Abstract
Glutathione S-transferase alpha 2 (GSTA2), a member of the glutathione S-transferase family, plays the role of cellular detoxification against oxidative stress. Although oxidative stress is related to ischemic injury, the role of GSTA2 against ischemia has not been elucidated. Thus, we studied whether GSTA2 prevents ischemic injury by using the PEP-1-GSTA2 protein which has a cell-permeable protein transduction domain. We revealed that cell-permeable PEP-1-GSTA2 transduced into HT-22 cells and markedly protected cell death via the inhibition of reactive oxygen species (ROS) production and DNA damage induced by oxidative stress. Additionally, transduced PEP-1-GSTA2 promoted mitogen-activated protein kinase (MAPK), and nuclear factor-kappaB (NF-κB) activation. Furthermore, PEP-1-GSTA2 regulated Bcl-2, Bax, cleaved Caspase-3 and -9 expression protein levels. An in vivo ischemic animal model, PEP-1-GSTA2, markedly prevented the loss of hippocampal neurons and reduced the activation of microglia and astrocytes. These findings indicate that PEP-1-GSTA2 suppresses hippocampal cell death by regulating the MAPK and apoptotic signaling pathways. Therefore, we suggest that PEP-1-GSTA2 will help to develop the therapies for oxidative-stress-induced ischemic injury.
1. Introduction
The multifunctional protein Glutathione S-transferase (GST) plays a key role in cellular detoxification and signal transduction [1,2]. Mammalian cytosolic GSTs have been identified such as alpha, theta, zeta, omega, sigma, pi and mu, and alpha class GSTs are the most abundant in mammalians [3,4]. Among the alpha classes, GST alpha 1 (GSTA1) and GST alpha 2 (GSTA2) are highly expressed in the liver and breast [5,6].
GSTA2 has a protective function against oxidative stress as one of the functional antioxidant response elements and Bousova et al. reported that the overexpression of GSTA2 showed a compensatory function against depending on the elevation of oxidative stress, whereas a reduction in GSTA2 impaired the defense system against oxidative stress [7,8]. Additionally, several studies have shown that GSTA2 inhibited the cell death of human erythroleukemia and lens epithelial cells, and these reports indicate that GSTA2 plays a key role in cellular detoxification under oxidative stress [9,10,11,12,13].
ROS are overexpressed under oxidative stress which leads to cell death due to lipid peroxidation, DNA, carbohydrate and protein damages [14,15,16], and the overproduction of ROS is related to neuronal diseases via the reaction with the apoptotic cell signaling pathway [17,18,19,20]. MAPK signaling is activated by ROS and involved in cell survival and apoptosis [21,22,23,24]. Therefore, it is important to study the regulation of oxidative stress and the MAPK signaling pathways for protecting neuronal cell damage.
The protein transduction domain’s (PTD) ability to deliver proteins into cells across the cell membrane and blood–brain barrier (BBB) without toxicity [25,26] was explored, and various PTD-attached proteins were used to investigate the functional roles [25,26,27,28,29,30,31,32,33,34,35]. We showed that the cell-permeable PEP-1-GSTA2 fusion protein protected against hippocampal neuronal cell death in HT-22 cells and an ischemic animal model.
2. Results
2.1. Preparation and Cell Permeation of Fusion Protein
To construct PEP-1-GSTA2 plasmid, the human GSTA2 gene was cloned in the PEP-1 expression vector. Figure 1A shows that the PEP-1-GSTA2 expression vector consists of six histidine, PEP-1-peptide and cDNA of human GSTA2. After transformation of the PEP-1-GSTA2 plasmid, overexpressed protein was purified and confirmed (Figure 1B,C).
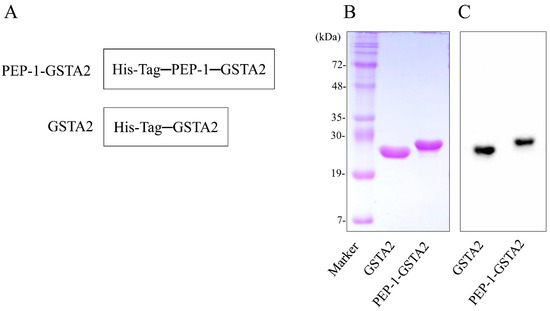
Figure 1.
Purification of PEP-1-GSTA2 protein. Diagrams of PEP-1-GSTA2 and control GSTA2 proteins (A). Purified PEP-1-GSTA2 and control GSTA2 proteins were identified by 15% SDS-PAGE (B) and were detected via Western blotting using an anti-histidine antibody (C).
To assess whether PEP-1-GSTA2 could transduce into cells, HT-22 cells were treated with different concentrations (0.5–3 μM) for 60 min of PEP-1-GSTA2 or for different times (10–60 min) of PEP-1-GSTA2 (3 μM), as shown in Western blot analysis presented in Figure 2. The PEP-1-GSTA2 protein transduces HT-22 cells and existed for up to 12 h, while no band was detected in the cells treated with GSTA2 protein without PEP-1 peptide. Additionally, the distributions of PEP-1-GSTA2 in the cells were determined using immunofluorescence staining (Figure 3A). Extensive green fluorescence was observed in both the cytoplasm and nucleus in the PEP-1-GSTA2-treated cells.
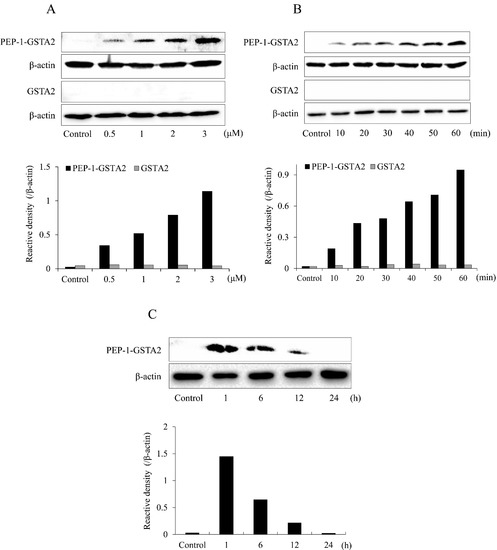
Figure 2.
Transduction of PEP-1-GSTA2 proteins into HT-22 cells. The cell culture media were treated with PEP-1-GSTA2 protein at different doses (0.5–3 μM) or control GSTA2 protein for 1 h (A). The cell culture media were treated with PEP-1-GSTA2 proteins (3 μM) or control GSTA2 protein for different time periods (10–60 min) (B). Intracellular stability of transduced PEP-1-GSTA2 protein. HT-22 cell culture media were incubated for 24 h after transduction of PEP-1-GSTA2 protein for 1 h (C). Then, transduction of PEP-1-GSTA2 protein was assessed via Western blotting using an anti-histidine antibody and the intensity of the bands was measured using a densitometer.
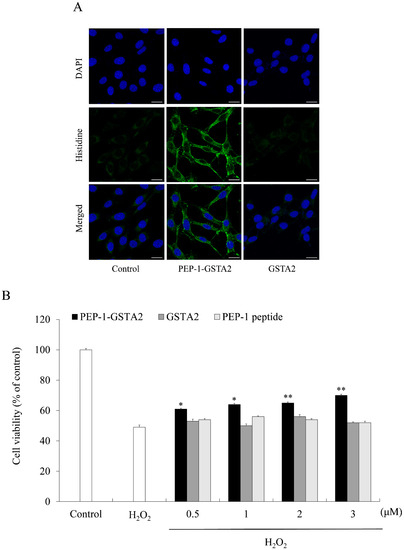
Figure 3.
Effects of transduced PEP-1-GSTA2 protein against H2O2-induced cell viability. HT-22 cell culture media were treated with PEP-1-GSTA2 protein (3 μM) or control GSTA2 protein for 1 h. Cellular localization of transduced PEP-1-GSTA2 proteins was confirmed via fluorescence microscopy (A). Scale bar = 20 μm. Effect of transduced PEP-1-GSTA2 protein on cell viability. HT-22 cells were pretreated with PEP-1-GSTA2 protein or control GSTA2 (0.5–3 μM) for 1 h and exposed to H2O2 (600 μM) for 2 h. Then, cell viability was assessed via MTT assay (B). * p < 0.05 and ** p < 0.01 compared with H2O2-treated cells.
2.2. Effects of PEP-1-GSTA2 on Oxidative Stress
To evaluate the effects of PEP-1-GSTA2 on cell viability, cell viability was determined using an MTT assay. Figure 3B showed that being exposed only to H2O2 markedly reduced HT-22 cell viability to 49% of control cells. However, treatment with PEP-1-GSTA2 increased cell viability up to 70%, while treatment with GSTA2 had no effect at all.
In order to investigate the effects of PEP-1-GSTA2 on ROS production and DNA damage in cells, DCF-DA and TUNEL staining were performed. The results showed that ROS and DNA damage were reduced after treatment of PEP-1-GSTA2, while treatment with GSTA2 had no effect (Figure 4).
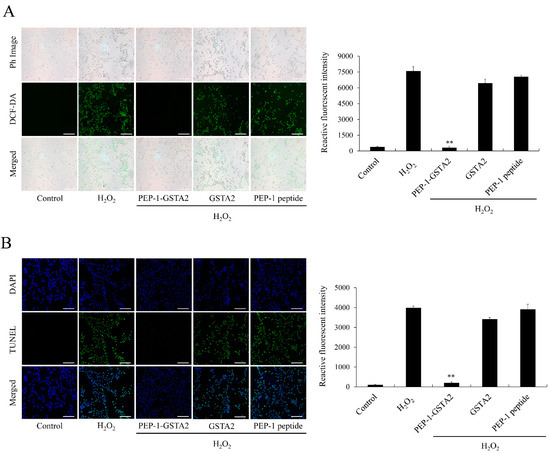
Figure 4.
Effects of PEP-1-GSTA2 protein against H2O2-induced ROS production and DNA fragmentation. HT-22 cells were treated with PEP-1-GSTA2 protein (3 μM) or control GSTA2 protein for 1 h before treatment with 600 μM H2O2 for 1 h or 4 h. Then, intracellular ROS levels (A) and DNA fragmentation (B) were determined via DCF-DA and TUNEL staining. Fluorescence intensity was quantified using an ELISA plate reader. Scale bar = 50 μm. ** p < 0.01 compared with H2O2-treated cells.
2.3. PEP-1-GSTA2-Activated Phosphorylation of Akt, MAPKs and p65
MAPKs and NF-κB phosphorylation are highly involved with oxidative-stress-induced ROS and lead to cell death [36,37]. We evaluated the expression of Akt, MAPKs and p65 to determine the signaling mechanism of PEP-1-GSTA2 in HT-22 cells. Akt, MAPKs and p65 phosphorylated levels were increased, whereas PEP-1-GSTA2 significantly reduced those levels in the H2O2 exposed cells. However, GSTA2 and PEP-1 peptide showed a similar pattern with cells exposed to H2O2 alone (Figure 5).
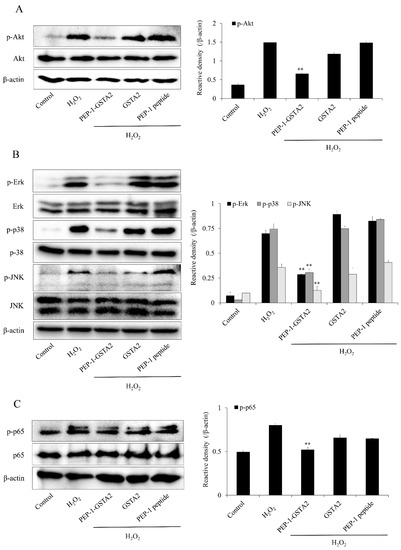
Figure 5.
Effects of PEP-1-GSTA2 protein on H2O2-induced MAPK activation in HT-22 cells. The cells were treated with PEP-1-GSTA2 protein (3 μM) or control GSTA2 protein for 1 h before being exposed to H2O2 (600 μM). Akt (A), MAPK (B) and NF-κB (C) activation were analyzed via Western blotting. Band intensity was measured using a densitometer. ** p < 0.01, compared with H2O2-treated cells.
2.4. PEP-1-GSTA2 Regulated Bcl-2, Bax, Cleaved Caspase-3 and -9 Expressions
To further evaluate whether the PEP-1-GSTA2 protein inhibited apoptosis, we assessed the Bax, Bcl-2, cleaved Caspase-3 and -9 expression in cells. As shown in Figure 6, PEP-1-GSTA2 markedly reduced these protein expression levels. In addition, this fusion protein increased the Bcl-2 expression level. However, GSTA2 and PEP-1 peptide had no effect on apoptosis.
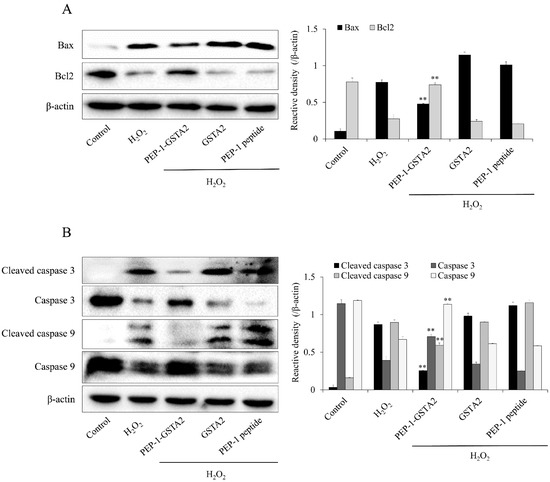
Figure 6.
Effects of PEP-1-GSTA2 protein against H2O2-induced Bcl-2, Bax, Caspase 3 and Caspase 9 expression in HT-22 cells. One hour pretreatment of HT-22 cells with PEP-1-GSTA2 protein (3 μM), control GSTA2 protein (3 μM) or PEP-1 peptide (3 μM) was followed by treatment with H2O2 (600 μM). The expression levels of Bcl-2 and Bax (A), caspase-3 and caspase-9 (B) were determined via Western blot analysis and band intensity was measured using a densitometer. ** p < 0.01 compared with H2O2-treated cells.
2.5. Effects of PEP-1-GSTA2 in an Ischemic Injury Animal Model
To investigate whether PEP-1-GSTA2 could protect against an ischemic animal model, an immunostaining experiment was performed. As shown in Figure 7, PEP-1-GSTA2 was transduced into the CA1 region of the brain. However, there was no difference between mice treated with GSTA2 compared to controls. Furthermore, we observed that CV-positive cells were significantly reduced following ischemia–reperfusion, while PEP-1-GSTA2 drastically increased CV-positive cells.
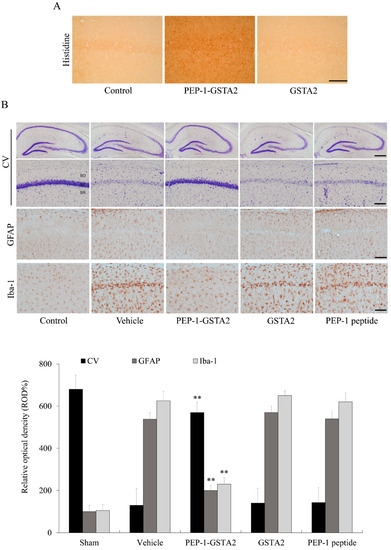
Figure 7.
Neuroprotective effects of PEP-1-GSTA2 protein against ischemic damage. Gerbils were treated with single injections of PEP-1-GSTA2 (2 mg/kg) protein and killed after 12 h. Transduction of PEP-1-GSTA2 into the CA1 region of the brain was determined via immunohistochemistry with an anti-histidine antibody (A). Scale bar = 100 μm. Gerbils were treated with single injections of PEP-1-GSTA2 (2 mg/kg) protein, control GSTA2 protein or PEP-1 peptide and killed after 7 days. Neuronal cell viability after ischemic insults was determined using CV, GFAP and Iba-1 immunostaining (B). Relative numeric analysis of CV, GFAP and Iba-1 positive neurons in the CA1 region. Scale bar = 400 and 50 μm. ** p < 0.01, significantly different from the vehicle group.
GFAP and Iba-1 staining showed that neuronal cells were markedly aggregated in the vehicle-treated group, whereas PEP-1-GSTA2 inhibited the aggregation of neuronal cells and similarly displayed the neuronal cells compared with the control group. There were no significant changes in neuronal cells found in GSTA2- and PEP-1-peptide-treated groups compared with the vehicle-treated group.
3. Discussion
GSTA2, one of the alpha classes of glutathione S-transferases, is known to be a major defensive protein against oxidative stress and lipid peroxidation [7,8], and this protein is predominantly expressed in the human liver [5,38]. Some reports have demonstrated that an overexpression of GSTA2 protected against cell damage by playing a role in defense under oxidative stress [8,9,10,11,12,13] and that this protein inhibited apoptosis and lipid peroxidation in K562 cells [11]. Recently, Liu et al. reported that capsaicin up-regulated GSTA2 in a Parkinson’s disease animal model and up-regulated GSTA2-inhibited apoptosis and cell death by regulating the autophagy and signaling pathways under oxidative stress. Therefore, the authors suggested that GSTA2 is promising as a therapeutic target for alleviating the progression of PD [39]. Another group also showed that the overexpression of GSTA2 has a protective effect against ROS-induced cell death in hepatocellular carcinoma (HCC) cells [40,41]. Since it is well known that ROS are associated with various diseases, these reports indicate that GSTA2 can be a main therapeutic target because this protein inhibits ROS-induced cell death via glutathione peroxidase activity [21,42,43,44].
The protein transduction domain (PTD) is a powerful tool to deliver the therapeutic protein into cells and tissues without side effects [25,26,45]. PEP-1 is a type of PTD and consists of 21 amino acids. In addition, PEP-1 PTD has some advantages of high stability, efficiency and rapid transduction with low toxicity [46,47]. The efficiency of the transduction of the PTD fusion protein depends on various factors, including the type of PTD, the size of the target protein, and cell types. Additionally, a problem to be solved with non-specific effects in animal experiments is that the intra-tissue delivery of the PTD fusion protein lacks target tissue specificity [26,31]. However, PTD offers an effective tool for transducing therapeutic target proteins into cells or tissues. Therefore, we constructed PEP-1-GSTA2 and showed that this fusion protein protected cell death. In K562 cells, overexpressed GSTA2 protected cells via a reduction in lipid peroxidation and cytotoxic effects in H2O2-exposed cells [10,11,12], and this protein significantly inhibited cataractogenesis in mice or lens epithelial cells under oxidative stress [48,49]. These reports suggest that GSTA2 has protective roles against the deleterious effects of oxidative stress.
It is well known that H2O2 induces neuronal cell death via the activation of Akt and MAPK signaling pathways [50,51]; however, the activation of Akt and MAPK are involved in cell survival in different cell types [52,53,54]. Therefore, we investigated the function of the signaling pathway of PEP-1-GSTA2 in H2O2-induced HT-22 cells and PEP-1-GSTA2 was shown to suppress Akt and MAPK phosphorylation. Consistent with our results, Lonicera japonica THUNB (LJ) suppressed the phosphorylated Akt and MAPKs in SH-SY5Y cells, suggesting that LJ protects against cell death induced by H2O2 [51]. It has been reported that the phosphorylation of Akt (ser-473) induced by oxidative stress leads to apoptosis and cell death [55]. In addition, the overexpression of GSTA2 in K562 cells caused by transfection significantly reduced JNK activation in H2O2-exposed cells [11].
The apoptotic activator (Bax) and inhibitor (Bcl-2) play important roles in cell death and the activations of Caspase-3 and Caspase-9 are known as makers of apoptotic cell death [56]. It has been reported that GSTs protect Caco-2 cells and hepatocytes cells via the inhibition of oxidative stress and apoptosis [10,57,58]. We also revealed that PEP-1-GSTA2 suppressed cell death via the regulation of apoptotic protein expression levels.
We further examined the effects of PEP-1-GSTA2 in an ischemic injury animal model. PEP-1-GSTA2 was delivered into the hippocampal CA1 region and protected against cell death in vivo. Other studies have already shown that the cell-permeable PTD fusion protein significantly reduced cell death in an animal model [59,60,61]. It has been reported that the activation of microglial and astrocytes is used as a marker for the detection of ischemic neuronal injury [62,63]. We showed that PEP-1-GSTA2 significantly inhibits ischemic injury by reducing the activation of microglia and astrocytes. The present result demonstrates that PEP-1-GSTA2 plays a protective role to prevent neuronal cell death in ischemia under oxidative stress.
In conclusion, we have reported here that PEP-1-GSTA2 was transduced into cells in vitro and in vivo and this protein significantly prevented neuronal cell death. Therefore, PEP-1-GSTA2 will help to develop the therapies for neuronal diseases including ischemic injury.
4. Materials and Methods
4.1. Purification of PEP-1-GSTA2 Proteins
To obtain the recombinant PEP-1-GSTA2 protein, the cDNA for human GSTA2 was cloned into the pET-15b expression vector as described previously [29]. Briefly, PEP-1-GSTA2 was constructed via PCR. The plasmid PEP-1-GSTA2 was transformed into E. coli BL21 (DE3) and induced by adding IPTG (Duchefa, Haarlem, The Netherlands). Then, PEP-1-GSTA2 was purified and the protein concentration was measured as described previously [29,64].
4.2. Cell Culture and Transduction of PEP-1-GSTA2
Cell culture, transduction of fusion protein and Western blot were performed via methods as described in a previous report [28,33,65].
4.3. Assessment of Cell Viability Using MTT Assay
A protective effect of PEP-1-GSTA2 against H2O2-induced cell death was determined by measuring the viability of cultured cells using an MTT assay (Abcam, Cambridge, MA, USA) [28,29,66]. The cells were divided as follows: (1) normal control cells; (2) only H2O2-treated cells; (3) H2O2 + proteins/peptide (0.5 μΜ)-treated cells; (4) H2O2 + proteins/peptide (1 μΜ)-treated cells; (5) H2O2 + proteins/peptide (2 μΜ)-treated cells; and (6) H2O2 + proteins/peptide (3 μΜ)-treated cells.
HT-22 cells were seeded at a density of 1 × 104 cells per well on a 96-well plate and incubated at 37 °C with 5% CO2 for 12 h. PEP-1-GSTA2 (0.5–3 μM) protein was treated for 1 h and washed three times with PBS and trypsin-EDTA. Then, H2O2 (600 μM) was treated for 6 h and the absorbance was measured at 570 nm using an ELISA microplate reader (Labsystems Multiskan MCC/340, Helsinki, Finland). Cell viability was expressed as a percentage of the normal control cells.
4.4. DCF-DA and TUNEL Staining
The levels of ROS and DNA damage were confirmed using DCF-DA (Sigma-Aldrich, St. Louis, MO, USA) and TUNEL (Roche Applied Science, Basel, Switzerland) staining as described in a previous report [28,29,67].
4.5. Experimental Animals
Male gerbils (65–75 g; 6 months old) were obtained from the Experimental Animal Center at Hallym University. All animal experiments were performed according to the ARRIVE guideline (https://www.nc3rs.org.uk/arrive-guidelines, accessed on July 2020) and approved by the Institutional Animal Care and Use Committee of Soonchunhyang University [SCH16-0004].
The ischemic injury animal models were prepared as described in a previous report [28,30].
4.6. Statistical Analysis
All statistical data used GraphPad Prism software (version 5.01; GraphPad Software Inc., San Diego, CA, USA). Values are shown as mean ± standard error of the mean from three experiments. Statistical comparisons between each group were performed using one-way analysis of variance with Bonferroni’s post hoc test. Difference at p < 0.05 was considered statistically significant.
Author Contributions
Y.J.C., M.J.S., W.S.E. and S.Y.C. conceived and designed the study. H.Y.J., D.W.K., G.S.Y., J.H.P., H.J.Y., E.J.Y. and H.J.K. performed experiments. L.R.L., N.Y.K. and S.Y.K. performed experiments and contributed the reagent. Y.-J.C., J.P., K.H.H., K.W.L., J.K.P., C.H.L., W.S.E. and S.Y.C. interpreted and analyzed the data. W.S.E. and S.Y.C. drafted the manuscript and provided the final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program (2019R1A6A1A11036849) through the National Research Foundation of Korea (NRF), funded by the Ministry of Education.
Institutional Review Board Statement
The animal study protocol was approved by Experimental Animal Center at Hallym University. All animal experiments were performed according to the ARRIVE guideline (https://www.nc3rs.org.uk/arrive-guidelines. Accessed on July 2020) and approved by the Institutional Animal Care and Use Committee of Soonchunhyang University [SCH16-0004].
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glisic, B.; Mihaljevic, I.; Popovic, M.; Zaja, R.; Loncar, J.; Fent, K.; Kovacevic, R.; Smital, T. Characterization of glutathione-S-transferases in zebrafish (Danio rerio). Aquat. Toxicol. 2015, 158, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, J.; Hou, N.; Yu, N.; Zhang, Y.; Liu, Z. Identification, genomic organization and expression pattern of glutathione transferase in Pardosa pseudoannulata. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100626. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.L.; Stauffer, G.; Gallagher, E.P. Induction of glutathione S-transferase activity and protein expression in brown bullhead (Ameiurus nebulosus) liver by ethoxyquin. Toxicol. Sci. 2001, 62, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione s-transferase supergene family: Regulation of gst and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef] [PubMed]
- Coles, B.F.; Kadlubar, F.F. Human alpha class glutathione Stransferases: Genetic polymorphism, expression, and susceptibility to disease. Methods Enzymol. 2005, 401, 9–42. [Google Scholar]
- Ahn, J.; Gammon, M.D.; Santella, R.M.; Gaudet, M.M.; Britton, J.A.; Teitelbaum, S.L.; Terry, M.B.; Neugut, A.I.; Eng, S.M.; Zhang, Y.; et al. Effects of glutathione Stransferase A1 (GSTA1) genotype and potential modifiers on breast cancer risk. Carcinogenesis 2006, 27, 1876–1882. [Google Scholar] [CrossRef]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular mechanism of Nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef]
- Bousova, I.; Kostakova, S.; Matouskova, P.; Bartikova, H.; Szotakova, B.; Skalova, L. Monosodium glutamate-induced obesity changed the expression and activity of glutathione S-transferases in mouse heart and kidney. Pharmazie 2017, 72, 257–259. [Google Scholar]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Sharma, R.; Yang, Y.; Sharma, A.; Awasthi, S.; Awasthi, Y.C. Antioxidant role of glutathione S-transferases: Protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox Signal. 2004, 6, 289–300. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.Z.; Singhal, S.S.; Saini, M.; Pandya, U.; Awasthi, S.; Awasthi, Y.C. Role of glutathione S-transferases in protection against lipid peroxidation. J. Biol. Chem. 2001, 276, 19220–19230. [Google Scholar] [CrossRef]
- Yang, Y.; Sharma, R.; Cheng, J.Z.; Saini, M.K.; Ansari, N.H.; Andley, U.P.; Awasthi, S.; Awasthi, Y.C. Protection of HLE B-3 Cells against Hydrogen Peroxide–and Naphthalene-Induced Lipid Peroxidation and Apoptosis by Transfection with hGSTA1 and hGSTA2. Investig. Ophthalmol. Vis. Sci. 2002, 43, 434–445. [Google Scholar]
- Tetlow, N.; Board, P.G. Functional polymorphism of human glutathione transferase A2. Pharmacogenetics 2004, 14, 111–116. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Sharma, R.K. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 2005, 60, 807–816. [Google Scholar] [CrossRef]
- Petito, C.K.; Torres-Munoz, J.; Roberts, B.; Olarte, J.P.; Nowak, T.S., Jr.; Pulsinelli, W.A. DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J. Cereb. Blood Flow Metab. 1997, 17, 967–976. [Google Scholar] [CrossRef]
- Frantseva, M.V.; Carlen, P.L.; Perez Velazquez, J.L. Dynamics of intracellular calcium and free radical production during ischemia in pyramidal neurons. Free Radic. Biol. Med. 2001, 31, 1216–1227. [Google Scholar] [CrossRef]
- Sayre, L.M.; Smith, M.A.; Perry, G. Chemistry and biochemistry of oxidative stress in neurodegenrative disease. Curr. Med. Chem. 2001, 8, 721–738. [Google Scholar] [CrossRef]
- Floyd, R.A. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990, 4, 2587–2597. [Google Scholar] [CrossRef]
- Guo, C.; Tong, L.; Xi, M.; Yang, H.; Dong, H.; Wen, A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar] [CrossRef]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Ang, Y.L.; Yong, W.P.; Tan, P. Translating gastric cancer genomics into targeted therapies. Crit. Rev. Oncol. Hematol. 2016, 100, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, L.; Qi, G.; Zhao, S.; Gao, Y.; Li, Y. Gypenoside Protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of mitogen-activated protein kinase mediated nuclear factor kappa B pathway in vitro and in vivo. Front. Pharmacol. 2016, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, J.; Zhang, Y.; Xiao, F.; Wang, J.; Gao, H.; Liu, Y.; Rong, S.; Yao, Y.; Xu, G.; et al. HMGB1-TLR4 signaling participates in renal ischemia reperfusion injury and could be attenuated by dexamethasone-mediated inhibition of the ERK/NF-kappaB pathway. Am. J. Transl. Res. 2016, 8, 4054–4067. [Google Scholar] [PubMed]
- Van den Berg, A.; Dowdy, S.F. Protein transduction domain delivery of therapeutic macromolecules. Curr. Opin. Biotechnol. 2011, 22, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Wadia, J.S.; Dowdy, S.F. Protein transduction technology. Curr. Opin. Biotechnol. 2002, 13, 52–56. [Google Scholar] [CrossRef]
- Zhou, G.; Shan, P.; Hu, X.; Zheng, X.; Zhou, S. Neuroprotective effect of TAT PTD-Ngb fusion protein on primary cortical neurons against hypoxia-induced apoptosis. Neurol. Sci. 2013, 34, 1771–1778. [Google Scholar] [CrossRef]
- Shin, M.J.; Kim, D.W.; Lee, Y.P.; Ahn, E.H.; Jo, H.S.; Kim, D.S.; Kwon, O.S.; Kang, T.C.; Cho, Y.J.; Park, J.; et al. Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic. Biol. Med. 2013, 67, 195–210. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, M.; Kim, D.W.; Shin, M.J.; Son, O.; Jo, H.S.; Yeo, H.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; et al. Transduced PEP-1-PON1 protein regulates microglial activation and dopaminergic neuronal death in a Parkinson’s model. Biomaterials 2015, 64, 45–56. [Google Scholar] [CrossRef]
- Yeo, H.J.; Shin, M.J.; Yeo, E.J.; Choi, Y.J.; Kim, D.W.; Kim, D.S.; Eum, W.S.; Choi, S.Y. Tat-CIAPIN1 inhibits hippocampal neuronal cell damage through the MAPK and apoptotic signaling pathways. Free Radic. Biol. Med. 2019, 135, 68–78. [Google Scholar] [CrossRef]
- Dietz, G.P. Cell-penetrating peptide technology to deliver chaperones and associated factors in diseases and basic research. Curr. Pharm. Biotechnol. 2010, 11, 167–174. [Google Scholar] [CrossRef]
- Kubo, E.; Fatma, N.; Akagi, Y.; Beier, D.R.; Singh, S.P.; Singh, D.P. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am. J. Physiol. Cell Physiol. 2008, 294, C842–C855. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, M.J.; Kim, D.W.; Yeo, H.J.; Yeo, E.J.; Choi, Y.J.; Sohn, E.J.; Han, K.H.; Park, J.; Lee, K.W.; et al. Tat-biliverdin reductase A exerts a protective role in oxidative stress-induced hippocampal neuronal cell damage by regulating the apoptosis and MAPK Signaling. Int. J. Mol. Sci. 2020, 21, 2672. [Google Scholar] [CrossRef]
- Shin, M.J.; Kim, D.W.; Choi, Y.J.; Cha, H.J.; Lee, S.H.; Park, J.; Han, K.H.; Eum, W.S.; Choi, S.Y. PEP-1-GLRX1 protein exhibits anti-inflammatory effects by inhibiting the activation of MAPK and NF-κB pathways in Raw 264.7 cells. BMB Rep. 2020, 53, 106–111. [Google Scholar] [CrossRef]
- Yeo, H.J.; Shin, M.J.; Kim, D.W.; Kwon, H.Y.; Eum, W.S.; Choi, S.Y. Tat-CIAPIN1 protein prevents against cytokine-induced cytotoxicity in pancreatic RINm5F β-cells. BMB Rep. 2021, 54, 458–463. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef]
- Rowe, J.D.; Nieves, E.; Listowsky, I. Subunit diversity and tissue distribution of human glutathione S-transferases: Interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem. J. 1997, 325, 481–486. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Zhao, Z.; Wang, J.; Guo, D.; Liu, Y. Regulation of Actg1 and Gsta2 is possible mechanism by which capsaicin alleviates apoptosis in cell model of 6-OHDA-induced Parkinson’s disease. Biosci. Rep. 2020, 40, BSR20191796. [Google Scholar] [CrossRef]
- Ng, K.T.; Yeung, O.W.; Lam, Y.F.; Liu, J.; Liu, H.; Pang, L.; Yang, X.X.; Zhu, J.Y.; Zhang, W.Y.; Lau, M.Y.; et al. Glutathion S-transferase A2 (GSTA2) promotes hepatocellular carcinoma recurrence after liver transplantation through modulating reactive oxygen species metabolism. Cell Death Discov. 2021, 7, 188. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, C.Y.; Yang, J.W.; Smith, C.; Kim, S.K.; Lee, E.Y.; Kim, S.G.; Kang, K.W. Induction of glutathione transferase in insulin-like growth factor type I receptor-overexpressed hepatoma cells. Mol. Pharmacol. 2007, 72, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.L.; Shi, Z.; Ou, X.; Wang, W.; Chen, X.; Liu, G. Transcriptional profiling of liver tissues in chicken embryo at day 16 and 20 using RNA sequencing reveals differential antioxidant enzyme activity. PLoS ONE 2018, 13, e0192253. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovich, V.V.; Vavilin, V.A.; Zenkov, N.K.; Menshchikova, E.B. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry 2006, 71, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef]
- Joliot, A.; Prochiantz, A. Transduction peptides: From technology to physiology. Nat. Cell Biol. 2004, 6, 189–196. [Google Scholar] [CrossRef]
- Luo, X.G.; Ma, D.Y.; Wang, Y.; Li, W.; Wang, C.X.; He, Y.Y.; Gu, X.C.; Li, X.M.; Zhou, H.; Zhang, T.C. Fusion with pep-1, a cell-penetrating peptide, enhances the transmembrane ability of human epidermal growth factor. Biosci. Biotechnol. Biochem. 2016, 80, 584–590. [Google Scholar] [CrossRef]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivatava, S.K.; Piper, J.T.; Singhal, S.S.; Chaubey, M.; Awasthi, Y.C. Curcumin protects against 4-hydroxy-2-trans-nonenal–induced cataract formation in rat lenses. Am. J. Clin. Nutr. 1996, 64, 761–766. [Google Scholar] [CrossRef]
- Pandya, U.; Chandra, A.; Awasthi, S.; Jin, G.F.; Piper, J.T.; Godley, B.F.; Awasthi, Y.C. Attenuation of galactose cataract by low levels of dietary curcumin. Nutr. Res. 2000, 20, 515–526. [Google Scholar] [CrossRef]
- Lahair, M.M.; Howe, C.J.; Rodriguez-Mora, O.; McCubrey, J.A.; Franklin, R.A. Molecular pathways leading to oxidative stress-induced phosphorylation of Akt. Antioxid. Redox Signal. 2006, 8, 1749–1756. [Google Scholar] [CrossRef]
- Kwon, S.H.; Hong, S.I.; Kim, J.A.; Jung, Y.H.; Kim, S.Y.; Kim, H.C.; Lee, S.Y.; Jang, C.G. The neuroprotective effects of Lonicera japonica THUNB. against hydrogen peroxide-induced apoptosis via phosphorylation of MAPKs and PI3K/Akt in SH-SY5Y cells. Food Chem. Toxicol. 2011, 49, 1011–1019. [Google Scholar] [CrossRef]
- Hwang, S.L.; Yen, G.C. Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J. Agric. Food Chem. 2009, 57, 2576–2582. [Google Scholar] [CrossRef]
- Yang, B.; Oo, T.N.; Rizzo, V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006, 20, 1501–1503. [Google Scholar] [CrossRef]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of EKR1/2 in H2O2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef]
- Yano, S.; Morioka, M.; Fukunaga, K.; Kawano, T.; Hara, T.; Kai, Y.; Hamada, J.; Miyamoto, E.; Ushio, Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J. Cereb. Blood Flow Metab. 2001, 21, 351–360. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Romero, L.; Andrews, K.; Ng, L.; O’Rourke, K.; Maslen, A.; Kirby, G. Human GSTA1-1 reduces c-Jun N-terminal kinase signaling and apoptosis in Caco-2 cells. Biochem. J. 2006, 400, 135–141. [Google Scholar] [CrossRef]
- Gilot, D.; Loyer, P.; Corlu, A.; Glaise, D.; Lagadic-Gossmann, D.; Atfi, A.; Morel, F.; Ichijo, H.; Guguen-Guillouzo, C. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J. Biol. Chem. 2002, 277, 49220–49229. [Google Scholar] [CrossRef]
- Pradeep, H.; Diya, J.B.; Shashikumar, S.; Rajanikant, G.K. Oxidative stress-assassin behind the ischemic stroke. Folia. Neuropathol. 2012, 50, 219–230. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, Q.; Liu, X.; Hu, W.; Wang, Y. Neuroprotective effect of TAT-14-3-3ε fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS ONE 2014, 26, e93334. [Google Scholar] [CrossRef]
- Lim, K.S.; Cha, M.J.; Kim, J.K.; Park, E.J.; Chae, J.W.; Rhim, T.; Hwang, K.C.; Kim, Y.H. Protective effects of protein transduction domain-metallothionein fusion proteins against hypoxia- and oxidative stress-induced apoptosis in an ischemia/reperfusion rat model. J. Control. Release 2013, 169, 306–312. [Google Scholar] [CrossRef]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2001, 32, 1208–1215. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Park, J.; Rahman, S.; Jin, J.; Choi, I.; Kim, J. Adipogenic function of tetranectin mediated by enhancing mitotic clonal expansion via ERK signaling. BMB Rep. 2021, 54, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Cho, I.J.; Kim, E.O.; Lee, D.G.; Jung, D.H.; Ki, S.H.; Ku, S.K.; Kim, S.C. Hemistepsin A inhibits T0901317-induced lipogenesis in the liver. BMB Rep. 2021, 54, 106–111. [Google Scholar] [CrossRef]
- Koo, B.H.; Lee, J.; Jin, Y.; Lim, H.K.; Ryoo, S. Arginase inhibition by rhaponticin increases L-arginine concentration that contributes to Ca2+-dependent eNOS activation. BMB Rep. 2021, 54, 516–521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).