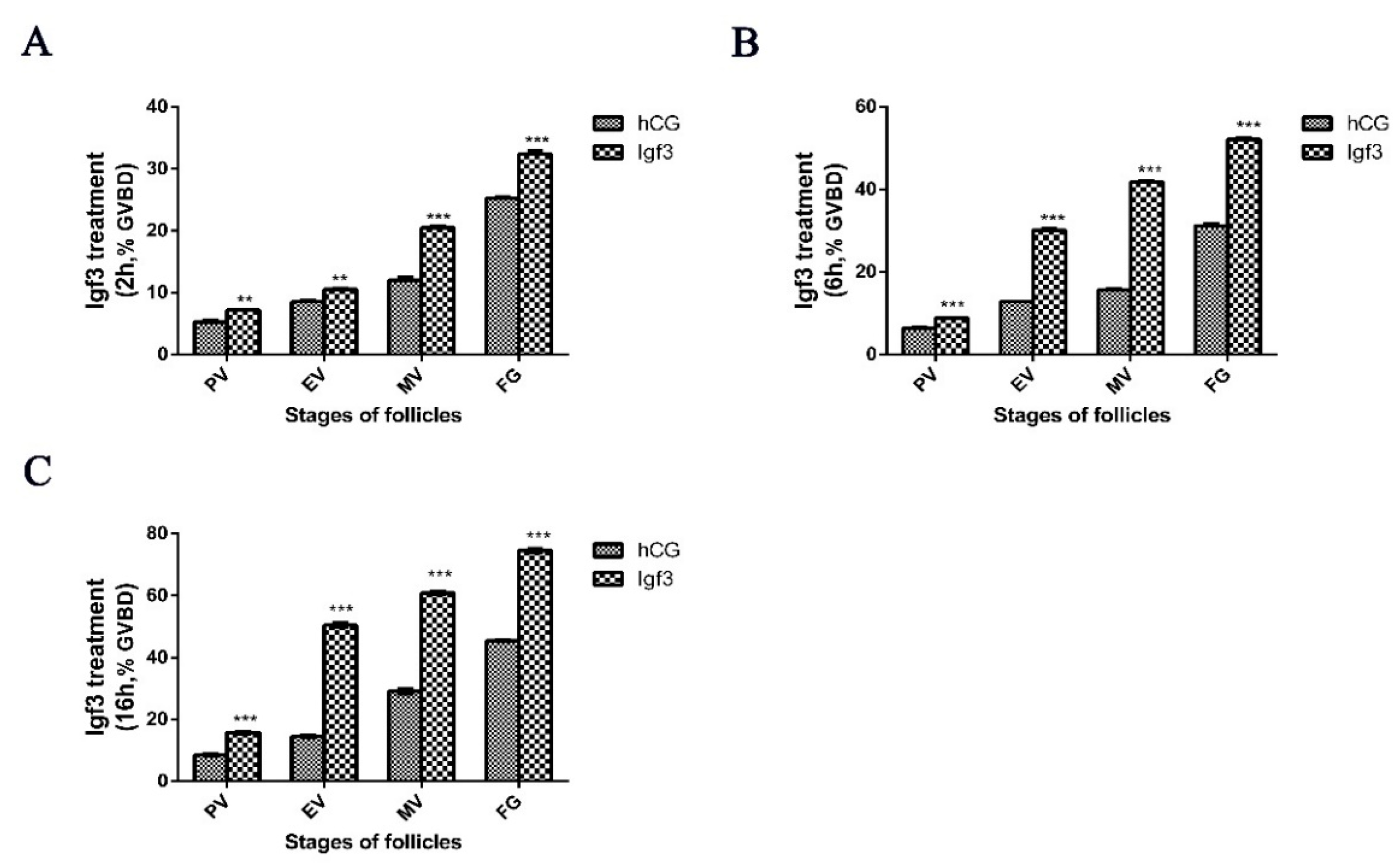
2.3. Igf3 Promotes the Development and Maturation of Follicular
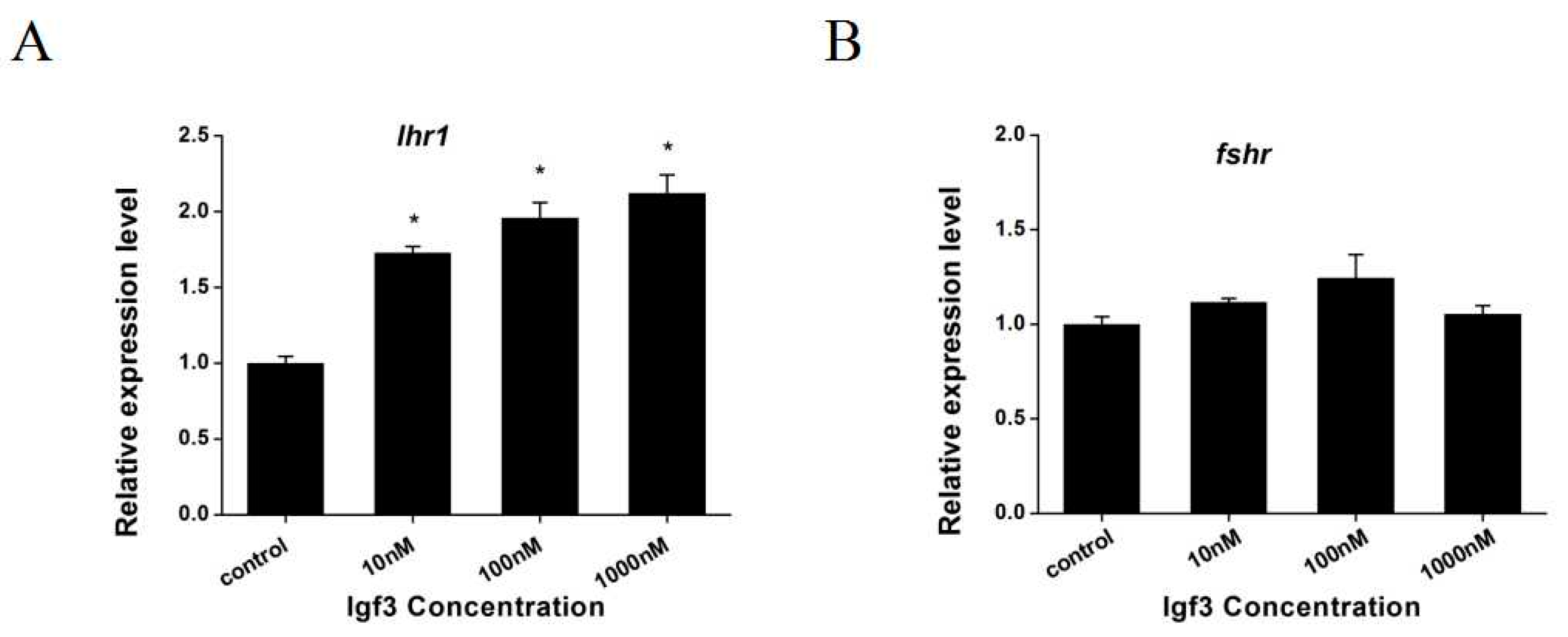
2.4. Effects of Igf3 on the Expression of Genes Related to Sex Determination and Reproduction
3. Discussion
4. Materials and Methods
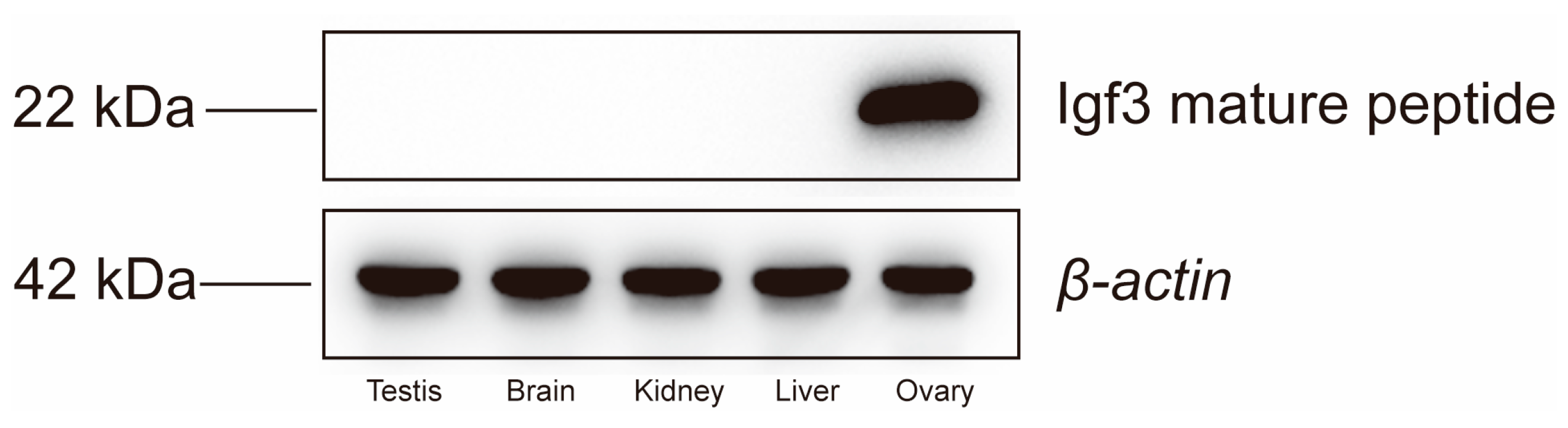
4.1. Fish, Cells Line and Igf3 Mature Peptide
4.2. RNA Extraction, cDNA Synthesis and Real-Time Polymerase Chain Reaction (qRT-PCR)
4.3. Ovarian Follicle Experiment
4.4. In Situ Hybridization
4.5. Western Blotting
4.6. Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.S.; Jiao, B.; Hu, C.; Huang, X.; Liu, Z.; Cheng, C.H. Discovery of a gonad-specific IGF subtype in teleost. Biochem. Biophys. Res. Commun. 2008, 367, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, F.; Gu, Y.; Wang, T.; Wang, H.; Yang, S.; Sun, Y.; Zhou, L.; Huang, X.; Jiao, B.; et al. Insulin-like growth factor 3 regulates expression of genes encoding steroidogenic enzymes and key transcription factors in the Nile tilapia gonad. Biol. Reprod. 2012, 86, 163. [Google Scholar] [CrossRef] [PubMed]
- Elabd, H.; Soror, E.; El-Asely, A.; El-Gawad, E.A.; Abbass, A. Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: Effect on growth and stress indices. Egypt. J. Aquat. Res. 2019, 45, 265–271. [Google Scholar] [CrossRef]
- Sparkman, A.M.; Schwartz, T.S.; Madden, J.A.; Boyken, S.E.; Ford, N.B.; Serb, J.M.; Bronikowski, A.M. Rates of molecular evolution vary in vertebrates for insulin-like growth factor-1 (IGF-1), a pleiotropic locus that regulates life history traits. Gen. Comp. Endocrinol. 2012, 178, 164–173. [Google Scholar] [CrossRef]
- Ozcan Gokcek, E.; Isik, R. Associations between genetic variants of the insulin-like growth factor I (IGF-I) gene and growth traits in European sea bass (Dicentrarchus labrax, L.). Fish Physiol. Biochem. 2020, 46, 1131–1138. [Google Scholar] [CrossRef]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth hormone and insulin-like growth factor action in reproductive tissues. Front. Endocrinol. (Lausanne) 2019, 10, 777. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.; Liu, X.; Liu, Q.; Liu, Y.; Zhang, Y.; Shi, B. Molecular characterization and expression profiles of insulin-like growth factors in yellowtail kingfish (Seriola lalandi) during embryonic development. Fish Physiol. Biochem. 2019, 45, 375–390. [Google Scholar] [CrossRef]
- Song, F.; Wang, L.; Zhu, W.; Fu, J.; Dong, J.; Dong, Z. A novel igf3 gene in common carp (Cyprinus carpio): Evidence for its role in regulating gonadal development. PLoS ONE 2016, 11, e0168874. [Google Scholar] [CrossRef]
- Prado, P.S.; Pinheiro, A.P.B.; Weber, A.A.; Bazzoli, N.; Rizzo, E. Expression patterns and immunolocalisation of IGF-I and IGF-II in male and female gonads of the Neotropical characid fish Astyanax fasciatus. Fish Physiol. Biochem. 2019, 45, 167–176. [Google Scholar] [CrossRef]
- Reinecke, M.; Björnsson, B.T.; Dickhoff, W.W.; McCormick, S.D.; Navarro, I.; Power, D.M.; Gutiérrez, J. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen. Comp. Endocrinol. 2005, 142, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Geba, K.; Martos-Sitcha, J.A.; Galal-Khallaf, A.; Mancera, J.M.; Martinez-Rodriguez, G. Insulin-like growth factor 1 (IGF-1) regulates prolactin, growth hormone, and IGF-1 receptor expression in the pituitary gland of the gilthead sea bream Sparus aurata. Fish Physiol. Biochem. 2016, 42, 365–377. [Google Scholar] [CrossRef]
- Picha, M.E.; Shi, B.; Thomas, P. Dual role of IGF-II in oocyte maturation in southern flounder Paralichthys lethostigma: Up-regulation of mPRα and resumption of meiosis. Gen Comp. Endocrinol. 2012, 177, 220–230. [Google Scholar] [CrossRef]
- Higuchi, K.; Gen, K.; Izumida, D.; Kazeto, Y.; Hotta, T.; Takashi, T.; Aono, H.; Soyano, K. Changes in gene expression and cellular localization of insulin-like growth factors 1 and 2 in the ovaries during ovary development of the yellowtail, Seriola quinqueradiata. Gen. Comp. Endocrinol. 2016, 232, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Berishvili, G.; D’Cotta, H.; Baroiller, J.F.; Segner, H.; Reinecke, M. Differential expression of IGF-I mRNA and peptide in the male and female gonad during early development of a bony fish, the tilapia Oreochromis niloticus. Gen. Comp. Endocrinol. 2006, 146, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, S.; Gessner, J.; Kirschbaum, F.; Kloas, W. Expression of IGF-I and IGF-I receptor in male and female sterlet, Acipenser ruthenus—Evidence for an important role in gonad maturation. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2007, 147, 223–230. [Google Scholar] [CrossRef]
- Schmid, A.; Rhyn-Näf, E.; Kloas, W.; Reinecke, M. Insulin-like growth factor-I and -II in the ovary of a bony fish, Oreochromis mossambicus, the tilapia: In situ hybridisation, immunohistochemical localisation, Northern blot and cDNA sequences. Mol. Cell. Endocrinol. 1999, 156, 141–149. [Google Scholar] [CrossRef]
- Nelson, S.N.; Van Der Kraak, G. Characterization and regulation of the insulin-like growth factor (IGF) system in the zebrafish (Danio rerio) ovary. Gen. Comp. Endocrinol. 2010, 168, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Kraak, G.V.D. Regulation of DNA synthesis in goldfish vitellogenic ovarian-follicles by hormones and growth factors. J. Exp. Zool. A Ecol. Genet. Physiol. 1994, 270, 263–272. [Google Scholar] [CrossRef]
- Lokman, P.M.; George, K.A.; Divers, S.L.; Algie, M.; Young, G. 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 2007, 133, 955–967. [Google Scholar] [CrossRef]
- Weber, G.M.; Sullivan, C.V. Insulin-like growth factor-I induces oocyte maturational competence but not meiotic resumption in white bass (Morone chrysops) follicles in vitro: Evidence for rapid evolution of insulin-like growth factor action. Biol. Reprod. 2005, 72, 1177–1186. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Wang, D.; Cheng, C.H. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol. Reprod. 2011, 84, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, H.; Zhao, H.; Liu, L.; Xie, Z.; Xiao, L.; Li, S.; Zhang, Y.; Lin, H. Molecular cloning of the insulin-like growth factor 3 and difference in the expression of igf genes in orange-spotted grouper (Epinephelus coioides). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 186, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef]
- Maitra, S.; Das, D.; Ghosh, P.; Hajra, S.; Roy, S.S.; Bhattacharya, S. High cAMP attenuation of insulin-stimulated meiotic G2-M1 transition in zebrafish oocytes: Interaction between the cAMP-dependent protein kinase (PKA) and the MAPK3/1 pathways. Mol. Cell. Endocrinol. 2014, 393, 109–119. [Google Scholar] [CrossRef]
- Berishvili, G.; Baroiller, J.F.; Eppler, E.; Reinecke, M. Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: Development and regulation of gene expression by growth hormone (GH) and 17alpha-ethinylestradiol (EE2). Gen. Comp. Endocrinol. 2010, 167, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.-F.; So, W.-K.; Wang, Y.; Ge, W. Zebrafish gonadotropins and their receptors: I. cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors-evidence for their distinct functions in follicle development 1. Biol. Reprod. 2005, 72, 1370–1381. [Google Scholar] [CrossRef]
- Weber, G.M.; Sullivan, C.V. Effects of insulin-like growth factor-I on in vitro final oocyte maturation and ovarian steroidogenesis in striped bass, Morone saxatilis. Biol. Reprod. 2000, 63, 1049–1057. [Google Scholar] [CrossRef]
- Kagawa, H.; Kobayashi, M.; Hasegawa, Y.; Aida, K. Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen. Comp. Endocrinol. 1994, 95, 293–300. [Google Scholar] [CrossRef]
- Mukherjee, D.; Mukherjee, D.; Sen, U.; Paul, S.; Bhattacharyya, S.P. In vitro effects of insulin-like growth factors and insulin on oocyte maturation and maturation-inducing steroid production in ovarian follicles of common carp, Cyprinus carpio. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 63–77. [Google Scholar] [CrossRef]
- Nelson, S.N.; Van Der Kraak, G. The role of the insulin-like growth factor (IGF) system in zebrafish (Danio rerio) ovarian development. Gen. Comp. Endocrinol. 2010, 168, 103–110. [Google Scholar] [CrossRef]
- Ottolenghi, C.; Omari, S.; Garcia-Ortiz, J.E.; Uda, M.; Crisponi, L.; Forabosco, A.; Pilia, G.; Schlessinger, D. Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 2005, 14, 2053–2062. [Google Scholar] [CrossRef]
- Wang, D.S.; Kobayashi, T.; Zhou, L.Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.; Nagahama, Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortiz, J.E.; Pelosi, E.; Omari, S.; Nedorezov, T.; Piao, Y.; Karmazin, J.; Uda, M.; Cao, A.; Cole, S.W.; Forabosco, A.; et al. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev. Biol. 2009, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Duffy, T.A.; Picha, M.E.; Won, E.T.; Borski, R.J.; McElroy, A.E.; Conover, D.O. Ontogenesis of gonadal aromatase gene expression in atlantic silverside (Menidia menidia) populations with genetic and temperature-dependent sex determination. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 421–431. [Google Scholar] [CrossRef]
- Conley, A.; Hinshelwood, M. Mammalian aromatases. Reproduction 2001, 121, 685–695. [Google Scholar] [CrossRef]
- Guo, I.-C.; Shih, M.-C.; Lan, H.-C.; Hsu, N.-C.; Hu, M.-C.; Chung, B.-C. Transcriptional regulation of human CYP11A1 in gonads and adrenals. J. Biomed. Sci. 2007, 14, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, D.; Chu, L.; Liu, Y.; Sun, X.; Li, J.; Cheng, C.H.K. Igf3 is essential for ovary differentiation in zebrafishdagger. Biol. Reprod. 2021, 104, 589–601. [Google Scholar] [CrossRef]
- Parker, K.L.; Schimmer, B.P. Steroidogenic factor 1: A key determinant of endocrine development and function. Endocr. Rev. 1997, 18, 361–377. [Google Scholar] [CrossRef]
- Zhang, B.; Shozu, M.; Okada, M.; Ishikawa, H.; Kasai, T.; Murakami, K.; Nomura, K.; Harada, N.; Inoue, M. Insulin-like growth factor I enhances the expression of aromatase P450 by inhibiting autophagy. Endocrinology 2010, 151, 4949–4958. [Google Scholar] [CrossRef]
- van der Kraak, G. Chapter 3 the GnRH system and the neuroendocrine regulation of reproduction. Fish Physiol. 2009, 28, 115–149. [Google Scholar]
- Yerramalla, U.L.; Nadimpalli, S.K.; Schu, P.; Figura, K.V.; Hille-Rehfeld, A. Conserved cassette structure of vertebrate M(r) 300 kDa mannose 6-phosphate receptors: Partial cDNA sequence of fish MPR 300. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 127, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Ge, W. Spatial distribution and receptor specificity of zebrafish Kit system-evidence for a Kit-mediated bi-directional communication system in the preovulatory ovarian follicle. PLoS ONE 2013, 8, e56192. [Google Scholar] [CrossRef] [PubMed]
- Saez, N.J.; Vincentelli, R. High-throughput expression screening and purification of recombinant proteins in E. coli. Methods Mol. Biol. 2014, 1091, 33–53. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-ΔΔC(T)) method. Methods 2012, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Wedlich, D. PCR-based RNA probes: A quick and sensitive method to improve whole mount embryo in situ hybridizations. Biotechniques 2001, 30, 769–772. [Google Scholar] [CrossRef]
- Shi, S.R.; Cote, R.J.; Taylor, C.R. Antigen retrieval immunohistochemistry: Past, present, and future. J. Histochem. Cytochem. 1997, 45, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: Evidence for their differential roles during sexual maturation and ovulatory cycle1. Biol. Reprod. 2004, 71, 2056–2064. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev. Biol. 2010, 342, 194–206. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | GenBank Accession Number | Full Gene Name | Primer Sequence(5′-3′) | Amplification Efficiency (%) |
|---|---|---|---|---|
| β-actin | AY510710 | Beta-actin | Forward: TTCACCACCACAGCCGAGA Reverse: TGGTCTCGTGGATTCCGCAG | 100 |
| igf3 | KU743244.1 | Insulin-like growth factor subtype 3 | Forward: GTGTGTATGTTCTACTCCAC Reverse: TCCTTCTGTAAGCTTCCCTC | 100 |
| foxl2 | KP688066.1 | Forkhead box L2 | Forward: CAGGTAGCCATAGCCGTCTTCT Reverse: CCTCCACCGACGCACTTC | 99 |
| dax1 | HQ455522.1 | Dosage-sensitive sex reversal adrenal hypoplasia critical region on chromosome x gene 1 | Forward: CTGCCTCCACTACATCCAGT Reverse: TGCTGACGGTCCCTATAACG | 98 |
| cyp19a1a | EU239953.1 | Cytochrome P450 family 19 subfamily member 1 a | Forward: GGAGACATTGTGAGAGTCTGGATC Reverse: TGACAGGTACATCCAGGAAGAGTC | 99 |
| cyp19a1b | JF420889.1 | Cytochrome P450 family 19 subfamily member 1 b | Forward: TGACACCTGGCAAACAGTTC Reverse: GATGGTGTCGTCTTCCAGAG | 97 |
| cyp11a1 | FJ807733.1 | Cytochrome P450 family 11 subfamily a member 1 a | Forward: CTCCCTCCTGCTCTGTTGA Reverse: GCTGGCCTTGATGTCTTC | 96 |
| lhr1 | HQ650770.1 | Luteinizing hormone receptor 1 | Forward: GACCTTCCCATTAATCCTGCATGG Reverse: CACGTTTCCAAAGCCTTCATCATCCTC | 99 |
| fshr | HQ650769.1 | Follicle-stimulating hormone receptor | Forward: CGAGGCTGACCCTTACTTCC Reverse: GATCCAGATGAGGACCCGTA | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, F.; Fu, B.; Yang, Y.; Xue, H.; Wu, Y.; Zhao, H.; Wang, Q.; Yang, H. The Expression Pattern of Insulin-Like Growth Factor Subtype 3 (igf3) in the Orange-Spotted Grouper Epinephelus coioides and Its Function on Ovary Maturation. Int. J. Mol. Sci. 2023, 24, 2868. https://doi.org/10.3390/ijms24032868
Jiao F, Fu B, Yang Y, Xue H, Wu Y, Zhao H, Wang Q, Yang H. The Expression Pattern of Insulin-Like Growth Factor Subtype 3 (igf3) in the Orange-Spotted Grouper Epinephelus coioides and Its Function on Ovary Maturation. International Journal of Molecular Sciences. 2023; 24(3):2868. https://doi.org/10.3390/ijms24032868
Chicago/Turabian StyleJiao, Fang, Bing Fu, Yan Yang, Huayi Xue, Yuanyuan Wu, Huihong Zhao, Qing Wang, and Huirong Yang. 2023. "The Expression Pattern of Insulin-Like Growth Factor Subtype 3 (igf3) in the Orange-Spotted Grouper Epinephelus coioides and Its Function on Ovary Maturation" International Journal of Molecular Sciences 24, no. 3: 2868. https://doi.org/10.3390/ijms24032868
APA StyleJiao, F., Fu, B., Yang, Y., Xue, H., Wu, Y., Zhao, H., Wang, Q., & Yang, H. (2023). The Expression Pattern of Insulin-Like Growth Factor Subtype 3 (igf3) in the Orange-Spotted Grouper Epinephelus coioides and Its Function on Ovary Maturation. International Journal of Molecular Sciences, 24(3), 2868. https://doi.org/10.3390/ijms24032868





