Adjuvant Injections Altered the Ileal and Fecal Microbiota Differently with Changes in Immunoglobulin Isotypes and Antimycobacterial Antibody Responses
Abstract
1. Introduction
2. Results
2.1. Decreased Alpha Diversities of the Fecal Microbiota in CFA-Injected Mice
2.2. Fecal, but Not Ileal, Microbiota Changes in CFA-Injected Mice
2.3. Microbiota Differs between the Ileum and Feces in Naïve Mice, but Not in CFA-Injected Mice
2.4. Alterations of the Microbiota Compositions in the Ileum and Feces
2.5. Gut Microbiota Associates with Antibody Isotypes and Antimycobacterial Antibody Responses
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Antibody ELISA
4.3. 16S rRNA Amplicon Sequencing
4.4. Bioinformatics Analyses
4.4.1. Alpha Diversity
4.4.2. PCA
4.4.3. Pattern Matching
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garçon, N.; Friede, M. Evolution of adjuvants across the centuries. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 61–74. ISBN 978-0-323-35761-6. [Google Scholar]
- Matsumura, N.; Shiro, R.; Tsunoda, I. Critical evaluation on roles of macrophagic myofasciitis and aluminum adjuvants in HPV vaccine-induced adverse events. Cancer Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Freund, J. The mode of action of immunologic adjuvants. Adv. Tuberc. Res. 1956, 7, 130–148. [Google Scholar]
- Stills, H.F., Jr. Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Riteau, N.; Radtke, A.J.; Shenderov, K.; Mittereder, L.; Oland, S.D.; Hieny, S.; Jankovic, D.; Sher, A. Water-in-oil–only adjuvants selectively promote T follicular helper cell polarization through a type I IFN and IL-6–dependent pathway. J. Immunol. 2016, 197, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.L.; Hoebe, K.; Duong, B.; Ota, T.; Martin, C.; Beutler, B.; Nemazee, D. Adjuvant-enhanced antibody responses occur without toll-like receptor signaling. Science 2006, 314, 1936–1938. [Google Scholar] [CrossRef]
- Pollack, K.E.; Meneveau, M.O.; Melssen, M.M.; Lynch, K.T.; Koeppel, A.F.; Young, S.J.; Turner, S.; Kumar, P.; Sol-Church, K.; Mauldin, I.S.; et al. Incomplete Freund’s adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment. J. Immunother. Cancer 2020, 8, e000544. [Google Scholar] [CrossRef]
- Sato, F.; Omura, S.; Martinez, N.E.; Tsunoda, I. Animal models of multiple sclerosis. In Neuroinflammation, 2nd ed.; Minagar, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 37–72. [Google Scholar] [CrossRef]
- Sato, F.; Nakamura, Y.; Katsuki, A.; Khadka, S.; Ahmad, I.; Omura, S.; Martinez, N.E.; Tsunoda, I. Curdlan, a microbial β-glucan, has contrasting effects on autoimmune and viral models of multiple sclerosis. Front. Cell. Infect. Microbiol. 2022, 12, 805302. [Google Scholar] [CrossRef]
- Rivers, T.M.; Sprunt, D.H.; Berry, G.P. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J. Exp. Med. 1933, 58, 39–53. [Google Scholar] [CrossRef]
- Freund, J.; Stern, E.R.; Pisani, T.M. Isoallergic encephalomyelitis and radiculitis in guinea pigs after one injection of brain and mycobacteria in water-in-oil emulsion. J. Immunol. 1947, 57, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Nian, D.; Shi, P.; Qu, H.; Sun, J.; Li, Q.; Li, Q.; Wang, C.; Ye, M. Pathogenic mechanism of intestinal microbiota involved in Guillain-Barre syndrome and with Bifidobacterium intervention. Arch. Med. Sci. 2021, 128103. [Google Scholar] [CrossRef]
- Johanson, D.M., II; Goertz, J.E.; Marin, I.A.; Costello, J.; Overall, C.C.; Gaultier, A. Experimental autoimmune encephalomyelitis is associated with changes of the microbiota composition in the gastrointestinal tract. Sci. Rep. 2020, 10, 15183. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Omura, S.; Sato, F.; Nishio, K.; Kakeya, H.; Tsunoda, I. Curcumin β-D-glucuronide modulates an autoimmune model of multiple sclerosis with altered gut microbiota in the ileum and feces. Front. Cell. Infect. Microbiol. 2021, 11, 772962. [Google Scholar] [CrossRef]
- Rinaldi, E.; Consonni, A.; Cordiglieri, C.; Sacco, G.; Crasà, C.; Fontana, A.; Morelli, L.; Elli, M.; Mantegazza, R.; Baggi, F. Therapeutic effect of Bifidobacterium administration on experimental autoimmune myasthenia gravis in lewis rats. Front. Immunol. 2019, 10, 2949. [Google Scholar] [CrossRef]
- Hu, X.-F.; Zhang, W.-Y.; Wen, Q.; Chen, W.-J.; Wang, Z.-M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.-X.; Yang, J.; et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharm. Res. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Barin, J.G.; Talor, M.V.; Diny, N.L.; Ong, S.; Schaub, J.A.; Gebremariam, E.; Bedja, D.; Chen, G.; Choi, H.S.; Hou, X.; et al. Regulation of autoimmune myocarditis by host responses to the microbiome. Exp. Mol. Pathol. 2017, 103, 141–152. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Xie, K.; Xu, Y.; He, R.; Guo, L.; Han, Y.; Wu, S.; Dong, X.; Lu, Y.; et al. Dynamic alterations in the gut microbiota of collagen-induced arthritis rats following the prolonged administration of total glucosides of paeony. Front. Cell. Infect. Microbiol. 2019, 9, 204. [Google Scholar] [CrossRef]
- Lu, C.; Chen, J.; Yi, C.; Han, J.; Shi, Q.; Li, J.; Liu, B.; Zhou, J.; Su, X. Gut microbiota mediated the protective effects of tuna oil on collagen-induced arthritis in mice. Food Funct. 2021, 12, 5387–5398. [Google Scholar] [CrossRef]
- Pan, H.; Guo, R.; Ju, Y.; Wang, Q.; Zhu, J.; Xie, Y.; Zheng, Y.; Li, T.; Liu, Z.; Lu, L.; et al. A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome 2019, 7, 107. [Google Scholar] [CrossRef]
- Kong, Y.M. Experimental autoimmune thyroiditis in the mouse. Curr. Protoc. Immunol. 2007, 78, 15.7.1–15.7.21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Gill, H.S. Immunostimulatory probiotic Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 do not induce pathological inflammation in mouse model of experimental autoimmune thyroiditis. Int. J. Food Microbiol. 2005, 103, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Metea, C.; Karstens, L.; Asquith, M.; Gruner, H.; Moscibrocki, C.; Lee, I.; Brislawn, C.J.; Jansson, J.K.; Rosenbaum, J.T.; et al. Gut microbial alterations associated with protection from autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 2016, 57, 3747–3758. [Google Scholar] [CrossRef] [PubMed]
- Musha, M.; Hirai, S.; Naito, M.; Terayama, H.; Qu, N.; Hatayama, N.; Itoh, M. The effects of adjuvants on autoimmune responses against testicular antigens in mice. J. Reprod. Dev. 2013, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Djedovic, N.; Stanisavljević, S.; Dimitrijević, M.; Stegnjaić, G.; Krishnamoorthy, G.; Mostarica Stojković, M.; Miljković, Đ.; Jevtić, B. Complete Freund’s adjuvant-free experimental autoimmune encephalomyelitis in dark agouti rats is a valuable tool for multiple sclerosis studies. J. Neuroimmunol. 2021, 354, 577547. [Google Scholar] [CrossRef] [PubMed]
- Heissigerova, J.; Seidler Stangova, P.; Klimova, A.; Svozilkova, P.; Hrncir, T.; Stepankova, R.; Kverka, M.; Tlaskalova-Hogenova, H.; Forrester, J.V. The microbiota determines susceptibility to experimental autoimmune uveoretinitis. J. Immunol. Res. 2016, 2016, 5065703. [Google Scholar] [CrossRef]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the microbiota in the modulation of vaccine immune responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef]
- Tsunoda, I. Lymphatic system and gut microbiota affect immunopathology of neuroinflammatory diseases, including multiple sclerosis, neuromyelitis optica and Alzheimer’s disease. Clin. Exp. Neuroimmunol. 2017, 8, 177–179. [Google Scholar] [CrossRef]
- Park, A.-M.; Omura, S.; Fujita, M.; Sato, F.; Tsunoda, I. Helicobacter pylori and gut microbiota in multiple sclerosis versus Alzheimer’s disease: 10 pitfalls of microbiome studies. Clin. Exp. Neuroimmunol. 2017, 8, 215–232. [Google Scholar] [CrossRef]
- Ungaro, F.; Massimino, L.; D’Alessio, S.; Danese, S. The gut virome in inflammatory bowel disease pathogenesis: From metagenomics to novel therapeutic approaches. United Eur. Gastroenterol. J. 2019, 7, 999–1007. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol. Res. Pr. 2016, 2016, 6951091. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef]
- Pérez-Brocal, V.; García-López, R.; Nos, P.; Beltrán, B.; Moret, I.; Moya, A. Metagenomic analysis of Crohn’s disease patients identifies changes in the virome and microbiome related to disease status and therapy, and detects potential interactions and biomarkers. Inflamm. Bowel Dis. 2015, 21, 2515–2532. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Verstraete, S.G.; Phan, T.G.; Deng, X.; Stekol, E.; LaMere, B.; Lynch, S.V.; Heyman, M.B.; Delwart, E. Enteric virome and bacterial microbiota in children with ulcerative colitis and crohn disease. J. Ppediatr. Gastroenterol. Nutr. 2019, 68, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Zawalna, N.; Nijakowski, K.; Muller, I.; Karpiński, T.; Salvi, M.; Ruchała, M. Microbiota alterations in patients with autoimmune thyroid diseases: A systematic review. Int. J. Mol. Sci. 2022, 23, 13450. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Denning, T.L. Segmented filamentous bacteria-induced immune responses: A balancing act between host protection and autoimmunity. Immunology 2018, 154, 537–546. [Google Scholar] [CrossRef]
- Gu, S.; Chen, D.; Zhang, J.-N.; Lv, X.; Wang, K.; Duan, L.-P.; Nie, Y.; Wu, X.-L. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE 2013, 8, e74957. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Chae, C.-S.; Kwon, H.-K.; Hwang, J.-S.; Kim, J.-E.; Im, S.-H. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS ONE 2012, 7, e52119. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.-C.; Yamane, H.; Honda, K.; et al. Microbiota-dependent activation of an autoreactive t cell receptor provokes autoimmunity in an immunologically privileged site. Immunity 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.A.; Mamon, H.J.; Fuchs, C.S.; Doyle, L.A.; Tirumani, S.H.; Ramaiya, N.H.; Rosenthal, M.H. Anorectal cancer: Critical anatomic and staging distinctions that affect use of radiation therapy. Radiographics 2015, 35, 2090–2107. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Hufeldt, M.R.; Nielsen, D.S.; Vogensen, F.K.; Midtvedt, T.; Hansen, A.K. Variation in the gut microbiota of laboratory mice is related to both genetic and environmental factors. Comp. Med. 2010, 60, 336–342. [Google Scholar]
- Constantinescu, C.S.; Hilliard, B.A. Adjuvants in EAE. In Experimental Models of Multiple Sclerosis; Lavi, E., Constantinescu, C.S., Eds.; Springer: Boston, MA, USA, 2005; pp. 73–84. ISBN 978-0-387-25518-7. [Google Scholar]
- Zhang, Y.; Ran, Z.; Tian, M.; Zhou, Y.; Yang, J.; Yin, J.; Lu, D.; Li, R.; Zhong, J. Commensal microbes affect host humoral immunity to Bordetella pertussis infection. Infect. Immun. 2019, 87, e00421-19. [Google Scholar] [CrossRef]
- Stražar, M.; Mourits, V.P.; Koeken, V.A.C.M.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Joosten, L.A.B.; van Crevel, R.; Vlamakis, H.; Netea, M.G.; Xavier, R.J. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. 2021, 22, 275. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Liao, Q.; Wang, Z.; Wan, C. Characterization of gut microbiota in children with pulmonary tuberculosis. BMC Pediatr. 2019, 19, 445. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Wu, P.; Luo, D.-X.; Sun, Q.; Zheng, H.; Hu, R.; Pandol, S.J.; Li, Q.-F.; Han, Y.-P.; et al. Alternation of gut microbiota in patients with pulmonary tuberculosis. Front. Physiol. 2017, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-R.; Lee, S.-H.; Kim, B.; Nam, M.H.; Ahn, Y.K.; Park, Y.M.; Jeong, S.-M.; Park, M.J.; Song, K.B.; Lee, S.-Y.; et al. Ruminococcus gnavus ameliorates atopic dermatitis by enhancing Treg cell and metabolites in BALB/c mice. Pediatr. Allergy Immunol. 2022, 33, e13678. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Im, S.-H. Of Men in Mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef]
- Parvataneni, K.C.; Iyer, S.; Khatib, R.; Saravolatz, L.D. Facklamia species and Streptococcus pneumoniae meningitis: A case report and review of the literature. Open Forum Infect. Dis. 2015, 2, ofv029. [Google Scholar] [CrossRef]
- Cotta, M.A.; Whitehead, T.R.; Collins, M.D.; Lawson, P.A. Atopostipes suicloacale gen. nov., sp. nov., isolated from an underground swine manure storage pit. Anaerobe 2004, 10, 191–195. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Sterbini, F.P.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: Time for microbial marker of gastrointestinal disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Ngom, I.I.; Hasni, I.; Lo, C.I.; Traore, S.I.; Fontanini, A.; Raoult, D.; Fenollar, F. Taxono-genomics and description of Gordonibacter massiliensis sp. nov., a new bacterium isolated from stool of healthy patient. New Microbes New Infect. 2019, 33, 100624. [Google Scholar] [CrossRef]
- Hosomi, K.; Shibata, N.; Shimoyama, A.; Uto, T.; Nagatake, T.; Tojima, Y.; Nishino, T.; Takeyama, H.; Fukase, K.; Kiyono, H.; et al. Lymphoid tissue-resident Alcaligenes establish an intracellular symbiotic environment by creating a unique energy shift in dendritic cells. Front. Microbiol. 2020, 11, 561005. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Hofstetter, H.H.; Shive, C.L.; Forsthuber, T.G. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J. Immunol. 2002, 169, 117–125. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of intestinal microbiota and targeted amplification of bacterial 16s rRNA genes for microbial ecologic analysis. Curr. Protoc. Immunol. 2014, 107, 7.41.1–7.41.11. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.E.; Sato, F.; Omura, S.; Kawai, E.; Takahashi, S.; Yoh, K.; Tsunoda, I. RORγt, but not T-bet, overexpression exacerbates an autoimmune model for multiple sclerosis. J. Neuroimmunol. 2014, 276, 142–149. [Google Scholar] [CrossRef]
- Omura, S.; Sato, F.; Park, A.-M.; Fujita, M.; Khadka, S.; Nakamura, Y.; Katsuki, A.; Nishio, K.; Gavins, F.N.E.; Tsunoda, I. Bioinformatics analysis of gut microbiota and CNS transcriptome in virus-induced acute myelitis and chronic inflammatory demyelination; potential association of distinct bacteria with CNS IgA upregulation. Front. Immunol. 2020, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Omura, S.; Sato, F.; Martinez, N.E.; Minagar, A.; Ramanathan, M.; Guttman, B.W.; Zivadinov, R.; Tsunoda, I.; Alexander, J.S. Inflammation induces neuro-lymphatic protein expression in multiple sclerosis brain neurovasculature. J. Neuroinflammation. 2013, 10, 125. [Google Scholar] [CrossRef]
- Omura, S.; Sato, F.; Martinez, N.E.; Park, A.-M.; Fujita, M.; Kennett, N.J.; Cvek, U.; Minagar, A.; Alexander, J.S.; Tsunoda, I. Bioinformatics analyses determined the distinct CNS and peripheral surrogate biomarker candidates between two mouse models for progressive multiple sclerosis. Front. Immunol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Kawai, E.; Martinez, N.E.; Omura, S.; Park, A.-M.; Takahashi, S.; Yoh, K.; Tsunoda, I. T-bet, but not Gata3, overexpression is detrimental in a neurotropic viral infection. Sci. Rep. 2017, 7, 10496. [Google Scholar] [CrossRef] [PubMed]
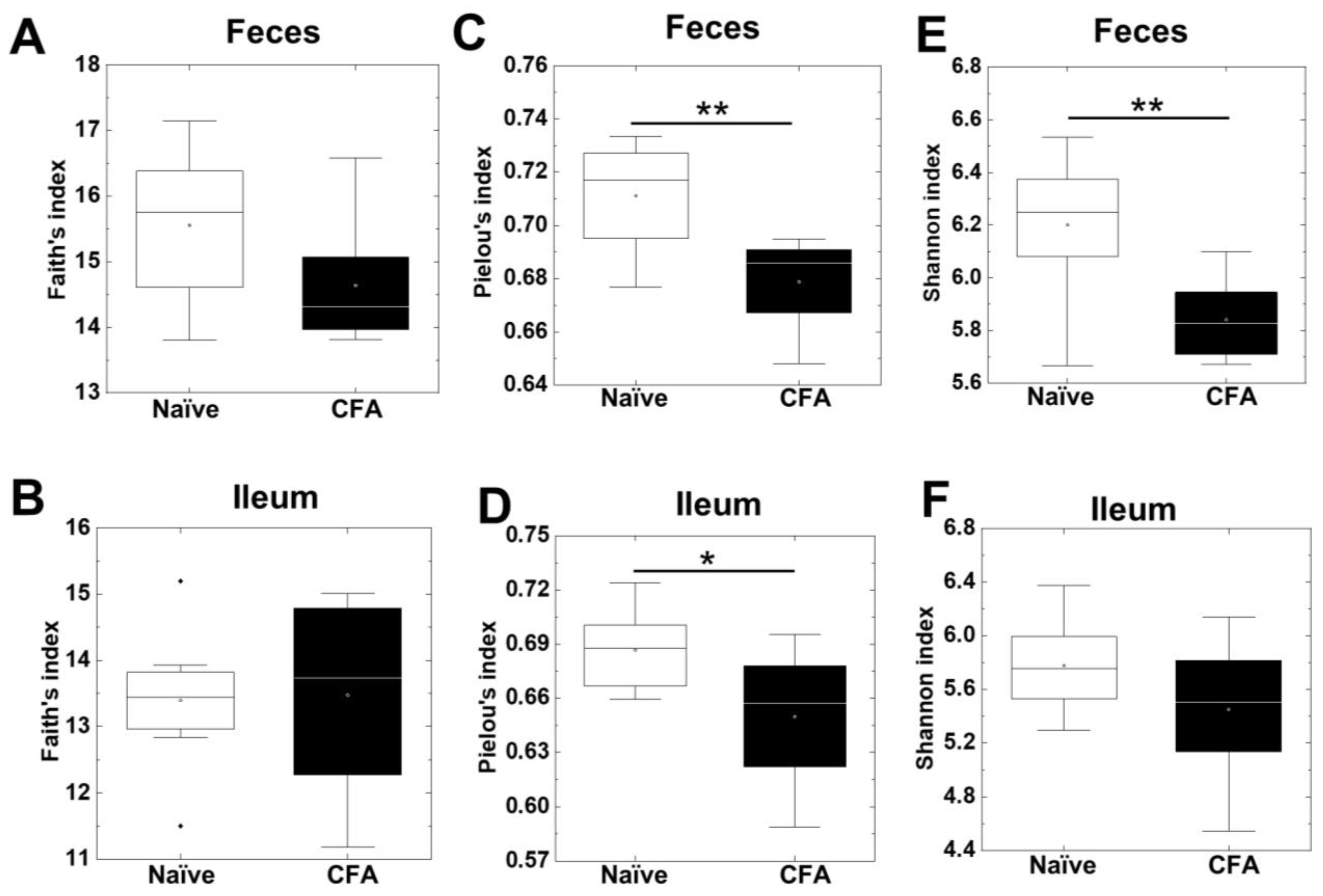
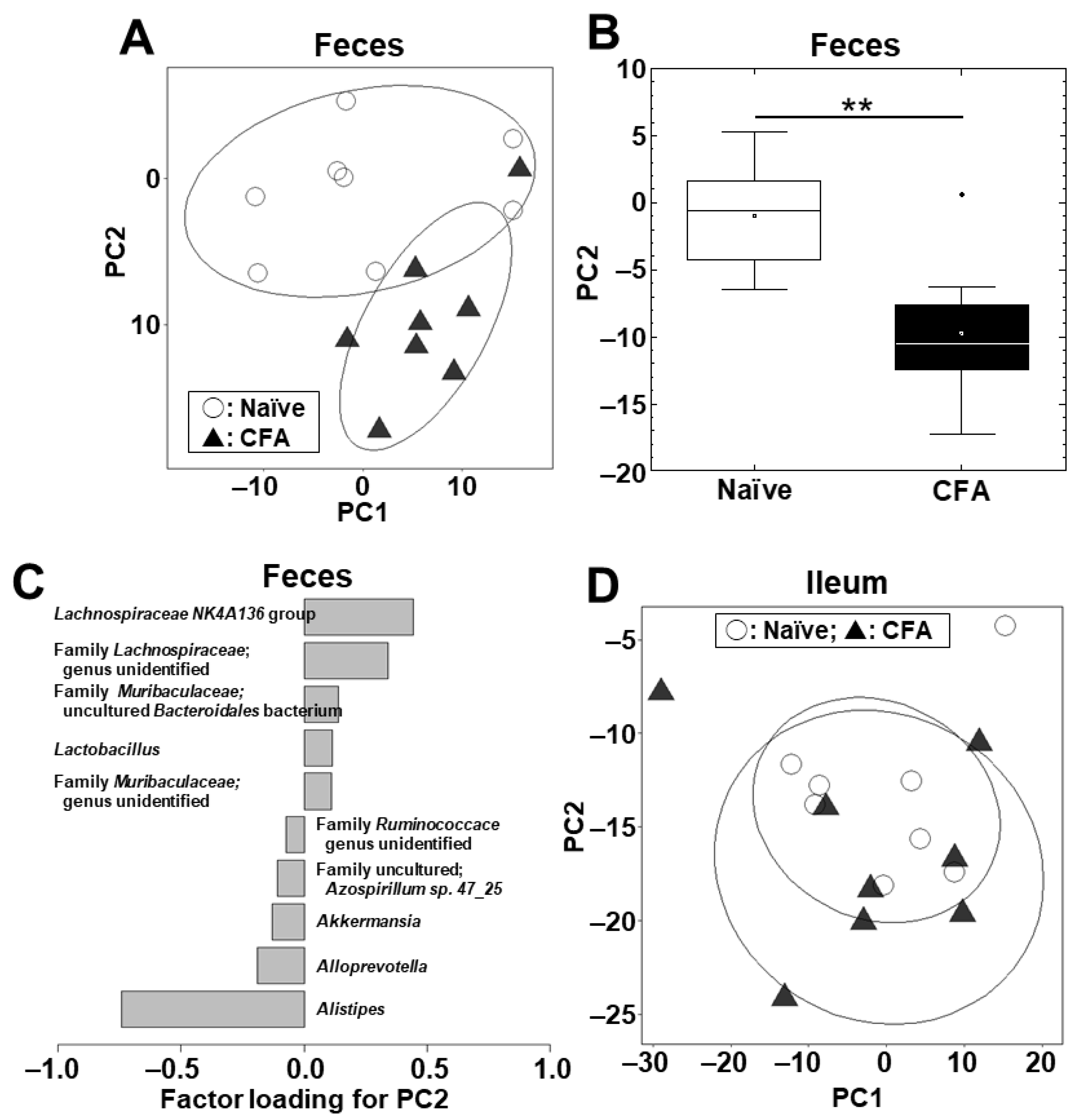
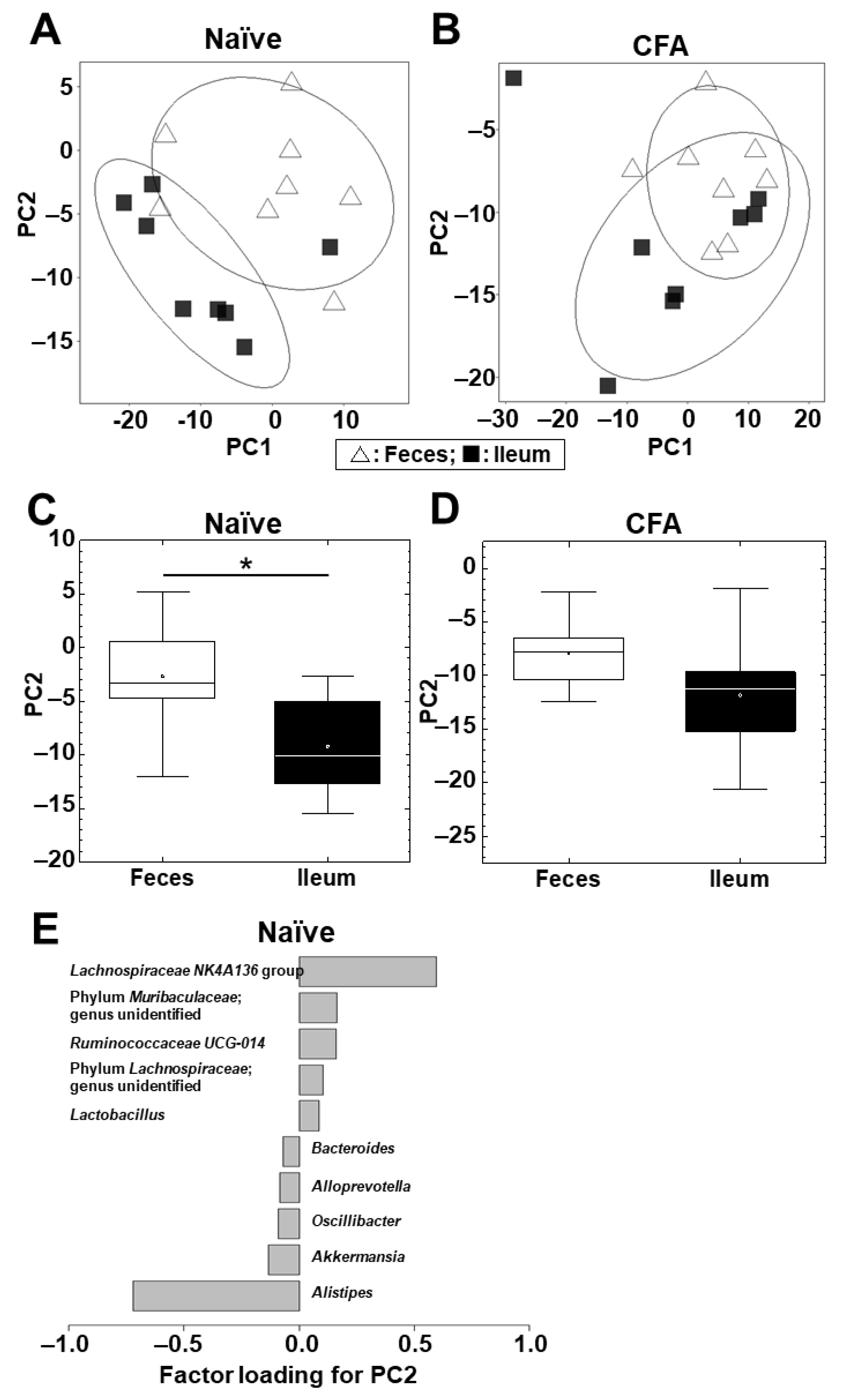
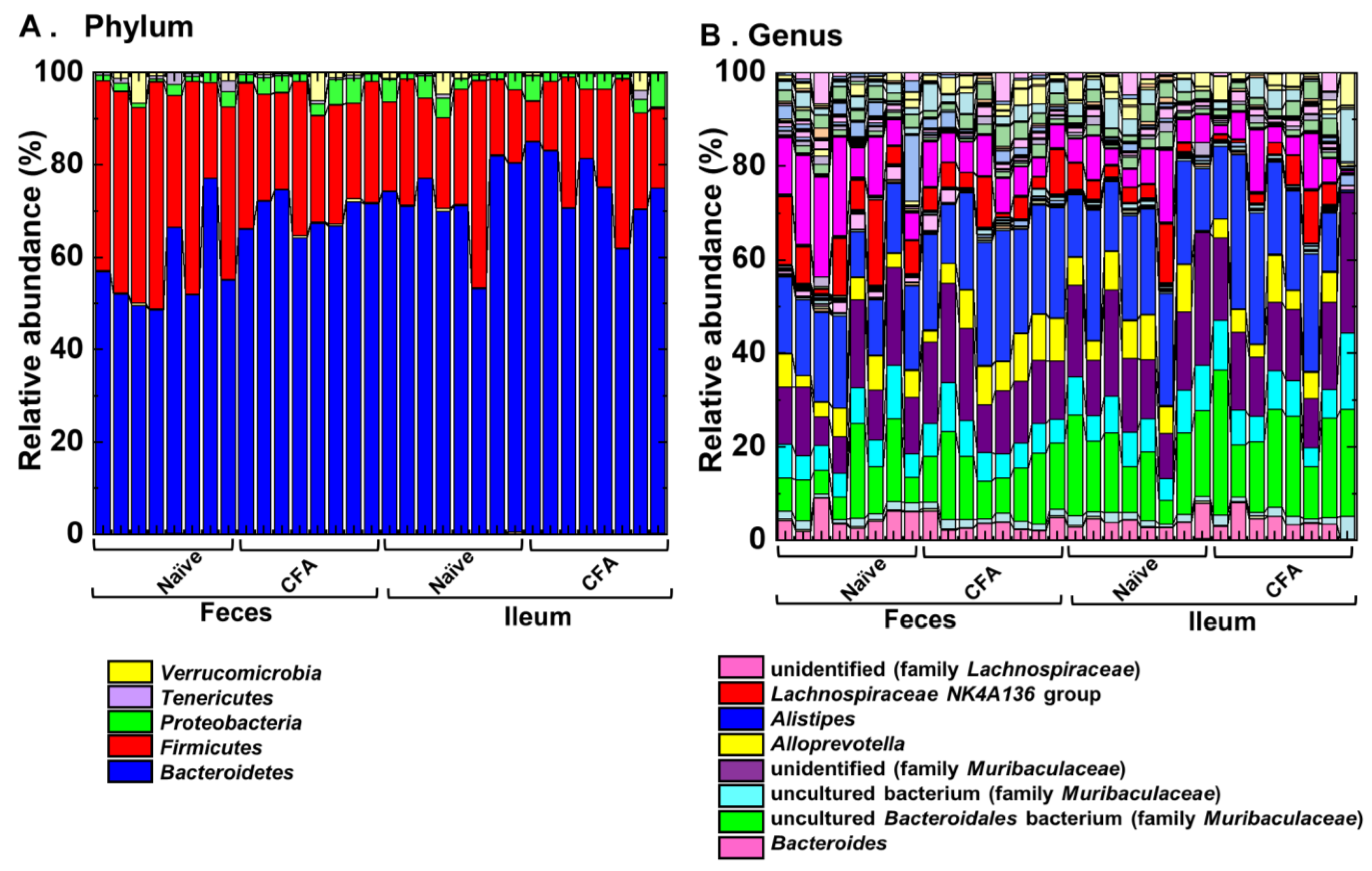

| Human Disease | Animal Model | Antigen/ Animal/PT * | Sample | Microbiota Findings | Other Findings | Ref |
|---|---|---|---|---|---|---|
| Guillain–Barré syndrome (GBS) | Experimental autoimmune neuritis (EAN) | myelin P2 protein/ Lewis rats/ PT | feces | ↑: Akkermansia, Escherichia, and Coprococcus; ↓: Lactobacillus, Ruminococcus, and Clostridium | Treatment with Bifidobacterium suppressed EAN | [14] |
| Multiple sclerosis (MS) | Experimental autoimmune encephalomyelitis (EAE) | MOG/ C57BL/6 mice/ PT | feces | ↑: Clostridiaceae, Ruminococcaceae, and Peptostreptococcaceae ↓: Lactobacillaceae ↓: alpha diversity (EAE vs. CFA-injected groups) | CFA injection alone altered microbiota ↑: Anaerostipes, Stomatobaculum, ↓: Akkermansia, Peptococcus−rc4−4 | [15] |
| MOG/ C57BL/6 mice/ PT | feces, ileal content, ileal mucosa | ↑: Ruminococcus bromii and Blautia (Ruminococcus) gnavus in feces ↑: Turicibacter sp. and Alistipes finegoldii in ileal contents ↑: Burkholderia spp. and Azoarcus spp. in ileal mucosa | Ileal, but not fecal, microbiota associated with EAE severity | [16] | ||
| Myasthenia gravis (MG) | Experimental Autoimmune myasthenia gravis (EAMG) | TAChR/ Lewis rats | feces | ↑: Ruminococcaceae/Lachnospiraceae ratio ↓: Tenericutes/Verrucomicrobia ratio | Treatment with Bifidobacterium suppressed EAMG | [17] |
| Myocarditis | Experimental autoimmune myocarditis (EAM) | MyHC-α/ BALB/c mice | feces | ↑: Firmicutes/Bacteroidetes ratio ↑: alpha diversity (EAM vs. saline-injected groups) | Treatment with FMT suppressed EAM without change in diversity | [18] |
| MyHC-α/ BALB/c mice/ PT | feces | ↑: Firmicutes ↓: Bacteroidetes, Proteobacteria | Treatment with antibiotics suppressed EAM pathology | [19] | ||
| Rheumatoid arthritis (RA) | Collagen-induced arthritis (CIA) | type II collagen /SD rats | feces | ↑: Proteobacteria, Actinobacteria ↓: Tenericutes | Treatment with total glucosides of paeony (TGP) suppressed CIA and restored gut dysbiosis | [20] |
| type II collagen /ICR mice | feces | ↑: Firmicutes, Bacteroidetes ↓: Proteobacteria, Actinobacteria alpha diversity: Chao1↑, Shannon ↓ (CIA vs. saline-injected groups) | Treatment with tuna oil suppressed CIA and restored gut dysbiosis | [21] | ||
| Adjuvant-induced arthritis (AIA) | Mycobacterium tuberculosis suspension/ SD rats | feces | ↑: Candidatus Arthromitus sp. SFB-rat Yit and Klebsiella pneumoniae ↓: Lactobacillus hominis, L. reuteri, and L. vaginalis | Treatment with Lactobacillus casei suppressed AIA and restored gut dysbiosis | [22] | |
| Thyroiditis | Experimental autoimmune thyroiditis (EAT) | thyroglobulin/ CBA/CaH mice | – | microbiota was not examined | Probiotic treatment with Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 had no effect on EAT | [24] |
| Uveoretinitis | Experimental autoimmune uveoretinitis (EAU) | IRBP/ B10.RIII mice | cecal content | ↑: Anaeroplasma, Turicibacter and Oscillospora ↓: Desulfovibrio, Clostridium, Staphylococcus, Adlecreutzia, and Lactobacillus ↓: alpha diversity (antibiotics-treated vs. untreated EAU groups) | Treatment with antibiotics suppressed EAU | [25] |
| Change | Feces | Ileum |
|---|---|---|
| ↑ | Bacteroides, Proteobacteria | – |
| ↓ | Firmicutes | Cyanobacteria |
| Change | Feces | Ileum |
|---|---|---|
| ↑ | Clostridium sensu stricto 1 Alistipes | Eubacterium coprostanoligenes group Facklamia Family Burkholderiaceae; genus unidentified Atopostipes |
| ↓ | Family Christensenellaceae; genus uncultured Gordonibacter Family Erysipelotrichaceae; genus uncultured bacterium Anaerovorax Eubacterium xylanophilum group ASF356 Lachnoclostridium Lachnospiraceae FCS020 group Family Lachnospiraceae; genus unidentified Peptococcus Family Peptococcaceae; genus uncultured Rikenella Ruminococcaceae UCG-009 Ruminococcaceae UCG-010 | Family XIII; genus unidentified Ruminococcaceae UCG-004 Ruminococcus 1 |
| Correlation | Feces | Ileum | ||
|---|---|---|---|---|
| Genus | r * | Genus | r* | |
| Positive (r ≥ 0.5) | Cloacibacterium Alicyclobacillus Family Burkholderiaceae; genus unidentified Acinetobacter Rhodanobacter | 0.59 0.58 0.58 0.58 0.58 | Facklamia ** Family Burkholderiaceae; genus unidentified ** Sporosarcina Family Desulfarculaceae; genus uncultured Family Erysipelotrichaceae; genus uncultured Family Erysipelotrichaceae; genus unidentified Family Prevotellaceae; genus unidentified | 0.82 0.67 0.60 0.59 0.58 0.54 0.52 |
| Negative (r ≤ −0.5) | Eubacterium xylanophilum group *** Family Christensenellaceae; genus uncultured *** Lachnoclostridium *** | −0.64 −0.56 −0.54 | – | |
| Correlation | Feces | Ileum | ||
|---|---|---|---|---|
| Genus | r * | Genus | r * | |
| Positive (r ≥ 0.5) | Ruminococcus 1 Micrococcus Family Saprospiraceae; genus unidentified Turicibacter | 0.53 0.53 0.53 0.51 | Atopostipes ** Family Bacillaceae; genus unidentified Dorea Faecalibaculum | 0.81 0.64 0.55 0.52 |
| Negative (r ≤ −0.5) | – | – | ||
| Correlation | Feces | Ileum | ||
|---|---|---|---|---|
| Genus | r * | Genus | r * | |
| Positive (r ≥ 0.5) | Turicibacter Ruminococcaceae UCG-004 Paenarthrobacter uncultured Sphingobacteriales bacterium Karenia brevis Family Cellvibrionaceae; genus unidentified Enhydrobacter Salinispira | 0.59 0.56 0.54 0.54 0.54 0.54 0.54 0.54 | Atopostipe s ** Family Erysipelotrichaceae; genus unidentified Hydrogenoanaerobacterium Candidatus Soleaferrea Ruminococcaceae NK4A214 group Dorea Lachnospiraceae UCG-001 Psychrobacter | 0.78 0.71 0.64 0.61 0.57 0.55 0.55 0.54 |
| Negative (r ≤ −0.5) | Rikenella *** ASF356 *** Gordonibacter *** Dorea Family Christensenellaceae; genus uncultured *** Family Peptococcaceae; genus uncultured *** Anaerovorax *** | −0.52 −0.52 −0.52 −0.54 −0.55 −0.56 −0.56 | – | |
| Correlation | Feces | Ileum | ||
|---|---|---|---|---|
| Genus | r * | Genus | r * | |
| Positive (r ≥ 0.5) | Dubosiella | 0.52 | Facklamia ** Hydrogenoanaerobacterium Family Bacillaceae; genus unidentified Family Prevotellaceae; genus unidentified Romboutsia | 0.66 0.62 0.58 0.57 0.52 |
| Negative (r ≤ −0.5) | – | – | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadka, S.; Omura, S.; Sato, F.; Tsunoda, I. Adjuvant Injections Altered the Ileal and Fecal Microbiota Differently with Changes in Immunoglobulin Isotypes and Antimycobacterial Antibody Responses. Int. J. Mol. Sci. 2023, 24, 2818. https://doi.org/10.3390/ijms24032818
Khadka S, Omura S, Sato F, Tsunoda I. Adjuvant Injections Altered the Ileal and Fecal Microbiota Differently with Changes in Immunoglobulin Isotypes and Antimycobacterial Antibody Responses. International Journal of Molecular Sciences. 2023; 24(3):2818. https://doi.org/10.3390/ijms24032818
Chicago/Turabian StyleKhadka, Sundar, Seiichi Omura, Fumitaka Sato, and Ikuo Tsunoda. 2023. "Adjuvant Injections Altered the Ileal and Fecal Microbiota Differently with Changes in Immunoglobulin Isotypes and Antimycobacterial Antibody Responses" International Journal of Molecular Sciences 24, no. 3: 2818. https://doi.org/10.3390/ijms24032818
APA StyleKhadka, S., Omura, S., Sato, F., & Tsunoda, I. (2023). Adjuvant Injections Altered the Ileal and Fecal Microbiota Differently with Changes in Immunoglobulin Isotypes and Antimycobacterial Antibody Responses. International Journal of Molecular Sciences, 24(3), 2818. https://doi.org/10.3390/ijms24032818









