Stimuli-Responsive Boron-Based Materials in Drug Delivery
Abstract
1. Introduction
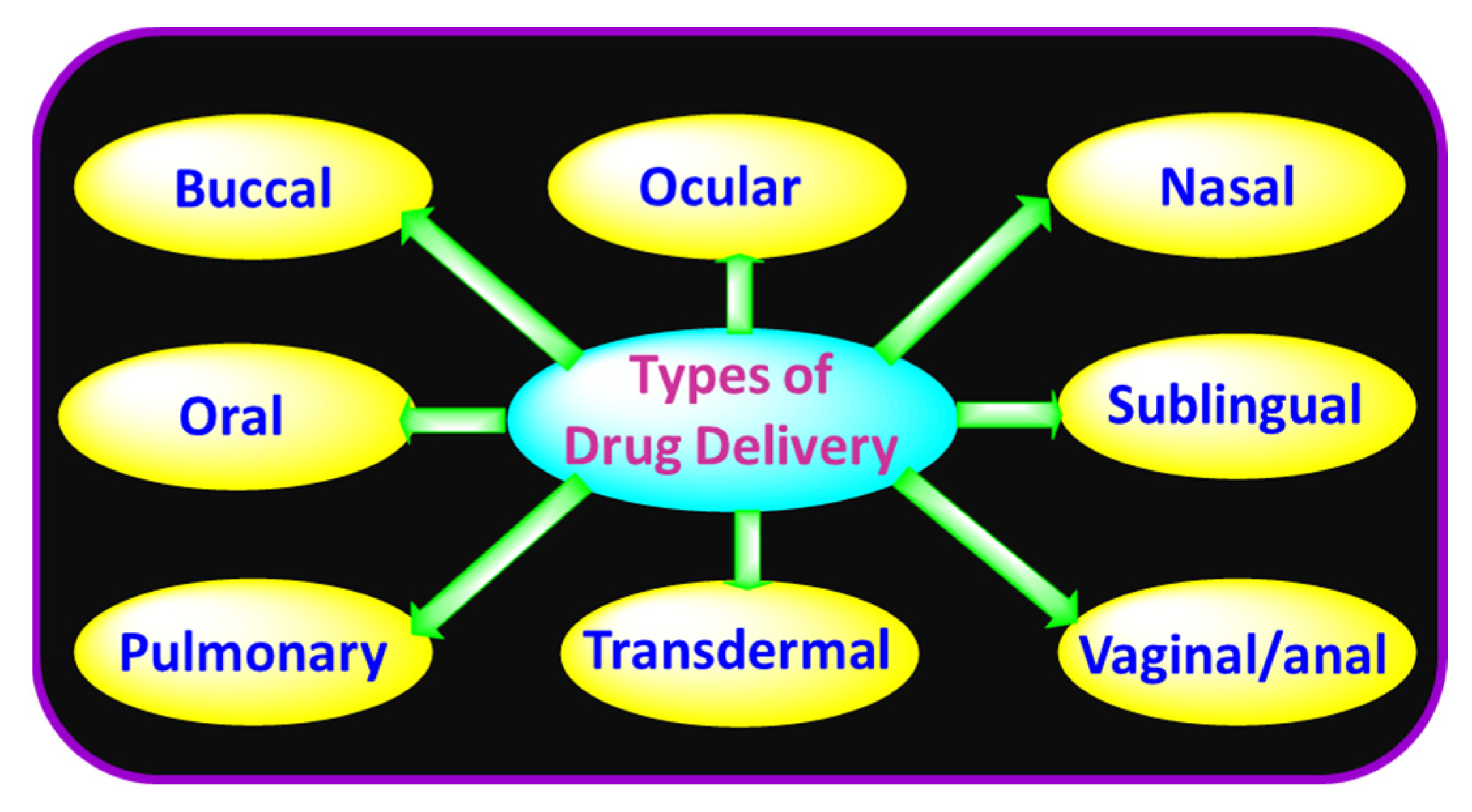
2. Types of Drug Delivery Methods
2.1. Buccal Drug Delivery
2.2. Nasal Drug Delivery
2.3. Ocular Drug Delivery
2.4. Oral Drug Delivery
2.5. Pulmonary Drug Delivery
2.6. Sublingual Drug Delivery
2.7. Transdermal Drug Delivery
2.8. Vaginal/Anal Drug Delivery
3. Types of Materials Used in Drug Delivery
3.1. Polymers
3.2. Nanoparticles
3.3. Nanocapsules
3.4. Nanotubes
3.5. Nanogels
3.6. Dendrimers
3.7. Novel Systems
4. Controlling Parameters for DDS
4.1. Drug Loading Strategies
4.1.1. Covalent Bonding
4.1.2. Non-Covalent Adsorption
4.1.3. Drug Encapsulation
5. Boron Nitride in Drug Delivery
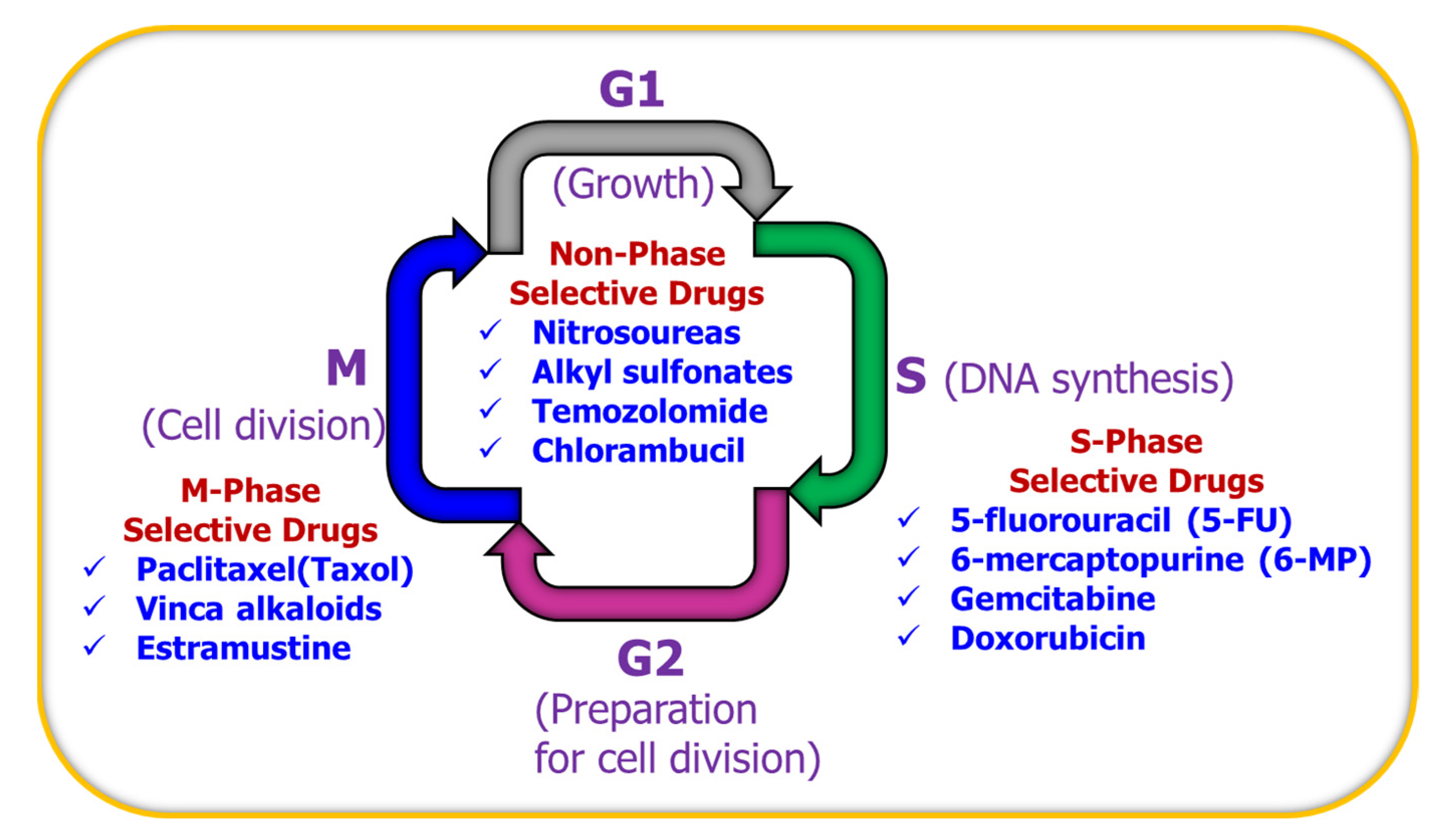
Anticancer Drug Delivery
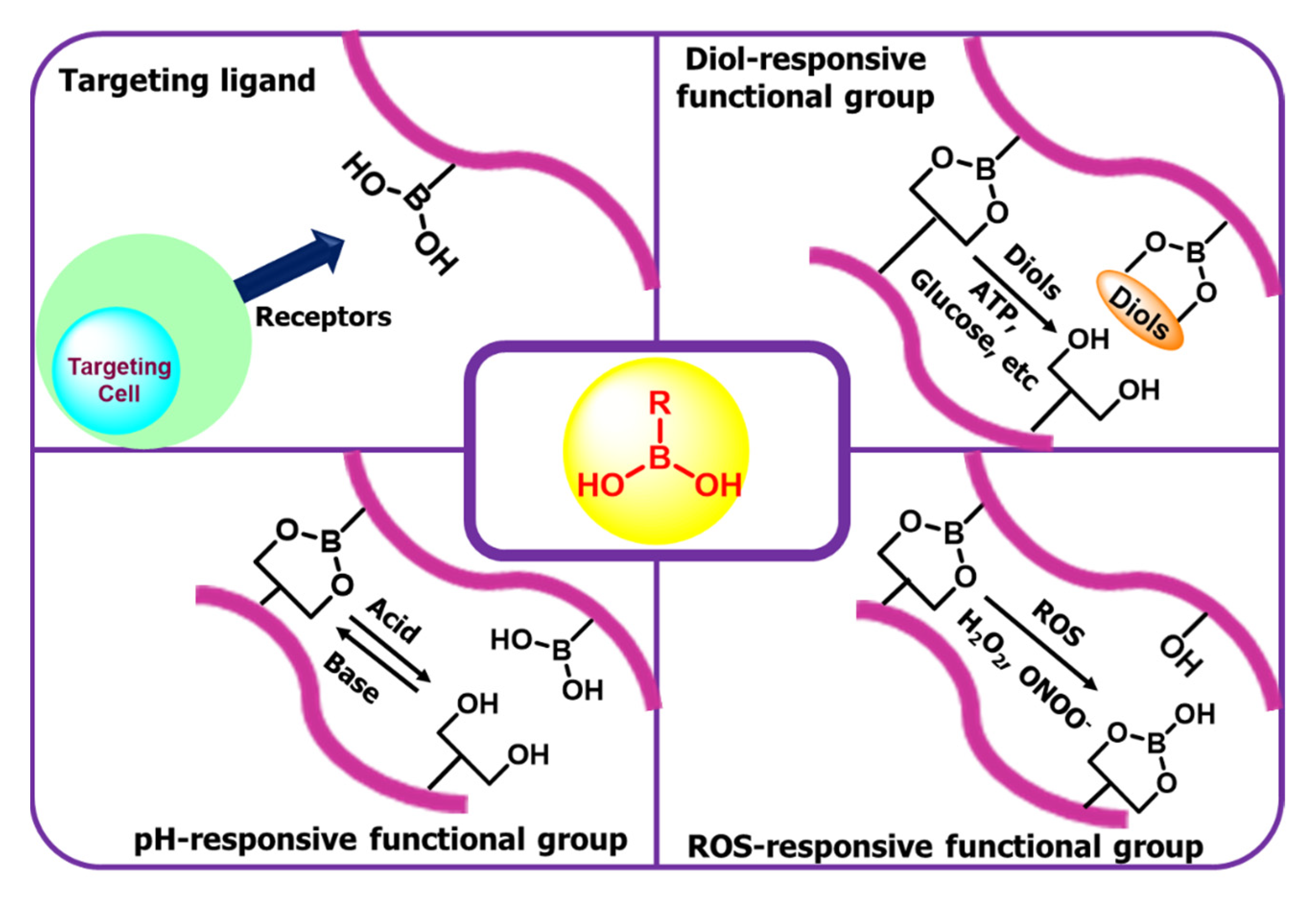
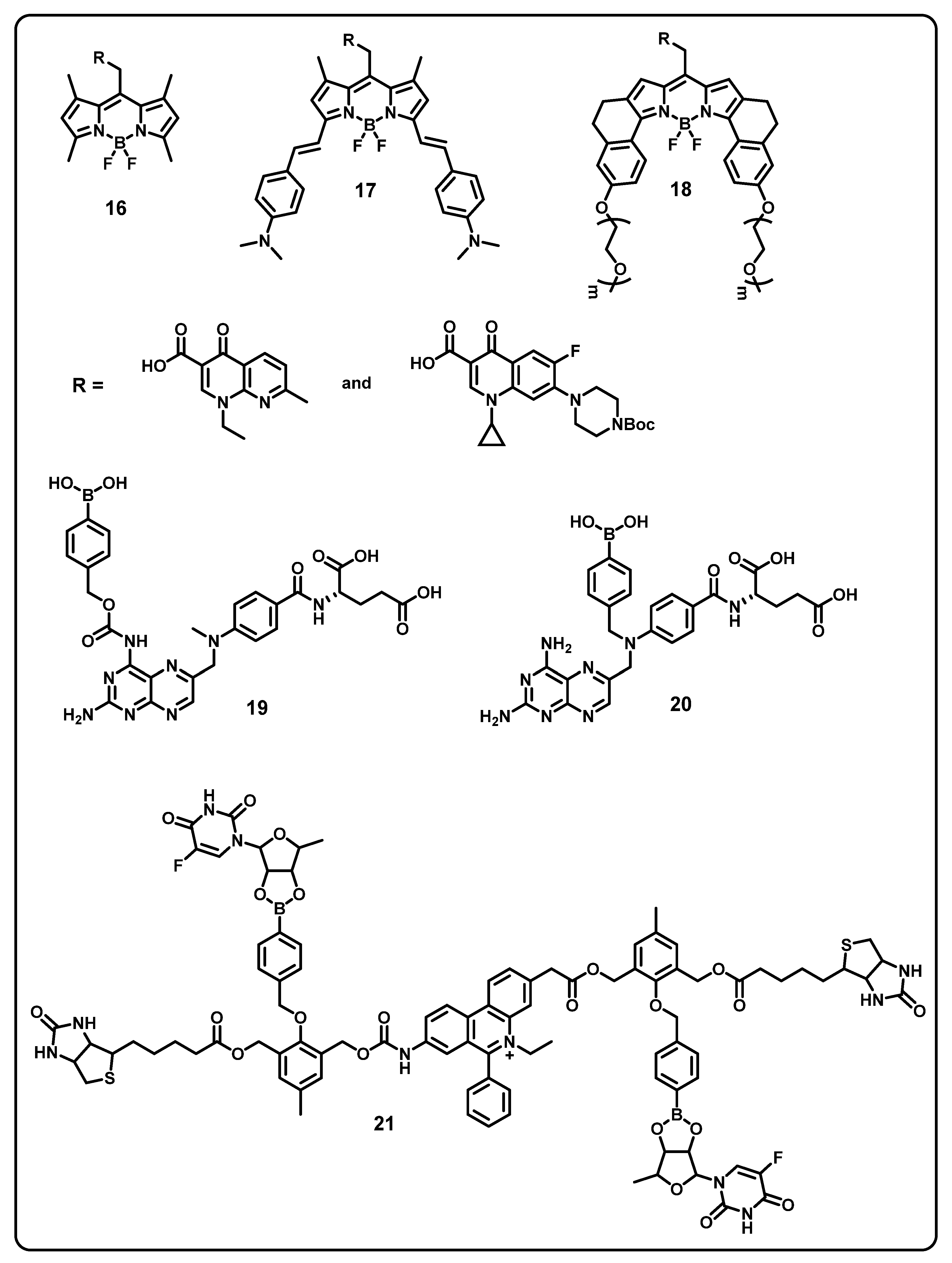
6. Boronic Acid-Based Drug Delivery
Insulin Delivery
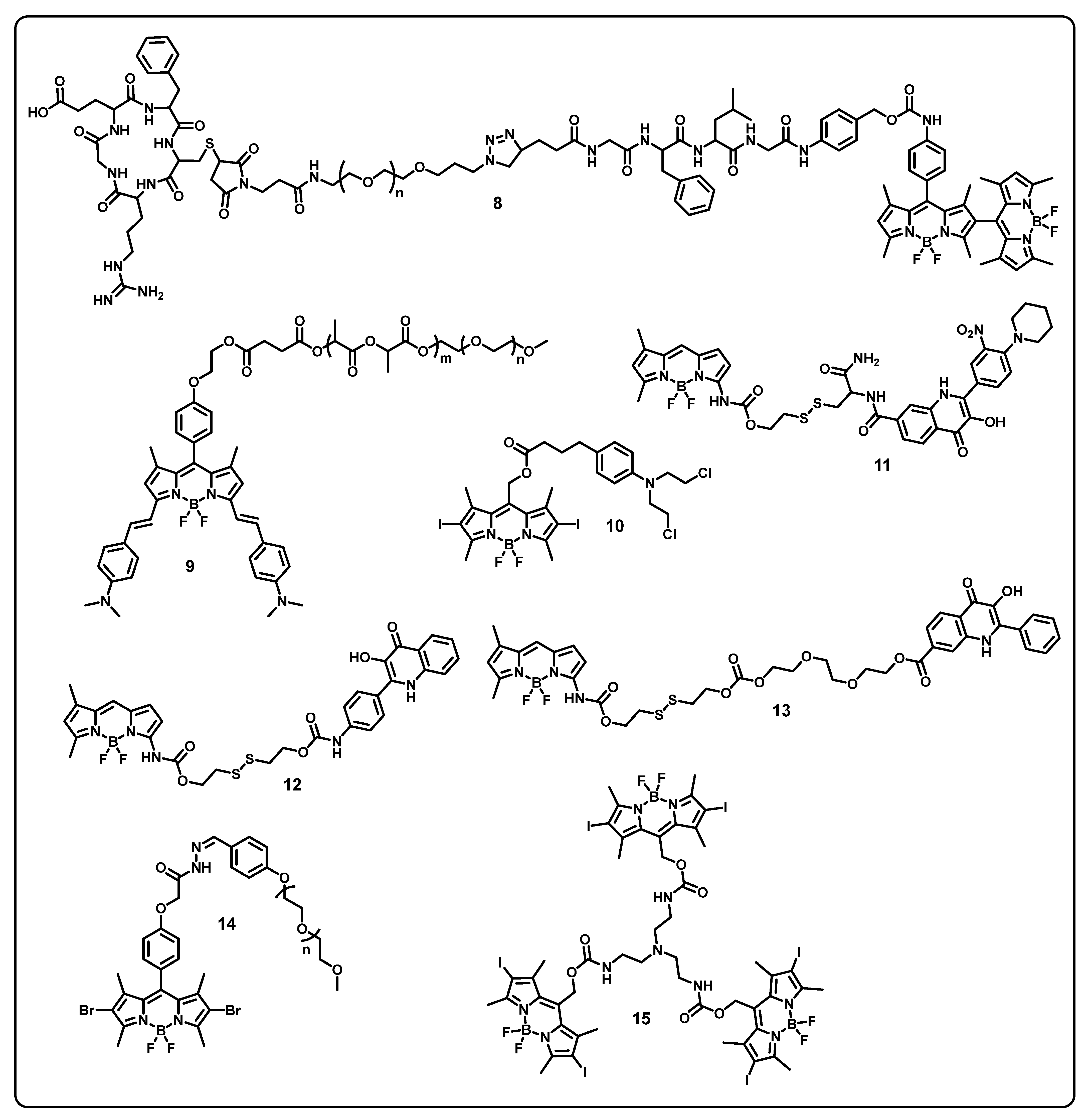
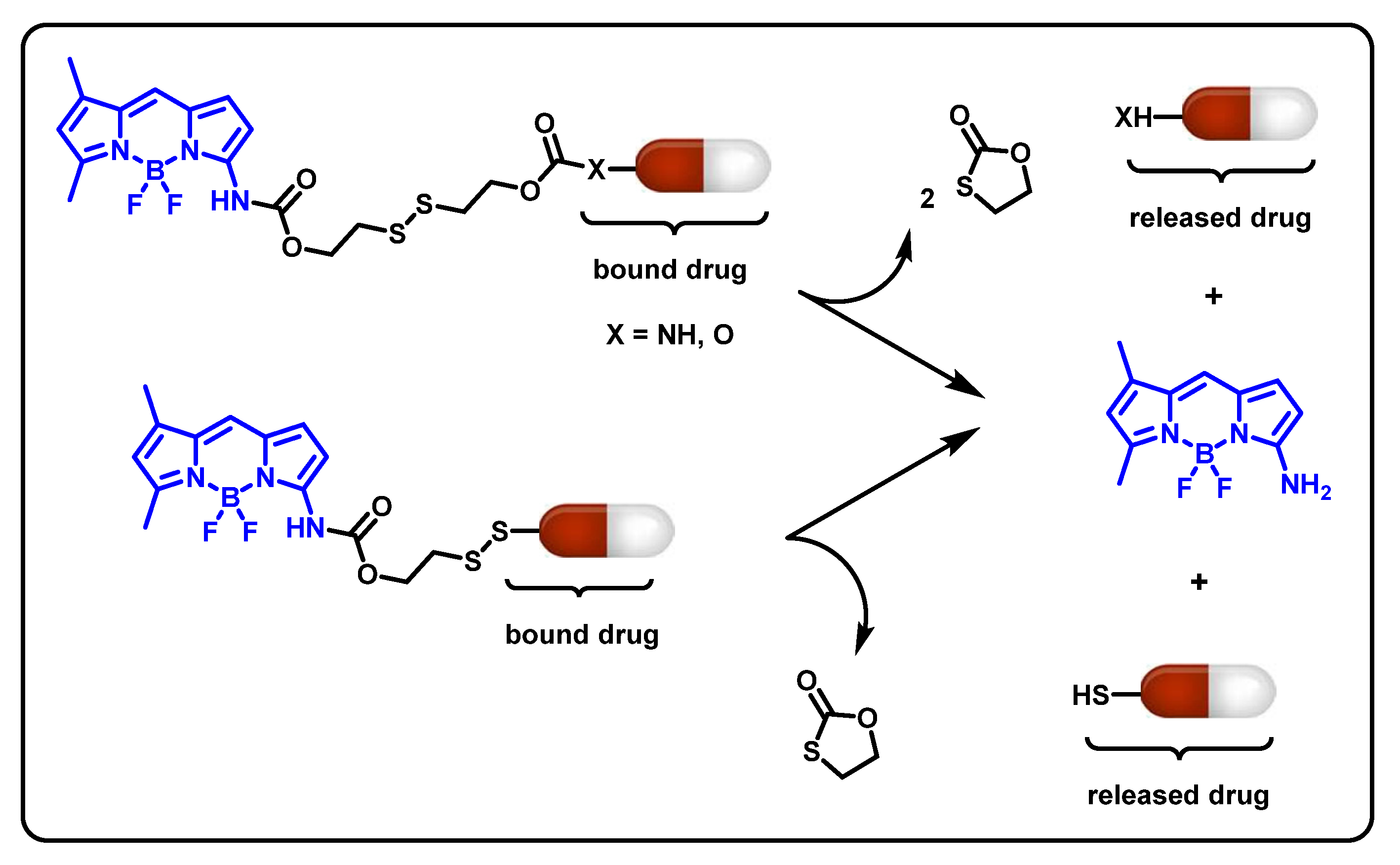
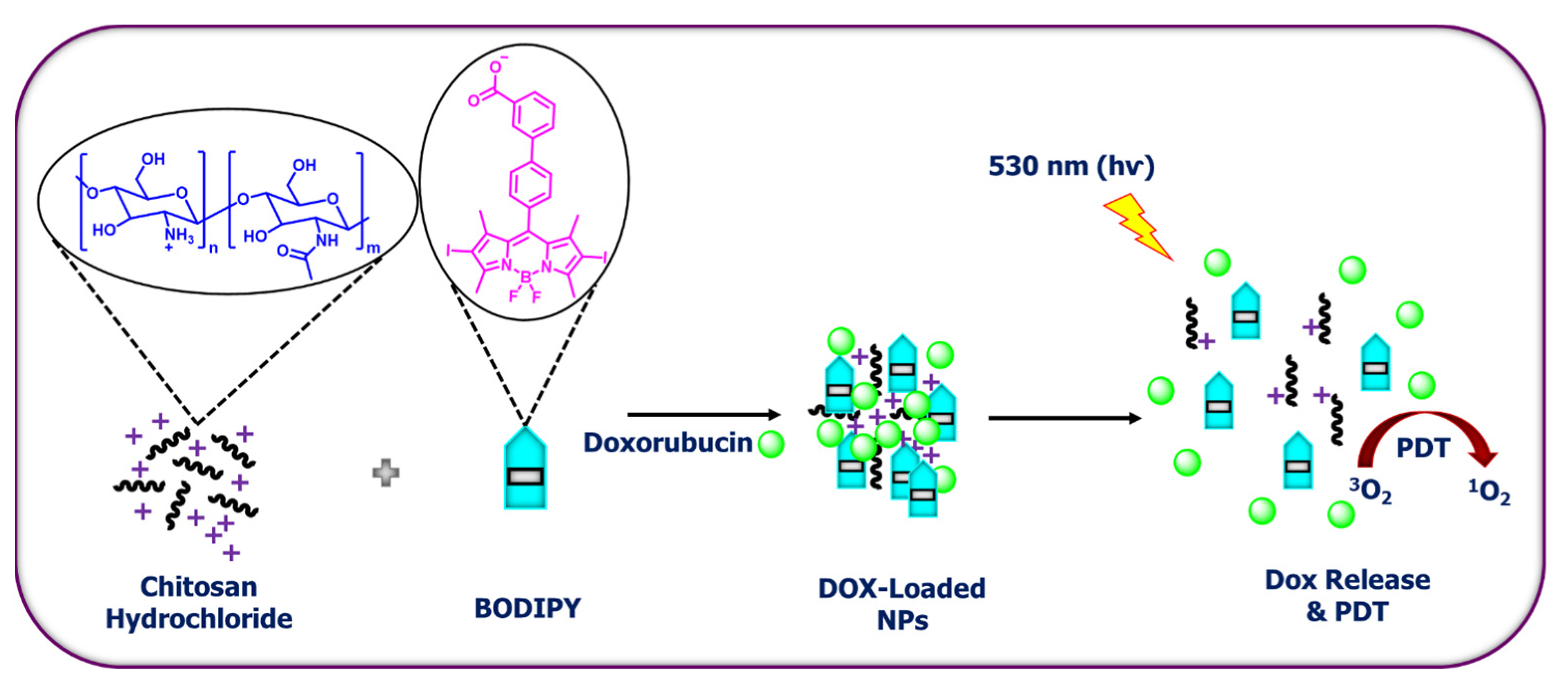
7. BODIPY-Based Drug Delivery
8. Boron-Based Materials for Other Diseases
9. Boron-Based Materials in Other Applications
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef]
- Smith, T.P.; Windsor, I.W.; Forest, K.T.; Raines, R.T. Stilbene boronic acids form a covalent bond with human transthyretin and inhibit its aggregation. J. Med. Chem. 2017, 60, 7820–7834. [Google Scholar] [CrossRef]
- Sai, K.K.S.; Das, B.C.; Sattiraju, A.; Almaguel, F.G.; Craft, S.; Mintz, A. Radiolabeling and initial biological evaluation of [18F] KBM-1 for imaging RAR-receptors in neuroblastoma. Bioorg. Med. Chem. Lett. 2017, 27, 1425–1427. [Google Scholar]
- Sattar, N.; Petrie, M.C.; Zinman, B.; Januzzi, J.L. Novel Diabetes Drugs and the Cardiovascular Specialist. J. Am. Coll. Cardiol. 2017, 69, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Robitzki, A.A.; Kurz, R. Biosensing and Drug Delivery at the Microscale. Handb. Exp. Pharmacol. 2010, 197, 87–112. [Google Scholar]
- Khaliq, N.U.; Sandra, F.C.; Park, D.Y.; Lee, J.Y.; Oh, K.S.; Kim, D.; Byun, Y.; Kim, I.S.; Kwon, I.C.; Kim, S.Y.; et al. Doxorubicin/heparin composite nanoparticles for caspase-activated prodrug chemotherapy. Biomaterials 2016, 101, 131–142. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar]
- Dubikovskaya, E.A.; Thorne, S.H.; Pillow, T.H.; Contag, C.H.; Wender, P.A. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 12128–12133. [Google Scholar] [CrossRef]
- Kunjachan, S.; Rychlik, B.; Storm, G.; Kiessling, F.; Lammers, T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv. Drug Deliv. Rev. 2013, 65, 1852–1865. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome Cancer Cell Drug Resistance Using Natural Products. J. Evid. -Based Complement. Altern. Med. 2015, 2015, 767136. [Google Scholar] [CrossRef]
- Mendoza, C.M.L.; Quintana, L.E.A. Smart Drug Delivery Strategies for Cancer Therapy. Front. Nanotechnol. 2022, 3, 753766. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Lu, Y.Y.; Burts, A.O.; Lim, Y.H.; Finn, M.G.; Koberstein, J.T.; Turro, N.J.; Tirrell, D.A.; Grubbs, R.H. Core-Clickable PEG-Branch-Azide Bivalent-Bottle-Brush Polymers by ROMP: Grafting-Through and Clicking-To. J. Am. Chem. Soc. 2011, 133, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Gois, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.; Florence, A.T. Towards more effective advanced drug delivery systems. Int. J. Pharm. 2013, 454, 496–511. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; He, Z.G.; Gao, J.Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, G.; Gu, J.C.; Xu, C.H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Thomas, T.; Li, M.H.; Kotlyar, A.; Desai, A.; Baker, J.R., Jr. Light-controlled release of caged doxorubicin from folate receptor-targeting PAMAM dendrimer nanoconjugate. Chem. Commun. 2010, 46, 2632–2634. [Google Scholar] [CrossRef]
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar]
- Miller, T.; Breyer, S.; van Colen, G.; Mier, W.; Haberkorn, U.; Geissler, S.; Voss, S.; Weigandt, M.; Goepferich, A. Premature drug release of polymeric micelles and its effects on tumor targeting. Int. J. Pharm. 2013, 445, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cai, K.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. Pseudo target release behavior of simvastatin through pH-responsive polymer based on dynamic imine bonds: Promotes rapid proliferation of osteoblasts. Mater. Sci. Eng. C 2020, 113, 110979. [Google Scholar] [CrossRef] [PubMed]
- Solomek, T.; Mercier, S.; Bally, T.; Bochet, C.G. Photolysis of ortho-nitrobenzylic derivatives: The importance of the leaving group. Photochem. Photobiol. Sci. 2012, 11, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Lee, J.H.; Lu, Q.; Lee, J.Y.; Choi, H.J. Polymer-Magnetic Composite Particles of Fe3O4/Poly(o-anisidine) and Their Suspension Characteristics under Applied Magnetic Fields. Polymers 2019, 11, 219. [Google Scholar] [CrossRef]
- Hariri, G.; Yan, H.; Wang, H.; Han, Z.; Hallahan, D.E. Radiation-Guided Drug Delivery to Mouse Models of Lung Cancer. Clin. Cancer Res. 2010, 16, 4968–4977. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Du, L.N.; Lu, C.T.; Jin, Y.G.; Ge, S.P. Potential and problems in ultrasound-responsive drug delivery systems. Int. J. Nanomed. 2013, 8, 1621–1633. [Google Scholar]
- Yonezawa, H.; Nishiyama, Y.; Takeo, K.; Iwatsubo, T.; Tomita, T.; Yokoshima, S.; Fukuyama, T. New photocleavable linker: α-Thioacetophenone-type linker. Bioorg. Med. Chem. Lett. 2014, 24, 2831–2833. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytow, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Majumdar, P.; Singh, S.P. Advances in BODIPY photocleavable protecting groups. Coord. Chem. Rev. 2021, 449, 214193. [Google Scholar] [CrossRef]
- Barrón-González, M.; Montes-Aparicio, A.V.; Cuevas-Galindo, M.E.; Orozco-Suárez, S.; Barrientos, R.; Alatorre, A.; Querejeta, E.; Trujillo-Ferrara, J.G.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Boron-containing compounds on neurons: Actions and potential applications for treating neurodegenerative diseases. J. Inorg. Biochem. 2023, 238, 112027. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, R.W. Boron-Containing Pyrazole Compounds as JAK Inhibitors for Treating Inflammation, Autoimmune Diseases, and Cancer. Acs Med. Chem. Lett. 2022, 13, 1554–1555. [Google Scholar] [CrossRef]
- Ciofani, G. Potential applications of boron nitride nanotubes as drug delivery systems. Expert Opin. Drug Deliv. 2010, 7, 889–893. [Google Scholar] [CrossRef]
- Joy, J.; George, E.; Haritha, P.; Thomas, S.; Anas, S. An overview of boron nitride based polymernanocomposites. J. Polym. Sci. 2020, 58, 3115–3141. [Google Scholar] [CrossRef]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2014, 5, 1503–1518. [Google Scholar] [CrossRef]
- Trippier, P.C.; Mcguigan, C. Boronic acids in medicinal chemistry: Anticancer, antibacterial and antiviral applications. Med. Chem. Commun. 2010, 1, 183–198. [Google Scholar] [CrossRef]
- Wei, R.; Bozorghi, S.J. γ-graphyne and its boron nitride analogue as nanocarriers for anti-cancer drug delivery. Mol. Phys. 2019, 118, e1691748. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J. Nanodiamond decorated liposomes as highly biocompatible delivery vehicles and a comparison with carbon nanotubes and graphene oxide. Nanoscale 2013, 5, 12375–12382. [Google Scholar] [CrossRef]
- Hu, Y.; Quan, H.; Cui, J.; Luo, W.; Zeng, W.; Chen, D. Carbon nanodot modified N, O-doped porous carbon for solid-state supercapacitor: A comparative study with carbon nanotube and graphene oxide. J. Alloys Compd. 2021, 877, 160237. [Google Scholar]
- Kim, J.; Oh, J.; Lee, K.Y.; Jung, I.; Park, M. Dispersion of graphene-based nanocarbon fillers in polyamide 66 by dry processing and its effect on mechanical properties. Compos. B. Eng. 2017, 114, 445–456. [Google Scholar]
- Kumar, V.; Nikhil, K.; Roy, P.; Lahiri, D.; Lahiri, I. Emergence of fluorescence in boron nitride nanoflakes and its application in bioimaging. RSC Adv. 2016, 6, 48025–48032. [Google Scholar]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [PubMed]
- Ciofani, G.; Danti, S.; Nitti, S.; Mazzolai, B.; Mattoli, V.; Giorgi, M. Biocompatibility of boron nitride nanotubes: An up-date of in vivo toxicological investigation. Int. J. Pharm. 2013, 444, 85–88. [Google Scholar] [PubMed]
- Wang, T.; Ou, D.; Liu, H.; Jiang, S.; Huang, W.; Fang, X.; Chen, X.; Lu, M. Thermally conductive boron nitride nanosheet composite paper as a flexible printed circuit board. ACS Appl. Nano Mater. 2018, 1, 1705–1712. [Google Scholar]
- Tapia, A.; Cab, C.; Hernández-Pérez, A.; Villanueva, C.; Peñuñuri, F.; Avilés, F. The bond force constants and elastic properties of boron nitride nanosheets and nanoribbons using a hierarchical modeling approach. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 183–193. [Google Scholar]
- Liu, Q.; Zhou, L.; Gao, J.; Wang, S.; Liu, L.; Liu, S. Surface chemistry-dependent activity and comparative investigation on the enhanced photocatalytic performance of graphitic carbon nitride modified with various nanocarbons. J. Colloid Interface Sci. 2020, 569, 12–21. [Google Scholar]
- Tiano, A.; Park, C.; Lee, J.; Luong, H.; Gibbons, L.; Chu, S.; Applin, S.; Gnoffo, P.; Lowther, S.; Kim, H.; et al. Boron nitride nanotube: Synthesis and applications. Nanosens. Biosens. Info-Tech Sens. Syst. 2014, 9060, 51–69. [Google Scholar]
- Lima, D.M.; Chinellato, A.C.; Champeau, M. Boron nitride-based nanocomposite hydrogels: Preparation, properties and applications. Soft Matter 2021, 17, 4475–4488. [Google Scholar]
- Sharker, S.M. Hexagonal Boron Nitrides (White Graphene): A Promising Method for Cancer Drug Delivery. Int. J. Nanomedicine. 2018, 14, 9983–9993. [Google Scholar] [CrossRef]
- Roosta, S.; Nikkhhah, S.J.; Sabzail, M.; Hashemianzadeh, S.M. Molecular dynamics simulation study of boron-nitride nanotubes as a drug carrier: From encapsulation to releasing. RSC Adv. 2016, 6, 9344–9351. [Google Scholar]
- Zhou, S.; Min, X.; Dou, H.; Sun, K.; Chen, C.Y.; Chen, C.T.; Zhang, Z.; Jin, Y.; Shen, Z. Facile fabrication of dextran-based fluorescent nanogels as potential glucose sensors. Chem. Commun. 2013, 49, 9473–9475. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; van Nostrum, C.F.; Mastrobattista, E.; Vermonden, T.; Hennink, W.E. Nanogels for intracellular delivery of biotherapeutics. J. Control. Release 2017, 259, 16–28. [Google Scholar] [PubMed]
- Zhao, L.; Ding, J.; Xiao, C.; Zhuang, X.; Chen, X. Phenylboronic acid-functionalized polypeptide nanogel for glucose-responsive insulin release under physiological pH. J. Control. Release 2015, 213, e69. [Google Scholar] [PubMed]
- Goponenko, A.V.; Dzenis, Y.A. Role of mechanical factors in applications of stimuli-responsive polymer gels—Status and prospects. Polymer 2016, 101, 415–449. [Google Scholar]
- Guan, Y.; Zhang, Y. Boronic acid-containing hydrogels: Synthesis and their applications. Chem. Soc. Rev. 2013, 42, 8106–8121. [Google Scholar]
- Zhao, L.; Huang, Q.; Liu, Y.; Wang, Q.; Wang, L.; Xiao, S.; Bi, F.; Ding, J. Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment. Materials 2017, 10, 170. [Google Scholar]
- Ulrich, G.; Ziessel, R.; Haefele, A. A general synthetic route to 3,5-substituted boron dipyrromethenes: Applications and properties. J. Org. Chem. 2012, 77, 4298–4311. [Google Scholar]
- Sitkowska, K.; Feringa, B.L.; Szymański, W. Green-Light-Sensitive BODIPY Photoprotecting Groups for Amines. J. Org. Chem. 2018, 83, 1819–1827. [Google Scholar]
- Pérez-Ojeda, M.E.; Thivierge, C.; Martin, V.; Costela, A.; Burgess, K.; Moreno, I.G. Highly efficient and photostable photonic materials from diiodinated BODIPY laser dyes. Opt. Mater. Express 2011, 1, 243–251. [Google Scholar]
- Kálai, T.; Hideg, K. Synthesis of new BODIPY-based sensors and labels. Tetrahedron 2006, 62, 10352–10360. [Google Scholar]
- Wang, L.; Cao, J.; Wang, J.W.; Chen, Q.; Cui, A.J.; He, M.Y. Facile synthesis of dimeric BODIPY and its catalytic activity for sulfide oxidation under visible light. RSC Adv. 2014, 4, 14786–14790. [Google Scholar]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [PubMed]
- Weinstain, R.; Slanina, T.; Kand, D.; Klan, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2015, 30, 6577–6595. [Google Scholar]
- Lu, P.; Chung, K.Y.; Stafford, A.; Kiker, M.; Kafle, K.; Page, Z.A. Boron dipyrromethene (BODIPY) in polymer chemistry. Polym. Chem. 2021, 12, 327–348. [Google Scholar]
- Duverger, E.; Balme, S.; Bechelany, M.; Miele, P.; Picaud, F. Natural payload delivery of the doxorubicin anticancer drug from boron nitride oxide nanosheets. Appl. Surf. Sci. 2019, 475, 666–675. [Google Scholar]
- Pasquale, D.D.; Marino, A.; Tapeinos, C.; Pucci, C.; Rocchiccioli, S.; Michelucci, E.; Finamore, F.; McDonnell, L.; Scarpellini, A.; Lauciello, S.; et al. Homotypic targeting and drug delivery in glioblastoma cells through cell membrane-coated boron nitride nanotubes. Mater. Des. 2020, 192, 108742. [Google Scholar]
- Zhang, Y.; Guo, R.; Wang, D.; Sun, X.; Xu, Z. Pd nanoparticle-decorated hydroxy boron nitride nanosheets as a novel drug carrier for chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 300–308. [Google Scholar]
- Khatti, Z.; Hashemianzadeh, S.M. Boron nitride nanotube as a delivery system for platinum drugs: Drug encapsulation and diffusion coefficient prediction. Eur. J. Pharm. Sci. 2016, 88, 291–297. [Google Scholar] [PubMed]
- Feng, S.; Zhang, H.; Xu, S.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated, mesoporous silica functionalized boron nitride nanospheres for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2019, 96, 552–560. [Google Scholar]
- Cheng, Y.; Han, Y.; Zhang, W.; Zeng, L.; Long, Y.; Wang, S.; Weng, Q. Gram-scale synthesis of boron nitride nanosheets by salt-template method for anticancer drug delivery. Chem. Eng. J. 2022, 437, 135304. [Google Scholar]
- Permyakova, E.S.; Sukhorukova, I.V.; Antipina, L.Y.; Konopatsky, A.S.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Golberg, D.V.; Manakhov, A.M.; Shtansky, D.V. Synthesis and Characterization of Folate Conjugated Boron Nitride Nanocarriers for Targeted Drug Delivery. J. Phys. Chem. C 2017, 121, 28096–28105. [Google Scholar]
- Sukhorukova, I.V.; Zhitnyak, I.Y.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Li, X.; Gloushankova, N.A.; Golberg, D.; Shtansky, D.V. Boron Nitride Nanoparticles with a Petal-Like Surface as Anticancer Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 17217–17225. [Google Scholar]
- Cheng, C.C.; Muhabie, A.A.; Huang, S.Y.; Wu, C.Y.; Gebeyehu, B.T.; Lee, A.W.; Lai, J.Y.; Lee, D.J. Dual Stimuli-Responsive Supramolecular Boron Nitride with Tunable Physical Properties for Controlled Drug Delivery. Nanoscale 2019, 11, 10393–10401. [Google Scholar]
- Feng, S.; Zhang, H.; Yan, T.; Huang, D.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated boron nitride nanospheres for targeted delivery of anticancer drugs. Int. J. Nanomed. 2016, 11, 4573–4582. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Zhi, C.; Gao, X.D.; Nakanishi, H. pH-responsive charge-reversal polymer-functionalized boron nitride nanospheres for intracellular doxorubicin delivery. Int. J. Nanomed. 2018, 13, 641–652. [Google Scholar] [CrossRef]
- Feng, S.; Ren, Y.; Li, H.; Tang, Y.; Yan, J.; Shen, Z.; Zhang, H.; Chen, F. Cancer Cell–Membrane Biomimetic Boron Nitride Nanospheres for Targeted Cancer Therapy. Int. J. Nanomed. 2021, 16, 2123–2136. [Google Scholar]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly Water-Soluble, Porous, and Biocompatible Boron Nitrides for Anticancer Drug Delivery. ACS Nano 2014, 8, 6123–6130. [Google Scholar]
- Chen, S.; Miyazaki, T.; Itoh, M.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Temperature-stable Boronate Gel based Microneedle Technology for Self-Regulated Insulin Delivery. ACS Appl. Polym. Mater. 2020, 2, 2781–2790. [Google Scholar]
- Zhang, X.; Guan, Y.; Zhang, Y. Dynamically bonded layer-by-layer films for self-regulated insulin release. J. Mater. Chem. 2012, 22, 16299–16305. [Google Scholar]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Microneedle-Array Patch Fabricated with Enzyme-Free Polymeric Components Capable of On-Demand Insulin Delivery. Adv. Funct. Mater. 2019, 29, 1807369. [Google Scholar]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar]
- Shen, D.; Yu, H.; Wang, L.; Khan, A.; Haq, F.; Chen, X.; Huang, Q.; Teng, L. Recent progress in design and preparation of glucose-responsive insulin delivery systems. J. Control. Release 2020, 321, 236–258. [Google Scholar]
- Cai, B.; Luo, Y.; Guo, Q.; Zhang, X.; Wu, Z. A glucose-sensitive block glycopolymer hydrogel based on dynamic boronic ester bonds for insulin delivery. Carbohyd. Res. 2017, 445, 32–39. [Google Scholar]
- Peng, H.; Ning, X.; Wei, G.; Wang, S.; Dai, G.; Ju, A. The preparations of novel cellulose/phenylboronic acid composite intelligent bio-hydrogel and its glucose, pH-responsive behaviors. Carbohyd. Polym. 2018, 195, 349–355. [Google Scholar]
- Zhi, X.; Zheng, C.; Xiong, J.; Li, J.; Zhao, C.; Shi, L.; Zhang, Z. Nanofilamentous Virus-Based Dynamic Hydrogels with Tunable Internal Structures, Injectability, Self-Healing, and Sugar Responsiveness at Physiological pH. Langmuir 2018, 34, 12914–12923. [Google Scholar] [CrossRef]
- Lee, J.; Ko, J.H.; Mansfield, K.M.; Nauka, P.C.; Bat, E.; Maynard, H.D. Glucose-Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery. Macromol. Biosci. 2018, 18, 1700372. [Google Scholar]
- Gu, S.; Yang, L.; Li, S.; Yang, J.; Zhang, B.; Yang, J. Thermo- and glucose-sensitivemicrogels with improved salt tolerance for controlled insulin release in a physiological environment. Polym. Int. 2018, 67, 1256–1265. [Google Scholar]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Elshaarani, T.; Yu, H.; Wang, L.; Feng, J.; Li, C.; Zhou, W.; Khan, A.; Usman, M.; Amin, B.U.; Khan, R. Chitosan reinforced hydrogels with swelling-shrinking behaviors in response to glucose concentration. Int. J. Biol. Macromol. 2020, 161, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.Q.; Luo, L.Z.; Xue, P.P.; Han, Y.H.; Wang, L.F.; Zhuge, D.L.; Yao, Q.; Chen, B.; Zhao, Y.Z.; Xu, H.L. Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats. Acta Biomater. 2021, 122, 111–132. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, X. Synthesized of glucose-responsive nanogels labeled with fluorescence molecule based on phenylboronic acid by RAFT polymerization. J. Biomat. Sci. Polym. Ed. 2019, 30, 815–831. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Shi, X.; Lu, X. Preparation of multiresponsive nanogels and their controlled release properties. Colloid Polym. Sci. 2019, 297, 613–621. [Google Scholar]
- Zhou, L.; Liu, C.; Zhang, H.; Han, J.; Liu, Z. Preparation and application of BODIPY-containing pillararenes based supramolecular systems. Dyes Pigment. 2021, 196, 109828. [Google Scholar]
- Zhang, J.; Huang, H.; Xue, L.; Zhong, L.; Ge, W.; Song, X.; Zhao, Y.; Wang, W.; Dong, X. On-demand drug release nanoplatform based on fluorinated aza-BODIPY for imaging-guided chemo-phototherapy. Biomaterials 2020, 256, 120211. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Du, Y.; Guo, W.; Su, Y.; Meng, Y.; Shan, Z.; Feng, Y.; Meng, S. Methotrexate coated AZA-BODIPY nanoparticles for chemotherapy, photothermal and photodynamic synergistic therapy. Dyes Pigment. 2020, 179, 108351. [Google Scholar] [CrossRef]
- Meng, Y.; Du, Y.; Lin, Y.; Su, Y.; Li, R.; Feng, Y.; Meng, S. A two-fold interpenetration pillar-layered metal-organic frameworks based on BODIPY for chemo-photodynamic therapy. Dyes Pigment. 2021, 188, 109174. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef]
- Song, X.; Wang, R.; Gao, J.; Han, X.; Jin, J.; Lv, C.; Yu, F. Construction of a biotin-targeting drug delivery system and its near-infrared theranostic fluorescent probe for real-time image-guided therapy of lung cancer. Chin. Chem. Lett. 2022, 33, 1567–1571. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Sun, Y.; Li, L.; Ye, L.; Zhang, W.; Zhou, N.; Zhang, Z.; Zhu, X. A novel BODIPY-based reductant-sensitive near-infrared fluorescent probe for real-time reporting azoreductase-triggered release. React. Funct. Polym. 2021, 165, 104951. [Google Scholar] [CrossRef]
- Porubsky, M.; Gurska, S.; Stankova, J.; Hajduch, M.; Dzubak, P.; Hlavac, J. AminoBODIPY Conjugates for Targeted Drug Delivery Systems and Real-Time Monitoring of Drug Release. Mol. Pharm. 2021, 18, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lei, X.; Ren, H.; Zheng, S.; Qiang, J.; Zhang, Z.; Chen, Y.; Wei, T.; Wang, F.; Chen, X. PEGylated Dimeric BODIPY Photosensitizer as Nanocarrier for Combined Chemotherapy and Cathepsin B-activated Photodynamic Therapy in 3D Tumor Spheroids. ACS Appl. Bio Mater. 2020, 3, 3835–3845. [Google Scholar] [CrossRef]
- Asem, H.; Zhang, W.; Nilsson, F.; Zhang, Y.; Hedenqvist, M.S.; Hassan, M.; Malmstrom, E. Functional Nanocarriers for Drug Delivery by Surface Engineering of Polymeric Nanoparticle Post-Polymerization-Induced Self-Assembly. ACS Appl. Bio Mater. 2021, 4, 1045–1056. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Lv, Y.; Dong, J.; Xuan, G.; Yang, J.; Wu, D.; Zhou, J.; Yu, G.; Tang, G.; et al. Nanomedicine Fabricated from A BODIPY-Embedded Amphiphilic Copolymer for Photothermal-Enhanced Chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 4463–4473. [Google Scholar] [CrossRef]
- Long, K.; Wang, Y.; Lv, W.; Yang, Y.; Xu, S.; Zhan, C.; Wang, W. Photoresponsive prodrug-dye nanoassembly for in-situ monitorable cancer therapy. Bioeng. Transl. Med. 2022, 7, e10311. [Google Scholar] [CrossRef]
- Porubsky, M.; Gurska, S.; Stankova, J.; Hajduch, M.; Dzubak, P.; Hlavac, J. Amino-BODIPY as the ratiometric fluorescent sensor for monitoring drug release or “power supply” selector for molecular electronics. RSC Adv. 2019, 9, 25075–25083. [Google Scholar] [CrossRef]
- Chang, Z.; Ye, J.H.; Qi, F.; Fang, H.; Lin, F.; Wang, S.; Mu, C.; Zhang, W.; He, W. A PEGylated photosensitizer-core pH-responsive polymeric nanocarrier for imaging-guided combination chemotherapy and photodynamic therapy. New J. Chem. 2021, 45, 6180–6185. [Google Scholar] [CrossRef]
- Ou, C.; Zhang, Y.; Ge, W.; Zhong, L.; Huang, Y.; Si, W.; Wang, W.; Zhao, Y.; Dong, X. Three-Dimensional BODIPY-Iron (III) Compound with Promoted H2O2-Response for NIR-II Photoacoustic Imaging Guided Chemodynamic/Photothermal Therapy. Chem. Commun. 2020, 56, 6281–6284. [Google Scholar] [CrossRef]
- Meng, L.B.; Zhang, W.; Li, D.; Li, Y.; Hu, X.Y.; Wang, L.; Li, G. pH-Responsive Supramolecular Vesicles Assembled by Water-Soluble Pillar [5]arene and BODIPY Photosensitizer for Chemo-Photodynamic Dual Therapy. Chem. Commun. 2015, 51, 14381–14384. [Google Scholar] [CrossRef]
- Long, K.; Han, H.; Kang, W.; Lv, W.; Wang, L.; Wang, Y.; Ge, L.; Wang, W. One-photon red light-triggered disassembly of small-molecule nanoparticles for drug delivery. J. Nanobiotechnol. 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, F.; Kucukoflaz, M.; Ergul, M.; Inan, Z.D.S.; Bozkurt, Y.; Taydas, D. Synthesis of Multifunctional Organic Nanoparticles Combining Photodynamic Therapy and Chemotherapeutic Drug Release. Macromol. Res. 2022, 30, 61–69. [Google Scholar] [CrossRef]
- Hao, J.; Heng, P. Buccal delivery systems. Drug Dev. Ind. Pharm. 2003, 29, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Smart, J. Buccal drug delivery. Expert Opin. Drug Deliv. 2005, 2, 507–517. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef]
- Kublik, H.; Vidgren, M. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.; Parenky, A.; Mitra, A. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Inamuddin, A.; Asiri, A.; Mohammad, A. Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Sawston, UK, 2018; pp. 509–573. [Google Scholar]
- Labiris, N.; Dolovich, M. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Narang, N.; Sharma, J. Sublingual mucosa as a route for systemic drug delivery. Int. J. Pharm. Pharm. 2011, 3, 18–22. [Google Scholar]
- Alkilani, A.; McCrudden, M.; Donnelly, R. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Alexander, N.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.K.; Nayak, P.K.; Sarangi, D.K.; Sahoo, T.K. Intra vaginal drug delivery system: An overview. Am. J. Adv. Drug Deliv. 2013, 1, 43–55. [Google Scholar]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nature Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated Nanoparticle–Biomolecule Hybrid Systems: Synthesis, Properties, and Applications. Angew. Chem. Int. Ed. Engl. 2004, 43, 6042–6108. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Y.; Bae, Y.H. Polymer Architecture and Drug Delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Peng, T.; Cheng, Y.L. PNIPAAm and PMAA co-grafted porous PE membranes: Living radical co-grafting mechanism and multi-stimuli responsive permeability. Polymer 2001, 42, 2091–2100. [Google Scholar] [CrossRef]
- Duncan, R.; Spreafico, F. Polymer conjugates. Pharmacokinetic considerations for design and development. Clin. Pharm. 1994, 27, 290–306. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef]
- Roney, C.; Kulkarni, P.; Arora, V.; Antich, P.; Bonte, F.; Wu, A.; Mallikarjuana, N.N.; Manohar, S.; Liang, H.F.; Kulkarni, A.R.; et al. Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease. J. Control. Release 2005, 108, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Bilati, U.; Allemann, E.; Doelker, E. Poly(D,L-lactide-co-glycolide) protein-loaded nanoparticles prepared by the double emulsion method--processing and formulation issues for enhanced entrapment efficiency. J. Microencapsul. 2005, 22, 205–214. [Google Scholar] [CrossRef]
- Mayer, C. Nanocapsules as drug delivery systems. Int. J. Artif. Organs. 2005, 28, 1163–1171. [Google Scholar] [CrossRef]
- Krol, S.; Diaspro, A.; Magrassi, R.; Ballario, P.; Grimaldi, B.; Filetici, P.; Ornaghi, P.; Ramoino, P.; Gliozzi, A. Nanocapsules: Coating for living cells. IEEE Trans. Nanobiosci. 2004, 3, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.N.J.; Staffhorst, R.W.H.M.; de Vijlder, H.C.; Velinova, M.J.; Bomans, P.H.; Frederik, P.M.; de Kruijff, B. Nanocapsules: Lipid-coated aggregates of cisplatin with high cytotoxicity. Nat. Med. 2002, 8, 81–84. [Google Scholar] [CrossRef]
- de Kroon, A.I.P.M.; Staffhorst, R.W.H.M.; de Kruijff, B.; Burger, K.N.J. Cisplatin Nanocapsules. Methods Enzymol. 1995, 391, 118–125. [Google Scholar]
- Ruysschaert, T.; Germain, M.; Gomes, J.F.; Fournier, D.; Sukhorukov, G.B.; Meier, W.M.W. Cucurbiturils: Molecular nanocapsules for time-resolved fluorescence-based assays. IEEE Trans. Nanobiosci. 2004, 3, 39–45. [Google Scholar]
- Haynie, D.T.; Palath, N.; Liu, Y.; Li, B.Y.; Pargaonkar, N. Biomimetic Nanostructured Materials: Inherent Reversible Stabilization of Polypeptide Microcapsules. Langmuir 2005, 21, 1136–1138. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Granick, S. Layered, Erasable Polymer Multilayers Formed by Hydrogen-Bonded Sequential Self-Assembly. Macromolecules 2002, 35, 301–310. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Quinn, J.F.; Caruso, F. Disulfide Cross-Linked Polymer Capsules: En Route to Biodeconstructible Systems. Biomacromolecules 2006, 7, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Kohli, P. The emerging field of nanotube biotechnology. Nat. Rev. Drug Discov. 2003, 2, 29–37. [Google Scholar] [CrossRef]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Soderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 296, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.T.; Lee, S.B.; Trofin, L.; Li, N.; Nevanen, T.K.; Soderlund, H.; Martin, C.R. Smart nanotubes for bioseparations and biocatalysis. J. Am. Chem. Soc. 2002, 124, 11864–11865. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Cepak, V.M.; Hulteen, J.C.; Che, G.L.; Jirage, K.B.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R. Fabrication and characterization of concentric-tubular composite micro- and nanostructures using the template-synthesis method. J. Mater. Res. 1998, 13, 3070–3080. [Google Scholar] [CrossRef]
- Nicewarner-Pena, S.R.; Freeman, R.G.; Reiss, B.D.; He, L.; Pena, D.J.; Walton, I.D.; Cromer, R.; Keating, C.D.; Natan, M.J. Submicrometer metallic barcodes. Science 2001, 294, 137–141. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. 2004, 1, 16–17. [Google Scholar] [CrossRef]
- ShiKam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into Mammalian cells. J. Am. Chem. Soc. 2004, 126, 6801–6851. [Google Scholar]
- Cai, D.; Mataraza, J.M.; Qin, Z.H.; Huang, Z.; Huang, J.; Chiles, T.C.; Carnahan, D.; Kempa, K.; Ren, Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2005, 2, 449–454. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Kruger, A.; Gansbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Endo, T.; Ohtomi, M.; Iwasaki, Y.; Akiyoshi, K. Hybrid nanogels with physical and chemical cross-linking structures as nanocarriers. Macromol. Biosci. 2005, 5, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Chang, J.H.; Liu, J.; Williford, R.; Shin, Y.K.; Exarhos, G.J. Hybrid nanogels for sustainable positive thermosensitive drug release. J. Control. Release 2001, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- McAllister, K.; Sazani, P.; Adam, M.; Cho, M.J.; Rubinstein, M.; Samulski, R.J.; DeSimone, J.M. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J. Am. Chem. Soc. 2002, 124, 15198–15207. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Zeman, A.D.; Batrakova, E.V.; Kabanov, A.V. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control. Release 2005, 107, 143–157. [Google Scholar] [CrossRef]
- Missirlis, D.; Tirelli, N.; Hubbell, J.A. Amphiphilic hydrogel nanoparticles. Preparation, characterization, and preliminary assessment as new colloidal drug carriers. Langmuir 2005, 21, 2605–2613. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Tomalia, D.A. Starburstr dendrimers—Nanoscopic supermolecules according to dendritic rules and principles. Macromol. Symp. 1996, 101, 243–255. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anti-Cancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug. Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, B.; Hill, R.A.; de Villiers, M.M. The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int. J. Pharm. 2004, 284, 133–140. [Google Scholar] [CrossRef]
- Khopade, A.J.; Caruso, F. Stepwise self-assembled poly(amidoamine) dendrimer and poly(styrenesulfonate) microcapsules as sustained delivery vehicles. Biomacromolecules 2002, 3, 1154–1162. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Sridevi, S.; Chalasani, K.B.; Jain, A.K.; Jain, S.K.; Jain, N.K.; Diwan, P.V. Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release 2003, 90, 335–343. [Google Scholar] [CrossRef]
- Lesinski, G.B.; Sharma, S.; Varker, K.A.; Sinha, P.; Ferrari, M.; Carson, W.E., III. Release of biologically functional interferon-alpha from a nanochannel delivery system. Biomed. Microdevices 2005, 7, 71–79. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl. Biochem. Biotechnol. 2005, 125, 147–157. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin–PEG–folate conjugate. J. Control. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zahr, A.S.; de Villiers, M.; Pishko, M.V. Encapsulation of drug nanoparticles in self-assembled macromolecular nanoshells. Langmuir 2005, 21, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chen, M.H.; Parmar, V.S.; Samuelson, L.A.; Kumar, J.; Nicolosi, R.; Yoganathan, S.; Watterson, A.C. Supramolecular assemblies based on copolymers of PEG600 and functionalized aromatic diesters for drug delivery applications. J. Am. Chem. Soc. 2004, 126, 10640–10644. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Sig. Transduct. Target. Ther. 2018, 3, 7. [Google Scholar]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef]
- West, K.R.; Otto, S. Reversible Covalent Chemistry in Drug Delivery. Curr. Drug Discov. Technol. 2005, 2, 123–160. [Google Scholar] [CrossRef]
- Doane, T.; Burda, C. Nanoparticle mediated non-covalent drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 607–621. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. Excli. J. 2014, 13, 265–286. [Google Scholar]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-J.; Cheng, J.; Jin, J.; Cheng, S.H.; Wong, W.-T. Development and evaluation of pH-responsive single-walled carbon nanotube-doxorubicin complexes in cancer cells. Int. J. Nanomed. 2011, 6, 2889–2898. [Google Scholar]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed]
- Zhitnyak, I.; Bychkov, I.; Sukhorukova, I.V.; Kovalskii, A.M.; Firestein, K.; Golberg, D.; Gloushankova, N.; Shtansky, D.V. Effect of BN nanoparticles loaded with doxorubicin on tumor cells with multiple drug resistance. ACS Appl. Mater. Interfaces 2017, 9, 32498–32508. [Google Scholar] [CrossRef]
- Khalifi, M.E.; Bentin, J.; Duverger, E.; Gharbi, T.; Boulahdour, H.; Picaud, F. Encapsulation capacity and natural payload delivery of an anticancer drug from boron nitride nanotube. Phys. Chem. Chem. Phys. 2016, 18, 24994–25001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, C.L.; Zhang, H.; Wang, L.Z. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Wu, L.; Qu, X.G. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Zheng, C.; Hao, Y.; Niu, M.; Hu, Y.; Chang, J.; Zhang, Z.; Wang, L. An intelligent dual stimuli-responsive photosensitizer delivery system with O2-supplying for efficient photodynamic therapy. Colloids Surf. B Biointerfaces 2018, 167, 299–309. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, H. Synthesis and Applications of Boronate Affinity Materials: From Class Selectivity to Biomimetic Specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Liu, Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, H.; Wang, H.; Liu, Z. Probing the interactions between boronic acids and cis-diol-containing biomolecules by affinity capillary electrophoresis. Anal. Chem. 2013, 85, 2361–2369. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols-it is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Stubelius, A.; Lee, S.; Almutairi, A. The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery. Acc. Chem. Res. 2019, 52, 3108–3119. [Google Scholar] [CrossRef]
- Matsumoto, A.; Cabral, H.; Sato, N.; Kataoka, K.; Miyahara, Y. Assessment of Tumor Metastasis by the Direct Determination of Cell-Membrane Sialic Acid Expression. Angew. Chem. Int. Ed. 2010, 49, 5494–5497. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Owens, D.R.; Zinman, B.; Bolli, G.B. Insulins Today and Beyond. Lancet 2001, 358, 739–746. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H. The Internal Secretion of The Pancreas. J. Lab. Clin. Med. 1922, 7, 251–266. [Google Scholar]
- Dunn, M.F. Zinc–Ligand Interactions Modulate Assembly and Stability of the Insulin Hexamer—A Review. BioMetals 2005, 18, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, S.J.; Cho, H.J.; Kim, D.D. Phenylboronic Acid-Decorated Chondroitin Sulfate A-Based Theranostic Nanoparticles for Enhanced Tumor Targeting and Penetration. Adv. Funct. Mater. 2015, 25, 3705–3717. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.Q.; Yi, X.Q.; Zhang, Q.; Cheng, S.X.; Zhuo, R.X.; Li, F. pH-Activated Targeting Drug Delivery System Based on the Selective Binding of Phenylboronic Acid. ACS Appl. Mater. Interfaces 2016, 8, 14845–14854. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.E.; Deming, T.J. Protein Complexation and pH Dependent Release Using Boronic Acid Containing PEG-Polypeptide Copolymers. Macromol. Biosci. 2017, 17, 1600136. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, N.; Uhida, S.; Dirisala, A.; Naito, M.; Osada, K.; Cabral, H.; Kataoka, K. mRNA loading into ATP-responsive polyplex micelles with optimal density of phenylboronate ester crosslinking to balance robustness in the biological milieu and intracellular translational efficiency. J. Control. Release 2021, 330, 317–328. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Uhida, S.; Dirisala, A.; Naito, M.; Koji, K.; Osada, K.; Cabral, H.; Kataoka, K. Bridging mRNA and Polycation Using RNA Oligonucleotide Derivatives Improves the Robustness of Polyplex Micelles for Efficient mRNA Delivery. Adv. Healthc. Mater. 2022, 11, e2102016. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Ishii, T.; Naito, M.; Endo, T.; Uhida, S.; Osada, H.K.; Kataoka, K. Polyplex Micelles with Phenylboronate/Gluconamide Cross-Linking in the Core Exerting Promoted Gene Transfection through Spatiotemporal Responsivity to Intracellular pH and ATP Concentration. J. Am. Chem. Soc. 2017, 139, 18567–18575. [Google Scholar] [CrossRef]
- Naito, M.; Ishii, T.; Matsumoto, A.; Miyata, K.; Miyahara, Y.; Kataoka, K. A Phenylboronate-Functionalized Polyion Complex Micelle for ATPTriggered Release of siRNA. Angew. Chem. Int. Ed. 2012, 51, 10751–10755. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Smith, B.D. Anionic Saccharides Activate Liposomes Containing Phospholipids Bearing a Boronic Acid for Ca2+-Dependent Fusion. J. Am. Chem. Soc. 1998, 120, 7141–7142. [Google Scholar] [CrossRef]
- Vandenburg, Y.R.; Zhang, Z.Y.; Fishkind, D.J.; Smith, B.D. Enhanced cell binding using liposomes containing an artificial carbohydrate-binding receptor. Chem. Commun. 2000, 2, 149–150. [Google Scholar] [CrossRef]
- Zhang, X.; Alves, D.S.; Lou, J.; Hill, S.D.; Barrerea, F.N.; Best, M.D. Boronic Acid Liposomes for Cellular Delivery and Content Release Driven by Carbohydrate Binding. Chem. Commun. 2018, 54, 6169–6172. [Google Scholar] [CrossRef] [PubMed]
- Qualla, M.L.; Hagewood, H.; Lou, J.; Mattern-Schain, S.I.; Zhang, X.; Mountain, D.J.; Best, M.D. Bis-Boronic Acid Liposomes for Carbohydrate Recognition and Cellular Delivery. ChemBioChem 2022, 23, e202200402. [Google Scholar]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. Therapeutic Vesicular Nanoreactors with Tumor-Specific Activation and Self-Destruction for Synergistic Tumor Ablation. Angew. Chem. Int. Ed. 2017, 129, 14213–14218. [Google Scholar] [CrossRef]
- Boensa, N.; Verbelena, B.; Ortizb, M.J.; Jiaoc, L.; Dehaena, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, H.; Peng, B.; Fang, B.; Baell, J.; Li, L.; Huang, W.; Voelcker, N.H. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021, 50, 4872–4931. [Google Scholar]
- Majumdar, P.; Yuan, X.; Li, S.; Le, B.; Guennic, J.; Ma, C.; Zhang, D.; Zhao Jacqueminde, J. Cyclometalated Ir(III) complexes with styryl-BODIPY ligands showing near IR absorption/emission: Preparation, study of photophysical properties and application as photodynamic/luminescence imaging materials. J. Mater. Chem. B 2014, 2, 2838–2854. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, R.P.; McCormick, T.M.; Lazarides, T.; Wilson, K.C.; Eisenberg, R.; McCamant, D.W. Intersystem crossing in halogenated bodipy chromophores used for solar hydrogen production. J. Phys. Chem. Lett. 2011, 2, 223–227. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Bonardi, L.; Kanaan, H.; Camerel, F.; Jolinat, P.; Retailleau, P.; Ziessel, R. Fine-tuning of yellow or red photo- and electroluminescence of functional difluoro boradiazaindacene films. Adv. Funct. Mater. 2008, 18, 401–413. [Google Scholar] [CrossRef]
- Findlay, N.J.; Bruckbauer, J.; Inigo, A.R.; Breig, B.; Arumugam, S.; Wallis, D.J.; Martin, R.W.; Skabara, P.J. An organic down-converting material for white-light emission from hybrid LEDs. Adv. Mater. 2014, 26, 7290–7294. [Google Scholar] [CrossRef]
- Mahmood, Z.; Zhao, J. Thiol-activatable triplet–triplet annihilation upconversion with maleimide-perylene as the caged triplet acceptor/emitter. J. Org. Chem. 2016, 81, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic Dendrimer–Peptide Conjugates for Early Multi-Target Therapy of Alzheimer’s Disease by Inflammatory Microenvironment Modulation. Adv. Mater. 2021, 33, 2100746. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Burch, Z.N.; Flaherty, D.B.; Larkin, J.D.; Dunbar, G.L. Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6664. [Google Scholar] [CrossRef] [PubMed]
- Kucukdogru, R.; Turkez, H.; Arslan, M.E.; Tozlu, O.O.; Sonmez, E.; Mardinoglu, A.; Cacciatore, I.; Stefano, A.D. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab. Brain Dis. 2020, 35, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Prommana, P.; Hosmane, N.S.; Coghi, P.; Uthaipibull, C.; Zhang, Y. Functionalized Boron Nanoparticles as Potential Promising Antimalarial Agents. ACS Omega 2022, 7, 5864–5869. [Google Scholar] [CrossRef]
- Garcia, E.C.; Lozano, C.; Iriepa, C.G.; Marazzi, M.; Winter, A.H.; Torres, C.; Sampedro, D. Controlling Antimicrobial Activity of Quinolones Using Visible/NIR Light-Activated BODIPY Photocages. Pharmaceutics 2022, 14, 1070. [Google Scholar] [CrossRef]
- Andersen, N.S.; Peiró Cadahía, J.; Previtali, V.; Bondebjerg, J.; Hansen, C.A.; Hansen, A.E.; Andresen, T.L.; Clausen, M.H. Methotrexate prodrugs sensitive to reactive oxygen species for the improved treatment of rheumatoid arthritis. Eur. J. Med. Chem. 2018, 156, 738–746. [Google Scholar] [CrossRef]
- Kumar, R.; Han, J.; Lim, H.-J.; Ren, W.X.; Lim, J.-Y.; Kim, J.-H.; Kim, J.S. Mitochondrial induced and self-monitored intrinsic apoptosis by antitumor theranostic prodrug: In Vivo imaging and precise cancer treatment. J. Am. Chem. Soc. 2014, 136, 17836–17843. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Reǵi, M.; Ruiz-Herńandez, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- WU, Y.Y.; Ye, S.; Yao, A.H.; Li, H.; Jia, W.T.; Huang, W.H.; Wang, D.P. Effect of gas-foaming porogen-NaHCO3 and citric acid on the properties of injectablemacroporous borate bioactive glass cement. J. Inorg. Mater. 2017, 32, 777–784. [Google Scholar]
- Li, H.B.; Wang, D.P.; Wu, Y.Y.; Yao, A.H.; Ye, S. Effect of citric acid concentration on the properties of borate glass bone cement. J. Inorg. Mater. 2017, 32, 831–836. [Google Scholar]
- Balasubramanian, P.; Büttner, T.; Miguez Pacheco, V.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Winet, H. The role of microvasculature in normal and perturbed bone healing as revealed by intravitalmicroscopy. Bone 1996, 19, 39S–57S. [Google Scholar] [CrossRef]
- Xia, L.; Ma, W.; Zhou, Y.; Gui, Z.; Yao, A.; Wang, D.; Takemura, A.; Uemura, M.; Lin, K.; Xu, Y. Stimulatory Effects of Boron Containing Bioactive Glass on Osteogenesis and Angiogenesis of Polycaprolactone: In Vitro Study. Biomed. Res. Int. 2019, 2019, 8961409. [Google Scholar] [CrossRef]
- Zheng, K.; Fan, Y.; Torre, E.; Balasubramanian, P.; Taccardi, N.; Cassinelli, C.; Morra, M.; Iviglia, G.; Boccaccini, A.R. Incorporation of Boron in Mesoporous Bioactive Glass Nanoparticles Reduces Inflammatory Response and Delays Osteogenic Differentiation. Part. Part. Syst. Charact. 2020, 37, 2000054. [Google Scholar] [CrossRef]
- Cal, F.; Arslan, T.S.; Derkus, B.; Kiran, F.; Cengiz, U.; Arslan, Y.E. Synthesis of Silica-Based Boron-Incorporated Collagen/Human Hair Keratin Hybrid Cryogels with the Potential Bone Formation Capability. ACS Appl. Bio Mater. 2021, 4, 7266–7279. [Google Scholar] [CrossRef]

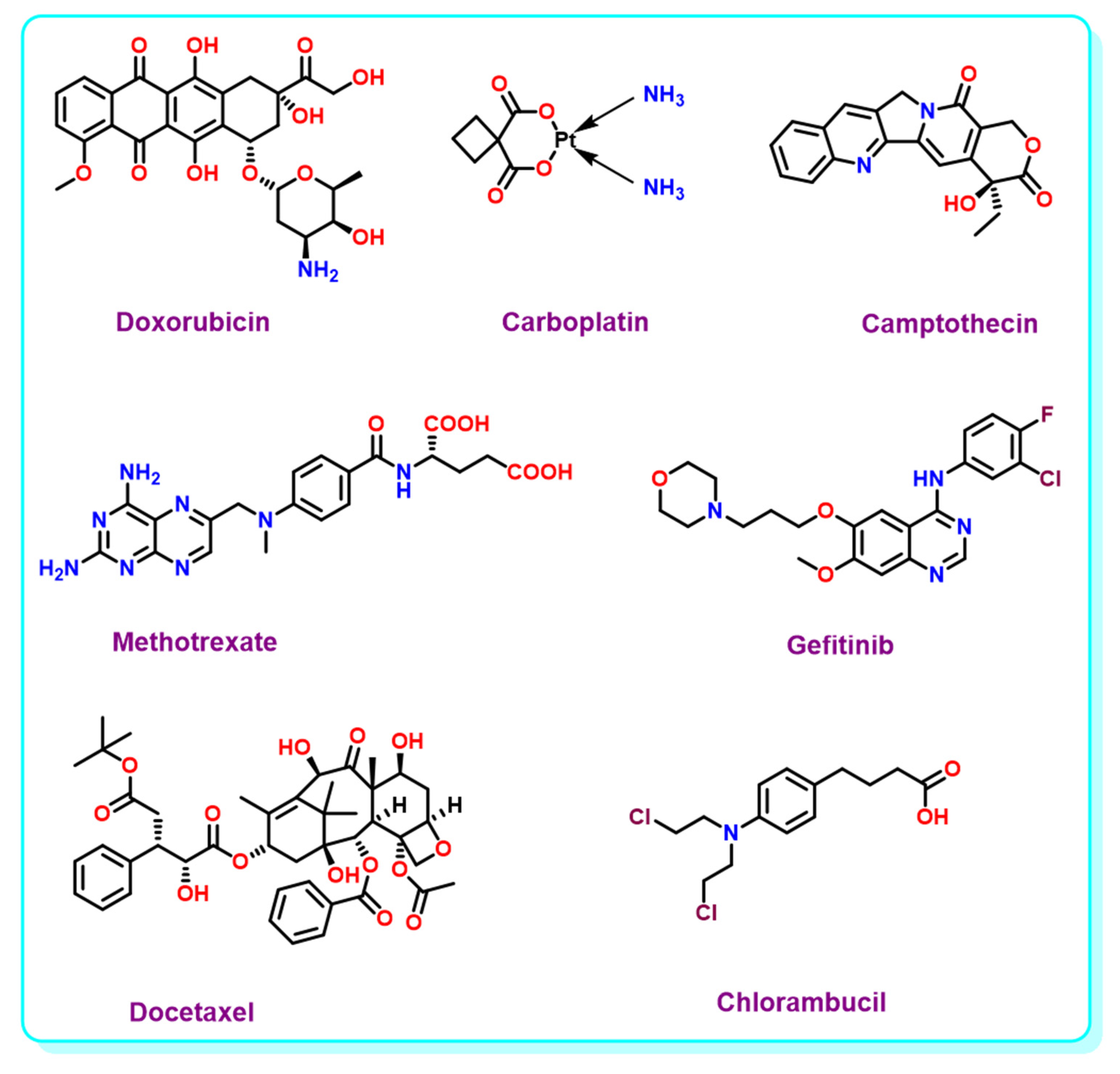
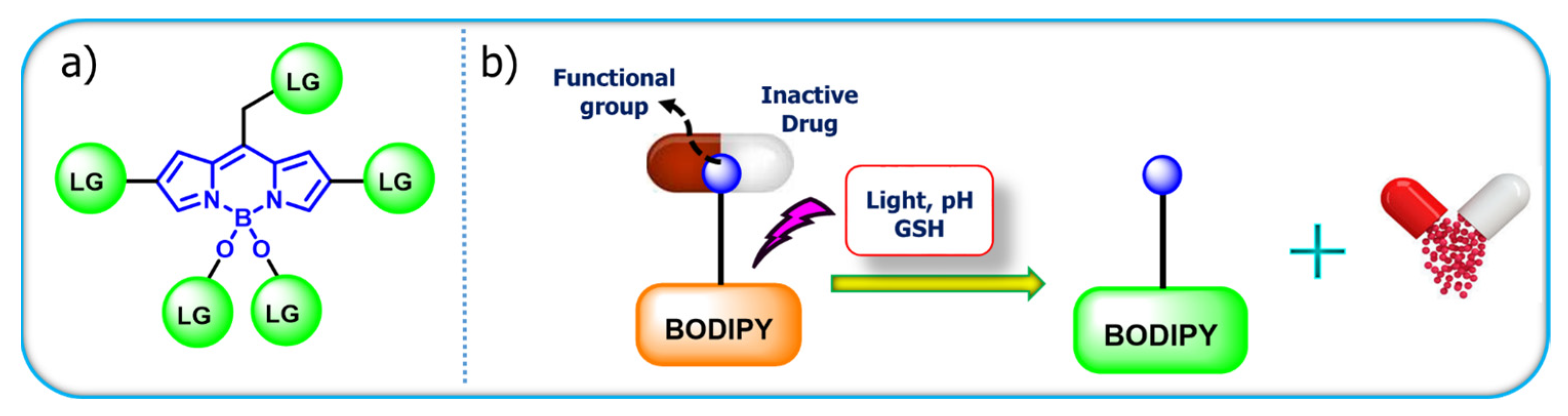
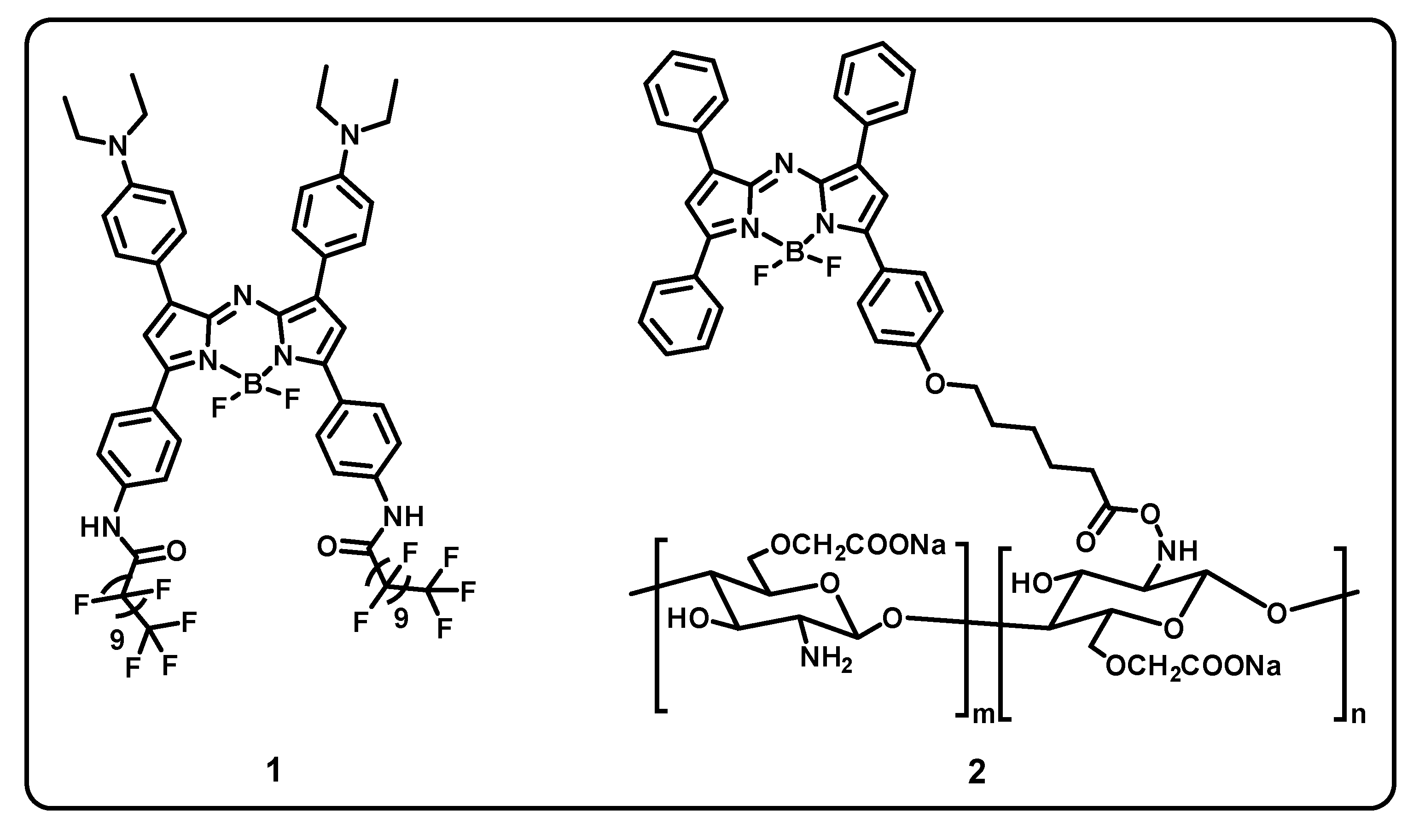
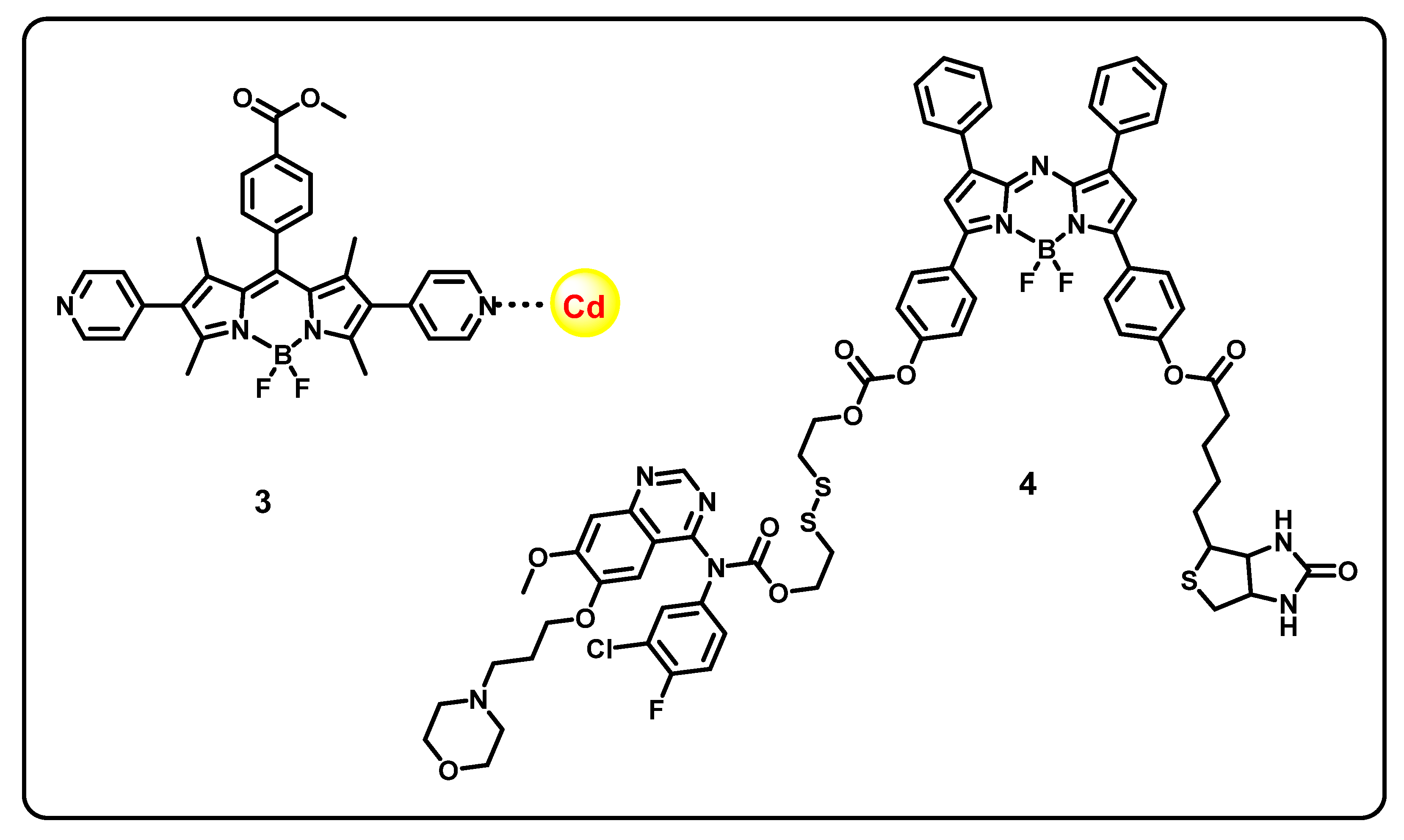
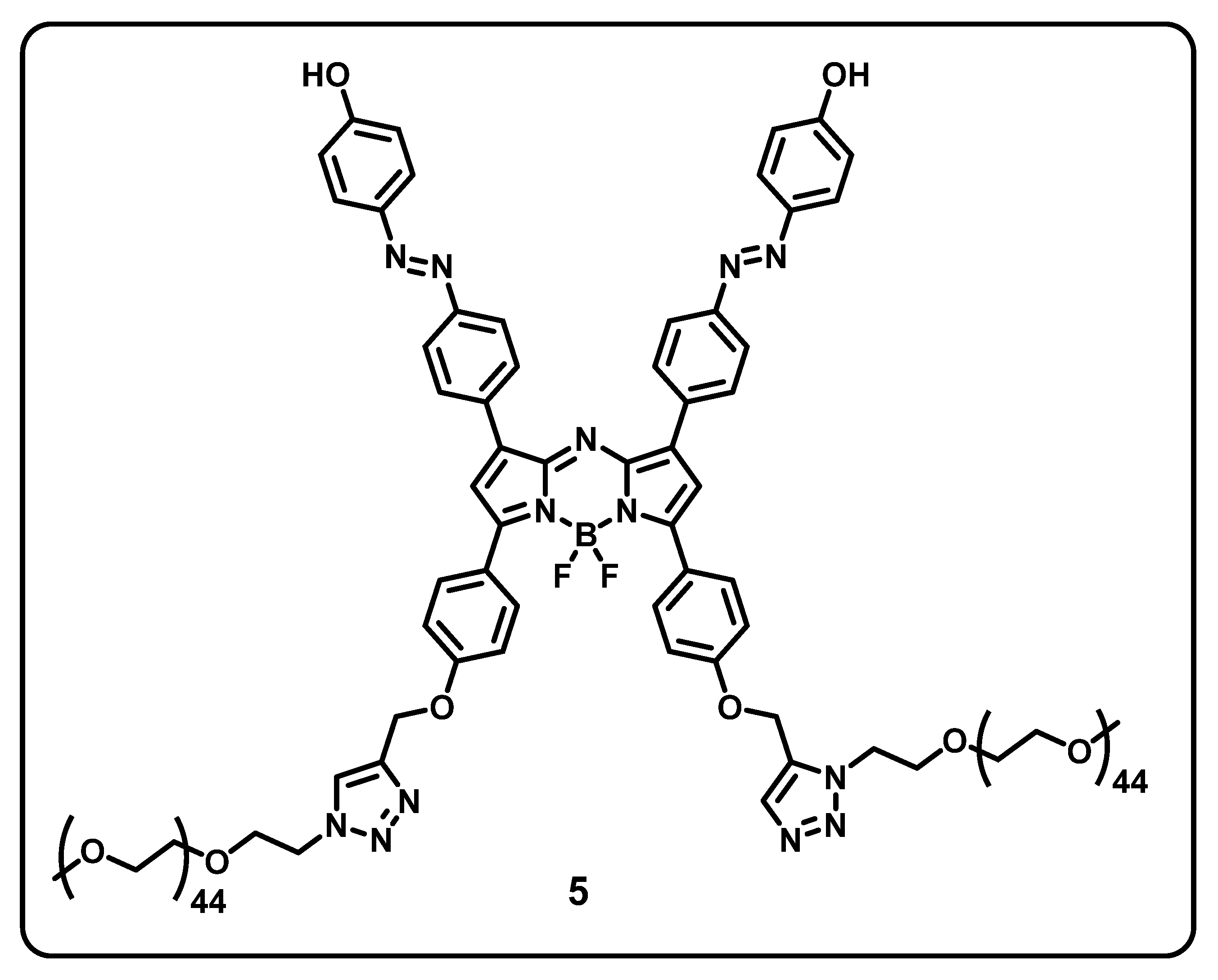

| Core Structure | Cargo | Application | Stimulus | Drug Loading Efficiency | Cell Line | Ref |
|---|---|---|---|---|---|---|
| Boron Nitride | Doxorubicin | NSs | - | - | Cancer cells | [68] |
| Doxorubicin | NTs | pH | 2.15% | U87 MG cells | [69] | |
| Doxorubicin | NSs | Light, pH, GSH | 32% | MCF-7 cells | [70] | |
| Carboplatin | NTs | - | - | - | [71] | |
| Doxorubicin | MS | pH | 5.26% | HeLa and MCF-7 cells | [72] | |
| Camptothecin | NSs | - | 30% | 4T1 cells | [73] | |
| Doxorubicin | NCs | pH | - | - | [74] | |
| Doxorubicin | NPs | pH | 5.50% | IAR-6-1 cells | [75] | |
| Doxorubicin | NSs | Temperature, pH | 36.20% | MCF-7 cells | [76] | |
| Doxorubicin | NSs | pH | 2.07% | HeLa cells | [77] | |
| Doxorubicin | NSs | pH | 7.26% | HEK 293, HeLa, MCF-7 | [78] | |
| Doxorubicin | NSs | pH | 86.20% | HeLa cells | [79] | |
| Doxorubicin | - | pH | - | LNCaP Cells | [80] | |
| Boronic Acid | Insulin | Hy | Glucose | - | - | [81] |
| Insulin | Film | pH | - | - | [82] | |
| Insulin | MNs | Glucose | - | - | [83] | |
| Insulin | MNs | Glucose | - | - | [84] | |
| Insulin | Hy | Glucose | - | - | [85] | |
| Insulin | Hy | Glucose, pH | 15.6% | NIH3T3 cells | [86] | |
| Insulin | Hy | Glucose, pH | - | - | [87] | |
| Insulin | Hy | Glucose | - | - | [88] | |
| Insulin | Hy | Glucose, pH | - | - | [89] | |
| Insulin | Microgel | Temperature, pH | - | - | [90] | |
| Insulin | Hy | Glucose, pH | - | L929 cells | [91] | |
| Insulin | Hy | pH | 62% | - | [92] | |
| Insulin | Hy | Glucose | - | NIH3T3 cells | [93] | |
| Insulin | Nanogel | Glucose | 8.2% | NIH3T3 cells | [94] | |
| Insulin | Nanogel | Temperature, Glucose, pH | 17.73% | L929 cells | [95] | |
| BODIPY | Doxorubicin | Vesicles | pH | - | A549 cells | [96] |
| Doxorubicin | NPs | Light | 25% | 4T1 cells | [97] | |
| Methotrexate | NPs | Light | - | HeLa cells | [98] | |
| Doxorubicin | MOF | Light | 49.70% | HeLa cells | [99] | |
| mRNA | DLNP | pH | - | IGROV1 cells | [100] | |
| Gefitinib | Fluorophore | GSH | - | PC9 cells | [101] | |
| Doxorubicin | Micelles | Azoreductase | - | L929 cells | [102] | |
| 2-phenyl-3-hydroxy-4(1H)-quinolinone | Fluorophore | GSH | - | HeLa cells | [103] | |
| 10-hydroxycamptothecin | NPs | Light | - | 4T1 cells | [104] | |
| Doxorubicin | NPs | pH | 28% | MCF-7, AW264.7 cells | [105] | |
| Docetaxel | NPs | Light | 34.20% | A549 cells | [106] | |
| Chlorambucil | NA | Light | 98.85% | HCT116 cells | [107] | |
| 2-phenyl-3-hydroxy-4(1H)-quinolinone based drug | Fluorophore | GSH | - | HeLa cells | [108] | |
| Doxorubicin | NC | pH | 6.73% | HeLa, MCF-7 cells | [109] | |
| Mertansine | Fluorophore | Light | - | MCF-7 cells | [110] | |
| Doxorubicin | Vesicles | pH | - | A549 cells | [111] | |
| Nalidixic acid, Ciprofloxacin | Fluorophore | Light | - | - | [112] | |
| Doxorubicin | NPs | Light | - | MCF7 cells | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.C.; Chokkalingam, P.; Masilamani, P.; Shukla, S.; Das, S. Stimuli-Responsive Boron-Based Materials in Drug Delivery. Int. J. Mol. Sci. 2023, 24, 2757. https://doi.org/10.3390/ijms24032757
Das BC, Chokkalingam P, Masilamani P, Shukla S, Das S. Stimuli-Responsive Boron-Based Materials in Drug Delivery. International Journal of Molecular Sciences. 2023; 24(3):2757. https://doi.org/10.3390/ijms24032757
Chicago/Turabian StyleDas, Bhaskar C., Parthiban Chokkalingam, Pavithra Masilamani, Srushti Shukla, and Sasmita Das. 2023. "Stimuli-Responsive Boron-Based Materials in Drug Delivery" International Journal of Molecular Sciences 24, no. 3: 2757. https://doi.org/10.3390/ijms24032757
APA StyleDas, B. C., Chokkalingam, P., Masilamani, P., Shukla, S., & Das, S. (2023). Stimuli-Responsive Boron-Based Materials in Drug Delivery. International Journal of Molecular Sciences, 24(3), 2757. https://doi.org/10.3390/ijms24032757





