High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery
Abstract
1. Introduction
2. Results
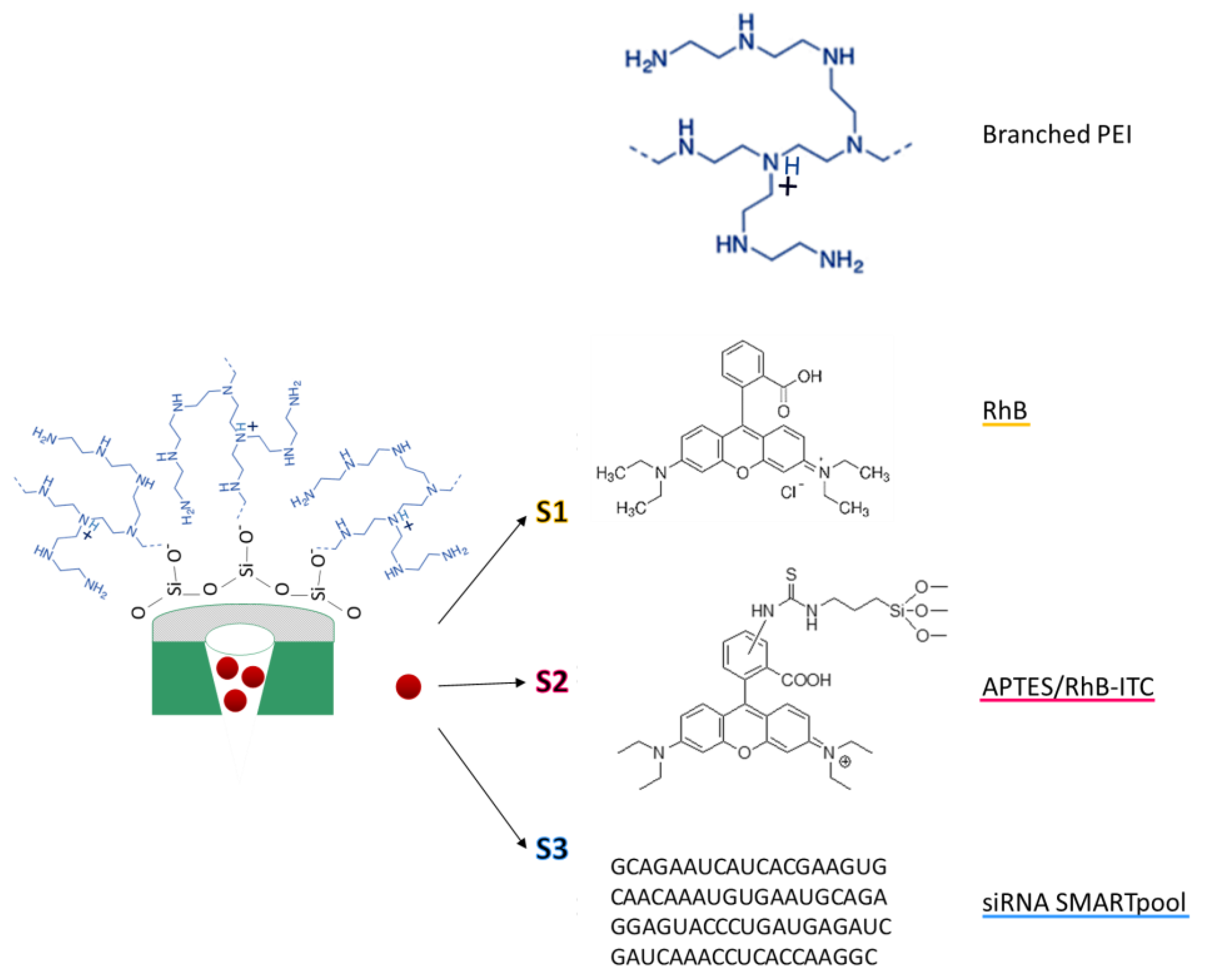
2.1. Design and Synthesis of Functionalised LP-MSNs
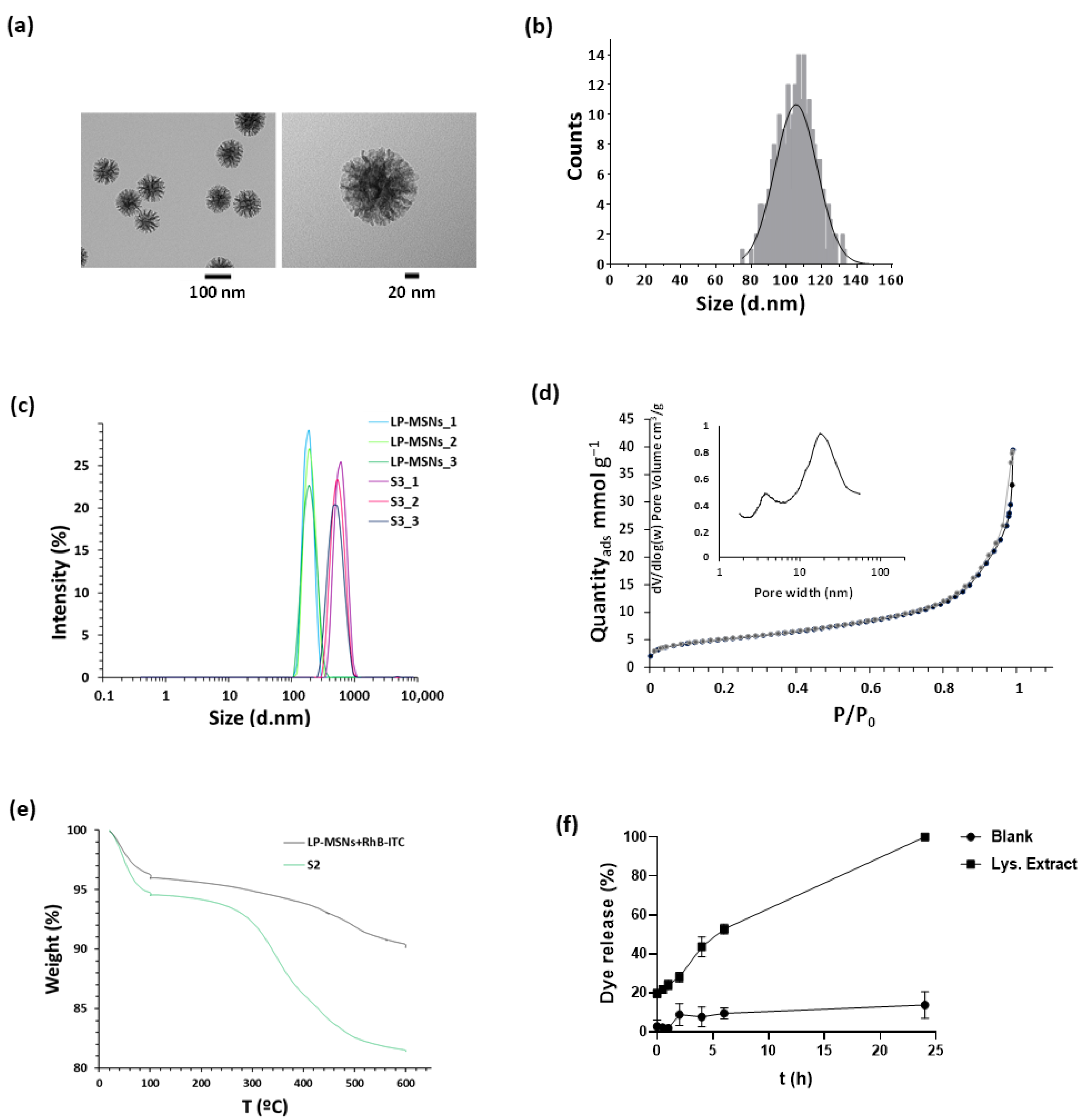
2.2. Nanoparticles Characterisation
2.3. Cargo Release
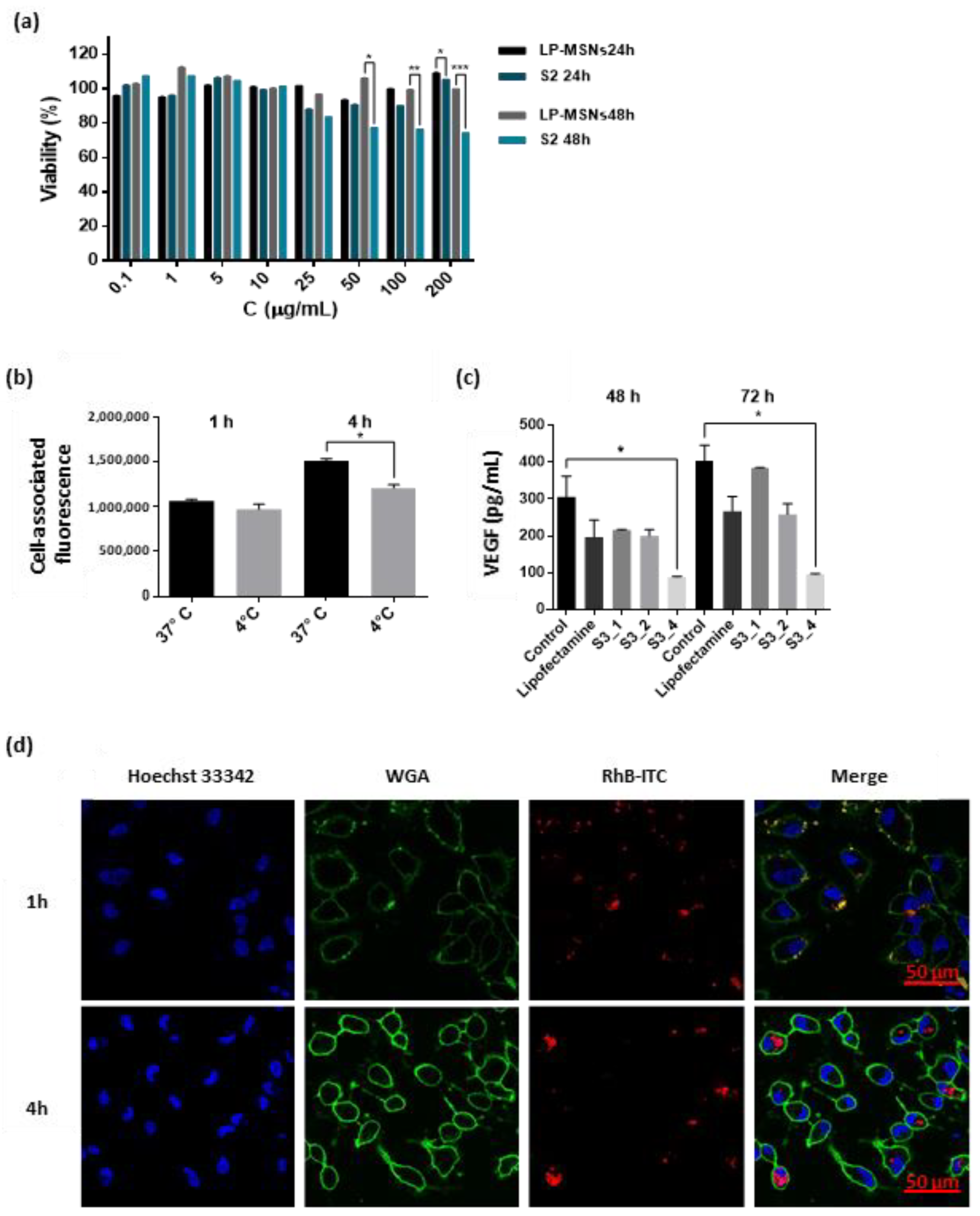
2.4. Nanoparticles Interaction and Activity in ARPE-19 Cells
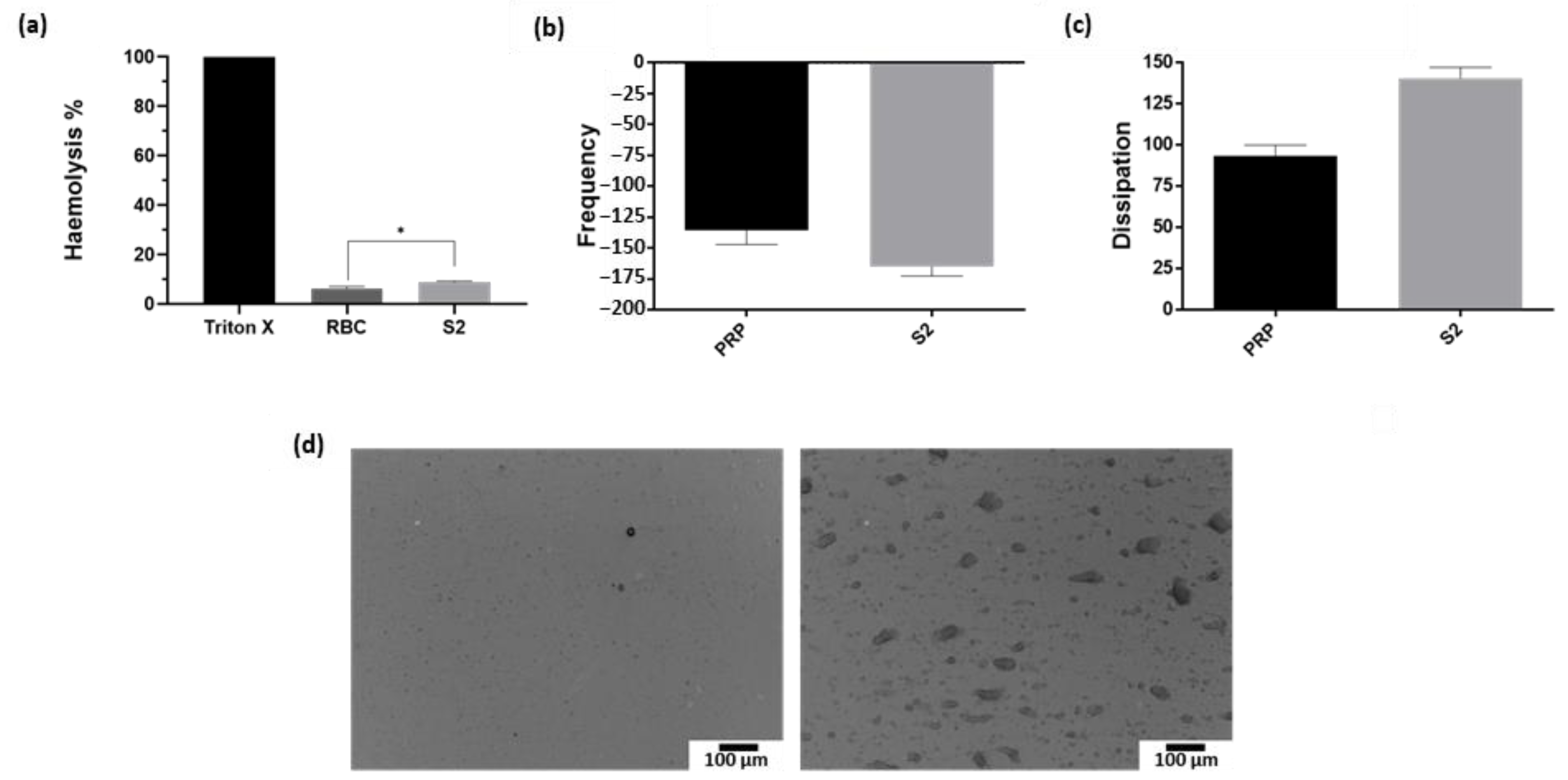
2.5. Nanoparticles Interaction with Red Blood Cells and Platelets
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of Large Pores MSNs (LP-MSNs)
4.3. Functionalisation of LP-MSNs
4.4. Characterisation
4.5. Cargo Release from S1 Nanoparticles
4.6. Cell Culture Conditions
4.7. Viability Assay
4.8. Cellular Uptake
4.9. S3 Activity Assay
4.10. Haemotoxicity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Kim, J.H. How to overcome retinal neuropathy: The fight against angiogenesis related blindness. Arch. Pharm. Res. 2010, 33, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Kamaleddin, M.A. Nano-ophthalmology: Applications and considerations. Nanomedicine 2017, 13, 1459–1472. [Google Scholar] [CrossRef]
- Shen, H.H.; Chan, E.C.; Lee, J.H.; Bee, Y.S.; Lin, T.W.; Dusting, G.J.; Guei-Sheung, L. Nanocarriers for treatment of ocular neovascularation in the back of the eye: New vehicles for ophthalmic drug delivery. Nanomedicine 2015, 10, 2093–2107. [Google Scholar] [CrossRef]
- Michalska-Małecka, K.; Kabiesz, A.; Nowak, M.; Spiewak, D. Age related macular degeneration-Challenge for future: Pathogenesis and new perspectives for the treatment. Eur. Geriatr. Med. 2015, 6, 69–75. [Google Scholar] [CrossRef]
- Bird, A.C. Therapeutic targets in age-related macular disease. J. Clin. Investig. 2010, 110, 3033–3041. [Google Scholar] [CrossRef]
- Birch, D.G.; Liang, F.Q. Age-related macular degeneration: A target for nanotechnology derived medicines. Int. J. Nanomed. 2007, 2, 65–77. [Google Scholar] [CrossRef]
- Volz, C.; Pauly, D. Antibody therapies and their challenges in the treatment of age-related macular degeneration. Eur. J. Pharm. Biopharm. 2015, 95, 158–172. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Seong, H.; Yoon, N.A.; Seo, S.W.; Park, J.W.; Kang, S.S.; Park, J.M.; Han, Y.S. Tristetraprolin regulates the decay of the hypoxia-induced vascular endothelial growth factor mRNA in ARPE-19 cells. Mol. Med. Rep. 2016, 14, 5395–5400. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine 2012, 8, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Silva, R. Subretinal Fluid. In Encyclopedia of Ophthalmology; Schmidt-Erfurth, U., Kohnen, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–3. [Google Scholar]
- Tong, J.; Yao, Y. Contribution of VEGF and PEDF to choroidal angiogenesis: A need for balanced expressions. Clin. Biochem. 2006, 39, 267–276. [Google Scholar] [CrossRef]
- Moleiro, A.F.; Conceição, G.; Leite-Moreira, A.F.; Rocha-Sousa, A. A Critical Analysis of the Available in Vitro and Ex Vivo Methods to Study Retinal Angiogenesis. J. Ophthalmol. 2017, 2017, 3034953. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.C.M.; Lai, T.Y.Y.; Gomi, F.; Ruamviboonsuk, P.; Koh, A.; Lee, W.K. Anti-VEGF Therapy for Neovascular AMD and Polypoidal Choroidal Vasculopathy. Asia Pac. J. Ophthalmol. 2017, 6, 527–534. [Google Scholar] [CrossRef]
- Sloan, F.A.; Hanrahan, B.W. The effects of technological advances on outcomes for elderly persons with exudative age-related macular degeneration. JAMA Ophthalmol. 2014, 132, 456–463. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Zhang, Y. Advance of the application of nano-controlled release system in ophthalmic drug delivery. Drug Deliv. 2016, 23, 2897–2901. [Google Scholar] [CrossRef]
- Kennedy, B.G.; Mangini, N.J. P-glycoprotein expression in human retinal pigment epithelium. Mol. Vis. 2002, 8, 422–430. [Google Scholar]
- Suri, R.; Neupane, Y.R.; Jain, G.K.; Kohli, K. Recent theranostic paradigms for the management of Age-related macular degeneration. Eur. J. Pharm. Sci. 2020, 153, 105489. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Unsal, E.; Cubuk, M.O. The results of aflibercept therapy as a first line treatment of age-related macular degeneration. J. Curr. Ophthalmol. 2019, 31, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sariyeva Ismayılov, A.; Esen, E.; Sızmaz, S.; Demircan, A.N. Aflibercept therapy in eyes with neovascular age-related macular degeneration and its effect on choroidal thickness. Clin. Exp. Optom. 2019, 102, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-related macular degeneration therapy: A review. Curr. Opin. Ophthalmol. 2020, 31, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.; Gallenga, C.E.; Bolletta, E.; Perri, P. The Role of Gene Therapy in the Treatment of Retinal Diseases: A Review. Curr. Gene Ther. 2017, 17, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Marano, R.J.; Wimmer, N.; Kearns, P.S.; Thomas, B.G.; Toth, I.; Brankov, M.; Rakoczy, P.E. Inhibition of in vitro VEGF expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Exp. Eye Res. 2004, 79, 525–535. [Google Scholar] [CrossRef]
- Fattal, E.; Bochot, A. Ocular delivery of nucleic acids: Antisense oligonucleotides, aptamers and siRNA. Adv. Drug Deliv. Rev. 2006, 58, 1203–1223. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Castro-López, V.; Pañeda, C.; Alonso, M.J. Synthetic nanocarriers for the delivery of polynucleotides to the eye. Eur. J. Pharm. Sci. 2017, 103, 5–18. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam. Clin. Pharmacol. 2021, 35, 4–24. [Google Scholar] [CrossRef] [PubMed]
- De Guimaraes, T.A.C.; Georgiou, M.; Bainbridge, J.W.B.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Akyol, E.; Lotery, A. Gene, cell and antibody-based therapies for the treatment of age-related macular degeneration. Biologics 2020, 14, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, E.; Hess, M.; Melen, G.J.; Theek, B.; Talelli, M.; Shi, Y.; Ozbakir, B.; Teunissen, E.A.; Ramírez, M.; Moeckel, D.; et al. PEG-pHPMAm-based polymeric micelles loaded with doxorubicin-prodrugs in combination antitumor therapy with oncolytic vaccinia viruses. Polym. Chem. 2014, 5, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.S.; Herron, C.C.; Hastings, C.L.; Deckers, R.; Lopez Noriega, A.; Kelly, H.M.; Hennink, W.E.; McDonnell, C.O.; O’Brien, F.J.; Ruiz-Hernández, E.; et al. A stimuli responsive liposome loaded hydrogel provides flexible on-demand release of therapeutic agents. Acta Biomater. 2017, 48, 110–119. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Rovira, M.; Galiana, I.; Giménez, C.; Lozano-Torres, B.; Paez-Ribes, M.; Llanos, S.; Chaib, S.; Muñoz-Martín, M.; Ucero, A.C.; et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018, 10, e9355. [Google Scholar] [CrossRef]
- de la Torre, C.; Casanova, I.; Acosta, G.; Coll, C.; Moreno, M.J.; Albericio, F.; Aznar, E.; Mangues, R.; Royo, M.; Sancenón, F.; et al. Gated Mesoporous Silica Nanoparticles Using a Double-Role Circular Peptide for the Controlled and Target-Preferential Release of Doxorubicin in CXCR4-Expresing Lymphoma Cells. Adv. Funct. Mater. 2015, 25, 687–695. [Google Scholar] [CrossRef]
- Ultimo, A.; Giménez, C.; Bartovsky, P.; Aznar, E.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Bernardo, A.R.; Martínez-Máñez, R.; Jiménez-Lara, A.M.; et al. Targeting Innate Immunity with dsRNA-Conjugated Mesoporous Silica Nanoparticles Promotes Antitumor Effects on Breast Cancer. Chem.-A Eur. J. 2016, 22, 1582–1586. [Google Scholar] [CrossRef]
- Knežević, N.Z.; Ruiz-Hernández, E.; Hennink, W.E.; Vallet-Regí, M. Magnetic mesoporous silica-based core/shell nanoparticles for biomedical applications. RSC Adv. 2013, 3, 9584–9593. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine-Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Mánez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; Lozano-Torres, B.; Bernardos, A.; Martínez-Máñez, R.; Sancenón, F. Mesoporous silica materials for controlled delivery based on enzymes. J. Mater. Chem. B 2017, 5, 3069–3083. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomed. 2018, 13, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Hocine, O.; Maynadier, M.; Gallud, A.; Da Silva, A.; Mongin, O.; Blanchard-Desce, M.; et al. Multifunctionalized mesoporous silica nanoparticles for the in vitro treatment of retinoblastoma: Drug delivery, one and two-photon photodynamic therapy. Int. J. Pharm. 2012, 432, 99–104. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. EpCAM antibody-conjugated mesoporous silica nanoparticles to enhance the anticancer efficacy of carboplatin in retinoblastoma. Mater. Sci. Eng. C 2017, 76, 646–651. [Google Scholar] [CrossRef]
- Liao, Y.T.; Lee, C.H.; Chen, S.T.; Lai, J.Y.; Wu, K.C.W. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013. [Google Scholar] [CrossRef]
- Sun, J.G.; Jiang, Q.; Zhang, X.P.; Shan, K.; Liu, B.H.; Zhao, C.; Yan, B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Yim, B.; Park, J.H.; Jeong, H.; Hong, J.; Shin, Y.J.; Chuck, R.S.; Park, C.Y. The effects of nonporous silica nanoparticles on cultured human keratocytes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, H.; Hong, J.; Chang, M.; Kim, M.; Chuck, R.S.; Lee, J.K.; Park, C.Y. The Effect of Silica Nanoparticles on Human Corneal Epithelial Cells. Sci. Rep. 2016, 6, 37762. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.H.; Park, C.Y. An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gong, L.; Xie, J.; Gu, X.; Li, Y.; Cao, Q.; Li, Q.; A., L.; Gu, Z.; Xu, H. Toxicity of silicon dioxide nanoparticles with varying sizes on the cornea and protein corona as a strategy for therapy. Sci. Bull. 2018, 63, 907–916. [Google Scholar] [CrossRef]
- González, B.; Ruiz-Hernández, E.; Feito, M.J.; López De Laorden, C.; Arcos, D.; Ramírez-Santillán, C.; Matesanz, C.; Portolés, M.T.; Vallet-Regí, M. Covalently bonded dendrimer-maghemite nanosystems: Nonviral vectors for in vitro gene magnetofection. J. Mater. Chem. 2011, 21, 4598–4604. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef]
- Du, X.; Xiong, L.; Dai, S.; Kleitz, F.; Qiao, S.Z. Intracellular Microenvironment-Responsive Dendrimer-Like Mesoporous Nanohybrids for Traceable, Effective, and Safe Gene Delivery. Adv. Funct. Mater. 2014, 24, 7627–7637. [Google Scholar] [CrossRef]
- Hom, C.; Lu, J.; Liong, M.; Luo, H.; Li, Z.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small 2010, 6, 1185–1190. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Snipstad, S.; Westrøm, S.; Mørch, Y.; Afadzi, M.; Åslund, A.K.O.; de Lange, D.C. Contact-mediated intracellular delivery of hydrophobic drugs from polymeric nanoparticles. Cancer Nanotechnol. 2014, 5, 8. [Google Scholar] [CrossRef]
- Kapara, A.; Brunton, V.; Graham, D.; Faulds, K. Investigation of cellular uptake mechanism of functionalised gold nanoparticles into breast cancer using SERS. Chem. Sci. 2020, 11, 5819–5829. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martinez, M.J. A Novel Method for the Measurement of Flow-Induced Platelet Activation at Nanoscale. Ph.D. Thesis, Trinity College Dublin, Dublin, Ireland, 2009. Available online: http://www.tara.tcd.ie/handle/2262/78201 (accessed on 2 February 2022).
- Santos-Martinez, M.J.; Medina, C.; Prina-Mello, A.; Conroy, J.; Samuels, S.P.; Volkov, Y.; Radomski, M.W. A nanoscale resolution assay of flow-induced platelet microaggregation Mikroagregacja płytek indukowana przepływem-badanie w nanorozdzielczości. Forum Eekspertow 2010, 7, 365–375. [Google Scholar]
- Santos-Martínez, M.J.; Prina-Mello, A.; Medina, C.; Radomski, M.W. Analysis of platelet function: Role of microfluidics and nanodevices. Analyst 2011, 136, 5120–5126. [Google Scholar] [CrossRef] [PubMed]
- Larkin, C.M.; Breen, E.P.; Tomaszewski, K.A.; Eisele, S.; Radomski, M.W.; Ryan, T.A.; Ryan, T.A.; Santos-Martínez, M.J. Platelet microaggregation in sepsis examined by quartz crystal microbalance with dissipation technology. Platelets 2018, 29, 301–304. [Google Scholar] [CrossRef]
- Santos-Martínez, M.J.; Inkielewicz-Stepniak, I.; Medina, C.; Rahme, K.; Arcy, D.; Fox, D.; Holmes, J.D.; Zhang, H.; Radomski, M.W. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int. J. Nanomed. 2012, 7, 243–255. [Google Scholar] [CrossRef]
- Santos-Martínez, M.J.; Rahme, K.; Corbalan, J.J.; Faulkner, C.; Holmes, J.D.; Tajber, L.; Medina, C.; Radomski, M.W. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1004–1015. [Google Scholar] [CrossRef]
- Medina, C.; Tomaszewski, K.; Gilmer, J.; Bazou, D.; Santos-Martínez, M.J.; Radomski, M.W. Pharmacological characterization of nanoparticle-induced platelet microaggregation using quartz crystal microbalance with dissipation: Comparison with light aggregometry. Int. J. Nanomed. 2015, 10, 5107–5119. [Google Scholar] [CrossRef]
- Samuel, S.P.; Santos-Martínez, M.J.; Medina, C.; Jain, N.; Radomski, M.W.; Prina-Mello, A.; Volkov, Y. CdTe quantum dots induce activation of human platelets: Implications for nanoparticle hemocompatibility. Int. J. Nanomed. 2015, 10, 2723–2734. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Fredriksson, C.; Kihlman, S.; Rodahl, M.; Kasemo, B. The piezoelectric quartz crystal mass and dissipation sensor: A means of studying cell adhesion. Langmuir 1998, 14, 248–251. [Google Scholar] [CrossRef]
- Kaji, H.; Nagai, N.; Nishizawa, M.; Abe, T. Drug delivery devices for retinal diseases. Adv. Drug Deliv. Rev. 2018, 128, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Hariprasad, S.M.; Ciulla, T.A. Treatment burden in neovascular AMD: Visual acuity outcomes are associated with anti-VEGF injection frequency. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Prado, D.A.; Acosta-Acero, M.; Maldonado, R.S. Gene therapy beyond luxturna: A new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 2020, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Baran-Rachwalska, P.; Torabi-Pour, N.; Sutera, F.M.; Ahmed, M.; Thomas, K.; Nesbit, M.A.; Welsh, M.; Moore, C.B.T.; Saffie-Siebert, S.R. Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles. J. Control. Release 2020, 326, 192–202. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Lajunen, T.; Hisazumi, K.; Kanazawa, T.; Okada, H.; Seta, Y.; Yliperttula, M.; Urtti, A.; Takashima, Y. Topical drug delivery to retinal pigment epithelium with microfluidizer produced small liposomes. Eur. J. Pharm. Sci. 2014, 62, 23–32. [Google Scholar] [CrossRef]
- Scheive, M.; Yazdani, S.; Hajrasouliha, A.R. The utility and risks of therapeutic nanotechnology in the retina. Ther. Adv. Ophthalmol. 2021, 13, 25158414211003380. [Google Scholar] [CrossRef]
- Möller, K.; Müller, K.; Engelke, H.; Bräuchle, C.; Wagner, E.; Bein, T. Highly efficient siRNA delivery from core–shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale 2016, 8, 4007–4019. [Google Scholar] [CrossRef]
- Wang, T.; Larcher, L.M.; Ma, L.; Veedu, R.N. Systematic Screening of Commonly Used Commercial Transfection Reagents towards Efficient Transfection of Single-Stranded Oligonucleotides. Molecules 2018, 23, 2564. [Google Scholar] [CrossRef]
- Li, X.; Xie, Q.R.; Zhang, J.; Xia, W.; Gu, H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials 2011, 32, 9546–9556. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, 73, e50166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ultimo, A.; Orzaez, M.; Santos-Martinez, M.J.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Ruiz-Hernández, E. High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery. Int. J. Mol. Sci. 2023, 24, 2753. https://doi.org/10.3390/ijms24032753
Ultimo A, Orzaez M, Santos-Martinez MJ, Martínez-Máñez R, Marcos MD, Sancenón F, Ruiz-Hernández E. High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery. International Journal of Molecular Sciences. 2023; 24(3):2753. https://doi.org/10.3390/ijms24032753
Chicago/Turabian StyleUltimo, Amelia, Mar Orzaez, Maria J. Santos-Martinez, Ramón Martínez-Máñez, María D. Marcos, Félix Sancenón, and Eduardo Ruiz-Hernández. 2023. "High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery" International Journal of Molecular Sciences 24, no. 3: 2753. https://doi.org/10.3390/ijms24032753
APA StyleUltimo, A., Orzaez, M., Santos-Martinez, M. J., Martínez-Máñez, R., Marcos, M. D., Sancenón, F., & Ruiz-Hernández, E. (2023). High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery. International Journal of Molecular Sciences, 24(3), 2753. https://doi.org/10.3390/ijms24032753






