MADS-Box Subfamily Gene GmAP3 from Glycine max Regulates Early Flowering and Flower Development
Abstract
1. Introduction
2. Results
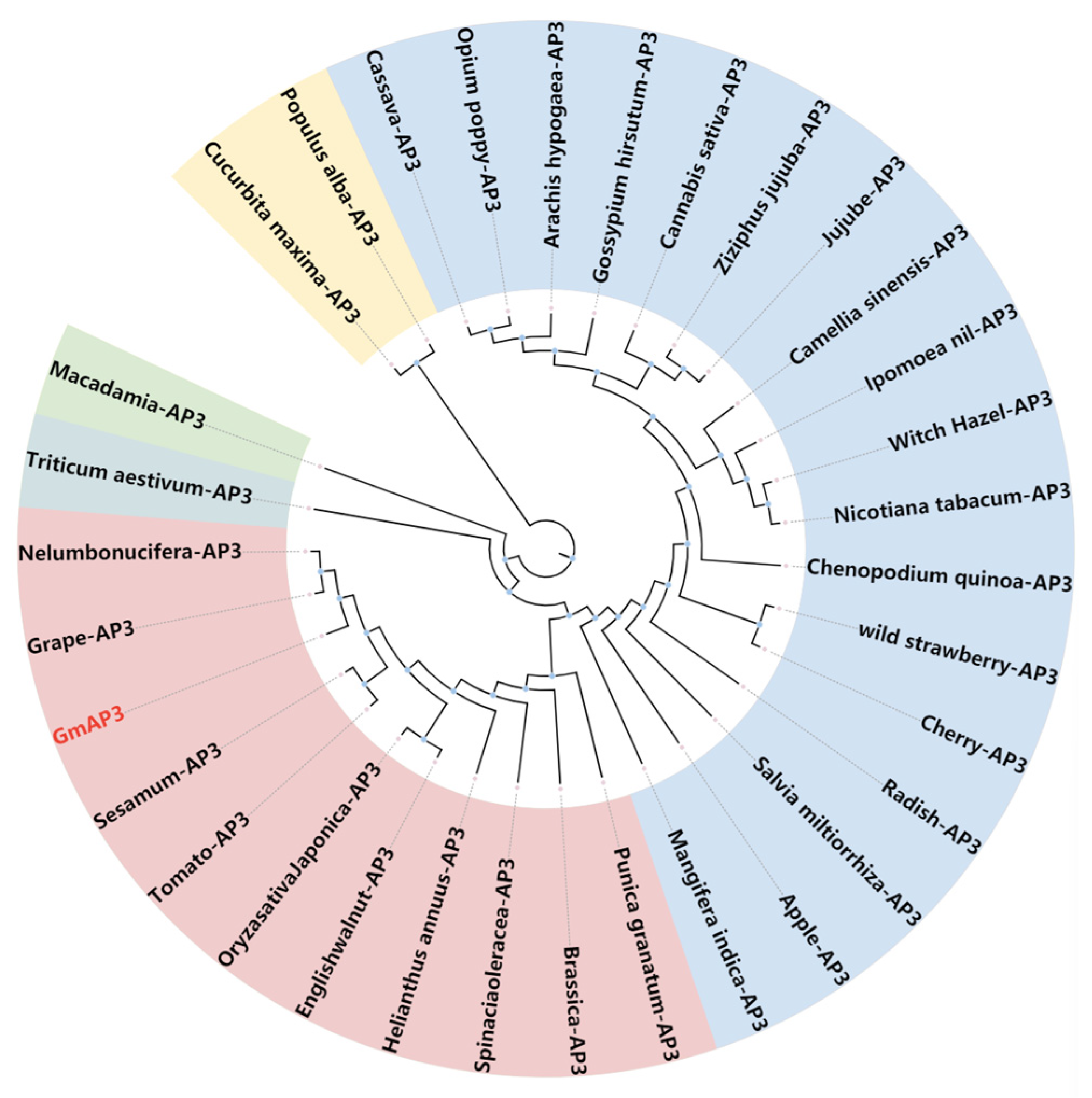
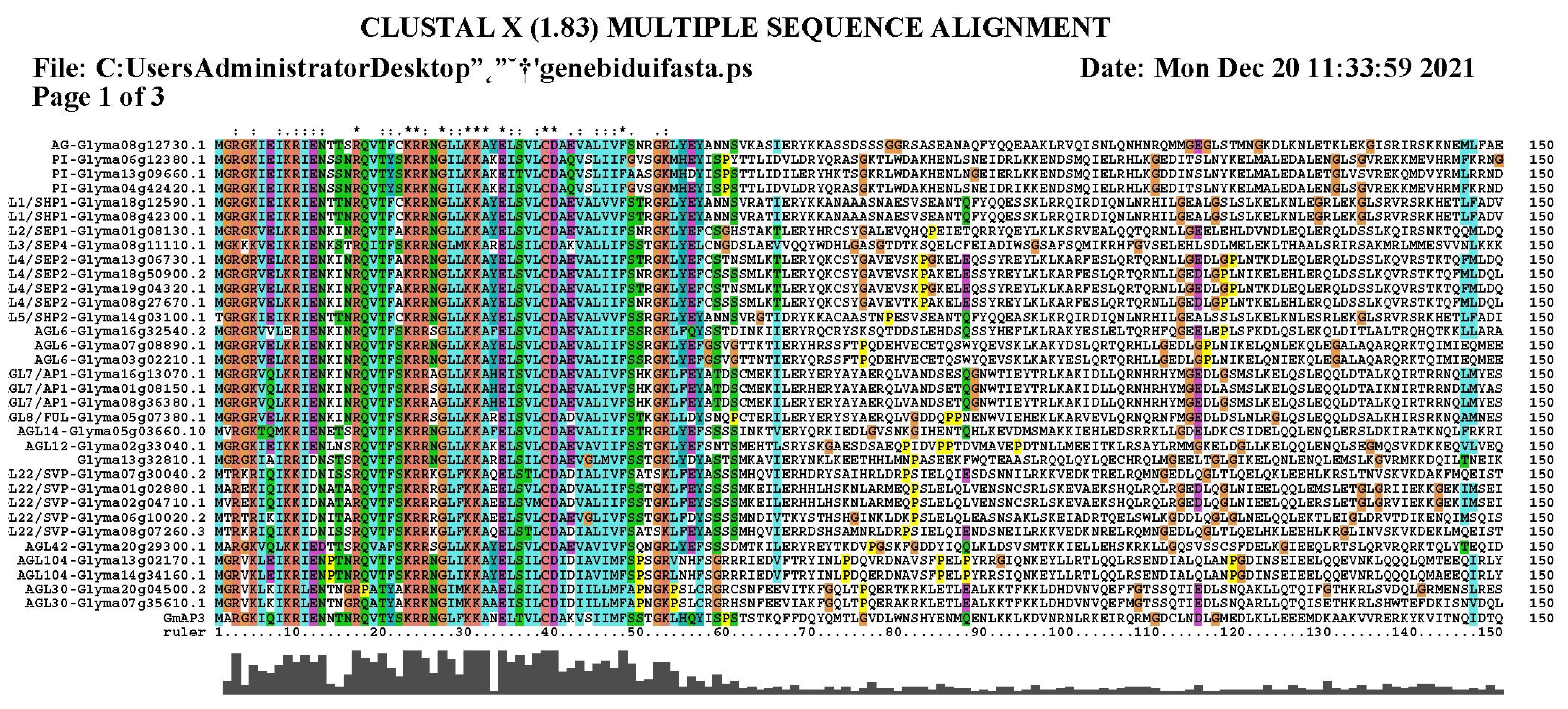
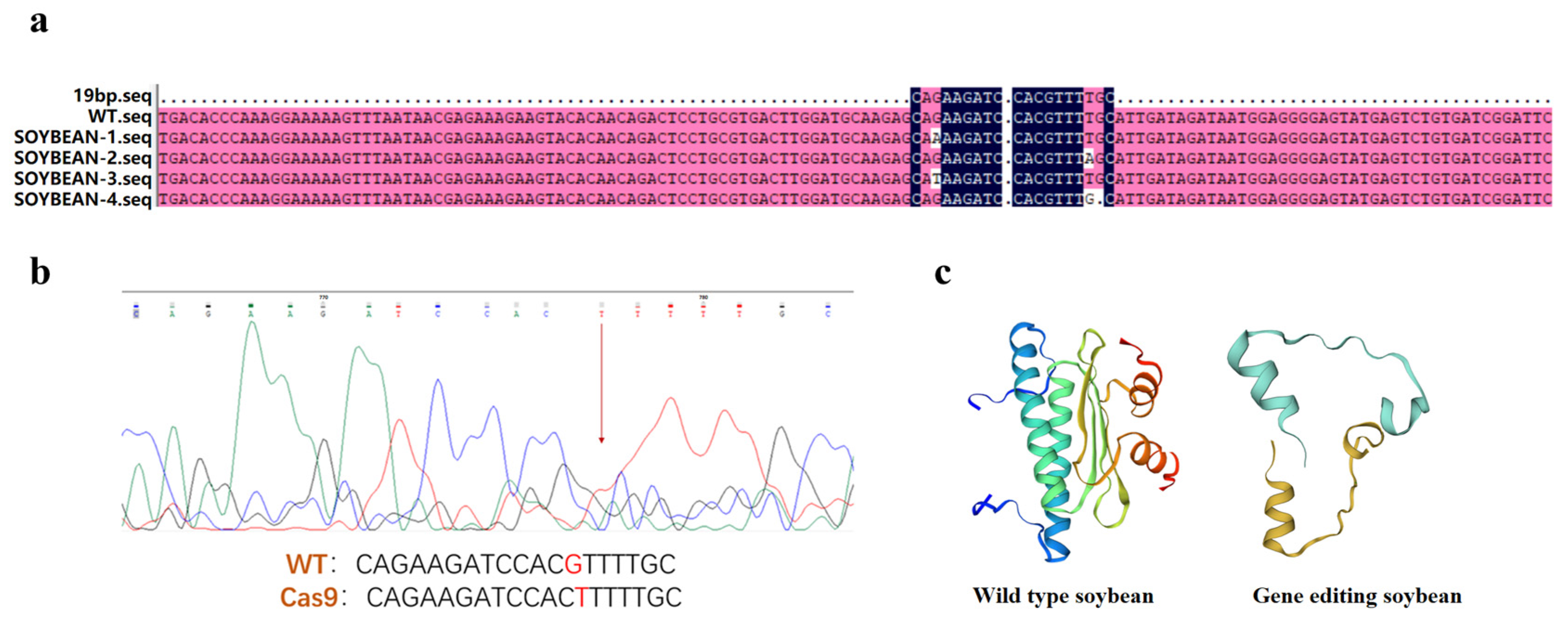
2.1. Gene Cloning and Sequence Analysis of GmAP3
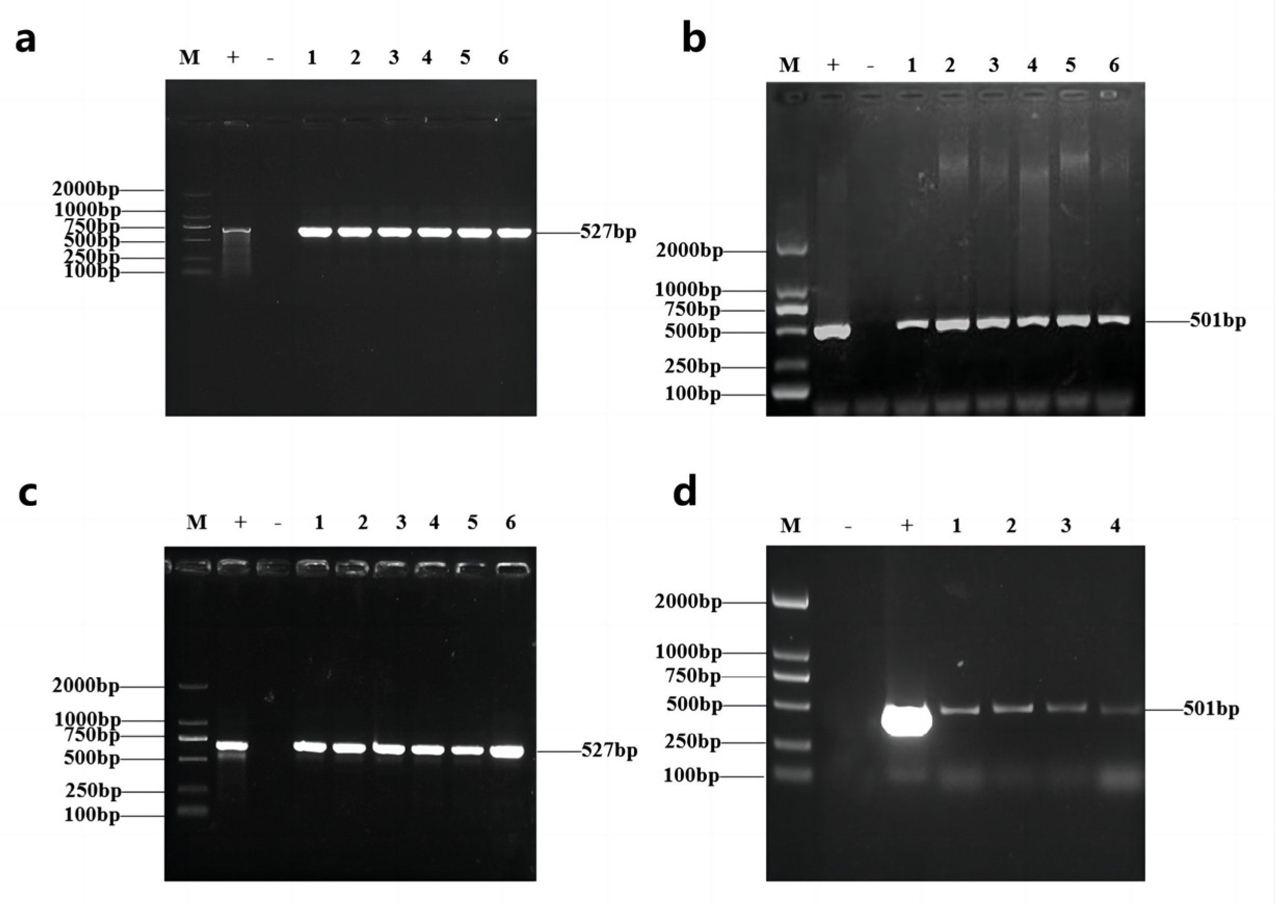
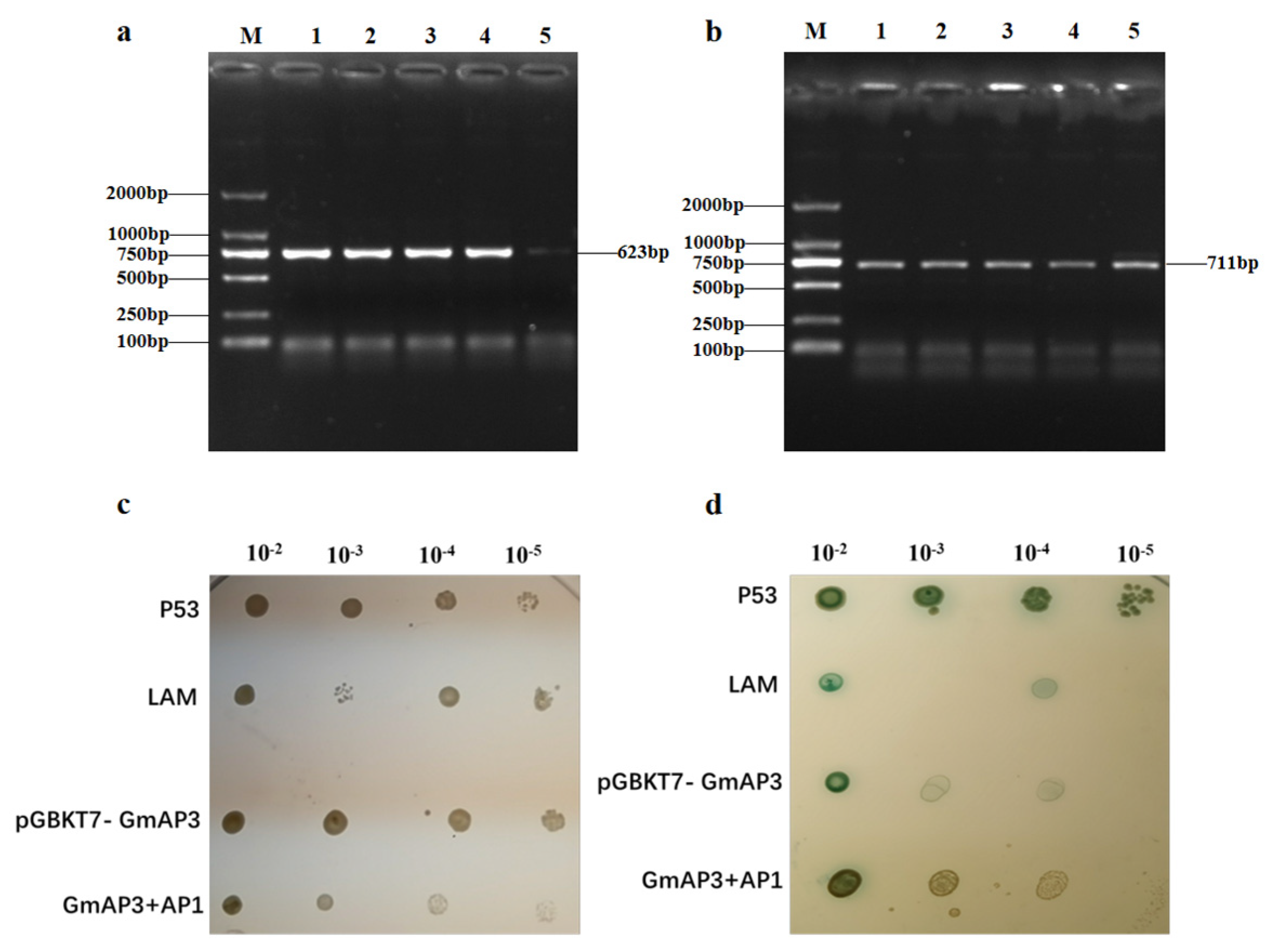
2.2. Genetic Transformation of GmAP3 Gene in Tobacco and Soybean
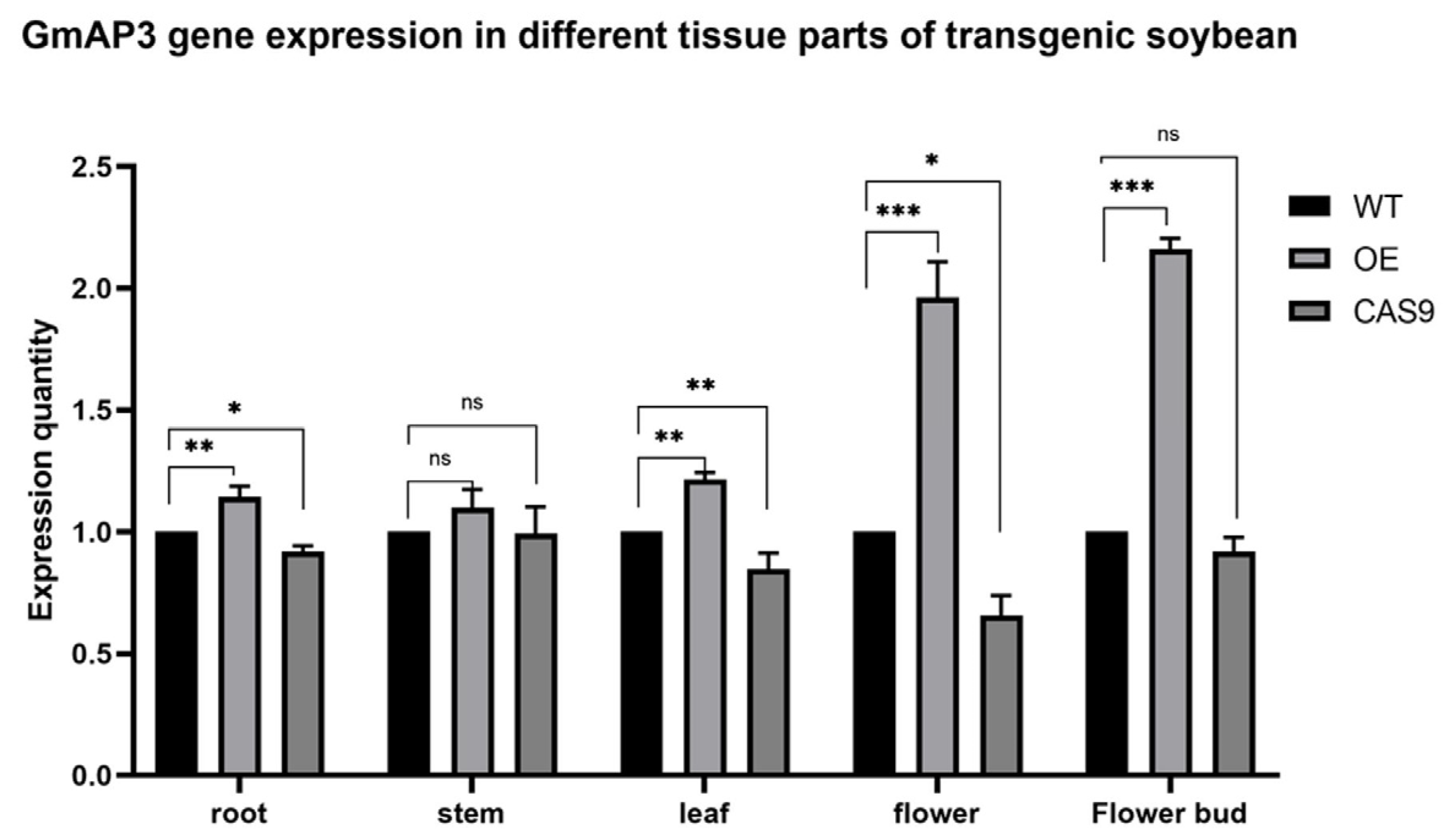
2.3. Potential Expression Profiles of GmAP3 in Flowering Regulation
2.4. GmAP3 Regulated the Expression of Genes Involved in Flower Development
2.5. GmAP3 Affected Flower Development by Regulating AP1
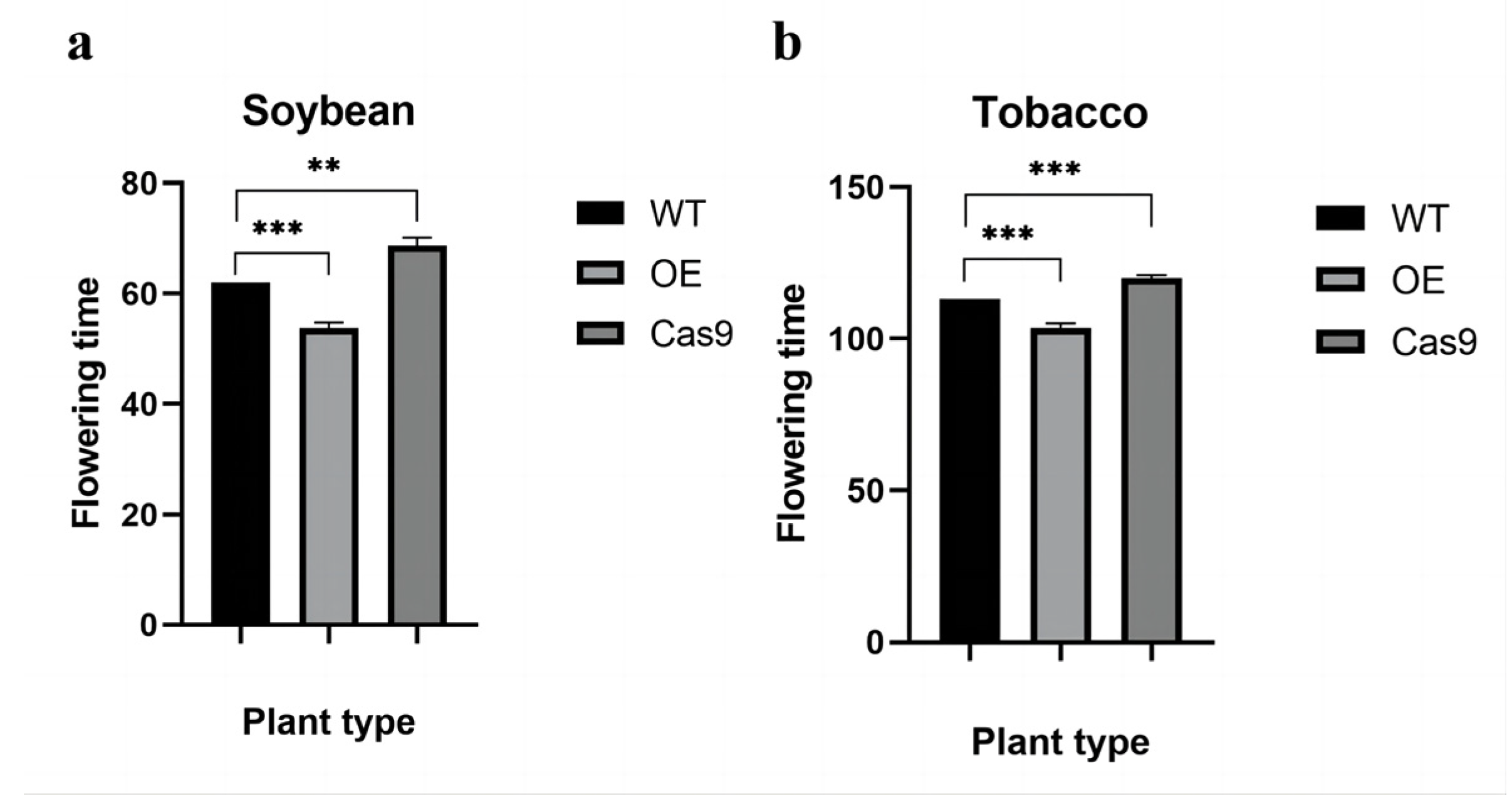
2.6. GmAP3 Advanced the Flowering Time
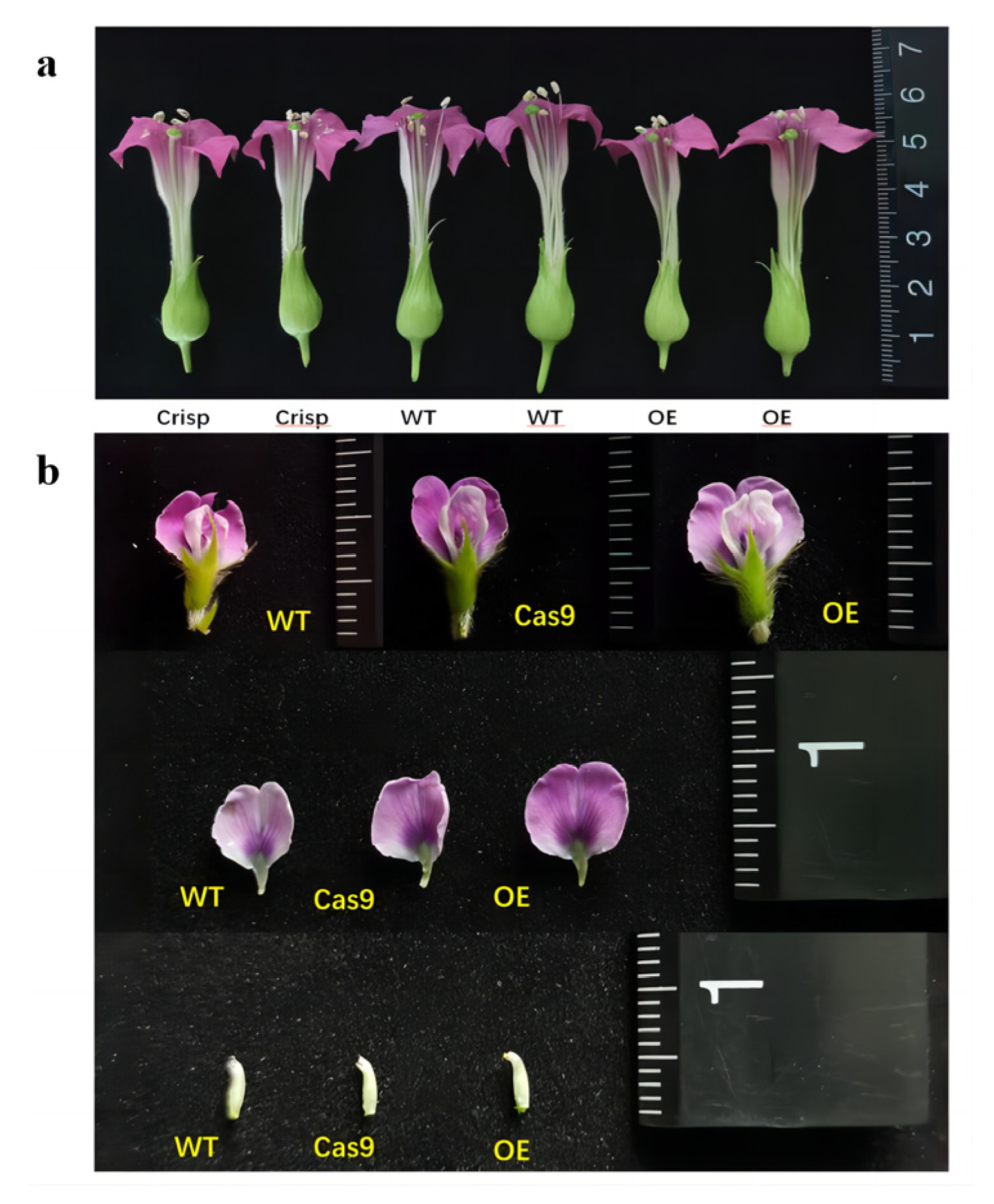
2.7. GmAP3 Advanced Flower Organs Development
2.8. Overexpression of GmAP3 Increased Seed Yield
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Isolation and Reverse Transcription
4.3. Cloning of Full-Length GmAP3 cDNA
4.4. Bioinformatics Analysis
4.5. Construction of Overexpression and Editing Vectors
4.6. Transformation of Soybean by Pollen Tube Pathway
4.7. Ectopic Expression in Tobacco
4.8. Quantitative Real-Time PCR Analysis
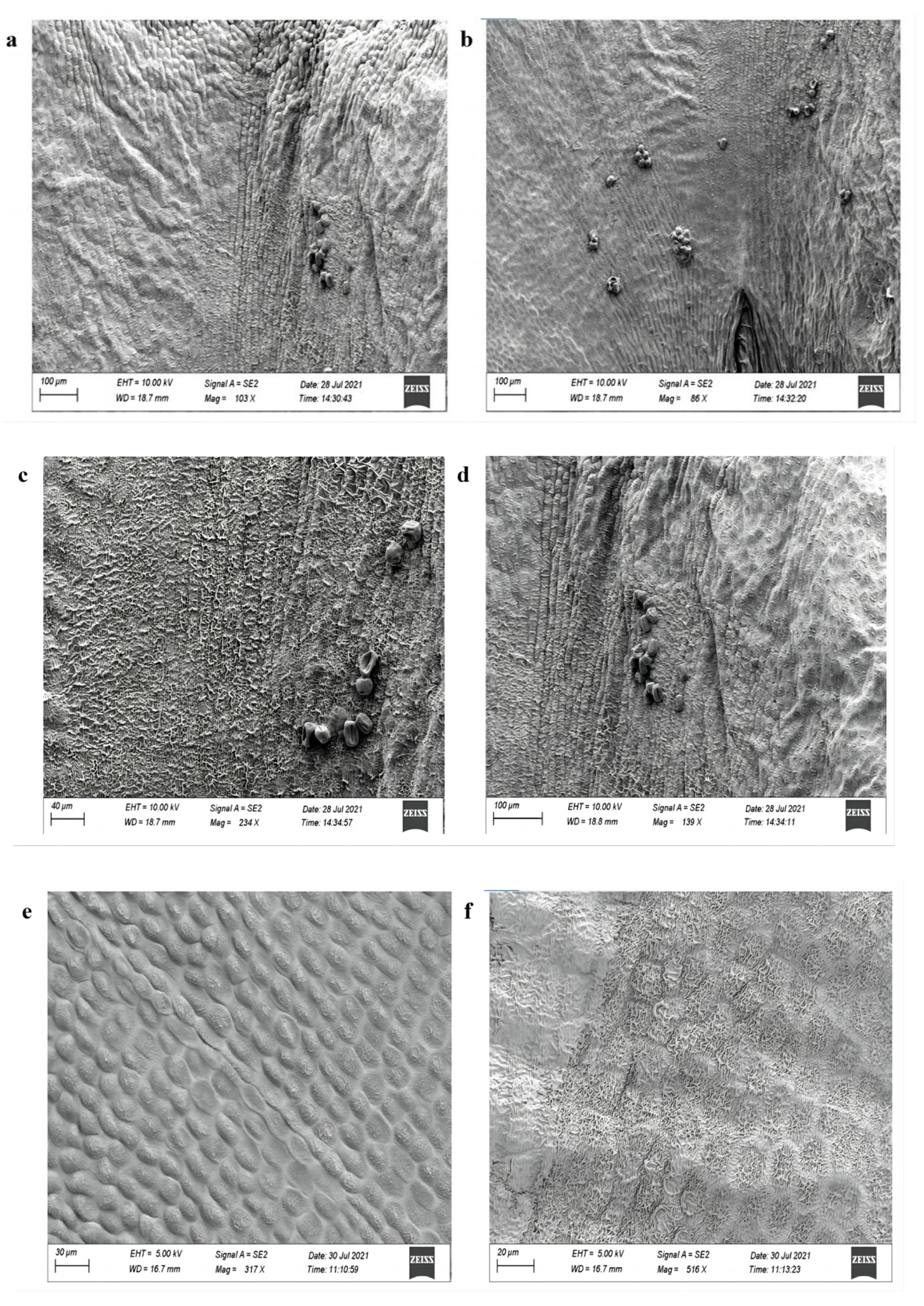
4.9. Electron Microscopy Analysis
4.10. Yeast Two-Hybrid Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, A.; Theissen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenetics Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Zhang, X.; Fatima, M.; Zhou, P.; Ma, Q.; Ming, R. Analysis of MADS-box genes revealed modified flowering gene network and diurnal expression in pineapple. BMC Genom. 2020, 21, 8–24. [Google Scholar] [CrossRef]
- Won, S.Y.; Jung, J.A.; Kim, J.S. Genome-wide analysis of the MADS-Box gene family in Chrysanthemum. Comput. Biol. Chem. 2021, 90, 107424–107434. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Wang, H.; Huang, J. Genome-Wide Identification and Expression Analysis of MADS-Box Family Genes in Litchi (Litchi chinensis Sonn.) and Their Involvement in Floral Sex Determination. Plants 2021, 10, 2142–2158. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; Fan, X. MawuAP1 promotes flowering and fruit development in the basal angiosperm Magnolia wufengensis (Magnoliaceae). Tree Physiol. 2020, 40, 1247–1259. [Google Scholar] [CrossRef]
- Tzeng, T.Y.; Yang, C.H. A MADS box gene from lily (Lilium Longiflorum) is sufficient to generate dominant negative mutation by interacting with PISTILLATA (PI) in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 1156–1168. [Google Scholar] [CrossRef]
- Joshi, S.; Keller, C.; Perry, S.E. The EAR Motif in the Arabidopsis MADS Transcription Factor AGAMOUS-Like 15 Is Not Necessary to Promote Somatic Embryogenesis. Plants 2021, 10, 758–770. [Google Scholar] [CrossRef]
- Wang, Y.S.; Guo, P.Y.; Zhang, J.L. Overexpression of the MADS-box gene SlMBP21 alters leaf morphology and affects reproductive development in tomato. J. Integr. Agr. 2021, 20, 3170–3185. [Google Scholar] [CrossRef]
- Yi, S.Y.; Rameneni, J.J.; Lee, M.; Song, S.G.; Choi, Y.; Lu, L.; Lee, H.; Lim, Y.P. Comparative Transcriptome-Based Mining of Senescence-Related MADS, NAC, and WRKY Transcription Factors in the Rapid-Senescence Line DLS-91 of Brassica rapa. Int. J. Mol. Sci. 2021, 22, 6017–6040. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Chen, W.H.; Shen, Y.H.; Hsu, W.H.; Mao, W.T.; Yang, C.H. Multifunctional evolution of B and AGL6 MADS box genes in orchids. Nat. Commun. 2021, 12, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, K.; Grierson, D. A critical evaluation of the role of ethylene and MADS transcription factors in the network controlling fleshy fruit ripening. New Phytol. 2019, 221, 1724–1741. [Google Scholar] [CrossRef]
- Dong, T.T.; Song, W.H.; Tan, C.T. Molecular characterization of nine sweet potato (Ipomoea batatas Lam.) MADS-box transcription factors during storage root development and following abiotic stress. Plant Breed. 2018, 137, 1–15. [Google Scholar] [CrossRef]
- Shi, S.Y.; Zhang, F.F.; Gao, S. Expression pattern and function analyses of the MADS thranscription factor genes in wheat (Triticum aestivum L.) under phosphorus-starvation condition. J. Integr. Agr. 2016, 8, 1703–1715. [Google Scholar] [CrossRef]
- Quinet, M.; Bataille, G.; Dobrev, P.I.; Capel, C.; Gómez, P.; Capel, J.; Lutts, S.; Motyka, V.; Angosto, T.; Lozano, R. Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B-class APETALA3 gene. J. Exp. Bot. 2014, 65, 2243–2256. [Google Scholar] [CrossRef]
- Ehlers, K.; Bhide, A.S.; Tekleyohans, D.G.; Wittkop, B.; Snowdon, R.J.; Becker, A. The MADS Box Genes ABS, SHP1, and SHP2 Are Essential for the Coordination of Cell Divisions in Ovule and Seed Coat Development and for Endosperm Formation in Arabidopsis thaliana. PLoS ONE 2016, 11, e0165075. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, R.; Wang, Y.; Li, X.; Ji, M.; Wang, X. NAC domain gene VvNAC26 interacts with VvMADS9 and influences seed and fruit development. Plant Physiol. Biochem. 2021, 164, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Xu, G.; Chi, Y. A soybean MADS-box protein modulates floral organ numbers, petal identity and sterility. BMC Plant Biol. 2014, 14, 89–102. [Google Scholar] [CrossRef]
- Lyu, J.; Cai, Z.; Li, Y. The Floral Repressor GmFLC-like Is Involved in Regulating Flowering Time Mediated by Low Temperature in Soybean. Int. J. Mol. Sci. 2020, 21, 1322. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.; Zhao, C. GmFULc Is Induced by Short Days in Soybean and May Accelerate Flowering in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 10333–10335. [Google Scholar] [CrossRef]
- Zhao, T.J.; Gai, J.Y. Discovery of new male-sterile cytoplasm sources and development of a new cytoplasmic-nuclear male-sterile line NJCMS3A in soybean. Euphytica 2006, 152, 387–396. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, H.; Zhang, D. Ectopic expression of a soybean SVP-like gene in tobacco causes abnormal floral organs and shortens the vegetative phase. Plant Growth Regul. 2016, 80, 345–353. [Google Scholar] [CrossRef]
- Yue, Y.; Sun, S.; Li, J. GmFULa improves soybean yield by enhancing carbon assimilation without altering flowering time or maturity. Plant Cell Rep. 2021, 40, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Jia, Y.; Wang, W.L. Evolutionary divergence of motifs in B-class MADS-box proteins of seed plants. J. Biol. Res. 2021, 28, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Sun, H.; Dai, S. Identification and Characterization of MIKCc-Type MADS-Box Genes in the Flower Organs of Adonis amurensis. Int. J. Mol. Sci. 2021, 22, 9362–9373. [Google Scholar] [CrossRef]
- Tyagi, S.; Sri, T.; Singh, A. SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 influences flowering time, lateral branching, oil quality, and seed yield in Brassica juncea cv. Varuna. Funct. Integr. Genom. 2019, 19, 43–60. [Google Scholar] [CrossRef]
- Chen, C.; Begcy, K.; Liu, K. Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. Plant Physiol. 2016, 171, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Chen, W.; Shi, M. Ectopic expression of an Eriobotrya japonica APETALA3 ortholog rescues the petal and stamen identities in Arabidopsis ap3-3 mutant. Biochem. Biophys. Res. Commun. 2020, 523, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.W.; Qi, R.; Li, X.F.; Liu, Z.X. Ectopic expression of FaesAP3, a Fagopyrum esculentum (Polygonaceae) AP3 orthologous gene rescues stamen development in an Arabidopsis ap3 mutant. Gene 2014, 550, 200–206. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.D.; Morabito, G.; Fiers, M.; van Ham, R.C.; Angenent, G.C.; Immink, R.G. Sequence motifs in MADS transcription factors responsible for specificity and diversification of protein-protein interaction. PLoS Comput. Biol. 2010, 6, e1001017. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Larson-Rabin, Z.; Li, D.Z.; Wang, G.Y.; Peng, S.; Li, C.Y. The expression and phylogenetic analysis of four AP3-like paralogs in the stamens, carpels, and single-whorl perianth of the paleoherb Asarum caudigerum. Mol. Biol. Rep. 2013, 40, 4691–4699. [Google Scholar] [CrossRef]
- Mao, W.T.; Hsu, H.F.; Hsu, W.H.; Li, J.Y.; Lee, Y.I.; Yang, C.H. The C-Terminal Sequence and PI motif of the Orchid (Oncidium Gower Ramsey) PISTILLATA (PI) Ortholog Determine its Ability to Bind AP3 Orthologs and Enter the Nucleus to Regulate Downstream Genes Controlling Petal and Stamen Formation. Plant Cell Physiol. 2015, 56, 2079–2099. [Google Scholar] [PubMed]
- Martín-Pizarro, C.; Triviño, J.C.; Posé, D. Functional analysis of the TM6 MADS-box gene in the octoploid strawberry by CRISPR/Cas9-directed mutagenesis. J. Exp. Bot. 2019, 70, 885–895. [Google Scholar] [CrossRef]
- Xing, M.; Li, H.; Liu, G.; Zhu, B.; Zhu, H.; Grierson, D.; Luo, Y.; Fu, D. A MADS-box transcription factor, SlMADS1, interacts with SlMACROCALYX to regulate tomato sepal growth. Plant Sci. 2022, 322, 111366. [Google Scholar] [CrossRef]
- Lu, Q.S.M.; Tian, L. An efficient and specific CRISPR-Cas9 genome editing system targeting soybean phytoene desaturase genes. BMC Biotechnol. 2022, 15, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Botella, J.R. Now for the hard ones: Is there a limit on CRISPR genome editing in crops? J. Exp. Bot. 2019, 70, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, R.; Palla, K.J.; Tuskan, G.A.; Yang, X. Advances and perspectives on the use of CRISPR/Cas9 systems in plant genomics research. Curr. Opin. Plant Biol. 2016, 30, 70–77. [Google Scholar] [CrossRef]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR screens in plants: Approaches, guidelines, and future prospects. Plant Cell. 2021, 33, 794–813. [Google Scholar] [CrossRef]
- Fauser, F.; Schiml, S.; Puchta, H. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J. 2014, 79, 348–359. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of CRISPR/Cas9 in Crop Quality Improvement. Int. J. Mol. Sci. 2021, 22, 4206–4221. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2020, 29, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Carrijo, J.; Illa-Berenguer, E.; LaFayette, P. Two efficient CRISPR/Cas9 systems for gene editing in soybean. Transgenic Res. 2021, 30, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.S.N.; Leguilloux, M.; Bellec, A.; Rodde, N.; Aubert, J.; Manicacci, D.; Damerval, C.; Berges, H.; Deveaux, Y. A MITE insertion abolishes the AP3-3 self-maintenance regulatory loop in apetalous flowers of Nigella damascena. J. Exp. Bot. 2022, 13, erac489. [Google Scholar] [CrossRef]
- Thomson, B.; Wellmer, F. Molecular regulation of flower development. Curr. Top. Dev. Biol. 2019, 131, 185–210. [Google Scholar] [PubMed]
- Li, Q.; Li, K.; Zhang, Z. Transcriptomic comparison sheds new light on regulatory networks for dimorphic flower development in response to photoperiod in Viola prionantha. BMC Plant Biol. 2022, 22, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Cao, Z.; Wu, P. Genome-wide identification, interaction of the MADS-box proteins in Zanthoxylum armatum and functional characterization of ZaMADS80 in floral development. Front. Plant Sci. 2022, 13, 1038828. [Google Scholar] [CrossRef]
- Liu, Y.; Dreni, L.; Zhang, H. A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 15711–15730. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.J.; Ye, L.X.; Ai, X.Y.; Hu, C.G.; Cheng, Z.P.; Zhang, J.Z. Functional analysis of a PISTILLATA-like gene CcMADS20 involved in floral organs specification in citrus. Plant Sci. 2022, 319, 111263. [Google Scholar] [CrossRef]
- Myat, A.A.; Zhou, Y.; Gao, Y. Overexpression of GhKTI12 Enhances Seed Yield and Biomass Production in Nicotiana Tabacum. Genes 2022, 13, 426–442. [Google Scholar] [CrossRef]
- Li, J.; Foster, R.; Ma, S. Identification of transcription factors controlling cell wall invertase gene expression for reproductive development via bioinformatic and transgenic analyses. Plant J. 2021, 106, 1058–1074. [Google Scholar] [CrossRef]


| Strain | Number of Branches | Number of Nodes on the Main Stem | Number of Four-Seed Pods | 100-Grain Weight/g | Height/cm | Number of Pods Per Plant |
|---|---|---|---|---|---|---|
| JN18CK | 7.00 ± 1.00 a | 18.67 ± 1.53 ab | 1.33 ± 0.58 b | 18.83 ± 0.29 a | 67.83 ± 1.61 ab | 97.67 ± 1.15 bc |
| Cas9-GmAP3-26 | 6.33 ± 0.58 a | 16.00 ± 2.65 b | 1.33 ± 0.58 b | 17.33 ± 2.08 a | 67.33 ± 3.79 ab | 114.33 ± 24.54 abc |
| Cas9-GmAP3-30 | 4.00 ± 0.00 a | 17.00 ± 1.00 ab | 1.33 ± 1.15 b | 17.33 ± 1.15 a | 68.5 ± 5.50 ab | 68.33 ± 15.95 c |
| Cas9-GmAP3-46 | 6.33 ± 1.15 a | 17.33 ± 0.58 ab | 2.33 ± 2.08 ab | 18.33 ± 1.15 a | 64.07 ± 5.25 bc | 106.00 ± 12.29 abc |
| Cas9-GmAP3-56 | 4.33 ± 1.15 a | 17.00 ± 1.00 ab | 0.67 ± 1.15 b | 16.67 ± 0.58 a | 67.33 ± 3.51 c | 98.00 ± 43.59 bc |
| Cas9-GmAP3-67 | 5.67 ± 1.15 a | 19.00 ± 1.00 ab | 0.33 ± 0.58 b | 18.67 ± 1.15 a | 65.33 ± 2.52 abc | 95.00 ± 22.65 bc |
| OE-GmAP3-41 | 5.33 ± 1.15 a | 17.00 ± 3.00 ab | 2.00 ± 1.00 b | 17.33 ± 0.58 a | 66.83 ± 4.54 ab | 107.33 ± 67.95 abc |
| OE-GmAP3-30 | 5.67 ± 1.53 a | 17.33 ± 1.53 ab | 1.00 ± 1.00 b | 16.67 ± 1.53 a | 68.33 ± 7.51 ab | 115.00 ± 25.16 abc |
| OE-GmAP3-61 | 6.33 ± 1.15 a | 19.67 ± 0.58 a | 5.00 ± 2.65 ab | 18.00 ± 1.00 a | 73.83 ± 3.62 a | 164.00 ± 32.23 ab |
| OE-GmAP3-74 | 4.33 ± 0.58 a | 18.33 ± 1.15 ab | 3.33 ± 2.08 ab | 18.33 ± 1.15 a | 72.67 ± 3.79 ab | 84.67 ± 7.23 bc |
| OE-GmAP3-82 | 6.33 ± 3.51 a | 18.67 ± 0.58 ab | 7.33 ± 6.51 a | 18.33 ± 4.04 a | 69.33 ± 3.06 ab | 193.33 ± 94.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; He, H.; Li, Y.; Wang, L.; Liu, Y.; Luan, X.; Wang, J.; Liu, H.; Liu, S.; Zhang, J.; et al. MADS-Box Subfamily Gene GmAP3 from Glycine max Regulates Early Flowering and Flower Development. Int. J. Mol. Sci. 2023, 24, 2751. https://doi.org/10.3390/ijms24032751
Zhang A, He H, Li Y, Wang L, Liu Y, Luan X, Wang J, Liu H, Liu S, Zhang J, et al. MADS-Box Subfamily Gene GmAP3 from Glycine max Regulates Early Flowering and Flower Development. International Journal of Molecular Sciences. 2023; 24(3):2751. https://doi.org/10.3390/ijms24032751
Chicago/Turabian StyleZhang, Aijing, Haobo He, Yue Li, Lixue Wang, Yixuan Liu, Xinchao Luan, Jiaxin Wang, Huijing Liu, Shuying Liu, Jun Zhang, and et al. 2023. "MADS-Box Subfamily Gene GmAP3 from Glycine max Regulates Early Flowering and Flower Development" International Journal of Molecular Sciences 24, no. 3: 2751. https://doi.org/10.3390/ijms24032751
APA StyleZhang, A., He, H., Li, Y., Wang, L., Liu, Y., Luan, X., Wang, J., Liu, H., Liu, S., Zhang, J., & Yao, D. (2023). MADS-Box Subfamily Gene GmAP3 from Glycine max Regulates Early Flowering and Flower Development. International Journal of Molecular Sciences, 24(3), 2751. https://doi.org/10.3390/ijms24032751






