Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing
Abstract
1. Introduction
2. Results and Discussion
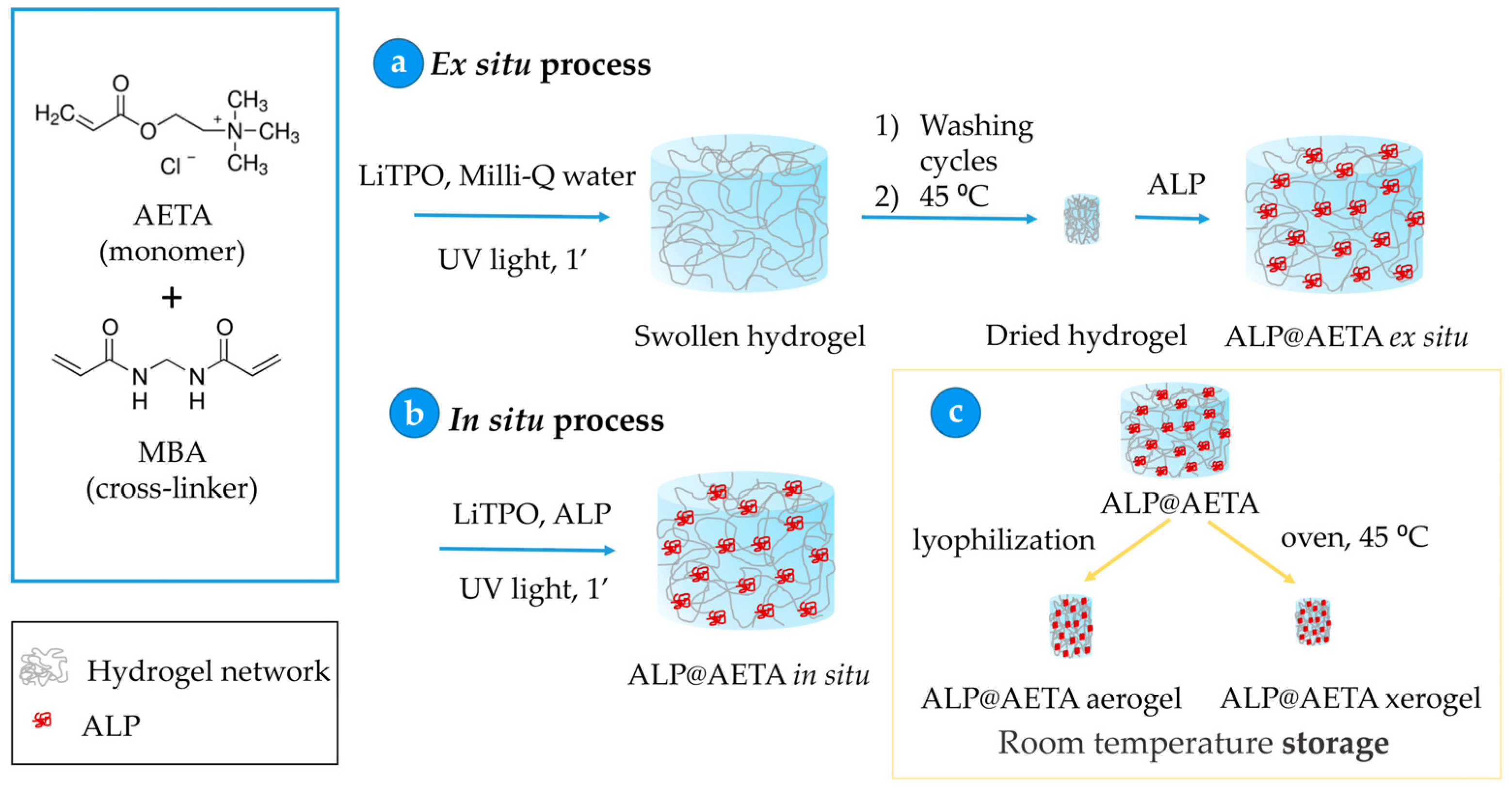
2.1. Immobilization of ALP in AETA Hydrogels
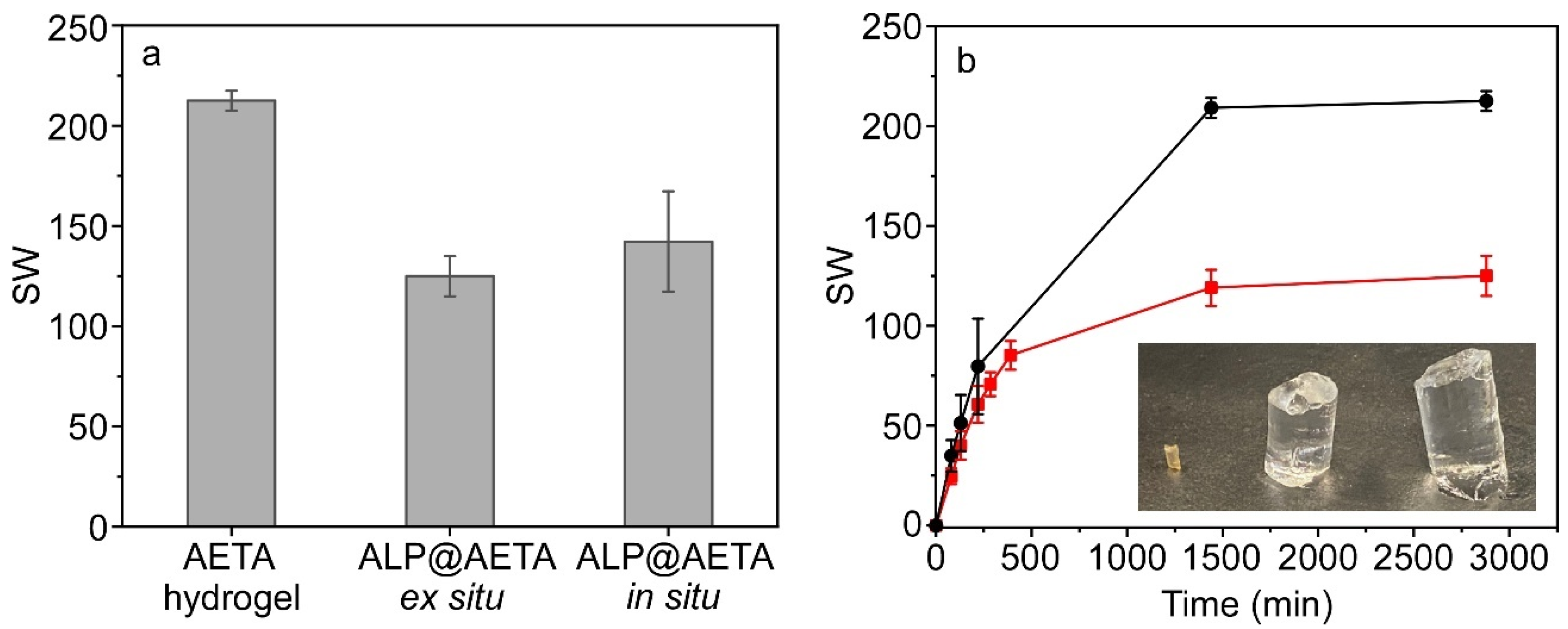
2.2. Swelling Study
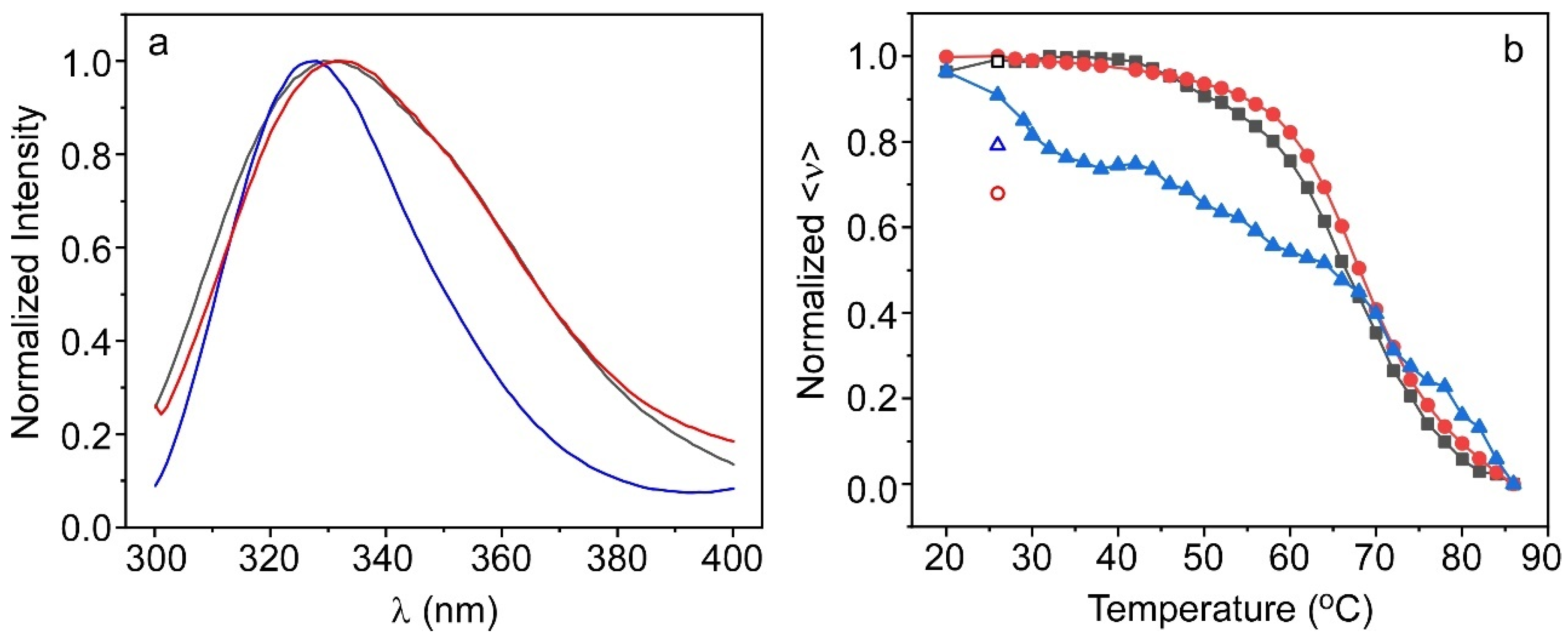
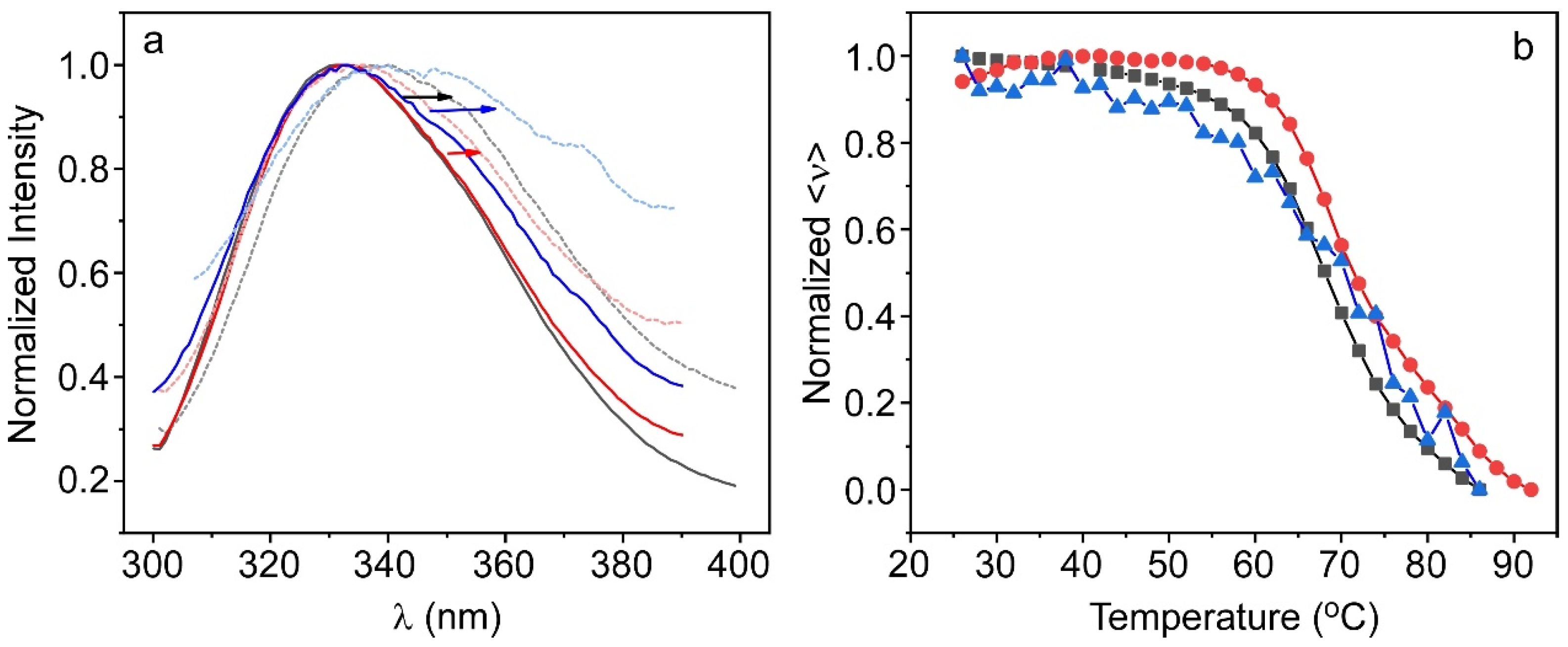
2.3. Characterization of the Protein Inside the Hydrogel
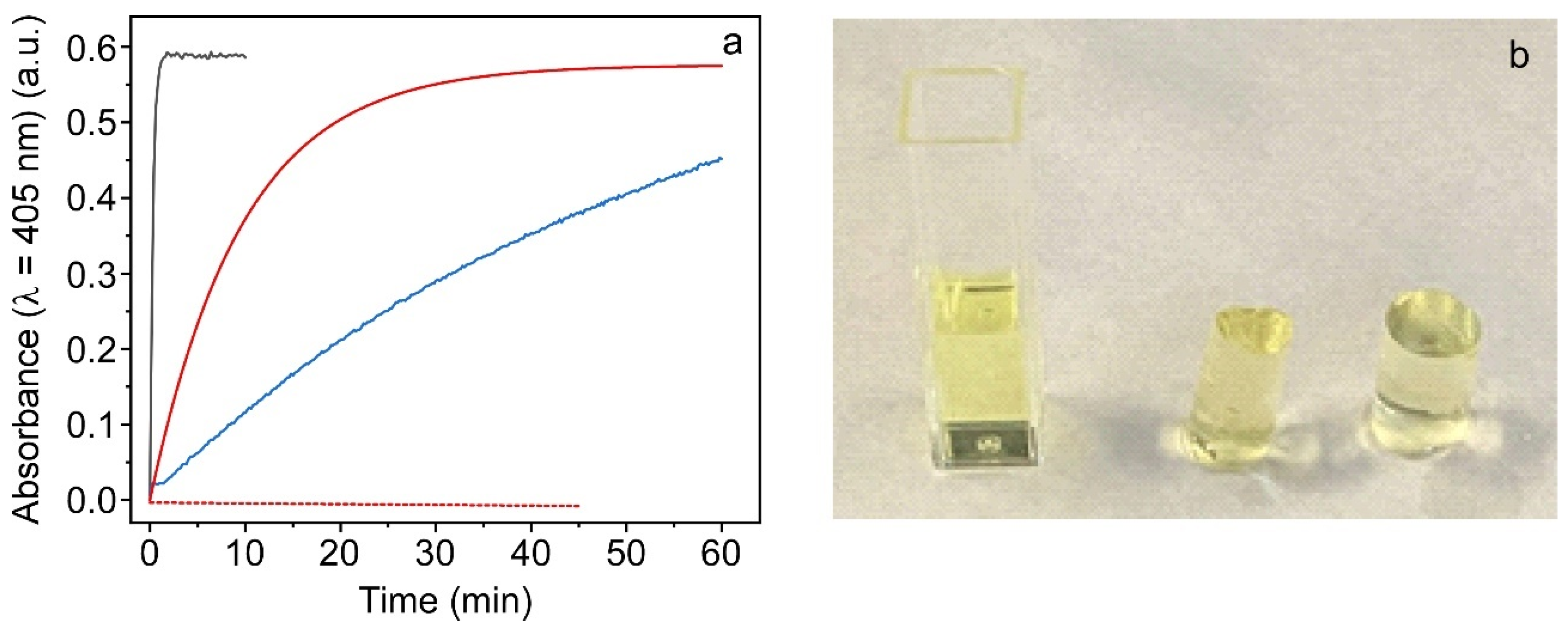
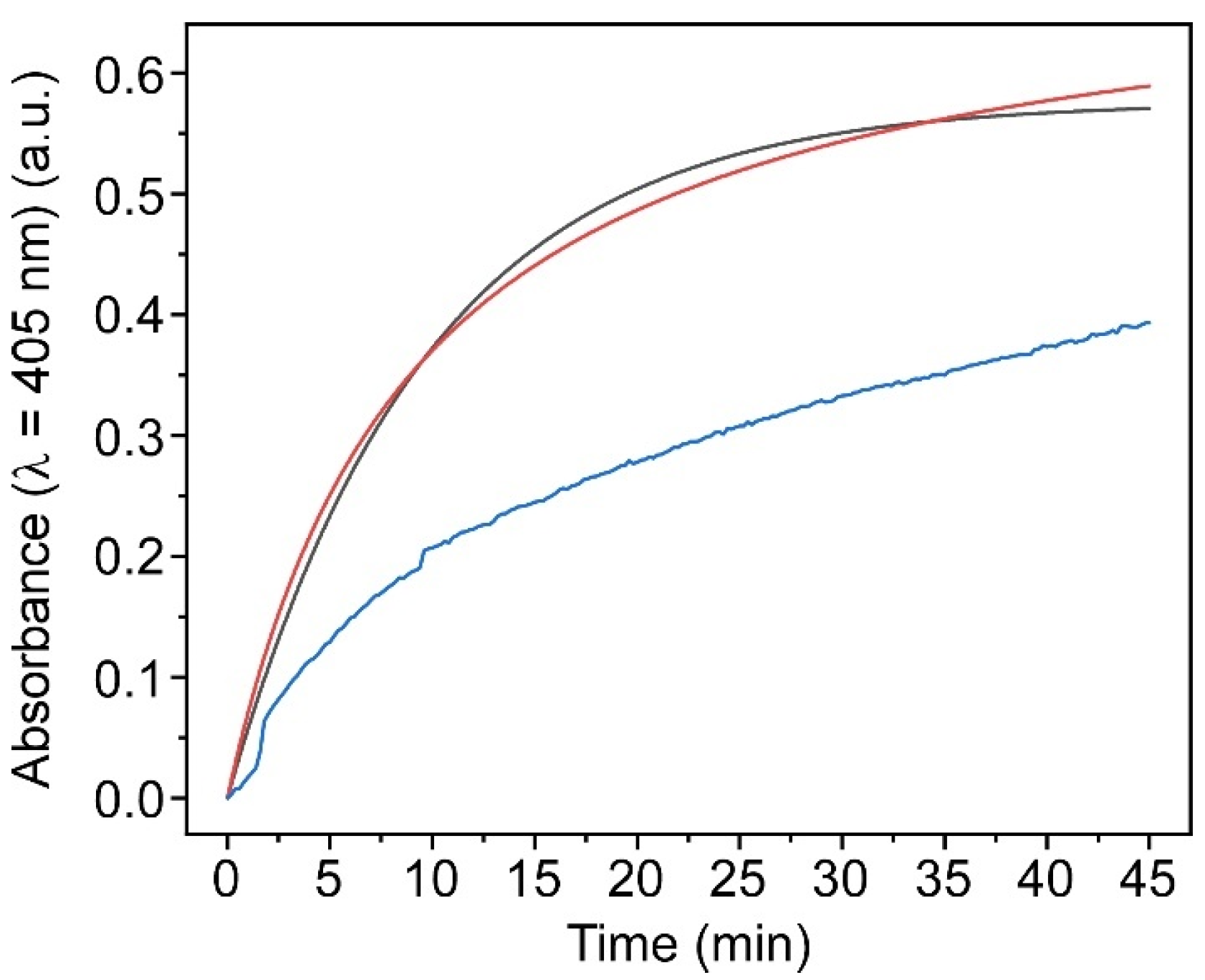
2.3.1. Enzymatic Activity Study of Ex Situ and In Situ ALP@AETA Hydrogels
2.3.2. ALP@AETA Stability Study
2.4. ALP@AETA Storage
2.4.1. Enzymatic Activity of ALP@AETA Aerogel and Xerogel
2.4.2. ALP@AETA Xerogel Storage
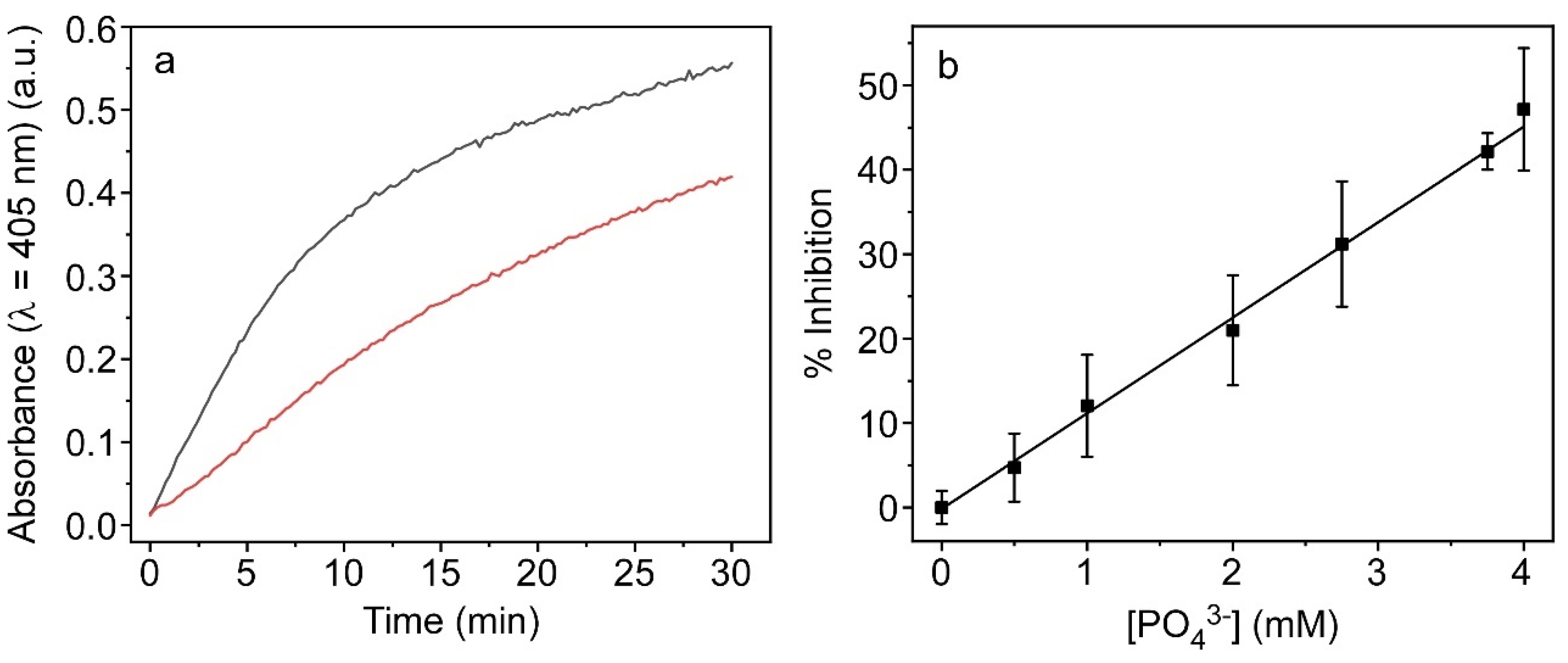
2.5. Application: Phosphate Detection
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of [2-(Acryloyloxy)ethyl]trimethylammonium Chloride Hydrogels
3.3. ALP Immobilization in AETA Hydrogels
- Ex situ process: Embedding ALP into the hydrogel. In this process, the previously washed and dried AETA hydrogel prepared by the above methods was immersed in ALP solution for 24 h and stored at 4 °C. This step allows loading ALP from the enzymatic solution into the hydrogel network to form ALP@AETA (Scheme 1a).
- In situ process: Incorporation of ALP into the hydrogel. Milli-Q water was replaced as solvent by 5 mL of ALP buffered solution before the polymerization process started. After irradiation with UV light, ALP@AETA hydrogels were obtained (Scheme 1b).
3.4. Swelling Measurements
3.5. Fluorescence Measurements
3.6. Colorimetric Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Alacid, Y.; Martínez-Tomé, M.J.; Mateo, C.R. Reusable Fluorescent Nanobiosensor Integrated in a Multiwell Plate for Screening and Quantification of Antidiabetic Drugs. ACS Appl. Mater. Interfaces 2021, 13, 25624–25634. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.M.O.; Falleiros, L.N.S.S.; de Resende, M.M.; Ribeiro, E.J.; Almeida, R.M.R.G.; da Silva, A.O.S. Immobilization of the enzyme invertase in SBA-15 with surfaces functionalized by different organic compounds. J. Porous Mater. 2019, 26, 77–89. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Baruch-Shpigler, Y.; Avnir, D. Enzymes in a golden cage. Chem. Sci. J. 2020, 11, 3965–3977. [Google Scholar] [CrossRef]
- Maldonado, N.; Amo-Ochoa, P. Advances and Novel Perspectives on Colloids, Hydrogels, and Aerogels Based on Coordination Bonds with Biological Interest Ligands. Nanomaterials 2021, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Schmieg, B.; Schimek, A.; Franzreb, M. Development and performance of a 3D-printable poly(ethylene glycol) diacrylate hydrogel suitable for enzyme entrapment and long-term biocatalytic applications. Eng. Life. Sci. 2018, 18, 659–667. [Google Scholar] [CrossRef]
- Meyer, J.; Meyer, L.E.; Kara, S. Enzyme immobilization in hydrogels: A perfect liaison for efficient and sustainable biocatalysis. Eng. Life Sci. 2022, 22, 165–177. [Google Scholar] [CrossRef]
- Millán, J.L. Alkaline Phosphatases: Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Campíns-Falcó, P.; Hall, E.A.H. Zein as biodegradable material for effective delivery of alkaline phosphatase and substrates in biokits and biosensors. Biosens. Bioelectron. 2016, 86, 14–19. [Google Scholar] [CrossRef]
- García Sánchez, F.; Navas Díaz, A.; Ramos Peinado, M.C.; Belledone, C. Free and sol–gel immobilized alkaline phosphatase-based biosensor for the determination of pesticides and inorganic compounds. Anal. Chim. Acta 2003, 484, 45–51. [Google Scholar] [CrossRef]
- Kahveci, Z.; Martínez-Tomé, M.J.; Mallavia, R.; Mateo, C.R. Fluorescent Biosensor for Phosphate Determination Based on Immobilized Polyfluorene-Liposomal Nanoparticles Coupled with Alkaline Phosphatase. ACS Appl. Mater. Interfaces 2017, 9, 136–144. [Google Scholar] [CrossRef]
- Alacid, Y.; Quintero Jaime, A.F.; Martínez-Tomé, M.J.; Mateo, C.R.; Montilla, F. Disposable Electrochemical Biosensor Based on the Inhibition of Alkaline Phosphatase Encapsulated in Acrylamide Hydrogels. Biosensors 2022, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pacheco, A.; Del Río Castillo, A.E.; Martín, C.; Herrero, M.A.; Merino, S.; García Fierro, J.L.; Díez-Barra, E.; Vázquez, E. Graphene Quantum Dot–Aerogel: From Nanoscopic to Macroscopic Fluorescent Materials. Sensing Polyaromatic Compounds in Water. ACS Appl. Mater. Interfaces 2018, 10, 18192–18201. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.; Martín, C.; López-Díaz, A.; Martín-Pacheco, A.; Rodríguez, A.M.; Patiño, F.J.; Herrero, M.A.; Vázquez, A.S.; Vázquez, E. Autonomous self-healing hydrogel with anti-drying properties and applications in soft robotics. Appl. Mater. Today 2020, 21, 100806. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B.; Verma, N. Recent advances in phosphate biosensors. Biotechnol. Lett. 2015, 37, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Park, D.-S.; Chang, S.-C.; McNeil, C.J.; Shim, Y.-B. The biosensor based on the pyruvate oxidase modified conducting polymer for phosphate ions determinations. Biosens. Bioelectron. 2006, 21, 1116–1124. [Google Scholar] [CrossRef]
- Kumru, B.; Shalom, M.; Antonietti, M.; Schmidt, B.V.K.J. Reinforced Hydrogels via Carbon Nitride Initiated Polymerization. Macromolecules 2017, 50, 1862–1869. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Hai, F.A.; Wahab, H.A.E.; Mahmoud, H. Synthesis, Characterization, Swelling Studies and Dye Removal of Chemically Crosslinked Acrylic Acid/Acrylamide/N,N-Dimethyl Acrylamide Hydrogels. Am. J. Appl. Chem. 2016, 4, 221. [Google Scholar] [CrossRef]
- Royer, C.A. Probing Protein Folding and Conformational Transitions with Fluorescence. Chem. Rev. 2006, 106, 1769–1784. [Google Scholar] [CrossRef]
- Bortolato, M.; Besson, F.; Roux, B. Role of metal ions on the secondary and quaternary structure of alkaline phosphatase from bovine intestinal mucosa. Proteins 1999, 37, 310–318. [Google Scholar] [CrossRef]
- Dumitrașcu, L.; Stănciuc, N.; Aprodu, I.; Ciuciu, A.-M.; Alexe, P.; Bahrim, G.E. Monitoring the heat-induced structural changes of alkaline phosphatase by molecular modeling, fluorescence spectroscopy and inactivation kinetics investigations. J. Food Sci. Technol. 2015, 52, 6290–6300. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Shimizu, T.; Korehisa, T.; Imanaka, H.; Ishida, N.; Imamura, K. Characteristics of proteinaceous additives in stabilizing enzymes during freeze-thawing and -drying. Biosci. Biotechnol. Biochem. 2017, 81, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ogabiela, E.E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.B.; Zhang, X.; Wu, Y. Electrochemical Biosensor based on Pt/Au Alloy Nanowire Arrays for Phosphate Detection. J. Electrochem. Soc. 2015, 162, B62. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Berchmans, S. Inorganic-Organic Composite Matrix for the Enzymatic Detection of Phosphate in Food Samples. J. Electrochem. Soc. 2013, 160, B73. [Google Scholar] [CrossRef]
- Esquembre, R.; Sanz, J.M.; Wall, J.G.; del Monte, F.; Mateo, C.R.; Ferrer, M.L. Thermal unfolding and refolding of lysozyme in deep eutectic solvents and their aqueous dilutions. Phys. Chem. Chem. Phys. 2013, 15, 11248–11256. [Google Scholar] [CrossRef]

| Tm (°C) ± SD | |||
|---|---|---|---|
| t (days) | ALP Solution | ALP@AETA | ALP@AETA Xerogel |
| 1 | 67.3 ± 0.2 | 69.2 ± 0.2 | 72.4 ± 0.5 |
| 21 | 67.8 ± 0.4 | 69.6 ± 0.2 | |
| 30 | 68.5 ± 0.4 | 68.9 ± 0.3 | 70.0 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alacid, Y.; Martínez-Tomé, M.J.; Esquembre, R.; Herrero, M.A.; Mateo, C.R. Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing. Int. J. Mol. Sci. 2023, 24, 2672. https://doi.org/10.3390/ijms24032672
Alacid Y, Martínez-Tomé MJ, Esquembre R, Herrero MA, Mateo CR. Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing. International Journal of Molecular Sciences. 2023; 24(3):2672. https://doi.org/10.3390/ijms24032672
Chicago/Turabian StyleAlacid, Yolanda, María José Martínez-Tomé, Rocío Esquembre, M. Antonia Herrero, and C. Reyes Mateo. 2023. "Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing" International Journal of Molecular Sciences 24, no. 3: 2672. https://doi.org/10.3390/ijms24032672
APA StyleAlacid, Y., Martínez-Tomé, M. J., Esquembre, R., Herrero, M. A., & Mateo, C. R. (2023). Portable Alkaline Phosphatase–Hydrogel Platform: From Enzyme Characterization to Phosphate Sensing. International Journal of Molecular Sciences, 24(3), 2672. https://doi.org/10.3390/ijms24032672






