Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy
Abstract
1. Introduction
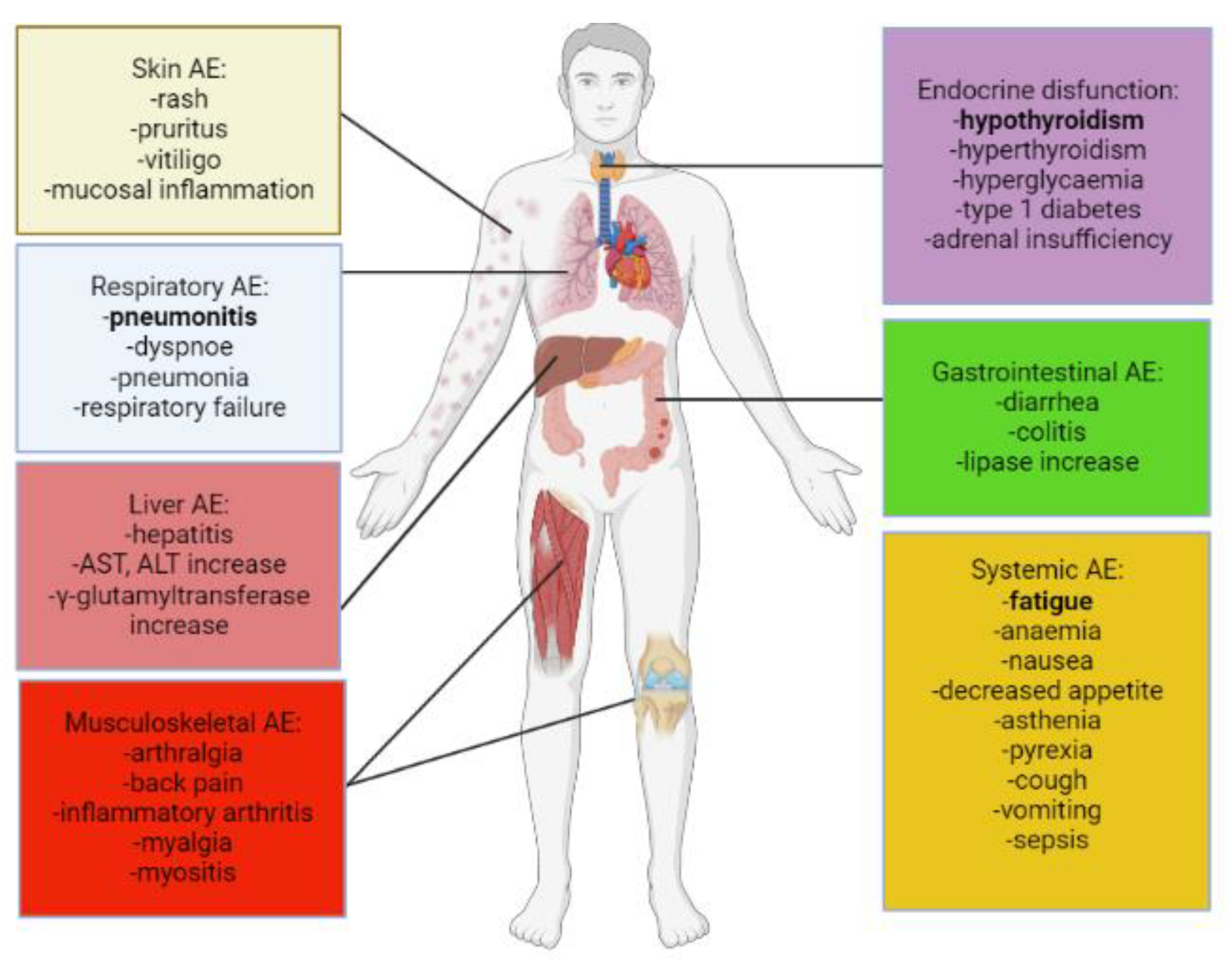
2. Adverse Events and Their Clinical Management in Anti-Cancer Immunotherapy
2.1. Dermatological irAEs
2.2. Gastrointestinal irAEs
2.3. Respiratory irAEs
2.4. Endocrine irAEs
2.5. Cardiovascular irAEs
2.6. Musculoskeletal irAEs
2.7. Neurological irAEs-Peripheral Nervous System
2.8. Neurological irAEs-Central Nervous System
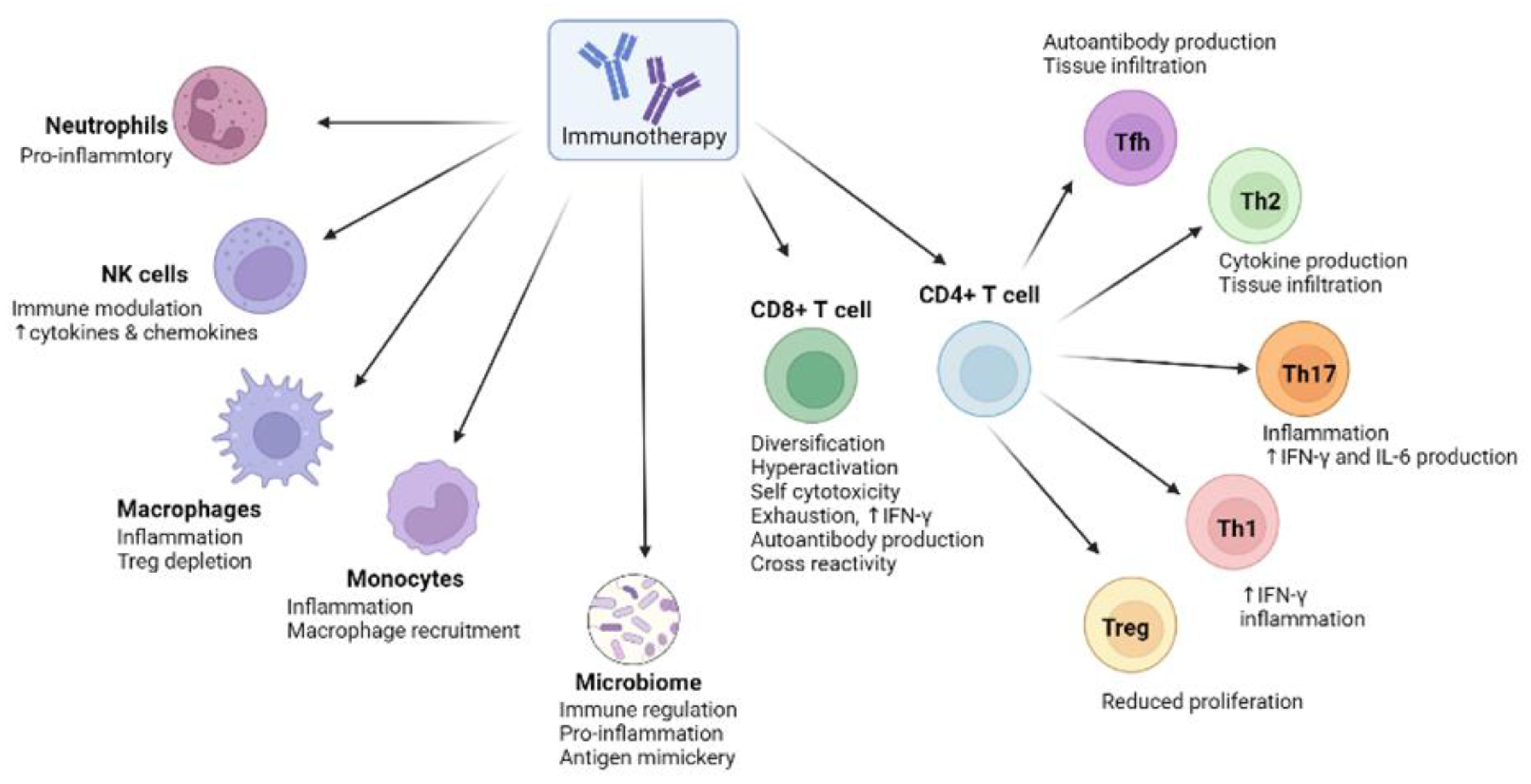
3. The Biological Basis of irAEs in Anti-Cancer Immunotherapies
4. Microbial Signatures Associated with irAEs
5. Microbial Metabolites and Metabolic Pathways Associated with irAEs
6. The Efficacy–Toxicity Coupling Effect: A Crucial Challenge for Future Clinical Practice
7. The Prospect of the Microbiome in Future Cancer Therapies
7.1. FMT
7.2. Nanotechnologies
7.3. CRISPR/Cas9
7.4. Viruses and Bacteriophages
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research status and outlook of pd-1/pd-l1 inhibitors for cancer therapy. Drug Des. Dev. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Chan, R.J.; Chan, A.; Keefe, D.; Costello, S.P.; Hart, N.H. Dual contribution of the gut microbiome to immunotherapy efficacy and toxicity: Supportive care implications and recommendations. Support Care Cancer 2022, 30, 6369–6373. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Pierrard, J.; Seront, E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy-a systematic review. Curr. Oncol. 2019, 26, 395–403. [Google Scholar] [CrossRef]
- Huang, C.; Li, M.; Liu, B.; Zhu, H.; Dai, Q.; Fan, X.; Mehta, K.; Huang, C.; Neupane, P.; Wang, F.; et al. Relating Gut Microbiome and Its Modulating Factors to Immunotherapy in Solid Tumors: A Systematic Review. Front. Oncol. 2021, 11, 642110. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Limeta, A.; Ji, B.; Levin, M.; Gatto, F.; Nielsen, J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight 2020, 5, e140940. [Google Scholar] [CrossRef]
- de Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front Immunol. 2014, 5, 60. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Coffin, P.; Maillet, D.; Gan, H.K.; Stelmes, J.-J.; You, B.; Dalle, S.; Péron, J. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Brown, V.T.; Antol, D.D.; Racsa, P.N.; Ward, M.A.; Naidoo, J. Real-World Incidence and Management of Immune-Related Adverse Events from Immune Checkpoint Inhibitors: Retrospective Claims-Based Analysis. Cancer Investig. 2021, 39, 789–796. [Google Scholar] [CrossRef]
- Ouyang, T.; Cao, Y.; Kan, X.; Chen, L.; Ren, Y.; Sun, T.; Yan, L.; Xiong, B.; Liang, B.; Zheng, C. Treatment-Related Serious Adverse Events of Immune Checkpoint Inhibitors in Clinical Trials: A Systematic Review. Front. Oncol. 2021, 11, 621639. [Google Scholar] [CrossRef]
- Okiyama, N.; Tanaka, R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol. Int. 2022, 71, 169–178. [Google Scholar] [CrossRef]
- Bardoscia, L.; Pasinetti, N.; Triggiani, L.; Cozzi, S.; Sardaro, A. Biological Bases of Immune-Related Adverse Events and Potential Crosslinks With Immunogenic Effects of Radiation. Front. Pharmacol. 2021, 12, 746853. [Google Scholar] [CrossRef]
- Khan, S.; Gerber, D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin. Cancer Biol. 2019, 64, 93–101. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 1–4. [Google Scholar] [CrossRef]
- Esfahani, K.; Meti, N.; Miller, W.H.; Hudson, M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. Can. Med Assoc. J. 2019, 191, E40–E46. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Kirkwood, J.M. PD-1 Immune Checkpoint Inhibitors and Immune-Related Adverse Events: Understanding the Upside of the Downside of Checkpoint Blockade. JAMA Oncol. 2019, 5, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Pahalyants, V.; Kalinich, M.; Murphy, W.; Yu, K.-H.; Wang, F.; Chen, S.T.; Reynolds, K.; Kwatra, S.G.; Semenov, Y.R. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A United States population-level analysis. J. Am. Acad. Dermatol. 2021, 86, 563–572. [Google Scholar] [CrossRef]
- Zhao, F.; Zhu, J.; Yu, R.; Shao, T.; Chen, S.; Zhang, G.; Shu, Q. Cutaneous adverse events in patients treated with PD-1/PD-L1 checkpoint inhibitors and their association with survival: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20038. [Google Scholar] [CrossRef]
- Phillips, G.S.; Wu, J.; Hellmann, M.D.; Postow, M.A.; Rizvi, N.A.; Freites-Martinez, A.; Chan, D.; Dusza, S.; Motzer, R.J.; Rosenberg, J.E.; et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J. Clin. Oncol. 2019, 37, 2746–2758. [Google Scholar] [CrossRef]
- Chadha, S.; Para, A.J.; Choi, J. Management of Immune-Related Cutaneous Adverse Reactions to PD-1 and PD-L1 Inhibitors for the Inpatient Dermatologist. Curr. Dermatol. Rep. 2020, 9, 231–243. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Lin, N.; Zhou, Y.; He, W.; Liu, J.; Ma, X. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Front. Immunol. 2021, 12, 800879. [Google Scholar] [CrossRef]
- de Malet, A.; Antoni, G.; Collins, M.; Soularue, E.; Marthey, L.; Vaysse, T.; Coutzac, C.; Chaput, N.; Mateus, C.; Robert, C.; et al. Evolution and recurrence of gastrointestinal immune-related adverse events induced by immune checkpoint inhibitors. Eur. J. Cancer 2019, 106, 106–114. [Google Scholar] [CrossRef]
- Shivaji, U.N.; Jeffery, L.; Gui, X.; Smith, S.C.L.; Ahmad, O.F.; Akbar, A.; Ghosh, S.; Iacucci, M. Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their management. Ther. Adv. Gastroenterol. 2019, 12, 1756284819884196. [Google Scholar] [CrossRef]
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405–418. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Vareki, S.M.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef]
- Kähler, K.C.; For the German Dermatologic Cooperative Oncology Group (DeCOG); Eigentler, T.K.; Gesierich, A.; Heinzerling, L.; Loquai, C.; Meier, F.; Meiss, F.; Pföhler, C.; Schlaak, M.; et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol. Immunother. 2018, 67, 825–834. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Hu, J.; Zhu, E.C.; Su, Q. Hepatotoxicity in patients with solid tumors treated with PD-1/PD-L1 inhibitors alone, PD-1/PD-L1 inhibitors plus chemotherapy, or chemotherapy alone: Systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1345–1354. [Google Scholar] [CrossRef]
- Winer, A.; Bodor, J.N.; Borghaei, H. Identifying and managing the adverse effects of immune checkpoint blockade. J. Thorac. Dis. 2018, 10, S480–S489. [Google Scholar] [CrossRef]
- Rogers, C.B.B.; Cuddahy, R.T.; Zawislak, M.C. Management of Acute Pancreatitis Associated With Checkpoint Inhibitors. J. Adv. Pract. Oncol. 2020, 11, 49–62. [Google Scholar] [CrossRef]
- Kakuwa, T.; Hashimoto, M.; Izumi, A.; Naka, G.; Takeda, Y.; Sugiyama, H. Pembrolizumab-related pancreatitis with elevation of pancreatic tumour markers. Respirol. Case Rep. 2020, 8, e00525. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Yang, X.; Li, D.; Zhang, L.; Li, Z.; Zhang, S.; Mao, Y.; Jin, C.; Zhao, Y. The Risk Ratio of Immune-Related Colitis, Hepatitis, and Pancreatitis in Patients with Solid Tumors Caused by PD-1/PD-L1 Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, Y.; Yang, X.; Gan, L.; Xue, J. Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism. Front. Immunol. 2022, 13, 830631. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, J.; Lee, J.S.; Kim, Y.J.; Kim, S.H.; Lee, Y.J.; Cho, Y.-J.; Yoon, H.I.; Lee, J.H.; Lee, C.-T.; et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018, 125, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Banavasi, H.; Kim, S.; Alkassis, S.; Daoud, A.; Laktineh, A.; Nagasaka, M.; Sukari, A.; Soubani, A.O. Immune checkpoint inhibitor-induced pneumonitis: Incidence, clinical characteristics, and outcomes. Hematol. Stem Cell Ther. 2021. [Google Scholar] [CrossRef]
- Tiu, B.C.; Zubiri, L.; Iheke, J.; Pahalyants, V.; Theodosakis, N.; Ugwu-Dike, P.; Seo, J.; Tang, K.; E Sise, M.; Sullivan, R.; et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: A multi-institutional cohort study. J. Immunother. Cancer 2022, 10, e004670. [Google Scholar] [CrossRef]
- A Muir, C.; Clifton-Bligh, R.J.; Long, G.V.; A Scolyer, R.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Bala-Hampton, J.E.; Bazzell, A.F.; Dains, J.E. Clinical Management of Pneumonitis in Patients Receiving Anti–PD-1/PD-L1 Therapy. J. Adv. Pract. Oncol. 2018, 9, 422. [Google Scholar]
- Yoon, J.H.; Hong, A.R.; Kim, H.K.; Kang, H.C. Characteristics of immune-related thyroid adverse events in patients treated with PD-1/PD-L1 inhibitors. Endocrinol. Metab. 2021, 36, 413–423. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. In Endocrine Reviews; Oxford University Press: Oxford, UK, 2018; Volume 40, pp. 17–65. [Google Scholar]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Kotwal, A.; Kottschade, L.; Ryder, M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020, 30, 177–184. [Google Scholar] [CrossRef]
- Baek, H.-S.; Jeong, C.; Shin, K.; Lee, J.; Suh, H.; Lim, D.-J.; Kang, M.I.; Ha, J. Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients. BMC Endocr. Disord. 2022, 22, 89. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Robert, L.; Tsoi, J.; Wang, X.; Emerson, R.; Homet, B.; Chodon, T.; Mok, S.; Huang, R.R.; Cochran, A.J.; Comin-Anduix, B.; et al. CTLA4 Blockade Broadens the Peripheral T-Cell Receptor Repertoire. Clin. Cancer Res. 2014, 20, 2424–2432. [Google Scholar] [CrossRef]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Friedman, C.F.; Navid-Azarbaijani, P.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018, 4, 98–101. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Patrizio, A.; Galdiero, M.R.; Baldini, E.; Ulisse, S.; Marone, G.; et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int. J. Mol. Sci. 2019, 20, 2560. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2021, 21, 495–508. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. New Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncol. 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Makunts, T.; Saunders, I.M.; Cohen, I.V.; Li, M.; Moumedjian, T.; Issa, M.A.; Burkhart, K.; Lee, P.; Patel, S.P.; Abagyan, R. Myocarditis occurrence with cancer immunotherapy across indications in clinical trial and post-marketing data. Sci. Rep. 2021, 11, 17324. [Google Scholar] [CrossRef] [PubMed]
- Matzen, E.; Bartels, L.E.; Løgstrup, B.; Horskær, S.; Stilling, C.; Donskov, F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: A case report and review of reported cases. Cardiooncology 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. A Systematic Review of the Mechanisms Involved in Immune Checkpoint Inhibitors Cardiotoxicity and Challenges to Improve Clinical Safety. Front. Cell Dev. Biol. 2022, 10, 851032. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Dong, H.; Qi, Y.; Kong, X.; Wang, Z.; Fang, Y.; Wang, J. PD-1/PD-L1 Inhibitor-Associated Myocarditis: Epidemiology, Characteristics, Diagnosis, Treatment, and Potential Mechanism. Front. Pharmacol. 2022, 13, 1082. [Google Scholar] [CrossRef]
- Safi, M.; Ahmed, H.; Al-Azab, M.; Xia, Y.L.; Shan, X.; Al-Radhi, M.; Al-Danakh, A.; Shopit, A.; Liu, J. PD-1/PDL-1 Inhibitors and Cardiotoxicity; Molecular, Etiological and Management Outlines. J. Adv. Res. 2021, 29, 45–54. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef]
- Angelopoulou, F.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Immune checkpoint inhibitor-induced musculoskeletal manifestations. Rheumatol. Int. 2020, 41, 33–42. [Google Scholar] [CrossRef]
- Chan, K.K.; Bass, A.R. Monitoring and Management of the Patient with Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis: Current Perspectives. J. Inflamm. Res. 2022, 15, 3105–3118. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O.; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. 2017, 69, 1751–1763. [Google Scholar] [CrossRef]
- Kao, J.C.; Liao, B.; Markovic, S.N.; Klein, C.; Naddaf, E.; Staff, N.P.; Liewluck, T.; Hammack, J.E.; Sandroni, P.; Finnes, H.; et al. Neurological Complications Associated with Anti–Programmed Death 1 (PD-1) Antibodies. JAMA Neurol. 2017, 74, 1216–1222. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur. J. Cancer 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Psimaras, D. Neuromuscular complications of immune checkpoint inhibitors. La Presse Médicale 2018, 47, e253–e259. [Google Scholar] [CrossRef]
- Kao, J.C.; Brickshawana, A.; Liewluck, T. Neuromuscular Complications of Programmed Cell Death-1 (PD-1) Inhibitors. Curr. Neurol. Neurosci. Rep. 2018, 18, 63. [Google Scholar] [CrossRef]
- Guptill, J.T.; Sanders, D.B.; Evoli, A. Anti-musk antibody myasthenia gravis: Clinical findings and response to treatment in two large cohorts. Muscle Nerve 2011, 44, 36–40. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis—Autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Neyns, B. Anti-CTLA-4 antibody-induced Guillain–Barré syndrome in a melanoma patient. Ann. Oncol. 2011, 22, 991–993. [Google Scholar] [CrossRef]
- Xu, M.; Nie, Y.; Yang, Y.; Lu, Y.-T.; Su, Q. Risk of Neurological Toxicities Following the Use of Different Immune Checkpoint Inhibitor Regimens in Solid Tumors: A Systematic Review and Meta-Analysis. Neurologist 2019, 24, 75–83. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
- Man, J.; Ritchie, G.; Links, M.; Lord, S.J.; Lee, C.K. Treatment-related toxicities of immune checkpoint inhibitors in advanced cancers: A meta-analysis. Asia-Pacific J. Clin. Oncol. 2018, 14, 141–152. [Google Scholar] [CrossRef]
- Johansen, A.; Christensen, S.J.; Scheie, D.; Højgaard, J.L.; Kondziella, D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies. Neurology 2019, 92, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Jacobs, B.C.; A van Doorn, P. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chmielowski, B.; Lao, C.D.; Hodi, F.S.; Sharfman, W.; Weber, J.; Suijkerbuijk, K.P.M.; Azevedo, S.; Li, H.; Reshef, D.; et al. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncology 2017, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef]
- Narumi, Y.; Yoshida, R.; Minami, Y.; Yamamoto, Y.; Takeguchi, S.; Kano, K.; Takahashi, K.; Saito, T.; Sawada, J.; Terui, H.; et al. Neuromyelitis optica spectrum disorder secondary to treatment with anti-PD-1 antibody nivolumab: The first report. BMC Cancer 2018, 18, 95. [Google Scholar] [CrossRef]
- Mancone, S.; Lycan, T.; Ahmed, T.; Topaloglu, U.; Dothard, A.; Petty, W.J.; Strowd, R.E. Severe neurologic complications of immune checkpoint inhibitors: A single-center review. J. Neurol. 2018, 265, 1636–1642. [Google Scholar] [CrossRef]
- Shimada, T.; Hoshino, Y.; Tsunemi, T.; Hattori, A.; Nakagawa, E.; Yokoyama, K.; Hattori, N. Neuromyelitis optica spectrum disorder after treatment with pembrolizumab. Mult. Scler. Relat. Disord. 2019, 37, 101447. [Google Scholar] [CrossRef]
- Garcia, C.R.; Jayswal, R.; Adams, V.; Anthony, L.B.; Villano, J.L. Multiple sclerosis outcomes after cancer immunotherapy. Clin. Transl. Oncol. 2019, 21, 1336–1342. [Google Scholar] [CrossRef]
- Läubli, H.; Hench, J.; Stanczak, M.; Heijnen, I.; Papachristofilou, A.; Frank, S.; Zippelius, A.; Stenner-Liewen, F. Cerebral vasculitis mimicking intracranial metastatic progression of lung cancer during PD-1 blockade. J. Immunother. Cancer 2017, 5, 46. [Google Scholar] [CrossRef]
- Bruno, F.; Palmiero, R.A.; Ferrero, B.; Franchino, F.; Pellerino, A.; Milanesi, E.; Soffietti, R.; Rudà, R. Pembrolizumab-Induced Isolated Cranial Neuropathy: A Rare Case Report and Review of Literature. Front. Neurol. 2021, 12, 669493. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kikuchi, R.; Iwai, Y.; Ito, M.; Tsukamoto, H.; Yamazaki, K.; Nakamura, H.; Aoshiba, K. Varicella zoster virus encephalitis mimicking nivolumab-induced autoimmune neuropathy in a patient with lung cancer. J. Thorac. Oncol. 2019, 14, e163–e165. [Google Scholar] [CrossRef]
- Johnson, D.B.; McDonnell, W.J.; Gonzalez-Ericsson, P.I.; Al-Rohil, R.N.; Mobley, B.C.; Salem, J.-E.; Wang, D.Y.; Sanchez, V.; Wang, Y.; Chastain, C.A.; et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 2019, 25, 1243–1250. [Google Scholar] [CrossRef]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neurological and related adverse events in immune checkpoint inhibitors: A pharmacovigilance study from the Japanese Adverse Drug Event Report database. J. Neurooncol. 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.N.; Allayous, C.; Callaghan, S.; Martínez-Vila, C.; Wallace, R.; Bhave, P.; Reijers, I.L.M.; et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann. Oncol. 2021, 32, 917–925. [Google Scholar] [CrossRef]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell RepertoireIpilimumab-Induced T-cell Diversity Predicts IRAEs. Cancer Res. 2017, 77, 1322–1330. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2019, 64, 29–35. [Google Scholar] [CrossRef]
- Denk, D.; Greten, F.R. Inflammation: The incubator of the tumor microenvironment. Trends Cancer 2022, 8, 901–914. [Google Scholar] [CrossRef]
- Milling, L.; Zhang, Y.; Irvine, D.J. Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 2017, 114, 79–101. [Google Scholar] [CrossRef]
- Yang, H.; Yao, Z.; Zhou, X.; Zhang, W.; Zhang, X.; Zhang, F. Immune-related adverse events of checkpoint inhibitors: Insights into immunological dysregulation. Clin. Immunol. 2020, 213, 108377. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Han, L.; Liu, H. Mechanisms underlying immune-related adverse events during checkpoint immunotherapy. Clin. Sci. 2022, 136, 771–785. [Google Scholar] [CrossRef]
- Young, A.; Quandt, Z.; Bluestone, J.A. The Balancing Act between Cancer Immunity and Autoimmunity in Response to Immunotherapy. Cancer Immunol. Res. 2018, 6, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e622. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Hur, J.Y.; Cho, J.; Ku, B.M.; Koh, J.; Koh, J.Y.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; et al. Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. Oncoimmunology 2020, 9, 1722023. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, E.; Chodon, T.; Attar, N.; Jalil, J.; Koya, R.C.; Comin-Anduix, B.; Ribas, A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J. Transl. Med. 2009, 7, 35. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakayama, T.; Ito, A.; Sato, E.; Kitano, S. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: Potential role of Th1-dominant immune response in immune-related adverse events: Two case reports. BMC Cancer 2019, 19, 1019. [Google Scholar] [CrossRef]
- Das, R.; Bar, N.; Ferreira, M.; Newman, A.M.; Zhang, L.; Bailur, J.K.; Bacchiocchi, A.; Kluger, H.; Wei, W.; Halaban, R.; et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Investig. 2018, 128, 715–720. [Google Scholar] [CrossRef]
- de Moel, E.C.; Rozeman, E.A.; Kapiteijn, E.H.; Verdegaal, E.M.; Grummels, A.; Bakker, J.A.; Huizinga, T.W.; Haanen, J.B.; Toes, R.E.; van der Woude, D. Autoantibody Development under Treatment with Immune-Checkpoint InhibitorsAutoantibodies under Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2019, 7, 6–11. [Google Scholar] [CrossRef]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H.; Calabrese, L. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef]
- Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; Sznol, M.; Young, A.; Rushakoff, R.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced with Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Sibille, A.; Alfieri, R.; Malaise, O.; Detrembleur, N.; Pirotte, M.; Louis, R.; Duysinx, B. Granulomatosis with polyangiitis in a patient on programmed death-1 inhibitor for advanced non-small-cell lung cancer. Front. Oncol. 2019, 9, 478. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, S.; Wei, X.; Liu, X.; Guan, J.; Zhu, H.; Chang, G.; Chen, Y.; Lu, H.; Qian, J.; et al. Highly activated TRAIL+ CD56bright NK cells are associated with the liver damage in HBV-LC patients. Immunol. Lett. 2021, 232, 9–19. [Google Scholar] [CrossRef]
- Matas-García, A.; Milisenda, J.C.; Selva-O’Callaghan, A.; Prieto-González, S.; Padrosa, J.; Cabrera, C.; Reguart, N.; Castrejón, N.; Solé, M.; Ros, J.; et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern. Autoimmun. Rev. 2019, 19, 102455. [Google Scholar] [CrossRef]
- Hu, H.; Zakharov, P.N.; Peterson, O.J.; Unanue, E.R. Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc. Natl. Acad. Sci. USA 2020, 117, 31319–31330. [Google Scholar] [CrossRef]
- Mihic-Probst, D.; Reinehr, M.; Dettwiler, S.; Kolm, I.; Britschgi, C.; Kudura, K.; Maggio, E.M.; Lenggenhager, D.; Rushing, E.J. The role of macrophages type 2 and T-regs in immune checkpoint inhibitor related adverse events. Immunobiology 2020, 225, 152009. [Google Scholar] [CrossRef]
- Curry, J.L.; Reuben, A.; Szczepaniak-Sloane, R.; Ning, J.; Milton, D.R.; Lee, C.H.; Hudgens, C.; George, S.; Torres-Cabala, C.; Johnson, D.; et al. Gene expression profiling of lichenoid dermatitis immune-related adverse event from immune checkpoint inhibitors reveals increased CD14 + and CD16 + monocytes driving an innate immune response. J. Cutan. Pathol. 2019, 46, 627–636. [Google Scholar] [CrossRef]
- Gudd, C.L.; Au, L.; Triantafyllou, E.; Shum, B.; Liu, T.; Nathwani, R.; Kumar, N.; Mukherjee, S.; Dhar, A.; Woollard, K.J.; et al. Activation and transcriptional profile of monocytes and CD8+ T cells are altered in checkpoint inhibitor-related hepatitis. J. Hepatol. 2021, 75, 177–189. [Google Scholar] [CrossRef]
- Soularue, E.; Lepage, P.; Colombel, J.F.; Coutzac, C.; Faleck, D.; Marthey, L.; Collins, M.; Chaput, N.; Robert, C.; Carbonnel, F. Enterocolitis due to immune checkpoint inhibitors: A systematic review. Gut 2018, 67, 2056–2067. [Google Scholar] [CrossRef]
- Al-Qadami, G.; Van Sebille, Y.; Le, H.; Bowen, J. Gut microbiota: Implications for radiotherapy response and radiotherapy-induced mucositis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2019, 30, 2012. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.-H.H.; del Rincón, S.V.; Miller, W.H. Potential therapies for immune-related adverse events associated with immune checkpoint inhibition: From monoclonal antibodies to kinase inhibition. J. Immunother. Cancer 2022, 10, e003551. [Google Scholar]
- Tan, B.; Liu, Y.; Tang, H.; Chen, D.; Xu, Y.; Chen, M.; Li, Y.; Wang, M.; Qian, J. Gut microbiota shed new light on the management of immune-related adverse events. Thorac. Cancer 2022, 13, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Xu, H.-M.; Zhou, Y.-L.; Xu, J.; Li, Y.-F.; Zhao, C.; Huang, H.-L.; Du, Y.-L.; He, J.; Zhou, Y.-J.; Nie, Y.-Q. Inhibition of PD-1 Protects against TNBS-Induced Colitis via Alteration of Enteric Microbiota. BioMed Res. Int. 2021, 2021, 4192451. [Google Scholar] [CrossRef]
- Davar, D.; Zarour, H.M. Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy. Clin. Cancer Res. 2022, 28, 4370–4384. [Google Scholar] [CrossRef]
- Hayase, E.; Jenq, R.R. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021, 13, 107. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Seo, S.U.; Kamada, N.; Muñoz-Planillo, R.; Kim, Y.G.; Kim, D.; Koizumi, Y.; Hasegawa, M.; Himpsl, S.D.; Browne, H.P.; Lawley, T.D.; et al. Distinct Commensals Induce Interleukin-1beta via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity 2015, 42, 744–755. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Naqash, A.R.; Ricciuti, B.; Owen, D.H.; Florou, V.; Toi, Y.; Cherry, C.; Hafiz, M.; De Giglio, A.; Muzaffar, M.; Patel, S.H.; et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: A pooled exploratory analysis from a global cohort. Cancer Immunol. Immunother. 2020, 69, 1177–1187. [Google Scholar] [CrossRef]
- Verzoni, E.; on behalf of the Italian Nivolumab Renal Cell Cancer Early Access Program group; Cartenì, G.; Cortesi, E.; Giannarelli, D.; De Giglio, A.; Sabbatini, R.; Buti, S.; Rossetti, S.; Cognetti, F.; et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: The Italian expanded access program. J. Immunother. Cancer 2019, 7, 99. [Google Scholar] [CrossRef]
- Littmann, E.R.; Lee, J.J.; Denny, J.E.; Alam, Z.; Maslanka, J.R.; Zarin, I.; Matsuda, R.; Carter, R.A.; Susac, B.; Saffern, M.S.; et al. Host immunity modulates the efficacy of microbiota transplantation for treatment of Clostridioides difficile infection. Nat. Commun. 2021, 12, 755. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2020, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Bernardes, M.; Hohl, T.M. Fungal Infections Associated with the Use of Novel Immunotherapeutic Agents. Curr. Clin. Microbiol. Rep. 2020, 7, 142–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.-X.; Zhang, X.-Z. Bacteriophage-mediated modulation of microbiota for diseases treatment. Adv. Drug Deliv. Rev. 2021, 176, 113856. [Google Scholar] [CrossRef]
- Sakai, K.; Sakurai, T.; De Velasco, M.A.; Nagai, T.; Chikugo, T.; Ueshima, K.; Kura, Y.; Takahama, T.; Hayashi, H.; Nakagawa, K. Intestinal Microbiota and Gene Expression Reveal Similarity and Dissimilarity Between Immune-Mediated Colitis and Ulcerative Colitis. Front. Oncol. 2021, 11, 763468. [Google Scholar] [CrossRef]
- Cascone, T.; William, N.; Weissferdt, A.; Leung, C.; Lin, H.; Pataer, A.; Godoy, M.; Carter, B.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.; Yadav, M.; Liu, B.; Furqan, M.; Dai, Q.; Shahi, S.; Gupta, A.; Mercer, K.N.; Eastman, E.; Hejleh, T.A.; et al. Prospective correlation between the patient microbiome with response to and development of immune-mediated adverse effects to immunotherapy in lung cancer. BMC Cancer 2021, 21, 808. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hao, H.; Li, X.; Wang, Z. The effect of intestinal flora on immune checkpoint inhibitors in tumor treatment: A narrative review. Ann. Transl. Med. 2020, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Bredin, P.; Naidoo, J. The gut microbiome, immune check point inhibition and immune-related adverse events in non-small cell lung cancer. Cancer Metastasis Rev. 2022, 41, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Kanjanapan, Y.; Yip, D. Characteristics and risk factors for microbial infections during cancer immune checkpoint therapy. Cancer Med. 2020, 9, 9027–9035. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Zhang, D.; Zhou, Q.; Liu, J.; Chen, M.; Xu, Y.; Zhao, J.; Zhong, W.; Wang, M. Risk factors for immune-related adverse events: What have we learned and what lies ahead? Biomark Res. 2021, 9, 79. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Lensu, S.; Pekkala, S. Gut Microbiota, Microbial Metabolites and Human Physical Performance. Metabolites 2021, 11, 716. [Google Scholar] [CrossRef]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.-X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Y.; Chen, X.; Wang, C.; Chen, X.; Yuan, X.; Liu, L.; Yang, J.; Zhou, X. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 1. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Batten, M.; Reijers, I.L.M.; Read, M.; Silva, I.P.; Versluis, J.M.; Ribeiro, R.; Angelatos, A.S.; Tan, J.; et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 2022, 28, 2344–2352. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Tao, R.; de Zoeten, E.F.; Özkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar] [CrossRef]
- Iraporda, C.; Romanin, D.; Bengoa, A.A.; Errea, A.J.; Cayet, D.; Foligne, B.; Sirard, J.-C.; Garrote, G.L.; Abraham, A.; Rumbo, M. Local Treatment with Lactate Prevents Intestinal Inflammation in the TNBS-Induced Colitis Model. Front. Immunol. 2016, 7, 651. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.; Kania, K.; Dastych, J. Lactate Stimulates IL-4 and IL-13 Production in Activated HuT-78 T Lymphocytes Through a Process That Involves Monocarboxylate Transporters and Protein Hyperacetylation. J. Interf. Cytokine Res. 2016, 36, 317–327. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine Inhibits Proinflammatory Cytokine Synthesis in Human Mononuclear Cells: A Counterregulatory Mechanism that Restrains the Immune Response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef]
- Renga, G.; Nunzi, E.; Pariano, M.; Puccetti, M.; Bellet, M.M.; Pieraccini, G.; D’Onofrio, F.; Santarelli, I.; Stincardini, C.; Aversa, F.; et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J. Immunother. Cancer 2022, 10, e003725. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rong, X.; Zhao, G.; Zhou, Y.; Xiao, Y.; Ma, D.; Jin, X.; Wu, Y.; Yan, Y.; Yang, H.; et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022, 34, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Gunasekera, D.C.; Ma, J.; Vacharathit, V.; Shah, P.; Ramakrishnan, A.; Uprety, P.; Shen, Z.; Sheh, A.; Brayton, C.F.; Whary, M.T.; et al. The development of colitis in Il10 mice is dependent on IL-22. Mucosal Immunol. 2020, 13, 493–506. [Google Scholar] [CrossRef]
- Nomura, M.; Nagatomo, R.; Doi, K.; Shimizu, J.; Baba, K.; Saito, T.; Matsumoto, S.; Inoue, K.; Muto, M. Association of Short-Chain Fatty Acids in the Gut Microbiome with Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw. Open 2020, 3, e202895. [Google Scholar] [CrossRef]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; Del Chierico, F.; Di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, Y.; Tomé, D.; Carrasco-Pozo, C.; Gotteland, M.; et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free. Radic. Biol. Med. 2015, 85, 219–227. [Google Scholar] [CrossRef]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.; Osman, I.; Ahn, J. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019, 11, 61. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef]
- Wind, T.T.; Gacesa, R.; Vila, A.V.; De Haan, J.J.; Jalving, M.; Weersma, R.K.; Hospers, G.A. Gut microbial species and metabolic pathways associated with response to treatment with immune checkpoint inhibitors in metastatic melanoma. Melanoma Res. 2020, 30, 235–246. [Google Scholar] [CrossRef]
- Johns, A.C.; Wei, L.; Grogan, M.; Hoyd, R.; Bridges, J.F.; Patel, S.H.; Li, M.; Husain, M.; Kendra, K.L.; Otterson, G.A.; et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer. J. Geriatr. Oncol. 2021, 12, 813–819. [Google Scholar] [CrossRef]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018, 115, 71–74, Erratum in: Lung Cancer 2018, 126, 230–231. [Google Scholar] [CrossRef]
- Grangeon, M.; Tomasini, P.; Chaleat, S.; Jeanson, A.; Souquet-Bressand, M.; Khobta, N.; Bermudez, J.; Trigui, Y.; Greillier, L.; Blanchon, M.; et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non–small-cell Lung Cancer. Clin. Lung Cancer 2018, 20, 201–207. [Google Scholar] [CrossRef]
- Ricciuti, B.; Genova, C.; De Giglio, A.; Bassanelli, M.; Bello, M.G.D.; Metro, G.; Brambilla, M.; Baglivo, S.; Grossi, F.; Chiari, R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J. Cancer Res. Clin. Oncol. 2018, 145, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Goyal, S.; Liu, Y.; Evans, S.T.; Olsen, T.A.; Case, K.; Magod, B.L.; Brown, J.T.; Yantorni, L.; Russler, G.A.; et al. Immune-Related Adverse Events as Clinical Biomarkers in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Oncol. 2021, 26, e1742–e1750. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ciombor, K.K.; Haraldsdottir, S.; Pumpalova, Y.; Sahin, I.H.; Pineda, G.; Shyr, Y.; Lin, E.; Hsu, C.-Y.; Chu, S.-K.; et al. Immune-Related Adverse Events and Immune Checkpoint Inhibitor Efficacy in Patients with Gastrointestinal Cancer with Food and Drug Administration-Approved Indications for Immunotherapy. Oncology 2020, 25, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Shoji, H.; Nagashima, K.; Yamamoto, S.; Ishikawa, M.; Imazeki, H.; Aoki, M.; Miyamoto, T.; Hirano, H.; Honma, Y.; et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019, 19, 974. [Google Scholar] [CrossRef]
- Foster, C.C.; Couey, M.A.; Ba, S.E.K.; Khattri, A.; Ms, R.K.A.; Tan, Y.C.; Brisson, R.J.; Leidner, R.S.; Seiwert, T.Y. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors. Cancer 2021, 127, 4565–4573. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, Y.; Wang, X.; Zhao, B. Guillain–Barré syndrome in patients treated with immune checkpoint inhibitors. J. Neurol. 2021, 268, 2169–2174. [Google Scholar] [CrossRef]
- Teraoka, S.; Fujimoto, D.; Morimoto, T.; Kawachi, H.; Ito, M.; Sato, Y.; Nagata, K.; Nakagawa, A.; Otsuka, K.; Uehara, K.; et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non–Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J. Thorac. Oncol. 2017, 12, 1798–1805. [Google Scholar] [CrossRef]
- Morehouse, C.; Abdullah, S.E.; Gao, C.; Dar, M.M.; Ranade, K.; Higgs, B.W. Early incidence of immune-related adverse events (irAEs) predicts efficacy in patients (pts) with solid tumors treated with immune-checkpoint inhibitors (ICIs). J. Clin. Oncol. 2019, 37, 2563. [Google Scholar] [CrossRef]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Rn, C.F.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Hodi, F.S.; Lee, S.; McDermott, D.F.; Rao, U.N.; Butterfield, L.H.; Tarhini, A.A.; Leming, P.; Puzanov, I.; Shin, D.; Kirkwood, J.M. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 2014, 312, 1744–1753. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.-E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating Nanoparticles with Gastric Epithelial Cell Membrane for Targeted Antibiotic Delivery against Helicobacter pylori Infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Kuo, T.T.; Levy-Nissenbaum, E.; Karnik, R.; Blumberg, R.S.; Langer, R.; Farokhzad, O.C. Transepithelial Transport of Fc-Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Sci. Transl. Med. 2013, 5, 213ra167. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806e12. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Poon, Z.; Chang, D.; Zhao, X.; Hammond, P.T. Layer-by-Layer Nanoparticles with a pH-Sheddable Layer for in Vivo Targeting of Tumor Hypoxia. ACS Nano 2011, 5, 4284–4292. [Google Scholar] [CrossRef]
- Song, W.; Shen, L.; Wang, Y.; Liu, Q.; Goodwin, T.J.; Li, J.; Dorosheva, O.; Liu, T.; Liu, R.; Huang, L. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018, 9, 2237. [Google Scholar] [CrossRef]
- Mimee, M.; Citorik, R.J.; Lu, T.K. Microbiome therapeutics—Advances and challenges. Adv. Drug Deliv. Rev. 2016, 105, 44–54. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2011, 6, 320–329. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, Inflammation, and Cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis Signature of Fecal Microbiota in Colorectal Cancer Patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef]
- Ramteke, S.; Ganesh, N.; Bhattacharya, S.; Jain, N.K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target. 2009, 17, 225–234. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. Erratum in: Trends Biotechnol. 2013, 31, 61–62. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.; Allaker, R.P. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. WIREs Nanomed. Nanobiotechnology 2014, 6, 532–547. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef]
- Lam, K.N.; Spanogiannopoulos, P.; Soto-Perez, P.; Alexander, M.; Nalley, M.J.; Bisanz, J.E.; Nayak, R.R.; Weakley, A.M.; Yu, F.B.; Turnbaugh, P.J. Phage-delivered CRISPR-Cas9 for strain-specific depletion and genomic deletions in the gut microbiome. Cell Rep. 2021, 37, 109930. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; McBride, S.W.; Hullahalli, K.; Palmer, K.L.; Duerkop, B.A. Conjugative Delivery of CRISPR-Cas9 for the Selective Depletion of Antibiotic-Resistant Enterococci. Antimicrob. Agents Chemother. 2019, 63, e01454-19. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.; Allard, N.; Roy, P.; Grenier, F.; Menendez, A.; Burrus, V.; Rodrigue, S. High-efficiency delivery of CRISPR-Cas9 by engineered probiotics enables precise microbiome editing. Mol. Syst. Biol. 2021, 17, e10335. [Google Scholar] [CrossRef] [PubMed]
- Rogovski, P.; Cadamuro, R.D.; da Silva, R.; de Souza, E.B.; Bonatto, C.; Viancelli, A.; Michelon, W.; Elmahdy, E.M.; Treichel, H.; Rodríguez-Lázaro, D.; et al. Uses of Bacteriophages as Bacterial Control Tools and Environmental Safety Indicators. Front. Microbiol. 2021, 12, 793135. [Google Scholar] [CrossRef]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as Solid Tumor Theragnostic Agents. Int. J. Mol. Sci. 2021, 23, 402. [Google Scholar] [CrossRef]
- Foglizzo, V.; Marchiò, S. Bacteriophages as Therapeutic and Diagnostic Vehicles in Cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef]
- Petrov, G.; Dymova, M.; Richter, V. Bacteriophage-Mediated Cancer Gene Therapy. Int. J. Mol. Sci. 2022, 23, 14245. [Google Scholar] [CrossRef]
- Ghaderi, S.S.; Riazi-Rad, F.; Qamsari, E.S.; Bagheri, S.; Rahimi-Jamnani, F.; Sharifzadeh, Z. Development of a human phage display-derived anti-PD-1 scFv antibody: An attractive tool for immune checkpoint therapy. BMC Biotechnol. 2022, 22, 22. [Google Scholar] [CrossRef]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.-G.; et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Wong, M.K.; Barbulescu, P.; Coburn, B.; Reguera-Nuñez, E. Therapeutic interventions and mechanisms associated with gut microbiota-mediated modulation of immune checkpoint inhibitor responses. Microbes Infect. 2021, 23, 104804. [Google Scholar] [CrossRef]
- Araya, D.V.; Quiroz, T.S.; Tobar, H.E.; Lizana, R.J.; Quezada, C.P.; Santiviago, C.A.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Deletion of a prophage-like element causes attenuation of Salmonella enterica serovar Enteritidis and promotes protective immunity. Vaccine 2010, 28, 5458–5466. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, R.; Wang, Y.; Zhu, M.; Zhang, X.; Dong, S.; Shi, H.; Wang, L. Recombinant Phage Elicits Protective Immune Response against Systemic S. globosa Infection in Mouse Model. Sci. Rep. 2017, 7, 42024. [Google Scholar] [CrossRef]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and their potential to modulate the microbiome and immunity. Cell. Mol. Immunol. 2020, 18, 889–904. [Google Scholar] [CrossRef]
- Mu, A.; McDonald, D.; Jarmusch, A.K.; Martino, C.; Brennan, C.; Bryant, M.; Humphrey, G.C.; Toronczak, J.; Schwartz, T.; Nguyen, D.; et al. Assessment of the microbiome during bacteriophage therapy in combination with systemic antibiotics to treat a case of staphylococcal device infection. Microbiome 2021, 9, 92. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Trovato, M.; Cuccaro, F.; Costa, V.; De Leo, M.G.; Marzullo, V.M.; Biondo, C.; D’Auria, S.; De Matteis, M.A.; et al. Antigen delivery by filamentous bacteriophage fd displaying an anti- DEC -205 single-chain variable fragment confers adjuvanticity by triggering a TLR 9-mediated immune response. EMBO Mol. Med. 2015, 7, 973–988. [Google Scholar] [CrossRef]
- Won, G.; Eo, S.K.; Park, S.-Y.; Hur, J.; Lee, J.H. A Salmonella Typhi ghost induced by the E gene of phage φX174 stimulates dendritic cells and efficiently activates the adaptive immune response. J. Veter. Sci. 2018, 19, 536–542. [Google Scholar] [CrossRef]
- Paule, A.; Frezza, D.; Edeas, M. Microbiota and Phage Therapy: Future Challenges in Medicine. Med. Sci. 2018, 6, 86. [Google Scholar] [CrossRef]
- Barr, J.J. Precision Engineers: Bacteriophages Modulate the Gut Microbiome and Metabolome. Cell Host Microbe 2019, 25, 771–773. [Google Scholar] [CrossRef]
- Baaziz, H.; Baker, Z.R.; Franklin, H.C.; Hsu, B.B. Rehabilitation of a misbehaving microbiome: Phages for the remodeling of bacterial composition and function. Iscience 2022, 25, 104146. [Google Scholar] [CrossRef]
| (a) Summary of the Microbial Taxonomic Units Associated with Decreased irAEs | |||||
|---|---|---|---|---|---|
| Bacteria Decreasing ICI Toxicity | Associated Disease/Intervention | Reported Adverse Reactions | Microbiome Analysis Method | Patient Number | Reference |
| Bacteroidetes (fragilis, Barnesiellaceae, ikenellaceae, Bacteroidaceae) | Kidney/Lung/Ovary/Stomach Cancer | Diarrhea, Bloody Stool | 16s rRNA | 30 | [141] |
| Akkermansia muciniphilia | Metastatic Melanoma | None | 16s rRNA | 42 | [8] |
| Metastatic Non-small Cell Lung Cancer | Pneumonia, Pneumonitis, Bronchopleural fistula | 16s V4 rRNA | 53 | [142] | |
| Bifidobacterium | Lung Cancer/PD-L1/Anti-PD-L1 | Colitis, Myositis Rash, Thrombocytopenia, Pneumonitis | 16s rRNA | 13 | [143] |
| Metastatic Melanoma | None | 16s rRNA | 42 | [8] | |
| Faecalibacterium | Metastatic Melanoma/Anti-CTLA-4 | Colitis | 16s rRNA | 26 | [134] |
| Lactobacillus | Metastatic Melanoma | None | 16s rRNA | 42 | [7,8] |
| Ruminococcaceae | Solid Tumors/Nivolumab + Ipilimumab | Pneumonia, Pneumonitis, Bronchopleural fistula | 16s V4 rRNA | 53 | [142] |
| Malignant Melanoma | None | 16s rRNA | 112 | [7] | |
| Burkholderia cepacia | Malignant Melanoma/Lung Cancer/GI tumors | Colitis | N/A | None | [144] |
| Dorea formicigenerans | Malignant Melanoma/Lung Cancer/GI tumors | Colitis | N/A | None | [144] |
| Caloramator coolhaasii | Cutaneous Melanoma, Lung Cancer | Colitis, Ileal Damage | 16s rRNA; Shotgun sequenceing | 77 | [126] |
| Anaerococcus vaginalis ATCC 51170 | Cutaneous Melanoma, Lung Cancer | Colitis, Ileal Damage | 16s rRNA; Shotgun sequenceing | 77 | [126] |
| Anaerotignum lactifermentans | Cutaneous Melanoma, Lung Cancer | Colitis, Ileal Damage | 16s rRNA; Shotgun sequenceing | 77 | [126] |
| Geosprorobacter unclassified | Cutaneous Melanoma, Lung Cancer | Colitis, Ileal Damage | 16s rRNA; Shotgun sequenceing | 77 | [126] |
| Proteobacteria Desulfovibrio | Lung Cancer/PD-L1/Anti-PD-L1 | Colitis, Myositis Rash, Thrombocytopenia, pneumonitis | 16s rRNA | 13 | [143] |
| Acinetobacter spp. | Solid Tumors (Non-Small Cell Lung Cancer (NSCLC), Small Cell Lung Cancer (SCLC), Hepatocellular Carcinoma (HCC), and Renal Cell Carcinoma (RCC) / N/A) | Colitis, Mysoitis, Neurologic, ICI Efficacy | N/A | 433 (across multiple studies) | [6] |
| Collinsella aerofaciens | Metastatic Melanoma | None | 16s rRNA | 42 | [8] |
| Enterococcus faecium | Metastatic Melanoma | None | 16s rRNA | 42 | [8] |
| Dialister unclassified | Non-Small Cell Lung Cancer | Colitis | 16s rRNA | 1010 | [145] |
| (b) Summary of the Microbial Taxonomic Units Associated with Increased irAEs | |||||
| Bacteria Increasing ICI Toxicity | Associated Disease/Intervention | Reported Adverse Reactions | Microbiome Analysis Method | Patient Number | Reference |
| Faecalibacterium prausnitzii and Gemmiger formicilis | Metastatic Melanoma | Colitis | Shotgun Sequencing; 16 s rRNA | 34 | [146] |
| Klebsiella pneumoniae | Metastatic Melanoma | N/A | 16s rRNA | 42 | [8] |
| Proteus Mirabilis | Solid Tumors (Cutaneous Melanoma/Non-small Cell Lung Cancer)/Anti-PD1/PDL1/Anti-CTLA4 | Rash, Respiratory, Genitourinary, Bacteremia | Routine Clinical Bacterium Testing | 327 | [147] |
| Lachnospiraceae, Streptococcus spp. | Melanoma | Adrenal, Arthritis, Dermatologic, Colitis, Hepatitis, Neurologic, Thyroid, Pneumonitis | 16s rRNA | 57 | [133] |
| Faecalibacterium | Solid Tumors (Cutaneous Melanoma/Non-small cell lung cancer)/Anti-PD1/PDL1/Anti-CTLA4 | Rash, Respiratory, Genitourinary, Bacteremia | Routine Clinical Microbial Test | 327 | [147] |
| Bacteroides Intestinalis | Melanoma/Anti-CTLA-4 | Colitis | 16s rRNA | 26 | [134] |
| Intestinibacter bartlettii | Cutaneous Melanoma, Lung Cancer | Colitis, Ileal Damage | 16s rRNA | 77 | [127] |
| Firmicutes | Melanoma | Colitis | 16s rRNA | 26 | [134,148] |
| Parabacteroides ditasonis | Melanoma | Colitis | N/A | N/A | [5] |
| Faecalibacterium prausnitzii and Gemmiger formicilis | Melanoma | Colitis | 16s rRNA | 26 | [134,148] |
| Metabolite/Metabolic Pathway | Disease/Model | Sample | Results | Reference |
|---|---|---|---|---|
| Butyrate | Mouse model | Stool | Low butyrate contributed to ICI-induced cardiotoxicity | [154] |
| Butyrogenesis | Metastatic melanoma | Stool, serum | Low butyrogenesis in patients with severe ICI- adverse effects Serum butyrate did not change | [155] |
| 3-IAld | Mouse model | Stool, serum | 3-IAld supplementation to mice with ICI-induced colitis protected from intestinal damage | [162] |
| TMAO | TNBC | Plasma | High plasma TMAO associated with longer PFS | [164] |
| Acetic acid Propionic acid Butyric acid Valeric acid Isovaleric acid | Solid tumors | Stool, Plasma | High SCFAs in feces (acetic acid, propionic acid, butyric acid and valeric acid) and plasma (isovaleric acid) associated with longer PFS | [169] |
| Propionate Nicotinic acid Lysine | Lung cancer | Stool | Low levels in patients with early progression | [170] |
| 2-Pentanone Tridecane p-cresol | Lung cancer | Stool | High levels in patients with early progression | [170] |
| Butyrate Propionate | Metastatic melanoma | Serum | Low levels associated with longer PFS | [172] |
| Polyamine transport system B vitamins biosynthesis | Metastatic melanoma | Stool | Abundant in colitis free patients | [146] |
| B vitamins biosynthesis Purine degradation Amino acid synthesis | Metastatic melanoma | Stool | Reduced in ICI-non responders who experienced severe adverse effects | [155] |
| B vitamins biosynthesis | Metastatic melanoma | Stool | Associated with shorter PFS | [174] |
| Fatty acid synthesis Inositol phosphate metabolism | Metastatic melanoma | Stool | Abundant in responders | [175] |
| Fatty acid synthesis Acarbose synthesis Polyketide sugar unit synthesis | Hepatobiliary cancer | Stool | Abundant in responders | [176] |
| Amino acid synthesis (arginine) | Hepatobiliary cancer | Stool | Abundant in non-responders | [176] |
| Carbohydrate metabolism Methanogenesis | Hepatocellular carcinoma | Stool | Abundant in responders | [177] |
| Methanogenesis | Metastatic melanoma | Stool | Abundant in non-responders | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dora, D.; Bokhari, S.M.Z.; Aloss, K.; Takacs, P.; Desnoix, J.Z.; Szklenárik, G.; Hurley, P.D.; Lohinai, Z. Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 2769. https://doi.org/10.3390/ijms24032769
Dora D, Bokhari SMZ, Aloss K, Takacs P, Desnoix JZ, Szklenárik G, Hurley PD, Lohinai Z. Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. International Journal of Molecular Sciences. 2023; 24(3):2769. https://doi.org/10.3390/ijms24032769
Chicago/Turabian StyleDora, David, Syeda Mahak Zahra Bokhari, Kenan Aloss, Peter Takacs, Juliane Zsuzsanna Desnoix, György Szklenárik, Patrick Deniz Hurley, and Zoltan Lohinai. 2023. "Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy" International Journal of Molecular Sciences 24, no. 3: 2769. https://doi.org/10.3390/ijms24032769
APA StyleDora, D., Bokhari, S. M. Z., Aloss, K., Takacs, P., Desnoix, J. Z., Szklenárik, G., Hurley, P. D., & Lohinai, Z. (2023). Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy. International Journal of Molecular Sciences, 24(3), 2769. https://doi.org/10.3390/ijms24032769









