Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D
Abstract
1. Introduction
2. Disorders Related to Metabolic Syndrome
2.1. Diabetes
2.2. Cardiovascular Diseases
2.3. Liver Disorders
2.4. Brain Disorders
3. Citrus and Grape Phytochemicals and Vitamin D
3.1. Phytochemicals Contained in Citruses
| Citrus Phytochemicals | Effects | Subjects | Ref |
|---|---|---|---|
| Hesperidin (flavonoids) | Endotoxin shock suppression | Mouse | [49] |
| Alleviating rheumatoid arthritis | Human/Mouse | [50] | |
| Hyperglycemia, triglyceride, high blood pressure | Human | [51] | |
| Reduction of blood pressure, blood glucose, cholesterol, TNF-α, hs-CPR | Human | [52] | |
| β-Cryptoxanthin (carotenoids) | Provitamin A effects: maintaining eyesight, helping growth and development | Human/Mouse | [53] |
| Anti-stress effects by anti-oxidative effects | Human | [54] | |
| Bone homeostasis, osteoporosis prevention, bone metabolism | Human/ Mouse cells | [55] | |
| Effects for liver disorders (NFALD/NASH) | Human, etc. | [56] | |
| Metabolic syndrome and type 2 diabetes | Rat | [57] | |
| Reducing body fat levels, anti-oxidative stress response, prevention of ageing | C. elegans | [58] | |
| Rutin (quercetin-glycoside: flavonoids) | Diabetes, blood glucose, anti-inflammatory effects, anti-oxidative effects | Human | [59] |
| Alleviating arthritis | Rat | [60] | |
| Depletion of AGEs | Rat/Human cells | [61] | |
| Stress-induced injury, oxytocin receptor activation | Rat/Human cells | [62] | |
| Decreasing LDL, increasing HDL, improving learning capability | Rat | [63] | |
| Naringin (flavanone-glycoside: flavonoids) | Enzyme activation related to tissue glucose intake from blood | Human/Rat/ Mouse/Cells | [64] |
| Therapy of diabetes | Rabbit/Rat/ Mouse/Cells | [65] | |
| Suppression of LPS-induced TNF-α production | Mouse | [66] | |
| Anti-inflammatory effects in arthritis | Mouse | [67] | |
| Prevention of atherosclerosis | Mouse | [68] | |
| Improvement of circulatory system disease | Rat | [69] | |
| Nobiletin (flavonoids) | Improving recognition, reducing soluble amyloid β | Mouse | [70] |
| Enhancing circadian rhythms | Mouse | [71] | |
| Reducing the risk of metabolic syndrome | Human/Rat/ Mouse/Cells | [72] | |
| Alleviating metabolic dysregulation | Mouse | [73] |
3.2. Phytochemicals in Grapes
3.3. Combination of Vitamin D and Phytochemicals
4. Adipokine, Myokine, Cytokine
4.1. Adipokine-Producing Adipocytes
4.2. Myokines Released from Skeletal Muscle
4.3. Cytokines Produced by a Variety of Cells in the Body
4.4. Effects of TNF-α and Adiponectin in Metabolic Syndrome
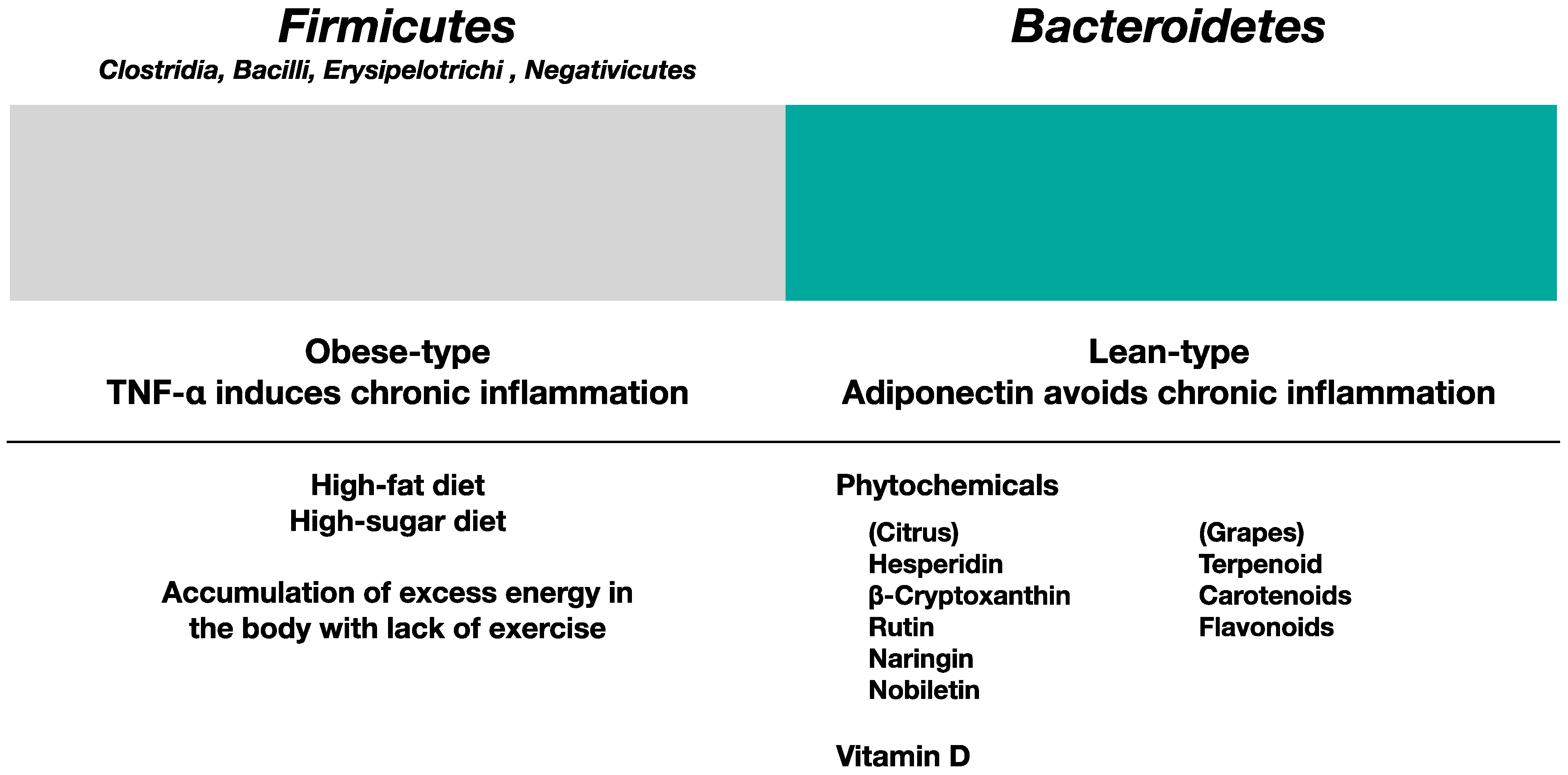
5. Phytochemicals and Vitamin D Prevent Metabolic Syndrome and Improve Gut Microbiota
5.1. Gut Microbiota in Obese Type and Lean Type
5.2. Influence of Phytochemicals and Vitamin D in Gut Microbiota
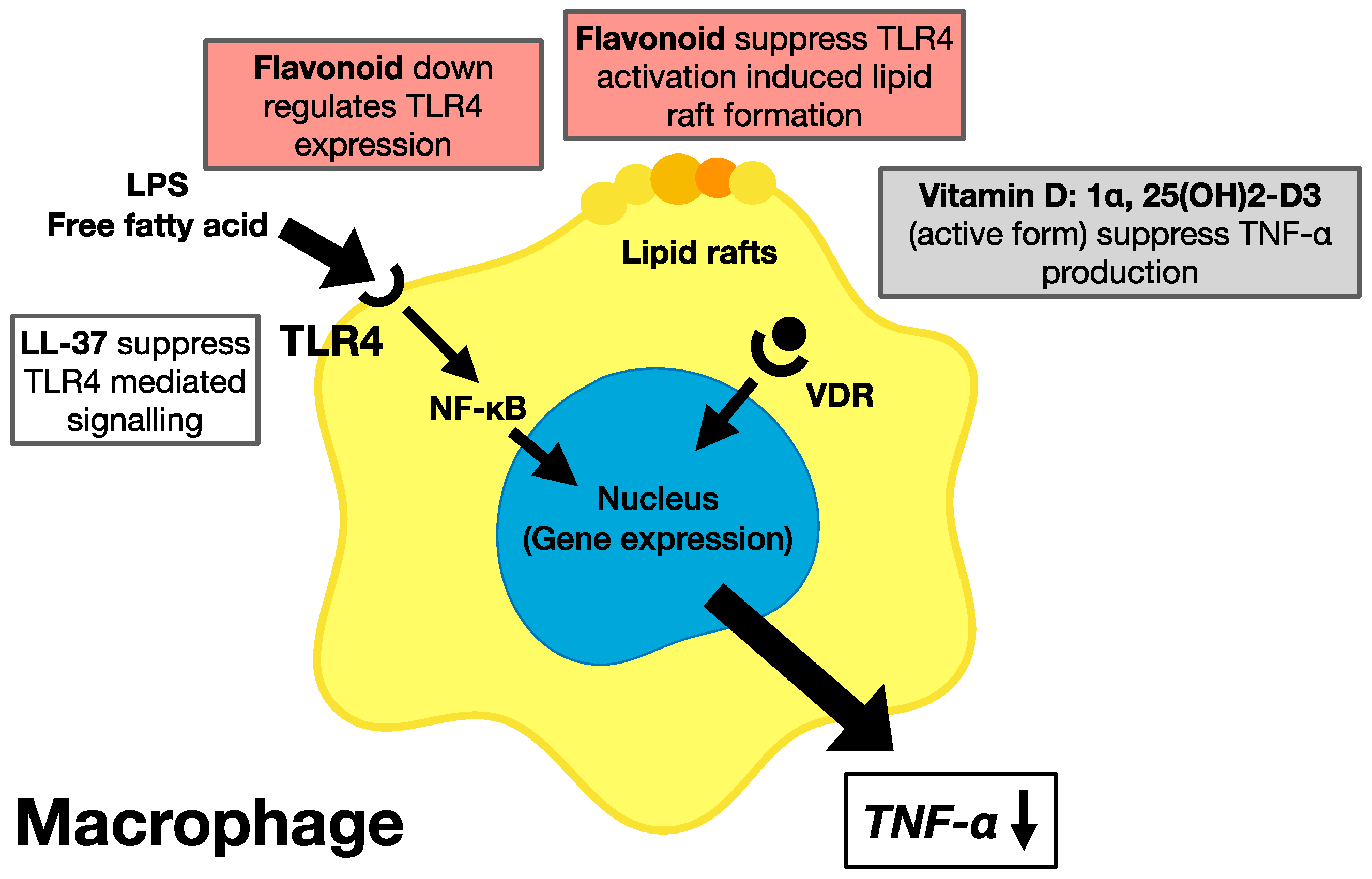
5.3. Effects of Phytochemicals and Vitamin D on the Suppression of Chronic Inflammation
5.4. Molecular Mechanism for the Prevention of Metabolic Syndrome Requiring the Suppression of TNF-α and Chronic Inflammation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Sethi, J.K. TNF-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Vatner, D.F.; Goedeke, L.; Hirabara, S.M.; Zhang, Y.; Perry, R.J.; Shulman, G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 32584–32593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Halle, M.; Berg, A.; Northoff, H.; Keul, J. Importance of TNF-alpha and leptin in obesity and insulin resistance: A hypothesis on the impact of physical exercise. Exerc. Immunol. Rev. 1998, 4, 77–94. [Google Scholar]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Owens, B. Cell physiology: The changing colour of fat. Nature 2014, 508, S52–S53. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Santa, K. Grape Phytochemicals and Vitamin D in Alleviation of Lung Disorders. Endocr. Metab. Immune. Disord. Drug Targets 2022, 22, 1276–1292. [Google Scholar] [CrossRef]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Matsumoto, T.; Kumazawa, Y. Effects of antioxidant polyphenols on TNF-alpha-related diseases. Curr. Top. Med. Chem. 2011, 11, 1767–1779. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell Endocrinol. 2017, 453, 68–78. [Google Scholar] [CrossRef]
- White, J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Watada, H. Type 2 Diabetes and Bacteremia. Ann. Nutr. Metab. 2017, 71, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jo, S.H.; Kwon, Y.I.; Hwang, J.K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int. J. Mol. Sci. 2011, 12, 3757–3769. [Google Scholar] [CrossRef]
- Hube, F.; Hauner, H. The two tumor necrosis factor receptors mediate opposite effects on differentiation and glucose metabolism in human adipocytes in primary culture. Endocrinology 2000, 41, 2582–2588. [Google Scholar] [CrossRef]
- Zou, Y.; Zhong, L.; Hu, C.; Zhong, M.; Peng, N.; Sheng, G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: A 5-year longitudinal cohort study. Lipids Health Dis. 2021, 20, 28. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Ganjali, S.; Gotto, A.M., Jr.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef]
- Suzuki, K.; Susaki, E.A.; Nagaoka, I. Lipopolysaccharides and Cellular Senescence: Involvement in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 11148. [Google Scholar] [CrossRef]
- Santos, G.M.; Ismael, S.; Morais, J.; Araújo, J.R.; Faria, A.; Calhau, C.; Marques, C. Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function. Microorganisms 2022, 10, 746. [Google Scholar] [CrossRef]
- Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. The role of mucoprotectants in the management of gastrointestinal disorders. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Duraisamy, P.; Ravi, S.; Krishnan, M.; Livya, C.M.; Manikandan, B.; Arunagirinathan, K.; Ramar, M. Dynamic Role of Macrophage Sub Types on Development of Atherosclerosis and Potential Use of Herbal Immunomodulators as Imminent Therapeutic Strategy. Cardiovasc. Hematol. Agents Med. Chem. 2022, 20, 2–12. [Google Scholar] [CrossRef]
- Hoving, L.R.; Katiraei, S.; Heijink, M.; Pronk, A.; van der Wee-Pals, L.; Streefland, T.; Giera, M.; Willems van Dijk, K.; van Harmelen, V. Dietary Mannan Oligosaccharides Modulate Gut Microbiota, Increase Fecal Bile Acid Excretion, and Decrease Plasma Cholesterol and Atherosclerosis Development. Mol. Nutr. Food Res. 2018, 62, e1700942. [Google Scholar] [CrossRef]
- Guan, B.; Tong, J.; Hao, H.; Yang, Z.; Chen, K.; Xu, H.; Wang, A. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta. Pharm. Sin. B 2022, 12, 2129–2149. [Google Scholar] [CrossRef]
- Lanthier, N.; Delzenne, N. Targeting the Gut Microbiome to Treat Metabolic Dysfunction-Associated Fatty Liver Disease: Ready for Prime Time? Cells 2022, 11, 2718. [Google Scholar] [CrossRef]
- Tough, I.R.; Schwartz, T.W.; Cox, H.M. Synthetic G protein-coupled bile acid receptor agonists and bile acids act via basolateral receptors in ileal and colonic mucosa. Neurogastroenterol. Motil. 2020, 32, e13943. [Google Scholar] [CrossRef]
- Sugiyama, N.; Uehara, O.; Morikawa, T.; Paudel, D.; Ebata, K.; Hiraki, D.; Harada, F.; Yoshida, K.; Kato, S.; Nagasawa, T.; et al. Gut flora alterations due to lipopolysaccharide derived from Porphyromonas gingivalis. Odontology 2022, 110, 673–681. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Breuer, D.A.; Pacheco, M.C.; Washington, M.K.; Montgomery, S.A.; Hasty, A.H.; Kennedy, A.J. CD8+ T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G211–G224. [Google Scholar] [CrossRef]
- Liu, Y.; Myojin, T.; Li, K.; Kurita, A.; Seto, M.; Motoyama, A.; Liu, X.; Satoh, A.; Munemasa, S.; Murata, Y.; et al. A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity. Int. J. Mol. Sci. 2022, 23, 1762. [Google Scholar] [CrossRef]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Kim, S.W.; Suda, W.; Kawasumi, M.; Onawa, S.; Taguchi-Atarashi, N.; Morita, H.; Taylor, T.D.; Hattori, M.; Ohno, H. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature 2020, 585, 102–106. [Google Scholar] [CrossRef]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The role of the gut microbiota in multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 544–558. [Google Scholar] [CrossRef]
- Shimamura, Y.; Sei, S.; Nomura, S.; Masuda, S. Protective effects of dried mature Citrus unshiu peel (Chenpi) and hesperidin on aspirin-induced oxidative damage. J. Clin. Biochem. Nutr. 2021, 68, 149–155. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and α-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kikuchi, S.; Hasunuma, R.; Maruyama, H.; Yoshikawa, T.; Kumazawa, Y. A citrus flavonoid hesperidin suppresses infection-induced endotoxin shock in mice. Biol. Pharm. Bull. 2004, 27, 679–683. [Google Scholar] [CrossRef]
- Kometani, T.; Fukuda, T.; Kakuma, T.; Kawaguchi, K.; Tamura, W.; Kumazawa, Y.; Nagata, K. Effects of alpha-glucosylhesperidin, a bioactive food material, on collagen-induced arthritis in mice and rheumatoid arthritis in humans. Immunopharmacol. Immunotoxicol. 2008, 30, 117–134. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Hekmatdoost, A. Flaxseed and/or hesperidin supplementation in metabolic syndrome: An open-labeled randomized controlled trial. Eur. J. Nutr. 2021, 60, 287–298. [Google Scholar] [CrossRef]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef]
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef]
- Unno, K.; Noda, S.; Nii, H.; Kawasaki, Y.; Iguchi, K.; Yamada, H. Anti-stress Effect of β-Cryptoxanthin in Satsuma Mandarin Orange on Females. Biol. Pharm. Bull. 2019, 42, 1402–1408. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J. Biomed. Sci. 2012, 19, 36. [Google Scholar] [CrossRef]
- Sodum, N.; Kumar, G.; Bojja, S.L.; Kumar, N.; Rao, C.M. Epigenetics in NAFLD/NASH: Targets and therapy. Pharmacol. Res. 2021, 167, 105484. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Gence, L.; Portet, K.; Tousch, D.; Poucheret, P. Preventive action of retinoids in metabolic syndrome/type 2 diabetic rats fed with citrus functional food enriched in β-cryptoxanthin. Food Funct. 2020, 11, 9263–9271. [Google Scholar] [CrossRef]
- Llopis, S.; Rodrigo, M.J.; González, N.; Genovés, S.; Zacarías, L.; Ramón, D.; Martorell, P. β-Cryptoxanthin Reduces Body Fat and Increases Oxidative Stress Response in Caenorhabditis elegans Model. Nutrients 2019, 11, 232. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Shah, M.R.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef]
- Chen, M.; Liu, P.; Zhou, H.; Huang, C.; Zhai, W.; Xiao, Y.; Ou, J.; He, J.; El-Nezami, H.; Zheng, J. Formation and metabolism of 6-(1-acetol)-8-(1-acetol)-rutin in foods and in vivo, and their cytotoxicity. Front. Nutr. 2022, 9, 973048. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Horita, S.; Yokota, S.; Ono, T.; Proks, P.; Yoshida-Komiya, H.; Ueta, Y.; Nishimori, K.; Misaka, S.; Shimomura, K. Identification of oxytocin receptor activating chemical components from traditional Japanese medicines. J. Food Drug Anal. 2021, 29, 653–675. [Google Scholar] [CrossRef] [PubMed]
- Micháliková, D.; Tyukos Kaprinay, B.; Lipták, B.; Švík, K.; Slovák, L.; Sotníková, R.; Knezl, V.; Gaspárová, Z. Natural substance rutin versus standard drug atorvastatin in a treatment of metabolic syndrome-like condition. Saudi. Pharm. J. 2019, 27, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kikuchi, S.; Hasegawa, H.; Maruyama, H.; Morita, H.; Kumazawa, Y. Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur. J. Pharmacol. 1999, 368, 245–250. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Maruyama, H.; Hasunuma, R.; Kumazawa, Y. Suppression of inflammatory responses after onset of collagen-induced arthritis in mice by oral administration of the Citrus flavanone naringin. Immunopharmacol. Immunotoxicol. 2011, 33, 723–729. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid Res. 2020, 61, 387–402. [Google Scholar] [CrossRef]
- Buja, L.M. The history, science, and art of wine and the case for health benefits: Perspectives of an oenophilic cardiovascular pathologist. Cardiovasc. Pathol. 2022, 60, 107446. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the gut microbiota with resveratrol: A demonstration of novel evidence for the management of hepatic steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Iijima, K.; Yoshizumi, M.; Ouchi, Y. Effect of red wine polyphenols on vascular smooth muscle cell function--molecular mechanism of the ‘French paradox’. Mech. Ageing. Dev. 2002, 123, 1033–1039. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, X.A.; Wang, B.; Yin, G.; Wang, J.; Wang, T.; Bi, K. Based on Multi-Activity Integrated Strategy to Screening, Characterization and Quantification of Bioactive Compounds from Red Wine. Molecules 2021, 26, 6750. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 29, e47. [Google Scholar] [CrossRef]
- Santa, K.; Kumazawa, Y.; Nagaoka, I. The Potential Use of Grape Phytochemicals for Preventing the Development of Intestine-Related and Subsequent Inflammatory Diseases. Endocr. Metab. Immune. Disord. Drug Targets 2019, 19, 794–802. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, J.X.; Liu, Y.N.; Ge, L.T.; Guan, Y.; Zhao, W.; Jia, Y.L.; Dong, X.W.; Sun, Y.; Xie, Q.M. Grape seed extract ameliorates bleomycin-induced mouse pulmonary fibrosis. Toxicol. Lett. 2017, 273, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Katano, Y. Cardiovascular Protective Effects of Polyphenols Contained in Passion Fruit Seeds Namely Piceatannol and Scirpusin B: A Review. Tokai J. Exp. Clin. Med. 2021, 46, 151–161. [Google Scholar] [PubMed]
- Kumazawa, Y.; Kawaguchi, K.; Takimoto, H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr. Pharm. Des. 2006, 12, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharmaceut. Med. 2022, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Albaik, M.; Khan, J.A.; Sindi, I.; Akesson, K.E.; McGuigan, F.E.A. Bone mass in Saudi women aged 20–40 years: The association with obesity and vitamin D deficiency. Arch. Osteoporos. 2022, 17, 123. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Alayed Albarri, E.M.; Sameer Alnuaimi, A.; Abdelghani, D. Effectiveness of vitamin D2 compared with vitamin D3 replacement therapy in a primary healthcare setting: A retrospective cohort study. Qatar Med. J. 2022, 2022, 29. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, M.; Pan, L.; Chen, Y.; Guo, S.; Luo, D.; Zhu, L.; Liu, Y.; Pan, L.; Xu, S.; et al. Vitamin D signaling maintains intestinal innate immunity and gut microbiota: Potential intervention for metabolic syndrome and NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G542–G553. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef]
- Su, D.; Nie, Y.; Zhu, A.; Chen, Z.; Wu, P.; Zhang, L.; Luo, M.; Sun, Q.; Cai, L.; Lai, Y.; et al. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front. Physiol. 2016, 7, 498. [Google Scholar] [CrossRef]
- Mabrouk, R.R.; Amer, H.A.; Soliman, D.; Mohamed, N.A.; El-Ghoneimy, D.H.; Hamdy, A.M.; Atef, S.A. Vitamin D Increases Percentages of Interleukin-10 Secreting Regulatory T Cells in Children with Cow’s Milk Allergy. Egypt. J. Immunol. 2019, 26, 15–29. [Google Scholar]
- Huang, F.; Ju, Y.H.; Wang, H.B.; Li, Y.N. Maternal vitamin D deficiency impairs Treg and Breg responses in offspring mice and deteriorates allergic airway inflammation. Allergy Asthma Clin. Immunol. 2020, 16, 89. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Makarewicz, A.; Jamka, M.; Geltz, J.; Śmidowicz, A.; Kokot, M.; Kaczmarek, N.; Mądry, E.; Walkowiak, J. Comparison of the Effect of Endurance, Strength, and Endurance-Strength Training on Inflammatory Markers and Adipokines Levels in Overweight and Obese Adults: Systematic Review and Meta-Analysis of Randomised Trials. Healthcare 2022, 10, 1098. [Google Scholar] [CrossRef]
- Bourebaba, L.; Marycz, K. Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review. J. Clin. Med. 2019, 8, 2033. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Haris, M.; Faheem, H.I.; Hamid, A.; Yousaf, R.; Rasul, A.; Shah, G.M.; Khalil, A.A.K.; Wahab, A.; Khan, H.; et al. Cross-Talk between Obesity and Diabetes: Introducing Polyphenols as an Effective Phytomedicine to Combat the Dual Sword Diabesity. Curr. Pharm. Des. 2022, 28, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Song, X.; Wang, H.; Yan, Y.; Liu, B. The role of exercise-induced myokines in promoting angiogenesis. Front. Physiol. 2022, 13, 981577. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2022, 204, 92–107. [Google Scholar] [CrossRef]
- Sun, W.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Kim, M.; Takayama, Y.; Takahashi, N.; Goto, T.; Wakabayashi, S.; Kawada, T.; et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016, 17, 383–399. [Google Scholar] [CrossRef]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef]
- Chen, Y.; Ikeda, K.; Yoneshiro, T.; Scaramozza, A.; Tajima, K.; Wang, Q.; Kim, K.; Shinoda, K.; Sponton, C.H.; Brown, Z.; et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 2019, 565, 180–185. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Liu, Y.; Huang, P.; Song, H.; Zheng, P. The potential function and clinical application of FGF21 in metabolic diseases. Front. Pharmacol. 2022, 13, 1089214. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Exercise Therapy for People with Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Wang, T.; Su, Y.; Li, L.; Lan, M.; Yu, Y.; Liu, F.; Xiong, L.; Wang, K.; et al. Immunoglobulin Superfamily Containing Leucine-Rich Repeat (Islr) Participates in IL-6-Mediated Crosstalk between Muscle and Brown Adipose Tissue to Regulate Energy Homeostasis. Int. J. Mol. Sci. 2022, 23, 10008. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Yoshiko, A.; Kaji, T.; Kozuka, T.; Sawazaki, T.; Akima, H. Evaluation of rehabilitation exercise effects by using gradation-based skeletal muscle echo intensity in older individuals: A one-group before-and-after trial study. BMC Geriatr. 2021, 21, 485. [Google Scholar] [CrossRef]
- Otsuka, Y.; Miyamoto, N.; Nagai, A.; Izumo, T.; Nakai, M.; Fukuda, M.; Arimitsu, T.; Yamada, Y.; Hashimoto, T. Effects of Quercetin Glycoside Supplementation Combined with Low-Intensity Resistance Training on Muscle Quantity and Stiffness: A Randomized, Controlled Trial. Front. Nutr. 2022, 9, 912217. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Satoh, M.; Takemura, Y.; Hamada, H.; Sekido, Y.; Kubota, S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E.; CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Santa, K.; Yahata, T.; Sato, N.; Ohta, A.; Ohmi, Y.; Sato, T.; Hozumi, K.; Habu, S. Involvement of IL-4-producing Vβ8.2+ CD4+ CD62L- CD45RB- T cells in non-MHC gene-controlled predisposition toward skewing into T helper type-2 immunity in BALB/c mice. J. Immunol. 1997, 158, 5698–56706. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Levin, S.G.; Pershina, E.V.; Bugaev-Makarovskiy, N.A.; Chernomorets, I.Y.; Konakov, M.V.; Arkhipov, V.I. Why Do Levels of Anti-inflammatory Cytokines Increase During Memory Acquisition? Neuroscience 2021, 473, 159–169. [Google Scholar] [CrossRef]
- Motomura, Y.; Kitamura, H.; Hijikata, A.; Matsunaga, Y.; Matsumoto, K.; Inoue, H.; Atarashi, K.; Hori, S.; Watarai, H.; Zhu, J.; et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011, 12, 450–459. [Google Scholar] [CrossRef]
- Lyu, M.; Suzuki, H.; Kang, L.; Gaspal, F.; Zhou, W.; Goc, J.; Zhou, L.; Zhou, J.; Zhang, W.; Shen, Z.; et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 2022, 610, 744–751. [Google Scholar] [CrossRef]
- Kedmi, R.; Najar, T.A.; Mesa, K.R.; Grayson, A.; Kroehling, L.; Hao, Y.; Hao, S.; Pokrovskii, M.; Xu, M.; Talbot, J.; et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 2022, 610, 737–743. [Google Scholar] [CrossRef]
- Okai, S.; Usui, F.; Yokota, S.; Hori-i, Y.; Hasegawa, M.; Nakamura, T.; Kurosawa, M.; Okada, S.; Yamamoto, K.; Nishiyama, E.; et al. High-affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat. Microbiol. 2016, 1, 16103. [Google Scholar] [CrossRef]
- Usami, K.; Niimi, K.; Matsuo, A.; Suyama, Y.; Sakai, Y.; Sato, S.; Fujihashi, K.; Kiyono, H.; Uchino, S.; Furukawa, M.; et al. The gut microbiota induces Peyer’s-patch-dependent secretion of maternal IgA into milk. Cell Rep. 2021, 36, 109655. [Google Scholar] [CrossRef]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L. Swimming intervention alleviates insulin resistance and chronic inflammation in metabolic syndrome. Exp. Ther. Med. 2019, 17, 57–62. [Google Scholar] [CrossRef]
- Hosseinkhani, F.; Heinken, A.; Thiele, I.; Lindenburg, P.W.; Harms, A.C.; Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021, 13, 1–22. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.Y.; Huang, H.F.; Zheng, T.T.; Li, J.; Wang, L.J.; Ma, X.C.; Xiao, H.T. Adiponectin Deficiency Suppresses Rhabdomyosarcoma Associated with Gut Microbiota Regulation. Biomed. Res. Int. 2021, 2021, 8010694. [Google Scholar] [CrossRef]
- Rotz, S.J.; Sangwan, N.; Nagy, M.; Tzeng, A.; Jia, M.; Moncaliano, M.; Majhail, N.S.; Eng, C. Fecal microbiota of adolescent and young adult cancer survivors and metabolic syndrome: An exploratory study. Pediatr. Hematol. Oncol. 2022, 39, 629–643. [Google Scholar] [CrossRef]
- Martino, C.; Dilmore, A.H.; Burcham, Z.M.; Metcalf, J.L.; Jeste, D.; Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat. Rev. Microbiol. 2022, 20, 707–720. [Google Scholar] [CrossRef]
- Nagata, N.; Nishijima, S.; Miyoshi-Akiyama, T.; Kojima, Y.; Kimura, M.; Aoki, R.; Ohsugi, M.; Ueki, K.; Miki, K.; Iwata, E.; et al. Population-level Metagenomics Uncovers Distinct Effects of Multiple Medications on the Human Gut Microbiome. Gastroenterology 2022, 163, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Gervasi, M.; Annibalini, G.; Amatori, S.; Ferrini, F.; Sisti, D.; Piccoli, G.; Barbieri, E.; Sestili, P.; et al. Mutual Interactions among Exercise, Sport Supplements and Microbiota. Nutrients 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Sahashi, Y.; Goto, A.; Takachi, R.; Ishihara, J.; Kito, K.; Kanehara, R.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Shoichiro, T.; et al. Inverse Association between Fruit and Vegetable Intake and All-Cause Mortality: Japan Public Health Center-Based Prospective Study. J. Nutr. 2022, 152, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Wilkerson, M.D.; Sampey, B.P.; Troester, M.A.; Hayes, D.N.; Makowski, L. Cafeteria diet-induced obesity causes oxidative damage in white adipose. Biochem. Biophys. Res. Commun. 2016, 473, 545–550. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Magrone, T. Effects of Plastics on Human Health and Mechanisms of Action. Endocr. Metab. Immune. Disord. Drug Targets 2022, 22, 663–664. [Google Scholar] [CrossRef]
- Santa, K.; Ohsawa, T.; Sakimoto, T. Para-Nonylphenol Induces Apoptosis of U937 Human Monocyte Leukemia Cells in vitro. Endocr. Metab. Immune. Disord. Drug Targets 2016, 16, 213–223. [Google Scholar] [CrossRef]
- Murro, I.; Lisco, G.; Di Noia, C.; Lampignano, L.; Zupo, R.; Giagulli, V.A.; Guastamacchia, E.; Triggiani, V.; De Pergola, G. Endocrine Disruptors and Obesity: An Overview. Endocr. Metab. Immune. Disord. Drug Targets 2022, 22, 798–806. [Google Scholar]
- Bibbò, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is there a role for gut microbiota in type 1 diabetes pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Murata, C.; Gutiérrez-Castrellón, P.; Pérez-Villatoro, F.; García-Torres, I.; Enríquez-Flores, S.; de la Mora-de la Mora, I.; Fernández-Lainez, C.; Werner, J.; López-Velázquez, G. Delivery mode-associated gut microbiota in the first 3 months of life in a country with high obesity rates: A descriptive study. Medicine 2020, 99, e22442. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; John Britto, J.S.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Kikut, J.; Konecka, N.; Ziętek, M.; Kulpa, D.; Szczuko, M. Diet supporting therapy for inflammatory bowel diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef]
- Hryckowian, A.J.; Van Treuren, W.; Smits, S.A.; Davis, N.M.; Gardner, J.O.; Bouley, D.M.; Sonnenburg, J.L. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat. Microbiol. 2018, 3, 662–669. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef]
- Tan, S.; Caparros-Martin, J.A.; Matthews, V.B.; Koch, H.; O’Gara, F.; Croft, K.D.; Ward, N.C. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci. Rep. 2018, 8, 10100. [Google Scholar] [CrossRef]
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Cano-Martínez, A.; Bautista-Pérez, R.; Castrejón-Téllez, V.; Carreón-Torres, E.; Pérez-Torres, I.; Díaz-Díaz, E.; Flores-Estrada, J.; Guarner-Lans, V.; Rubio-Ruíz, M.E. Resveratrol and Quercetin as Regulators of Inflammatory and Purinergic Receptors to Attenuate Liver Damage Associated to Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 8939. [Google Scholar] [CrossRef]
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008, 283, 9424–9434. [Google Scholar] [CrossRef]
- Ishisaka, A.; Kawabata, K.; Miki, S.; Shiba, Y.; Minekawa, S.; Nishikawa, T.; Mukai, R.; Terao, J.; Kawai, Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS ONE 2013, 8, e80843. [Google Scholar] [CrossRef]
- Kawai, Y. Understanding metabolic conversions and molecular actions of flavonoids in vivo: Toward new strategies for effective utilization of natural polyphenols in human health. J. Med. Investig. 2018, 65, 162–165. [Google Scholar] [CrossRef]
- Gurkan, N. Vitamin D supplementation during pregnancy inhibits the activation of fetal membrane NF-κB pathway. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5926–5931. [Google Scholar] [PubMed]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on diabetic nephropathy: Advances and therapeutic opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Kawaguchi, K.; Kanesaka, M.; Kawauchi, H.; Jirillo, E.; Kumazawa, Y. Suppression of type-I allergic responses by oral administration of grape marc fermented with Lactobacillus plantarum. Immunopharmacol. Immunotoxicol. 2010, 32, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr. Pharm. Des. 2014, 20, 857–863. [Google Scholar] [CrossRef]
- Kaneko, M.; Takimoto, H.; Sugiyama, T.; Seki, Y.; Kawaguchi, K.; Kumazawa, Y. Suppressive effects of the flavonoids quercetin and luteolin on the accumulation of lipid rafts after signal transduction via receptors. Immunopharmacol. Immunotoxicol. 2008, 30, 867–882. [Google Scholar] [CrossRef]
- Molhoek, E.M.; den Hertog, A.L.; de Vries, A.M.; Nazmi, K.; Veerman, E.C.; Hartgers, F.C.; Yazdanbakhsh, M.; Bikker, F.J.; van der Kleij, D. Structure-function relationship of the human antimicrobial peptide LL-37 and LL-37 fragments in the modulation of TLR responses. Biol. Chem. 2009, 390, 295–303. [Google Scholar] [CrossRef]

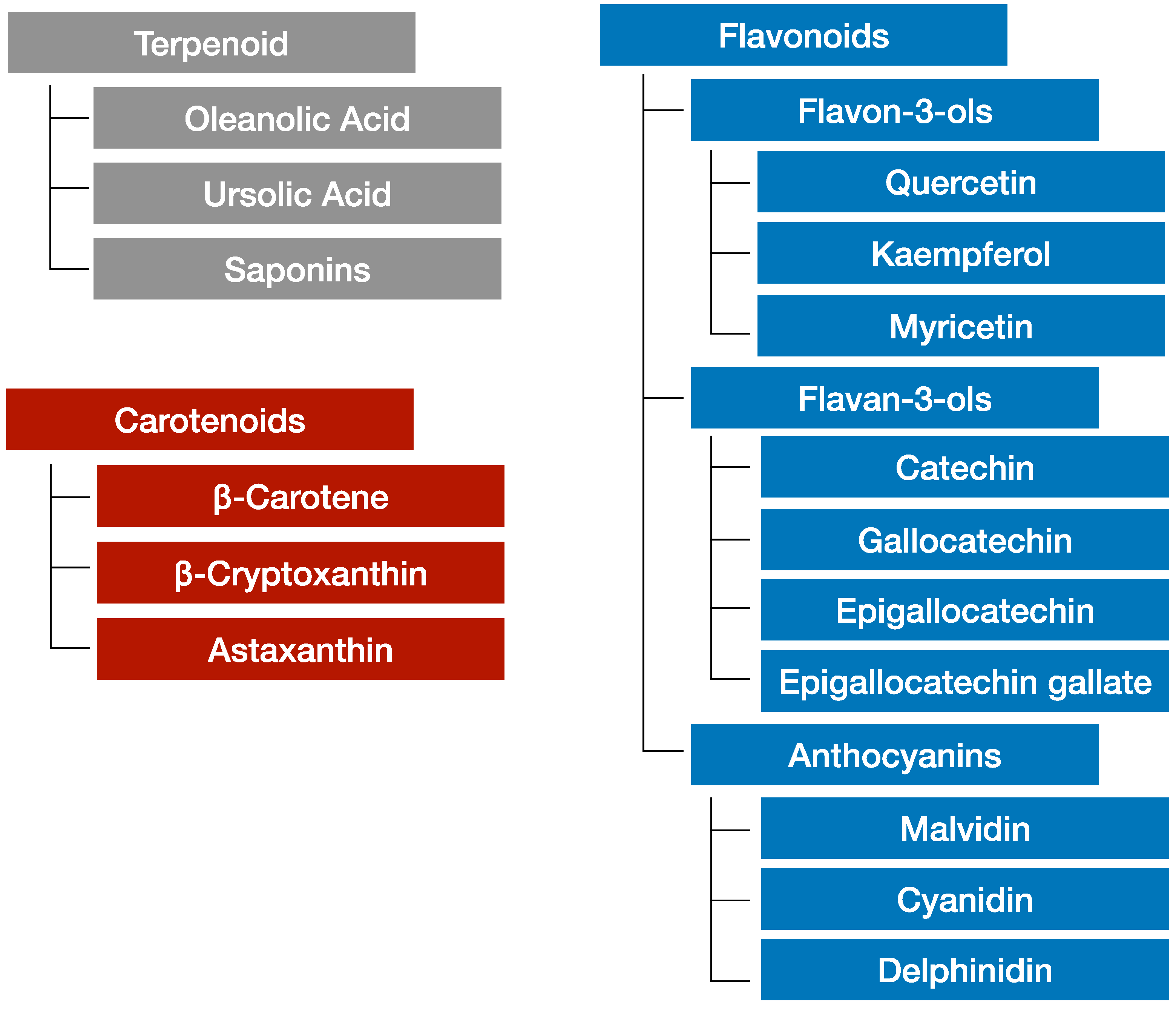

| Grape Phytochemicals | Effects | Subjects | Ref |
|---|---|---|---|
| Wine | Health-improving effects of wine | Human | [74] |
| Resveratrol | Maintaining health condition in high calorie intake | Mouse/ D. melanogaster/ C. elegans/ S. cerevisiae | [75] |
| Reducing risks of NAFLD and gut dysbiosis | Mouse | [76] | |
| Atherosclerosis prevention | Human | [83] | |
| Red wine polyphenol | Atherosclerosis prevention, effects in vascular smooth muscle cells | Human and Bovine endothelial cells | [77] |
| Red wine bioactive compound | Anti-oxidative, thrombin inhibition, lipase inhibition | Cells/Activity screening kit | [78] |
| Flavonoids | Anti-oxidative, anti-inflammatory, anti-carcinogenesis, circulatory system disease prevention | Human | [79] |
| Alleviating collagen-induced arthritis | Mouse | [84] | |
| Anti-ageing | Human | [85] | |
| Grape phytochemicals, GSE, K-FGF | Alleviating intestine related disordered | Human/ Rat/Mouse | [80] |
| Grape seed extract (GSE) | Lung fibrosis prevention | Mouse | [81] |
| Grape seed flan-3-ols | Analysis of biosynthetic pathways in nutraceuticals | Physical analysis | [82] |
| Procyanidin | Preventing senescence | Mouse/ Human cells | [86] |
| Effects | Subjects | Ref |
|---|---|---|
| Boosting natural immunity, maintaining diversity of gut microbiota | Human | [92] |
| Antibacterial peptide LL-37 induction, upregulation of innate immunity | Human | [93] |
| Strengthen natural immunity by the induction of antibacterial peptide | Human | [94] |
| Gut microbiota modification, insulin-resistance, NAFLD by defensins | Mouse | [95] |
| Treg activation by IL-10 production, suppression of inflammatory immune response | Human/ Human cells | [96] |
| Suppression of chronic inflammation related disorders by Treg activation | Mouse | [97] |
| Vascular vessel protection, anti-oxidative, proinflammatory cytokine suppression | Human | [98] |
| Upregulation of immunity against COVID-19 infection | Human | [99] |
| Source | Effects | Subjects | Ref |
|---|---|---|---|
| Polyphenols | Microbiota in metabolic disorders | Human/ Rat/Mouse | [167] |
| Quercetin | Improvement of obesity and NAFLD | Mouse | [168] |
| Correct F/B ratio, obesity | Mouse | [169] | |
| Insulin resistance, increases Faecalibaculum rodentium, improves F/B ratio, increases GULT4 | Mouse | [170] | |
| Vitamin D | Gut microbiota modification | Human | [171] |
| Increasing Akkermansia and Faecalibacterium (in multiple sclerosis) | Human | [172] | |
| Improvement of gut dysbiosis | Human | [173] | |
| Antimicrobial peptide release, gut microbiota interaction | Human/Mouse | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa, K.; Kumazawa, Y.; Nagaoka, I. Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D. Int. J. Mol. Sci. 2023, 24, 2627. https://doi.org/10.3390/ijms24032627
Santa K, Kumazawa Y, Nagaoka I. Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D. International Journal of Molecular Sciences. 2023; 24(3):2627. https://doi.org/10.3390/ijms24032627
Chicago/Turabian StyleSanta, Kazuki, Yoshio Kumazawa, and Isao Nagaoka. 2023. "Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D" International Journal of Molecular Sciences 24, no. 3: 2627. https://doi.org/10.3390/ijms24032627
APA StyleSanta, K., Kumazawa, Y., & Nagaoka, I. (2023). Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D. International Journal of Molecular Sciences, 24(3), 2627. https://doi.org/10.3390/ijms24032627







