Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females
Abstract
1. Introduction
2. Results
2.1. Elevated Serum Levels of CCL5 in Whole DJD-TMJ Patients
2.2. Correlations of Serum CCL5 with Bone Markers and Inflammatory Markers Whole DJD-TMJ Subjects
2.3. Correlations of Serum CCL5 with Bone Markers in the Younger DJD-TMJ Subjects
2.4. Independency of the Serum CCL5 Level from Changes in Bone Turnover Markers and Female Hormones
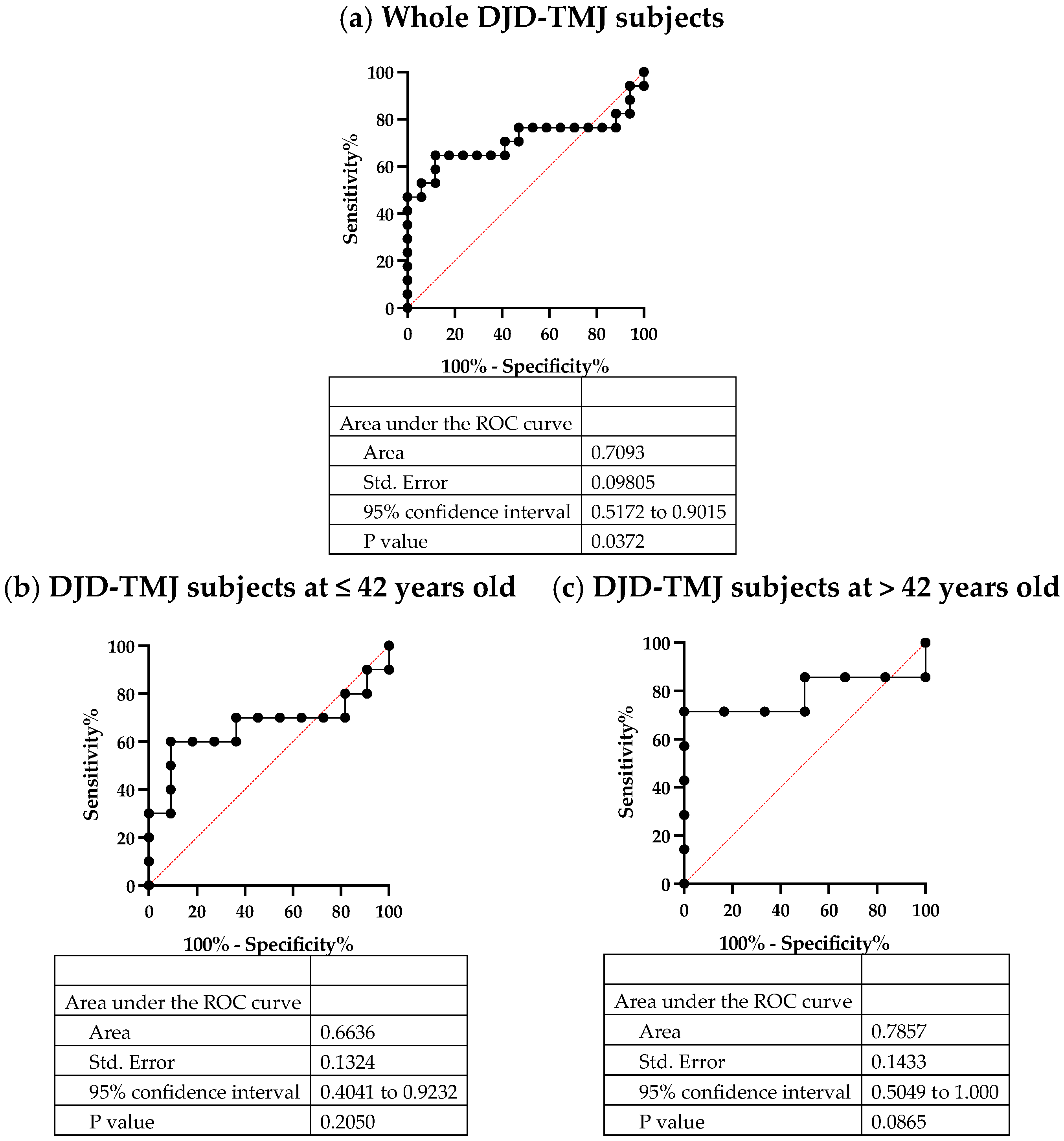
2.5. Serum CCL5, a Potent Biomarker of DJD-TMJ
3. Discussion
4. Materials & Methods
4.1. Clinical Subjects
- Bone resorption markers
- ○
- CTX/Creatinine equivalent (CTX/Cr)
- ○
- Urinary type I collagen cross-linked C-terminal telopeptide (Urine NTX)
- ○
- Serum tartrate-resistant acid phosphatase (TRAP5b)
- Osteogenic markers
- ○
- Serum bone alkaline phosphatase (BAP)
- ○
- Serum osteocalcin (OCN)
- Inflammatory markers
- ○
- Serum tumor necrosis factor-alpha (TNF-α)
4.2. Animals and Experimental Design
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantoja, L.L.Q.; De Toledo, I.P.; Pupo, Y.M.; Porporatti, A.L.; Canto, G.D.L.; Zwir, L.F.; Guerra, E.N.S. Prevalence of degenerative joint disease of the temporomandibular joint: A systematic review. Clin. Oral Investig. 2019, 23, 2475–2488. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J. Oral. Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Maruoka, Y.; Kanaya, F.; Hoshino, A.; Iimura, T.; Imai, H.; Otsuka, R.; Ueha, S.; Fujioca, K.; Katsuragawa, Y.; Shimbo, T.; et al. Study of Osteo-/Chondropenia Caused by Impaired Chemokine Receptor and for Progressive/Idiopathic Condylar Resorption. Jpn. J. Jaw Deform. 2012, 22, S15–S22. [Google Scholar] [CrossRef]
- Asakawa-Tanne, Y.; Su, S.; Kunimatsu, R.; Hirose, N.; Mitsuyoshi, T.; Okamoto, Y.; Tanaka, E.; Tanne, K.; Tanimoto, K. Effects of Enzymatic Degradation after Loading in Temporomandibular Joint. J. Dent. Res. 2014, 94, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Iwasaki, L.; Gonzalez, Y.; Gallo, L.; Yao, H. Mechanobehavior and Ontogenesis of the Temporomandibular Joint. J. Dent. Res. 2018, 97, 1185–1192. [Google Scholar] [CrossRef]
- de Melo, D.P.; Melo, S.L.S.; Oliveira, L.S.D.A.F.; de Moraes Ramos-Perez, F.M.; Campos, P.S.F. Evaluation of temporomandibular joint disk displacement and its correlation with pain and osseous abnormalities in symptomatic young patients with magnetic resonance imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 107–112. [Google Scholar] [CrossRef]
- Dias, I.; Cordeiro, P.; Devito, K.; Tavares, M.; Leite, I.; Tesch, R. Evaluation of temporomandibular joint disc displacement as a risk factor for osteoarthrosis. Int. J. Oral Maxillofac. Surg. 2015, 45, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Han, J.; Liu, M.; Zhang, Y.; Yap, A.U.-J.; Fu, K.-Y. Degenerative temporomandibular joint changes associated with recent-onset disc displacement without reduction in adolescents and young adults. J. Cranio-Maxillofac. Surg. 2017, 45, 408–413. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.Y.; Huh, K.-H.; Park, J.W. Long-term Changes of Temporomandibular Joint Osteoarthritis on Computed Tomography. Sci. Rep. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Tanaka, E.; Detamore, M.; Mercuri, L. Degenerative Disorders of the Temporomandibular Joint: Etiology, Diagnosis, and Treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef]
- Catherine, Z.; Breton, P.; Bouletreau, P. Condylar resorption after orthognathic surgery: A systematic review. Rev. Stomatol. Chir. Maxillo-Faciale Chir. Orale 2016, 117, 3–10. [Google Scholar] [CrossRef]
- Munjal, A.; Bapat, S.; Hubbard, D.; Hunter, M.; Kolhe, R.; Fulzele, S. Advances in Molecular biomarker for early diagnosis of Osteoarthritis. Biomol. Concepts 2019, 10, 111–119. [Google Scholar] [CrossRef]
- Sato, J.; Segami, N.; Kaneyama, K.; Mashiyama, Y.; Fujimura, K.; Yoshitake, Y. Vascular endothelial growth factor concentrations in synovial fluids of patients with symptomatic internal derangement of the temporomandibular joint. J. Oral Pathol. Med. 2005, 34, 170–177. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Gierman, L.; Van Spil, W.E.; Van Osch, G.; Huizinga, T.; Saris, D.; Creemers, L.; Zuurmond, A.-M. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr. Cartil. 2013, 21, 918–922. [Google Scholar] [CrossRef]
- Vos, L.M.; Kuijer, R.; Slater, J.J.H.; Stegenga, B. Alteration of Cartilage Degeneration and Inflammation Markers in Temporomandibular Joint Osteoarthritis Occurs Proportionally. J. Oral Maxillofac. Surg. 2013, 71, 1659–1664. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Ghanem, A.; Malmstrom, H.; Romanos, G.E.; Javed, F. Cytokine profile in the synovial fluid of patients with temporomandibular joint disorders: A systematic review. Cytokine 2016, 77, 98–106. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Hoshino, A.; Iimura, T.; Ueha, S.; Hanada, S.; Maruoka, Y.; Mayahara, M.; Suzuki, K.; Imai, T.; Ito, M.; Manome, Y.; et al. Deficiency of Chemokine Receptor CCR1 Causes Osteopenia Due to Impaired Functions of Osteoclasts and Osteoblasts. J. Biol. Chem. 2010, 285, 28826–28837. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Ueha, S.; Hanada, S.; Imai, T.; Ito, M.; Yamamoto, K.; Matsushima, K.; Yamaguchi, A.; Iimura, T. Roles of chemokine receptor CX3CR1 in maintaining murine bone homeostasis through the regulation of both osteoblasts and osteoclasts. J. Cell Sci. 2012, 126, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Hoshino, A.; Inoue, K.; Saitou, T.; Uehara, S.; Kobayashi, Y.; Ueha, S.; Matsushima, K.; Yamaguchi, A.; Imai, Y.; et al. The HIV co-receptor CCR5 regulates osteoclast function. Nat. Commun. 2017, 8, 2226. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lei, J.; Chen, H.; Wang, Y.; Yap, A.U.; Fu, K. Increased chemokine RANTES in synovial fluid and its role in early-stage degenerative temporomandibular joint disease. J. Oral Rehabil. 2020, 47, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Kisand, K.; Tamm, A.; Lintrop, M. New insights into the natural course of knee osteoarthritis: Early regulation of cytokines and growth factors, with emphasis on sex-dependent angiogenesis and tissue remodeling. A pilot study. Osteoarthr. Cartil. 2018, 26, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Klose-Jensen, R.; Hartlev, L.; Boel, L.; Laursen, M.; Stengaard-Pedersen, K.; Keller, K.; Hauge, E.-M. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthr. Cartil. 2015, 23, 2167–2173. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, D.-J.; Lee, S.-G.; Chung, J.-W. A longitudinal study on the osteoarthritic change of the temporomandibular joint based on 1-year follow-up computed tomography. J. Cranio-Maxillofac. Surg. 2012, 40, e223–e228. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Favero, L.; Gregorini, G.; Cocilovo, F.; Guarda-Nardini, L. Natural course of temporomandibular disorders with low pain-related impairment: A 2-to-3-year follow-up study. J. Oral Rehabil. 2013, 40, 436–442. [Google Scholar] [CrossRef]
- Kumm, J.; Tamm, A.; Lintrop, M. Diagnostic and prognostic value of bone biomarkers in progressive knee osteoarthritis: A 6-year follow-up study in middle-aged subjects. Osteoarthr. Cartil. 2013, 21, 815–822. [Google Scholar] [CrossRef]
- Schiffman, E.; Ahmad, M.; Hollender, L.; Kartha, K.; Ohrbach, R.; Truelove, E.; Zhang, L.; Hodges, J.; Sommers, E.; Anderson, G.; et al. Longitudinal Stability of Common TMJ Structural Disorders. J. Dent. Res. 2016, 96, 270–276. [Google Scholar] [CrossRef]
- Yang, D.W.; Qian, G.B.; Jiang, M.J.; Wang, P.; Wang, K.Z. Inhibition of microRNA-495 suppresses chondrocyte apoptosis through activation of the NF-kappaB signaling pathway by regulating CCL4 in osteoarthritis. Gene Ther. 2019, 26, 217–229. [Google Scholar] [CrossRef]
- Matijašević, M.I.; Flegar, D.; Kovačić, N.; Katavić, V.; Kelava, T.; Šućur, A.; Ivčević, S.; Cvija, H.; Mosler, E.L.; Kalajzić, I.; et al. Increased chemotaxis and activity of circulatory myeloid progenitor cells may contribute to enhanced osteoclastogenesis and bone loss in the C57BL/6 mouse model of collagen-induced arthritis. Clin. Exp. Immunol. 2016, 186, 321–335. [Google Scholar] [CrossRef]
- Hampel, U.; Sesselmann, S.M.; Iserovich, P.; Sel, S.; Paulsen, F.; Sack, R. Chemokine and cytokine levels in osteoarthritis and rheumatoid arthritis synovial fluid. J. Immunol. Methods 2013, 396, 134–139. [Google Scholar] [CrossRef]
- Pierzchala, A.W.; Kusz, D.J.; Hajduk, G. CXCL8 and CCL5 Expression in Synovial Fluid and Blood Serum in Patients with Osteoarthritis of the Knee. Arch. Immunol. Ther. Exp. 2011, 59, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Agere, S.A.; Akhtar, N.; Watson, J.M.; Ahmed, S. RANTES/CCL5 Induces Collagen Degradation by Activating MMP-1 and MMP-13 Expression in Human Rheumatoid Arthritis Synovial Fibroblasts. Front. Immunol. 2017, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Tanaka, M.; Masuko-Hongo, K.; Yudoh, K.; Kato, T.; Beppu, M.; Nishioka, K. Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol. Int. 2006, 26, 984–990. [Google Scholar] [CrossRef]
- Gunson, M.J.; Arnett, G.W.; Formby, B.; Falzone, C.; Mathur, R.; Alexander, C. Oral contraceptive pill use and abnormal menstrual cycles in women with severe condylar resorption: A case for low serum 17beta-estradiol as a major factor in progressive condylar resorption. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2017, 8, a031211. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Kousteni, S.; Jilka, R.L. Sex steroids and bone. Recent Prog. Horm. Res. 2002, 57, 385–409. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Constâncio, C.; Pagani, B.T.; De Azevedo, R.M.G.; Grion, D.P.; Marques, L.; Kinoshita, A. Effect of ovariectomy in bone structure of mandibular condyle. Acta Cir. Bras. 2017, 32, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, T.; Nakashima, M.; Okuda, T.; Tatematsu, N. Effect of estrogen replacement on temporomandibular joint remodeling in ovariectomized rats. J. Oral Maxillofac. Surg. 2000, 58, 189–196. [Google Scholar] [CrossRef]
- Wang, X.; Kou, X.; Meng, Z.; Bi, R.; Liu, Y.; Zhang, J.; Zhou, Y.; Gan, Y. Estrogen Aggravates Iodoacetate-induced Temporomandibular Joint Osteoarthritis. J. Dent. Res. 2013, 92, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Sun, D.; Mu, T.; Chu, Y.; Miao, H.; Zhang, M.; Yang, H.; Liu, Q.; Lu, L.; Xing, X.; et al. Differential effects of high-physiological oestrogen on the degeneration of mandibular condylar cartilage and subchondral bone. Bone 2018, 111, 9–22. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Figueroba, S.; Desjardins, M.P.; Ferreira, L.; Berto, L.A.; Valdrighi, H.C.; Groppo, F.C. The influence of altered occlusion on pro-inflammatory cytokine levels in the TMJ synovial tissues of rats. Arch. Oral Biol. 2014, 59, 1164–1171. [Google Scholar] [CrossRef]
- Jung, J.-K.; Sohn, W.-J.; Lee, Y.; Bae, Y.C.; Choi, J.-K.; Kim, J.-Y. Morphological and cellular examinations of experimentally induced malocclusion in mice mandibular condyle. Cell Tissue Res. 2013, 355, 355–363. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, K.; Zhang, M.; Zhou, T.; Liu, X.-D.; Yu, S.-B.; Lu, L.; Jing, L.; Yang, T.; Zhang, Y.; et al. Occlusal Effects on Longitudinal Bone Alterations of the Temporomandibular Joint. J. Dent. Res. 2013, 92, 253–259. [Google Scholar] [CrossRef]
- Wu, Y.; Kadota-Watanabe, C.; Ogawa, T.; Moriyama, K. Combination of estrogen deficiency and excessive mechanical stress aggravates temporomandibular joint osteoarthritis in vivo. Arch. Oral Biol. 2019, 102, 39–46. [Google Scholar] [CrossRef]
- Alaaeddine, N.; Olee, T.; Hashimoto, S.; Creighton-Achermann, L.; Lotz, M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001, 44, 1633–1643. [Google Scholar] [CrossRef]
- Takebe, K.; Rai, M.; Schmidt, E.; Sandell, L. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthr. Cartil. 2014, 23, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Exposto, C.R.; Stoustrup, P.; Kristensen, K.D.; Dalstra, M.; Pedersen, T.K. Condylar changes in patients with idiopathic condylar resorption: Retrospective 2-year follow-up CBCT-based case–control study. Eur. J. Orthod. 2020, 42, 619–625. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Lin, Q.; Lu, L.; Li, M.; Li, Q.; Xue, J.; Xu, Y. Morphologic changes in idiopathic condylar resorption with different degrees of bone loss. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.D.; Schmidt, B.; Stoustrup, P.; Pedersen, T.K. Idiopathic condylar resorptions: 3-dimensional condylar bony deformation, signs and symptoms. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Alsabban, L.; Amarista, F.J.; Mercuri, L.G.; Perez, D. Idiopathic Condylar Resorption: A Survey and Review of the Literature. J. Oral Maxillofac. Surg. 2018, 76, 2316.e1–2316.e13. [Google Scholar] [CrossRef]
- Zarb, G.A.; Carlsson, G.E. Temporomandibular disorders: Osteoarthritis. J. Orofac. Pain 1999, 13, 295–306. [Google Scholar]
- Bäck, K.; Ahlqwist, M.; Hakeberg, M.; Dahlström, L. Occurrence of signs of osteoarthritis/arthrosis in the temporomandibular joint on panoramic radiographs in Swedish women. Community Dent. Oral Epidemiol. 2017, 45, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
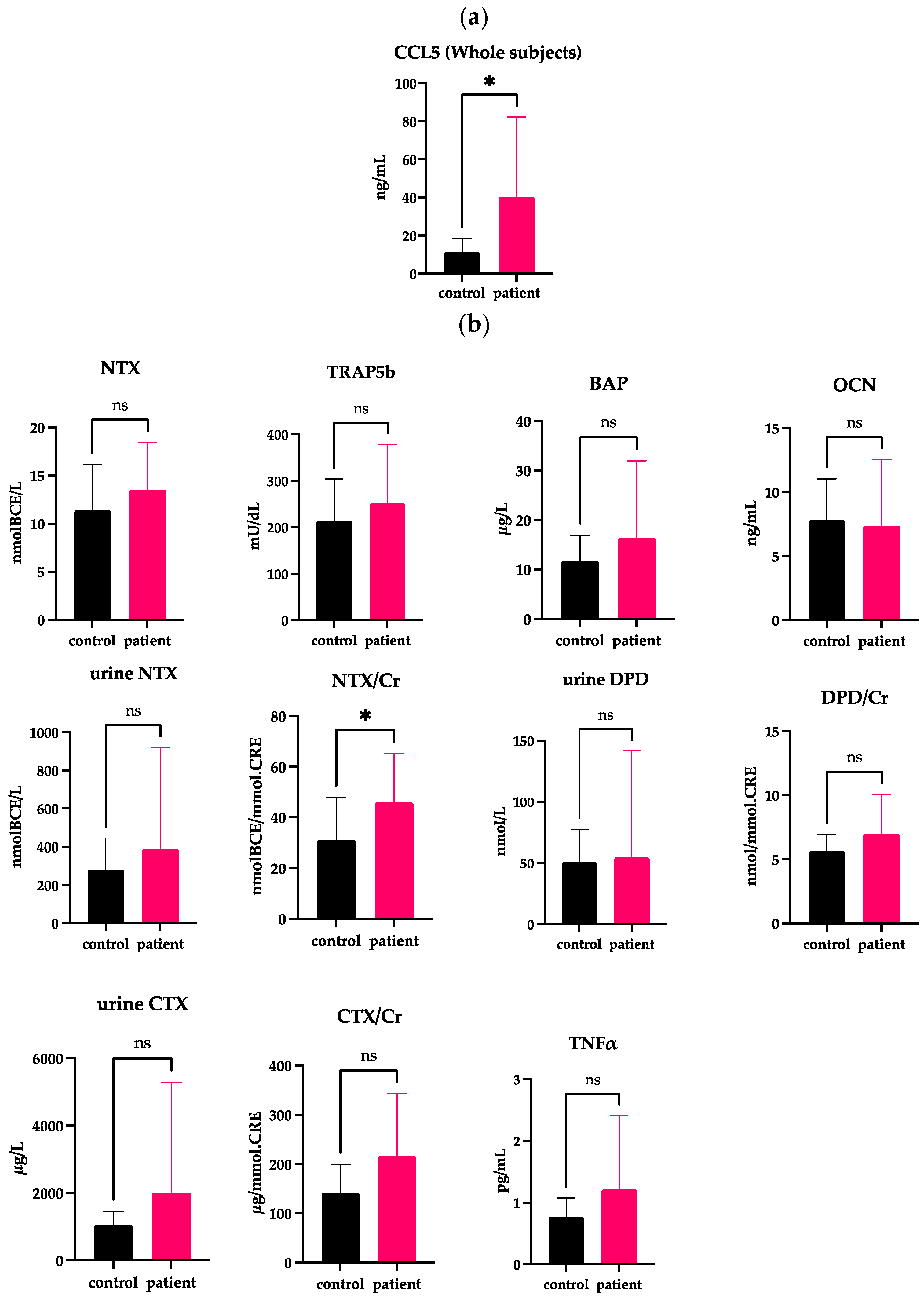

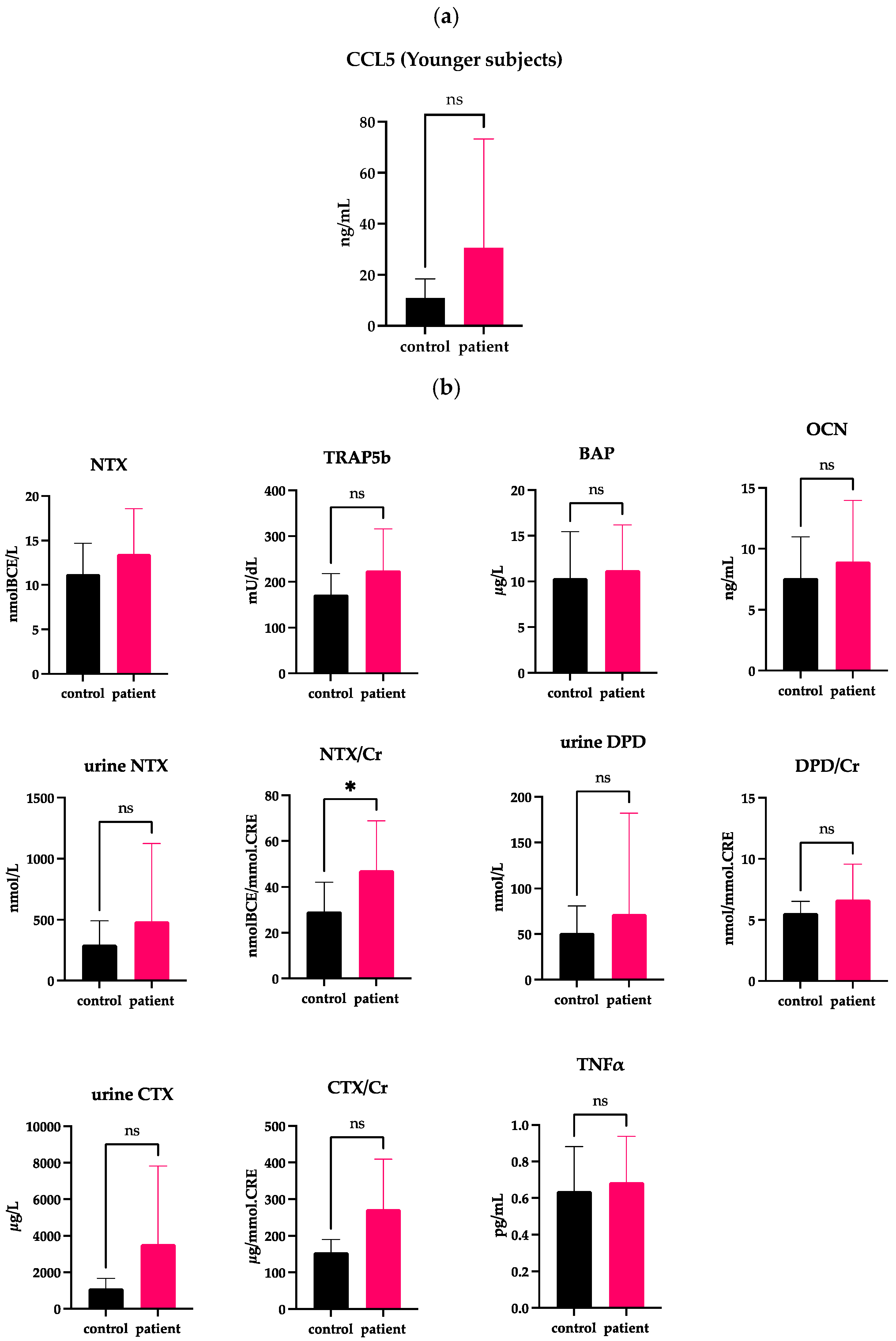
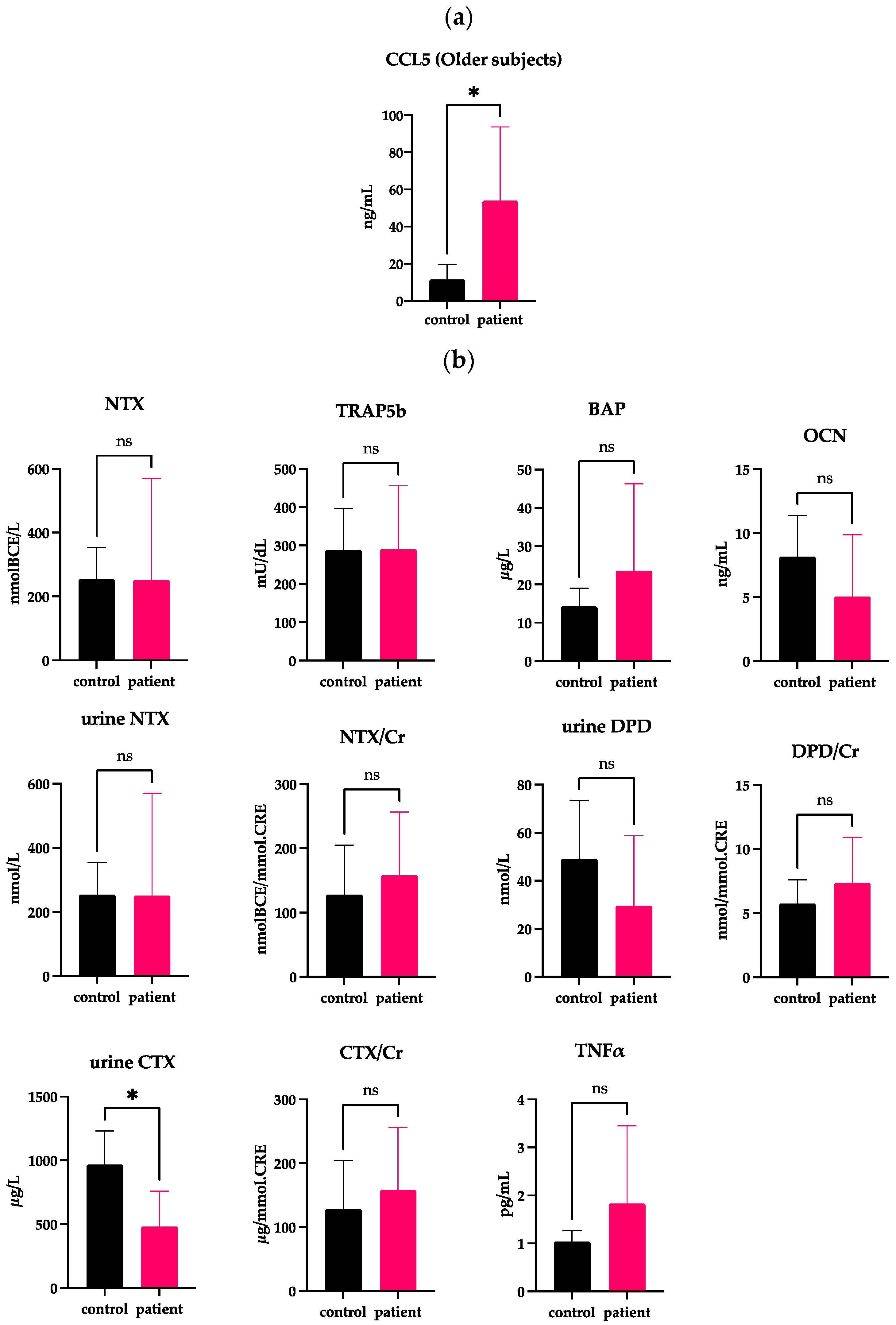


| Age | Sex | CCL5 | NTX | Urine NTX | Urine NTX/Cr | CTX | CTX/Cr | DPD | DPD/Cr | BAP | TRAP5b | OCN | TNFα | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patient | 1 | 10’s | F | 146.00 | 17.2 | 2155.0 | 74.9 | 10,691.0 | 373.0 | 377.0 | 13.1 | 18.7 | 375.0 | 8.3 | |
| patient | 2 | 10’s | F | 27.90 | 9.9 | 957.0 | 71.1 | 4334.0 | 314.0 | 100.5 | 7.5 | 20.0 | 221.0 | 9.8 | |
| patient | 3 | 20’s | F | 31.58 | 19.2 | 225.0 | 75.4 | 1215.0 | 414.0 | 26.6 | 8.9 | 15.5 | 381.0 | 20.6 | 0.82 |
| patient | 4 | 20’s | F | 11.30 | 11.9 | 69.0 | 34.3 | 12.1 | 6.1 | 10.8 | 205.0 | 8.5 | 0.80 | ||
| patient | 5 | 20’s | F | 2.76 | 10.9 | 256.0 | 29.9 | 34.1 | 4.0 | 7.3 | 147.0 | 4.9 | 1.0 | ||
| patient | 6 | 20’s | F | 1.59 | 7.4 | 310.0 | 28.1 | 49.7 | 4.5 | 6.6 | 253.0 | 5.9 | 0.50 | ||
| patient | 7 | 20’s | F | 3.40 | 10.2 | 336.0 | 51.0 | 49.1 | 7.5 | 7.9 | 233.0 | 4.3 | |||
| patient | 8 | 30’s | F | 16.50 | 9.2 | 37.0 | 12.7 | 16.3 | 5.6 | 8.7 | 136.0 | 4.6 | 0.70 | ||
| patient | 9 | 30’s | F | 42.57 | 15.7 | 241.0 | 41.7 | 958.0 | 167.0 | 38.1 | 6.6 | 8.6 | 168.0 | 13.8 | 0.3 |
| patient | 10 | 30’s | F | 21.58 | 23.2 | 282.0 | 52.3 | 498.0 | 93.0 | 16.3 | 3.0 | 8.3 | 133.0 | 8.9 | |
| patient | 11 | 50’s | F | 96.40 | 9.5 | 957.0 | 71.1 | 785.0 | 206.0 | 28.1 | 7.3 | 74.3 | 139.0 | 1.0 | |
| patient | 12 | 50’s | F | 36.40 | 8.6 | 183.0 | 38.8 | 492.0 | 103.0 | 30.3 | 6.4 | 11.6 | 150.0 | 1.2 | 1.0 |
| patient | 13 | 60’s | F | 80.00 | 21.3 | 70.0 | 26.1 | 19.4 | 7.3 | 15.1 | 396.0 | 2.7 | 4.70 | ||
| patient | 14 | 60’s | F | 100.00 | 11.1 | 51.0 | 48.8 | 100.0 | 97.0 | 6.1 | 5.8 | 21.1 | 140.0 | 1.3 | |
| patient | 15 | 60’s | F | 10.20 | 15.9 | 133.0 | 58.3 | 699.0 | 309.0 | 15.1 | 6.6 | 10.5 | 506.0 | 10.0 | 1.0 |
| patient | 16 | 70’s | F | 2.12 | 18.6 | 258.0 | 41.2 | 92.9 | 14.8 | 14.9 | 481.0 | 13.0 | 1.5 | ||
| patient | 17 | 70’s | F | 52.30 | 10.2 | 110.0 | 24.7 | 341.0 | 75.0 | 15.2 | 3.4 | 17.6 | 216.0 | 6.2 | 1.00 |
| control | 1 | 10’s | F | 12.70 | 10.0 | 91.0 | 18.8 | 502.0 | 107.0 | 38.4 | 7.9 | 22.5 | 243.0 | 8.1 | |
| control | 2 | 20’s | F | 11.20 | 17.1 | 383.0 | 41.7 | 1346.0 | 149.0 | 44.7 | 4.9 | 11.0 | 174.0 | 11.0 | 0.4 |
| control | 3 | 20’s | F | 9.80 | 16.6 | 396.0 | 28.7 | 1934.0 | 143.0 | 67.6 | 4.9 | 6.4 | 124.0 | 11.6 | 0.30 |
| control | 4 | 20’s | F | 16.18 | 8.1 | 143.0 | 32.7 | 854.0 | 201.0 | 23.4 | 5.4 | 6.9 | 121.0 | 9.2 | 0.49 |
| control | 5 | 20’s | F | 5.28 | 14.0 | 223.0 | 40.0 | 947.0 | 175.0 | 32.3 | 5.8 | 10.7 | 151.0 | 13.7 | 0.49 |
| control | 6 | 20’s | F | 3.19 | 12.8 | 67.0 | 22.9 | 13.8 | 4.7 | 7.0 | 166.0 | 6.0 | 1.00 | ||
| control | 7 | 20’s | F | 1.79 | 10.8 | 531.0 | 51.1 | 46.0 | 4.4 | 7.2 | 193.0 | 4.5 | 0.80 | ||
| control | 8 | 30’s | F | 9.65 | 7.4 | 289.0 | 6.4 | 77.3 | 6.4 | 17.3 | 203.0 | 5.3 | 1.00 | ||
| control | 9 | 30’s | F | 14.50 | 8.5 | 341.0 | 22.1 | 84.6 | 5.5 | 8.7 | 128.0 | 2.9 | 0.70 | ||
| control | 10 | 40’s | F | 28.80 | 8.0 | 109.0 | 21.1 | 26.8 | 5.2 | 8.0 | 142.0 | 5.2 | 0.50 | ||
| control | 11 | 40’s | F | 6.65 | 10.2 | 679.0 | 37.8 | 109.0 | 6.1 | 8.3 | 250.0 | 6.2 | 0.70 | ||
| control | 12 | 50’s | F | 3.83 | 22.3 | 357.0 | 77.9 | 577.0 | 128.0 | 35.3 | 7.7 | 20.0 | 352.0 | 12.0 | 1.00 |
| control | 13 | 50’s | F | 26.10 | 2.4 | 343.0 | 38.7 | 50.5 | 5.7 | 14.8 | 191.0 | 6.4 | 1.1 | ||
| control | 14 | 70’s | F | 5.96 | 8.1 | 155.0 | 12.0 | 850.0 | 65.0 | 40.7 | 3.2 | 6.8 | 182.0 | 5.0 | 1.4 |
| control | 15 | 70’s | F | 12.20 | 8.1 | 177.0 | 19.2 | 1015.0 | 109.0 | 37.1 | 4.0 | 12.8 | 210.0 | 5.2 | 0.90 |
| control | 16 | 70’s | F | 13.30 | 12.0 | 335.0 | 22.4 | 1222.0 | 82.0 | 97.0 | 6.5 | 12.4 | 361.0 | 8.5 | 0.80 |
| control | 17 | 70’s | F | 8.08 | 16.6 | 157.0 | 34.4 | 1175.0 | 258.0 | 34.0 | 7.5 | 18.7 | 436.0 | 12.0 |
| Age | Sex | CCL5 | NTX | Urine NTX | Urine NTX/Cr | CTX | CTX/Cr | DPD | DPD/Cr | BAP | TRAP5b | OCN | TNF-α | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patient | 1 | 10’s | F | 146.00 | 17.2 | 2155.0 | 74.9 | 10,691.0 | 373.0 | 377.0 | 13.1 | 18.7 | 375.0 | 8.3 | |
| patient | 2 | 10’s | F | 27.90 | 9.9 | 957.0 | 71.1 | 4334.0 | 314.0 | 100.5 | 7.5 | 20.0 | 221.0 | 9.8 | |
| patient | 3 | 20’s | F | 31.58 | 19.2 | 225.0 | 75.4 | 1215.0 | 414.0 | 26.6 | 8.9 | 15.5 | 381.0 | 20.6 | 0.82 |
| patient | 4 | 20’s | F | 11.30 | 11.9 | 69.0 | 34.3 | 12.1 | 6.1 | 10.8 | 205.0 | 8.5 | 0.80 | ||
| patient | 5 | 20’s | F | 2.76 | 10.9 | 256.0 | 29.9 | 34.1 | 4.0 | 7.3 | 147.0 | 4.9 | 1.0 | ||
| patient | 6 | 20’s | F | 1.59 | 7.4 | 310.0 | 28.1 | 49.7 | 4.5 | 6.6 | 253.0 | 5.9 | 0.50 | ||
| patient | 7 | 20’s | F | 3.40 | 10.2 | 336.0 | 51.0 | 49.1 | 7.5 | 7.9 | 233.0 | 4.3 | |||
| patient | 8 | 30’s | F | 16.50 | 9.2 | 37.0 | 12.7 | 16.3 | 5.6 | 8.7 | 136.0 | 4.6 | 0.70 | ||
| patient | 9 | 30’s | F | 42.57 | 15.7 | 241.0 | 41.7 | 958.0 | 167.0 | 38.1 | 6.6 | 8.6 | 168.0 | 13.8 | 0.3 |
| patient | 10 | 30’s | F | 21.58 | 23.2 | 282.0 | 52.3 | 498.0 | 93.0 | 16.3 | 3.0 | 8.3 | 133.0 | 8.9 | |
| control | 1 | 10’s | F | 12.70 | 10.0 | 91.0 | 18.8 | 502.0 | 107.0 | 38.4 | 7.9 | 22.5 | 243.0 | 8.1 | |
| control | 2 | 20’s | F | 11.20 | 17.1 | 383.0 | 41.7 | 1346.0 | 149.0 | 44.7 | 4.9 | 11.0 | 174.0 | 11.0 | 0.4 |
| control | 3 | 20’s | F | 9.80 | 16.6 | 396.0 | 28.7 | 1934.0 | 143.0 | 67.6 | 4.9 | 6.4 | 124.0 | 11.6 | 0.30 |
| control | 4 | 20’s | F | 16.18 | 8.1 | 143.0 | 32.7 | 854.0 | 201.0 | 23.4 | 5.4 | 6.9 | 121.0 | 9.2 | 0.49 |
| control | 5 | 20’s | F | 5.28 | 14.0 | 223.0 | 40.0 | 947.0 | 175.0 | 32.3 | 5.8 | 10.7 | 151.0 | 13.7 | 0.49 |
| control | 6 | 20’s | F | 3.19 | 12.8 | 67.0 | 22.9 | 13.8 | 4.7 | 7.0 | 166.0 | 6.0 | 1.00 | ||
| control | 7 | 20’s | F | 1.79 | 10.8 | 531.0 | 51.1 | 46.0 | 4.4 | 7.2 | 193.0 | 4.5 | 0.80 | ||
| control | 8 | 30’s | F | 14.50 | 8.5 | 341.0 | 22.1 | 84.6 | 5.5 | 8.7 | 128.0 | 2.9 | 0.70 | ||
| control | 9 | 30’s | F | 9.65 | 7.4 | 289.0 | 6.4 | 77.3 | 6.4 | 17.3 | 203.0 | 5.3 | 1.00 | ||
| control | 10 | 40’s | F | 28.80 | 8.0 | 109.0 | 21.1 | 26.8 | 5.2 | 8.0 | 142.0 | 5.2 | 0.50 | ||
| control | 11 | 40’s | F | 6.65 | 10.2 | 679.0 | 37.8 | 109.0 | 6.1 | 8.3 | 250.0 | 6.2 | 0.70 |
| Age | Sex | CCL5 | NTX | Urine NTX | Urine NTX/Cr | CTX | CTX/Cr | DPD | DPD/Cr | BAP | TRAP5b | OCN | TNF-α | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patient | 1 | 50’s | F | 96.40 | 9.5 | 957.0 | 71.1 | 785.0 | 206.0 | 28.1 | 7.3 | 74.3 | 139.0 | 1.0 | |
| patient | 2 | 50’s | F | 36.40 | 8.6 | 183.0 | 38.8 | 492.0 | 103.0 | 30.3 | 6.4 | 11.6 | 150.0 | 1.2 | 1.0 |
| patient | 3 | 60’s | F | 80.00 | 21.3 | 70.0 | 26.1 | 19.4 | 7.3 | 15.1 | 396.0 | 2.7 | 4.7 | ||
| patient | 4 | 60’s | F | 100.00 | 11.1 | 51.0 | 48.8 | 100.0 | 97.0 | 6.1 | 5.8 | 21.1 | 140.0 | 1.3 | |
| patient | 5 | 60’s | F | 10.20 | 15.9 | 133.0 | 58.3 | 699.0 | 309.0 | 15.1 | 6.6 | 10.5 | 506.0 | 10.0 | 1.0 |
| patient | 6 | 70’s | F | 2.12 | 18.6 | 258.0 | 41.2 | 92.9 | 14.8 | 14.9 | 481.0 | 13.0 | 1.5 | ||
| patient | 7 | 70’s | F | 52.30 | 10.2 | 110.0 | 24.7 | 341.0 | 75.0 | 15.2 | 3.4 | 17.6 | 216.0 | 6.2 | 1.00 |
| control | 1 | 50’s | F | 3.83 | 22.3 | 357.0 | 77.9 | 577.0 | 128.0 | 35.3 | 7.7 | 20.0 | 352.0 | 12.0 | 1.00 |
| control | 2 | 50’s | F | 26.10 | 2.4 | 343.0 | 38.7 | 50.5 | 5.7 | 14.8 | 191.0 | 6.4 | 1.1 | ||
| control | 3 | 70’s | F | 5.96 | 8.1 | 155.0 | 12.0 | 850.0 | 65.0 | 40.7 | 3.2 | 6.8 | 182.0 | 5.0 | 1.4 |
| control | 4 | 70’s | F | 12.20 | 8.1 | 177.0 | 19.2 | 1015.0 | 109.0 | 37.1 | 4.0 | 12.8 | 210.0 | 5.2 | 0.90 |
| control | 5 | 70’s | F | 13.30 | 12.0 | 335.0 | 22.4 | 1222.0 | 82.0 | 97.0 | 6.5 | 12.4 | 361.0 | 8.5 | 0.80 |
| control | 6 | 70’s | F | 8.08 | 16.6 | 157.0 | 34.4 | 1175.0 | 258.0 | 34.0 | 7.5 | 18.7 | 436.0 | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Iori, T.; Lee, J.-W.; Kajii, T.S.; Takakura, A.; Takao-Kawabata, R.; Kitagawa, Y.; Maruoka, Y.; Iimura, T. Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. Int. J. Mol. Sci. 2023, 24, 2775. https://doi.org/10.3390/ijms24032775
Watanabe H, Iori T, Lee J-W, Kajii TS, Takakura A, Takao-Kawabata R, Kitagawa Y, Maruoka Y, Iimura T. Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. International Journal of Molecular Sciences. 2023; 24(3):2775. https://doi.org/10.3390/ijms24032775
Chicago/Turabian StyleWatanabe, Haruhisa, Takashi Iori, Ji-Won Lee, Takashi S. Kajii, Aya Takakura, Ryoko Takao-Kawabata, Yoshimasa Kitagawa, Yutaka Maruoka, and Tadahiro Iimura. 2023. "Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females" International Journal of Molecular Sciences 24, no. 3: 2775. https://doi.org/10.3390/ijms24032775
APA StyleWatanabe, H., Iori, T., Lee, J.-W., Kajii, T. S., Takakura, A., Takao-Kawabata, R., Kitagawa, Y., Maruoka, Y., & Iimura, T. (2023). Association between an Increased Serum CCL5 Level and Pathophysiology of Degenerative Joint Disease in the Temporomandibular Joint in Females. International Journal of Molecular Sciences, 24(3), 2775. https://doi.org/10.3390/ijms24032775






