Macrophage Phenotyping in Atherosclerosis by Proteomics
Abstract
1. Introduction
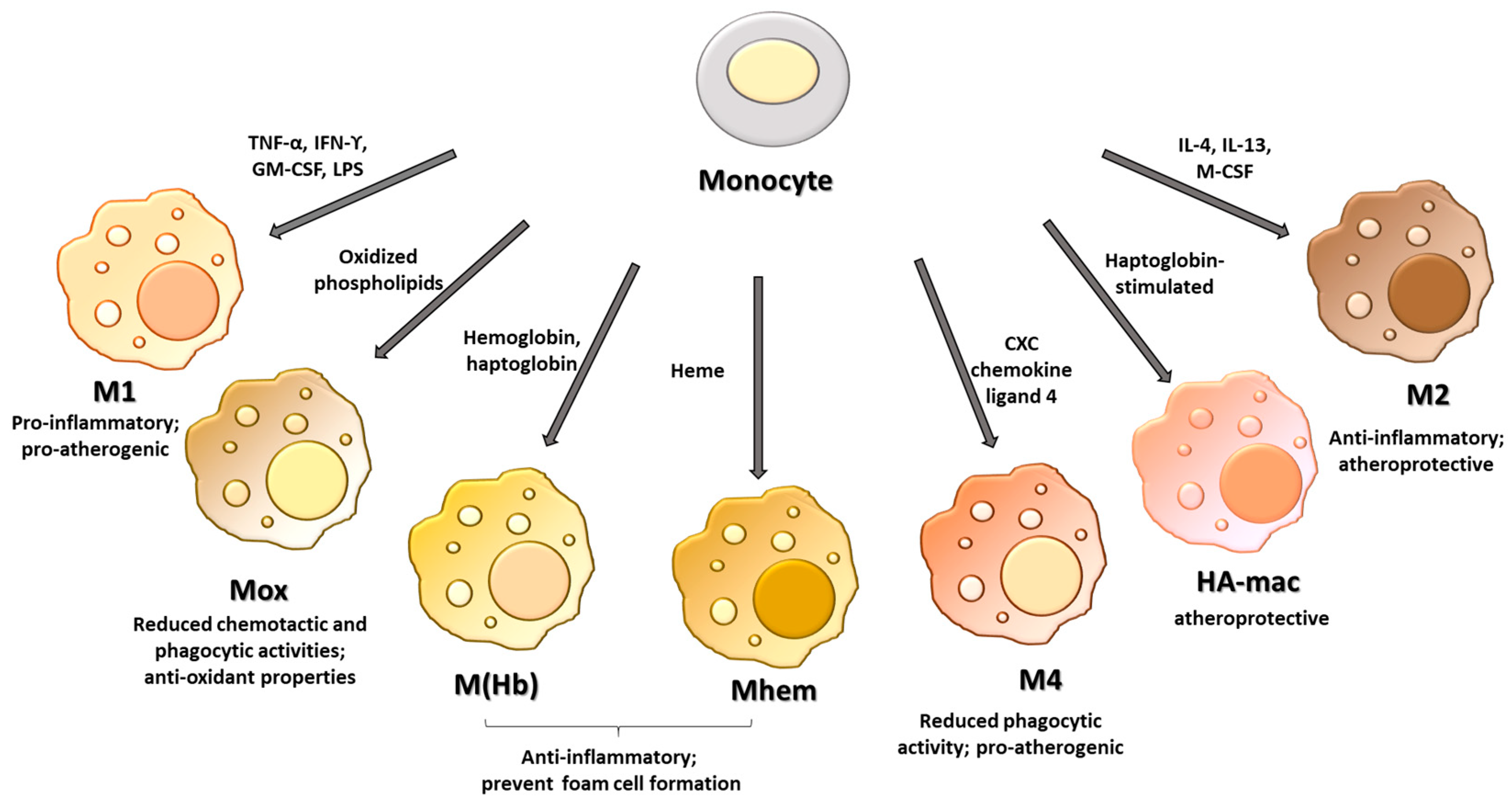
2. Macrophage Phenotypes
3. Role of Macrophage Phenotypes in the Atherosclerotic Plaque
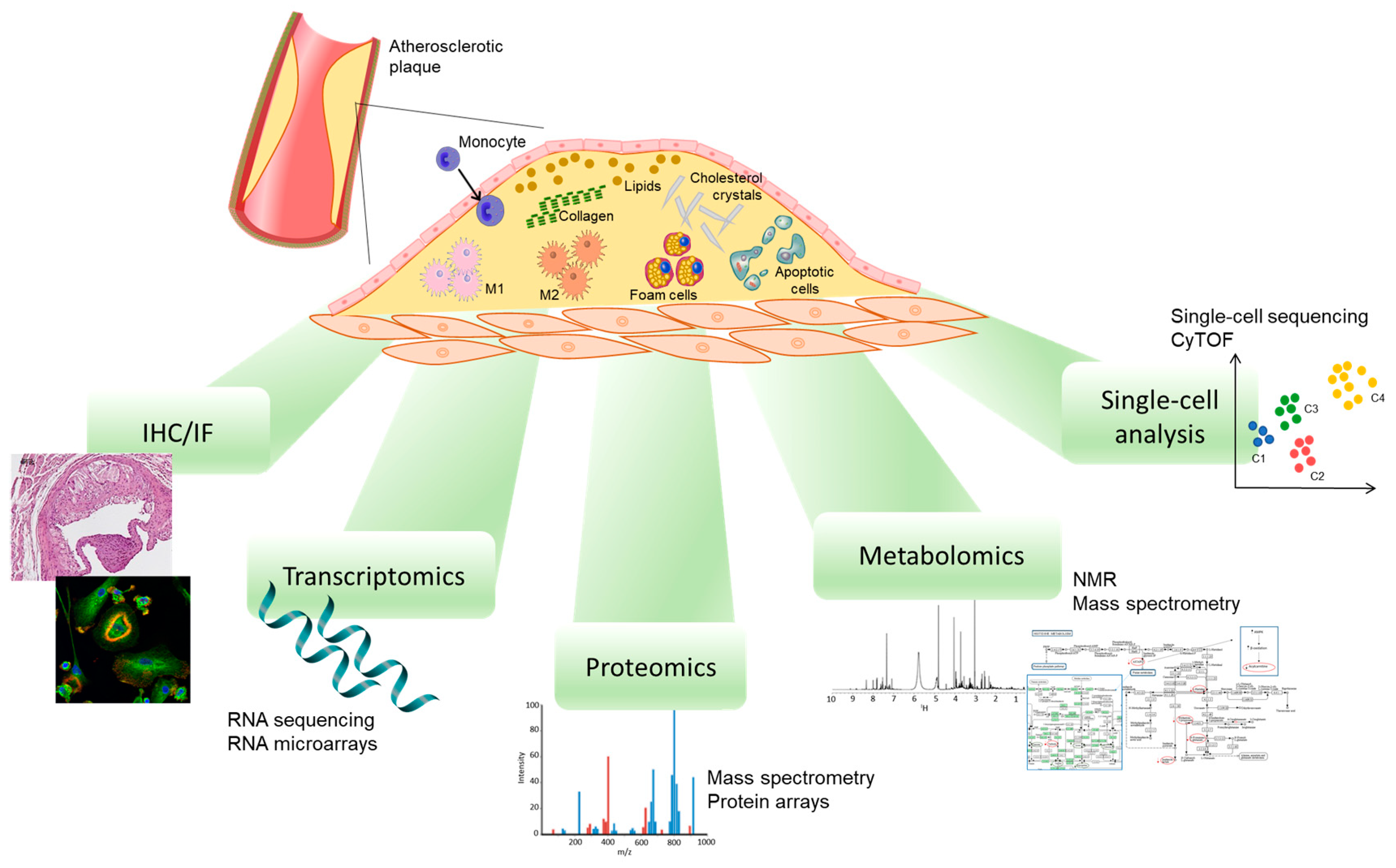
4. Methods to Study Plaque Macrophage Phenotypes
5. The Proteomic Analysis of Atherosclerotic Plaque Highlights the Contribution of Macrophages
6. The Analysis of the Atherosclerotic Plaque Secretome Reveals the Contribution of Macrophages
7. Proteomic Analysis of In Vitro-Cultured Macrophages
8. Single-Cell Proteomics of Macrophages
9. Macrophage Proteins as Therapeutic Targets
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V. Monocyte recruitment and foam cell formation in atherosclerosis. Micron 2006, 37, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Farahi, L.; Sinha, S.K.; Lusis, A.J. Roles of Macrophages in Atherogenesis. Front. Pharm. 2021, 12, 785220. [Google Scholar] [CrossRef]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Gui, T.; Shimokado, A.; Sun, Y.; Akasaka, T.; Muragaki, Y. Diverse roles of macrophages in atherosclerosis: From inflammatory biology to biomarker discovery. Mediat. Inflamm. 2012, 2012, 693083. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arter. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef]
- Grinberg, S.; Hasko, G.; Wu, D.; Leibovich, S.J. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am. J. Pathol. 2009, 175, 2439–2453. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 2013, 36, 921–931. [Google Scholar] [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 2010, 107, 737–746. [Google Scholar] [CrossRef]
- Eligini, S.; Brambilla, M.; Banfi, C.; Camera, M.; Sironi, L.; Barbieri, S.S.; Auwerx, J.; Tremoli, E.; Colli, S. Oxidized phospholipids inhibit cyclooxygenase-2 in human macrophages via nuclear factor-kappaB/IkappaB- and ERK2-dependent mechanisms. Cardiovasc. Res. 2002, 55, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Fazio, S. Cyclooxygenase-2 and inflammation in atherosclerosis. Curr. Opin. Pharm. 2004, 4, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Moller, H.J.; Moestrup, S.K. Hemoglobin and heme scavenger receptors. Antioxid. Redox. Signal. 2010, 12, 261–273. [Google Scholar] [CrossRef]
- Boyle, J.J. Heme and haemoglobin direct macrophage Mhem phenotype and counter foam cell formation in areas of intraplaque haemorrhage. Curr. Opin. Lipidol. 2012, 23, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Johns, M.; Kampfer, T.; Nguyen, A.T.; Game, L.; Schaer, D.J.; Mason, J.C.; Haskard, D.O. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 2012, 110, 20–33. [Google Scholar] [CrossRef]
- Philippidis, P.; Mason, J.C.; Evans, B.J.; Nadra, I.; Taylor, K.M.; Haskard, D.O.; Landis, R.C. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004, 94, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Tyka, M.; Helmes, C.M.; Akhavanpoor, M.; Rupp, G.; Domschke, G.; Linden, F.; Wolf, A.; Doesch, A.; Lasitschka, F.; et al. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015, 21, 255–265. [Google Scholar] [CrossRef]
- Gleissner, C.A.; Shaked, I.; Erbel, C.; Bockler, D.; Katus, H.A.; Ley, K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 2010, 106, 203–211. [Google Scholar] [CrossRef]
- Stoger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Cho, K.Y.; Miyoshi, H.; Kuroda, S.; Yasuda, H.; Kamiyama, K.; Nakagawara, J.; Takigami, M.; Kondo, T.; Atsumi, T. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J. Stroke Cereb. Dis. 2013, 22, 910–918. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-Type Macrophage Responses Are Predictive of Adverse Outcomes in Human Atherosclerosis. Front. Immunol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Landis, R.C.; Philippidis, P.; Domin, J.; Boyle, J.J.; Haskard, D.O. Haptoglobin Genotype-Dependent Anti-Inflammatory Signaling in CD163(+) Macrophages. Int. J. Inflam. 2013, 2013, 980327. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Vulf, M.; Khaziakhmatova, O.; Malashchenko, V.; Komar, A.; Shunkin, E.; Shupletsova, V.; Goncharov, A.; Urazova, O.; Litvinova, L. Tissue-Specific Role of Macrophages in Noninfectious Inflammatory Disorders. Biomedicines 2020, 8, 400. [Google Scholar] [CrossRef]
- Eligini, S.; Brioschi, M.; Fiorelli, S.; Tremoli, E.; Banfi, C.; Colli, S. Human monocyte-derived macrophages are heterogenous: Proteomic profile of different phenotypes. J. Proteom. 2015, 124, 112–123. [Google Scholar] [CrossRef]
- Eligini, S.; Brioschi, M.; Fiorelli, S.; Tremoli, E.; Colli, S.; Banfi, C. Data for proteomic analysis of Human monocyte-derived macrophages. Data Brief 2015, 4, 177–179. [Google Scholar] [CrossRef]
- Lee, C.W.; Hwang, I.; Park, C.S.; Lee, H.; Park, D.W.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; Park, S.J. Macrophage heterogeneity of culprit coronary plaques in patients with acute myocardial infarction or stable angina. Am. J. Clin. Pathol. 2013, 139, 317–322. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Njoroge, J.M.; Mitchell, L.B.; Centola, M.; Kastner, D.; Raffeld, M.; Miller, J.L. Characterization of viable autofluorescent macrophages among cultured peripheral blood mononuclear cells. Cytometry 2001, 44, 38–44. [Google Scholar] [CrossRef]
- Albaghdadi, M.S.; Ikegami, R.; Kassab, M.B.; Gardecki, J.A.; Kunio, M.; Chowdhury, M.M.; Khamis, R.; Libby, P.; Tearney, G.J.; Jaffer, F.A. Near-Infrared Autofluorescence in Atherosclerosis Associates With Ceroid and Is Generated by Oxidized Lipid-Induced Oxidative Stress. Arter. Thromb. Vasc. Biol. 2021, 41, e385–e398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ostblom, M.; Xu, L.H.; Hellsten, A.; Leanderson, P.; Liedberg, B.; Brunk, U.T.; Eaton, J.W.; Yuan, X.M. Cytocidal effects of atheromatous plaque components: The death zone revisited. Faseb J. 2006, 20, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Htun, N.M.; Chen, Y.C.; Lim, B.; Schiller, T.; Maghzal, G.J.; Huang, A.L.; Elgass, K.D.; Rivera, J.; Schneider, H.G.; Wood, B.R.; et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques. Nat. Commun. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Eijgelaar, W.J.; Horrevoets, A.J.; Bijnens, A.P.; Daemen, M.J.; Verhaegh, W.F. Equivalence testing in microarray analysis: Similarities in the transcriptome of human atherosclerotic and nonatherosclerotic macrophages. Physiol. Genom. 2010, 41, 212–223. [Google Scholar] [CrossRef]
- Chai, J.T.; Ruparelia, N.; Goel, A.; Kyriakou, T.; Biasiolli, L.; Edgar, L.; Handa, A.; Farrall, M.; Watkins, H.; Choudhury, R.P. Differential Gene Expression in Macrophages From Human Atherosclerotic Plaques Shows Convergence on Pathways Implicated by Genome-Wide Association Study Risk Variants. Arter. Thromb. Vasc. Biol. 2018, 38, 2718–2730. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.R.; Tang, X.; Lin, C.P.; Yan, D.; Xue, S.; Qian, R.Z.; Guo, D.Q. Identification of potential therapeutic targets for atherosclerosis by analysing the gene signature related to different immune cells and immune regulators in atheromatous plaques. Bmc Med. Genom. 2021, 14, 145. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Narang, R.; Bisoi, A.K.; Mitra, D.K. Signature transcriptome analysis of stage specific atherosclerotic plaques of patients. Bmc Med. Genom. 2022, 15, 99. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Changes in transcriptome of macrophages in atherosclerosis. J. Cell Mol. Med. 2015, 19, 1163–1173. [Google Scholar] [CrossRef]
- Willemsen, L.; de Winther, M.P. Macrophage subsets in atherosclerosis as defined by single-cell technologies. J. Pathol. 2020, 250, 705–714. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Eberhardt, N.; Giannarelli, C. How Single-Cell Technologies Have Provided New Insights Into Atherosclerosis. Arter. Thromb. Vasc. Biol. 2022, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.Y.; Kastenmuller, W.; Ifrim, I.; Kabat, J.; Germain, R.N. Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 2012, 37, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Macklin, A.; Khan, S.; Kislinger, T. Recent advances in mass spectrometry based clinical proteomics: Applications to cancer research. Clin. Proteom. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Polati, R.; Bossi, A.M.; Girelli, D. Monocyte/macrophage proteomics: Recent findings and biomedical applications. Expert Rev. Proteom. 2012, 9, 201–215. [Google Scholar] [CrossRef]
- Banfi, C.; Baetta, R.; Gianazza, E.; Tremoli, E. Technological advances and proteomic applications in drug discovery and target deconvolution: Identification of the pleiotropic effects of statins. Drug Discov. Today 2017, 22, 848–869. [Google Scholar] [CrossRef]
- Matallana-Surget, S.; Leroy, B.; Wattiez, R. Shotgun proteomics: Concept, key points and data mining. Expert Rev. Proteom. 2010, 7, 5–7. [Google Scholar] [CrossRef]
- Meyer, J.G. Qualitative and Quantitative Shotgun Proteomics Data Analysis from Data-Dependent Acquisition Mass Spectrometry. Methods Mol. Biol. 2021, 2259, 297–308. [Google Scholar] [CrossRef]
- Gevaert, K.; Impens, F.; Ghesquiere, B.; Van Damme, P.; Lambrechts, A.; Vandekerckhove, J. Stable isotopic labeling in proteomics. Proteomics 2008, 8, 4873–4885. [Google Scholar] [CrossRef]
- Old, W.M.; Meyer-Arendt, K.; Aveline-Wolf, L.; Pierce, K.G.; Mendoza, A.; Sevinsky, J.R.; Resing, K.A.; Ahn, N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell Proteom. 2005, 4, 1487–1502. [Google Scholar] [CrossRef]
- Gianazza, E.; Tremoli, E.; Banfi, C. The selected reaction monitoring/multiple reaction monitoring-based mass spectrometry approach for the accurate quantitation of proteins: Clinical applications in the cardiovascular diseases. Expert Rev. Proteom. 2014, 11, 771–788. [Google Scholar] [CrossRef]
- Tuomisto, T.T.; Riekkinen, M.S.; Viita, H.; Levonen, A.L.; Yla-Herttuala, S. Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading--cDNA and protein array study. Atherosclerosis 2005, 180, 283–291. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta, F.; Alvarez-Llamas, G.; Gil-Dones, F.; Martin-Rojas, T.; Zubiri, I.; Pastor, C.; Barderas, M.G.; Vivanco, F. Tissue proteomics in atherosclerosis: Elucidating the molecular mechanisms of cardiovascular diseases. Expert Rev. Proteom. 2009, 6, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Schrijvers, D.M.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. Western array analysis of human atherosclerotic plaques: Downregulation of apoptosis-linked gene 2. Cardiovasc. Res. 2003, 60, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Elasbali, A.B.; Miguel Turu, M.; Krupinski, J.; Badimon, L.; Gaffney, J. Identification of differential protein expression associated with development of unstable human carotid plaques. Am. J. Pathol. 2006, 168, 1004–1021. [Google Scholar] [CrossRef]
- Seimon, T.; Tabas, I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 2009, 50, S382–S387. [Google Scholar] [CrossRef]
- Bagnato, C.; Thumar, J.; Mayya, V.; Hwang, S.I.; Zebroski, H.; Claffey, K.P.; Haudenschild, C.; Eng, J.K.; Lundgren, D.H.; Han, D.K. Proteomics analysis of human coronary atherosclerotic plaque: A feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol. Cell. Proteom. 2007, 6, 1088–1102. [Google Scholar] [CrossRef]
- Bao, M.H.; Zhang, R.Q.; Huang, X.S.; Zhou, J.; Guo, Z.; Xu, B.F.; Liu, R. Transcriptomic and Proteomic Profiling of Human Stable and Unstable Carotid Atherosclerotic Plaques. Front. Genet. 2021, 12, 755507. [Google Scholar] [CrossRef] [PubMed]
- Farokhzadian, J.; Mangolian Shahrbabaki, P.; Bagheri, V. S100A12-CD36 axis: A novel player in the pathogenesis of atherosclerosis? Cytokine 2019, 122, 154104. [Google Scholar] [CrossRef]
- Stakhneva, E.M.; Meshcheryakova, I.A.; Demidov, E.A.; Starostin, K.V.; Sadovski, E.V.; Peltek, S.E.; Voevoda, M.I.; Chernyavskii, A.M.; Volkov, A.M.; Ragino, Y.I. A Proteomic Study of Atherosclerotic Plaques in Men with Coronary Atherosclerosis. Diagnostics 2019, 9, 177. [Google Scholar] [CrossRef]
- Haversen, L.; Sundelin, J.P.; Mardinoglu, A.; Rutberg, M.; Stahlman, M.; Wilhelmsson, U.; Hulten, L.M.; Pekny, M.; Fogelstrand, P.; Bentzon, J.F.; et al. Vimentin deficiency in macrophages induces increased oxidative stress and vascular inflammation but attenuates atherosclerosis in mice. Sci. Rep. 2018, 8, 16973. [Google Scholar] [CrossRef]
- Vaisar, T.; Hu, J.H.; Airhart, N.; Fox, K.; Heinecke, J.; Nicosia, R.F.; Kohler, T.; Potter, Z.E.; Simon, G.M.; Dix, M.M.; et al. Parallel Murine and Human Plaque Proteomics Reveals Pathways of Plaque Rupture. Circ. Res. 2020, 127, 997–1022. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M.; Van Eyk, J.E. Secreted proteins as a fundamental source for biomarker discovery. Proteomics 2012, 12, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, M.; Lento, S.; Tremoli, E.; Banfi, C. Proteomic analysis of endothelial cell secretome: A means of studying the pleiotropic effects of Hmg-CoA reductase inhibitors. J. Proteom. 2013, 78, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016, 5, e004099. [Google Scholar] [CrossRef]
- Duran, M.C.; Martin-Ventura, J.L.; Mohammed, S.; Barderas, M.G.; Blanco-Colio, L.M.; Mas, S.; Moral, V.; Ortega, L.; Tunon, J.; Jensen, O.N.; et al. Atorvastatin modulates the profile of proteins released by human atherosclerotic plaques. Eur. J. Pharm. 2007, 562, 119–129. [Google Scholar] [CrossRef]
- Duran, M.C.; Martin-Ventura, J.L.; Mas, S.; Barderas, M.G.; Darde, V.M.; Jensen, O.N.; Egido, J.; Vivanco, F. Characterization of the human atheroma plaque secretome by proteomic analysis. Methods Mol. Biol. 2007, 357, 141–150. [Google Scholar] [CrossRef]
- Li, W.; Dalen, H.; Eaton, J.W.; Yuan, X.M. Apoptotic death of inflammatory cells in human atheroma. Arter. Thromb. Vasc. Biol. 2001, 21, 1124–1130. [Google Scholar] [CrossRef]
- Auguet, T.; Aragones, G.; Guiu-Jurado, E.; Berlanga, A.; Curriu, M.; Martinez, S.; Alibalic, A.; Aguilar, C.; Camara, M.L.; Hernandez, E.; et al. Adipo/cytokines in atherosclerotic secretomes: Increased visfatin levels in unstable carotid plaque. Bmc Cardiovasc. Disord. 2016, 16, 149. [Google Scholar] [CrossRef]
- Dahl, T.B.; Yndestad, A.; Skjelland, M.; Oie, E.; Dahl, A.; Michelsen, A.; Damas, J.K.; Tunheim, S.H.; Ueland, T.; Smith, C.; et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation 2007, 115, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pan, Y.; Huang, Z.; Jia, Y.; Zhao, X.; Chen, Y.; Diao, J.; Wan, Q.; Cui, X. Visfatin induces cholesterol accumulation in macrophages through up-regulation of scavenger receptor-A and CD36. Cell Stress Chaperones 2013, 18, 643–652. [Google Scholar] [CrossRef]
- Aragones, G.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Curriu, M.; Martinez, S.; Alibalic, A.; Aguilar, C.; Hernandez, E.; Camara, M.L.; et al. Proteomic Profile of Unstable Atheroma Plaque: Increased Neutrophil Defensin 1, Clusterin, and Apolipoprotein E Levels in Carotid Secretome. J. Proteome Res. 2016, 15, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.L.; Henriques, M.; Tabuchi, A.; Han, B.; Yang, H.; Cheng, W.E.; Tole, S.; Yu, H.; Luo, A.; Charbonney, E.; et al. Human neutrophil peptides mediate endothelial-monocyte interaction, foam cell formation, and platelet activation. Arter. Thromb. Vasc. Biol. 2011, 31, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Yanni, A.E.; Agrogiannis, G.; Gkekas, C.; Perrea, D. Clusterin/Apolipoprotein J immunolocalization on carotid artery is affected by TNF-alpha, cigarette smoking and anti-platelet treatment. Lipids Health Dis. 2014, 13, 70. [Google Scholar] [CrossRef]
- Bellosta, S.; Mahley, R.W.; Sanan, D.A.; Murata, J.; Newland, D.L.; Taylor, J.M.; Pitas, R.E. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J. Clin. Investig. 1995, 96, 2170–2179. [Google Scholar] [CrossRef]
- Li, P.; Hao, Z.; Wu, J.; Ma, C.; Xu, Y.; Li, J.; Lan, R.; Zhu, B.; Ren, P.; Fan, D.; et al. Comparative Proteomic Analysis of Polarized Human THP-1 and Mouse RAW264.7 Macrophages. Front. Immunol. 2021, 12, 700009. [Google Scholar] [CrossRef]
- He, L.; Jhong, J.H.; Chen, Q.; Huang, K.Y.; Strittmatter, K.; Kreuzer, J.; DeRan, M.; Wu, X.; Lee, T.Y.; Slavov, N.; et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell. Rep. 2021, 37, 109955. [Google Scholar] [CrossRef]
- Zhang, L.; Lun, Y.; Yan, D.; Yu, L.; Ma, W.; Du, B.; Zhu, X. Proteomic analysis of macrophages: A new way to identify novel cell-surface antigens. J. Immunol. Methods 2007, 321, 80–85. [Google Scholar] [CrossRef]
- Becker, L.; Liu, N.C.; Averill, M.M.; Yuan, W.; Pamir, N.; Peng, Y.; Irwin, A.D.; Fu, X.; Bornfeldt, K.E.; Heinecke, J.W. Unique proteomic signatures distinguish macrophages and dendritic cells. PLoS ONE 2012, 7, e33297. [Google Scholar] [CrossRef]
- Yu, Y.L.; Huang, Z.Y.; Yang, P.Y.; Rui, Y.C.; Yang, P.Y. Proteomic studies of macrophage-derived foam cell from human U937 cell line using two-dimensional gel electrophoresis and tandem mass spectrometry. J. Cardiovasc. Pharm. 2003, 42, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, H.T.; Choi, M.S.; Lee, W.H.; Huh, T.L.; Park, Y.B.; Moon, B.J.; Kwon, O.S. Proteome analysis of human monocytic THP-1 cells primed with oxidized low-density lipoproteins. Proteomics 2006, 6, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Court, M.; Petre, G.; Atifi, M.E.; Millet, A. Proteomic Signature Reveals Modulation of Human Macrophage Polarization and Functions Under Differing Environmental Oxygen Conditions. Mol. Cell Proteom. 2017, 16, 2153–2168. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; D’Amico, F.; Mazzarino, M.C.; Malaguarnera, L. Modulation of chitotriosidase during macrophage differentiation. Cell. Biochem. Biophys. 2013, 66, 239–247. [Google Scholar] [CrossRef]
- Li, W.; Sultana, N.; Siraj, N.; Ward, L.J.; Pawlik, M.; Levy, E.; Jovinge, S.; Bengtsson, E.; Yuan, X.M. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J. Cell. Mol. Med. 2016, 20, 1664–1672. [Google Scholar] [CrossRef]
- Magnusson, L.U.; Lundqvist, A.; Karlsson, M.N.; Skalen, K.; Levin, M.; Wiklund, O.; Boren, J.; Hulten, L.M. Arachidonate 15-lipoxygenase type B knockdown leads to reduced lipid accumulation and inflammation in atherosclerosis. PLoS ONE 2012, 7, e43142. [Google Scholar] [CrossRef]
- Wigren, M.; Rattik, S.; Yao Mattisson, I.; Tomas, L.; Gronberg, C.; Soderberg, I.; Alm, R.; Sundius, L.; Ljungcrantz, I.; Bjorkbacka, H.; et al. Lack of Ability to Present Antigens on Major Histocompatibility Complex Class II Molecules Aggravates Atherosclerosis in ApoE(-/-) Mice. Circulation 2019, 139, 2554–2566. [Google Scholar] [CrossRef]
- Murugesan, G.; Davidson, L.; Jannetti, L.; Crocker, P.R.; Weigle, B. Quantitative Proteomics of Polarised Macrophages Derived from Induced Pluripotent Stem Cells. Biomedicines 2022, 10, 239. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Lin, J.D.; Nishi, H.; Poles, J.; Niu, X.; McCauley, C.; Rahman, K.; Brown, E.J.; Yeung, S.T.; Vozhilla, N.; Weinstock, A.; et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. Jci Insight 2019, 4, e124574. [Google Scholar] [CrossRef]
- Olsen, L.R.; Leipold, M.D.; Pedersen, C.B.; Maecker, H.T. The anatomy of single cell mass cytometry data. Cytom. A 2019, 95, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.D.; Baranov, V.I.; Ornatsky, O.I.; Bandura, D.R.; George, T.C. An introduction to mass cytometry: Fundamentals and applications. Cancer Immunol. Immunother. 2013, 62, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Winkels, H.; Ehinger, E.; Vassallo, M.; Buscher, K.; Dinh, H.Q.; Kobiyama, K.; Hamers, A.A.J.; Cochain, C.; Vafadarnejad, E.; Saliba, A.E.; et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 2018, 122, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, A.; Cave, L.; Hyde, G.; Moestrup, S.K.; Carling, D.; Mason, J.C.; Haskard, D.O.; Boyle, J.J. Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase. Cardiovasc. Res. 2021, 117, 1295–1308. [Google Scholar] [CrossRef]
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc. Res. 2019, 115, 419–431. [Google Scholar] [CrossRef]
- Hara, T.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis 2015, 242, 639–646. [Google Scholar] [CrossRef]
- Brenner, C.; Franz, W.M.; Kuhlenthal, S.; Kuschnerus, K.; Remm, F.; Gross, L.; Theiss, H.D.; Landmesser, U.; Krankel, N. DPP-4 inhibition ameliorates atherosclerosis by priming monocytes into M2 macrophages. Int. J. Cardiol. 2015, 199, 163–169. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Granfeldt, A.; Hvas, C.L.; Graversen, J.H.; Christensen, P.A.; Petersen, M.D.; Anton, G.; Svendsen, P.; Solling, C.; Etzerodt, A.; Tonnesen, E.; et al. Targeting dexamethasone to macrophages in a porcine endotoxemic model. Crit. Care Med. 2013, 41, e309–e318. [Google Scholar] [CrossRef]
- Adair, J.R.; Howard, P.W.; Hartley, J.A.; Williams, D.G.; Chester, K.A. Antibody-drug conjugates—A perfect synergy. Expert Opin. Biol. Ther. 2012, 12, 1191–1206. [Google Scholar] [CrossRef]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.L.; Moller, H.J.; Graversen, J.H.; Magnusson, N.E.; Moestrup, S.K.; Vilstrup, H.; Gronbaek, H. Anti-CD163-dexamethasone conjugate inhibits the acute phase response to lipopolysaccharide in rats. World J. Hepatol. 2016, 8, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, P.; Graversen, J.H.; Etzerodt, A.; Hager, H.; Roge, R.; Gronbaek, H.; Christensen, E.I.; Moller, H.J.; Vilstrup, H.; Moestrup, S.K. Antibody-Directed Glucocorticoid Targeting to CD163 in M2-type Macrophages Attenuates Fructose-Induced Liver Inflammatory Changes. Mol. Ther. Methods Clin. Dev. 2017, 4, 50–61. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, Q.; Bie, J.; Wallace, R.L.; Yannie, P.J.; Wang, J.; Lancina, M.G., 3rd; Zolotarskaya, O.Y.; Korzun, W.; Yang, H.; et al. Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: Use of this platform to modulate atherosclerosis. Transl. Res. 2018, 193, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Reichel, D.; Bae, Y.; Pennypacker, K.R. Leukemia Inhibitory Factor-Loaded Nanoparticles with Enhanced Cytokine Metabolic Stability and Anti-Inflammatory Activity. Pharm. Res. 2018, 35, 6. [Google Scholar] [CrossRef] [PubMed]
- Hristodorov, D.; Mladenov, R.; von Felbert, V.; Huhn, M.; Fischer, R.; Barth, S.; Thepen, T. Targeting CD64 mediates elimination of M1 but not M2 macrophages in vitro and in cutaneous inflammation in mice and patient biopsies. MAbs 2015, 7, 853–862. [Google Scholar] [CrossRef]
- Akinrinmade, O.A.; Chetty, S.; Daramola, A.K.; Islam, M.U.; Thepen, T.; Barth, S. CD64: An Attractive Immunotherapeutic Target for M1-type Macrophage Mediated Chronic Inflammatory Diseases. Biomedicines 2017, 5, 56. [Google Scholar] [CrossRef]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins. Arter. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef]
- Bories, G.F.P.; Leitinger, N. Macrophage metabolism in atherosclerosis. Febs Lett. 2017, 591, 3042–3060. [Google Scholar] [CrossRef]
| Method | DNA/RNA Analysis | Proteins Analysis | Wide Range of Source Material | Labor Time | Cost | Notes |
|---|---|---|---|---|---|---|
| Laser microdissection | V | V | V | High | High | Long-term sample storage is not recommended. Visualization of the specimen is hampered by the absence of a coverslip. Careful handling of samples is necessary and adequate sample processing is essential for reproducible results. |
| Cell sorting | V | V | V | Medium | High | Measures single cells keeping the cell alive. Allows the simultaneous analysis of multiple parameters. Requires highly trained specialists. Tissue structure is lost. Little information on intracellular distribution. |
| Drug | Proteins Target | Signaling | Metabolic Pathway | Reference |
|---|---|---|---|---|
| Metformin | HO-1 | AMPK/ATF | Oxidative stress, M2 phenotype | Seneviratne A. et al., 2021 |
| Pioglitazone | MCP1/CCR2 | PPARγ | Inflammation, M2 phenotype | Tokutome M. et al., 2019 |
| Rivaroxaban | MMP9, TNFα | FXa/PARs | Inflammation | Hara T. et al., 2015 |
| Sitagliptin | CXCR4 | SDF-1 | M2 phenotype | Brenner C. et al., 2015 |
| Anti-CD163-dexamethasone | α-2-macroglobulin | CD163 | Inflammation | Thomsen K.L. et al., 2016 |
| Anti-CD163-dexamethasone | Il-1β, TNF-α | CD163 | Inflammation | Svendsen P. et al., 2017 |
| Mannose functionalized dendrimeric nanoparticles | ABCA1, ABCG1 | CD206/LXR | Lipid metabolism | He H. et al., 2018 |
| Sample | Species | Technique | Number of Proteins Identified | Most Relevant Proteins Identified | Pathway | Reference |
|---|---|---|---|---|---|---|
| Carotid plaque | Human | Western blot | 823 | ALG-2, TSP-2, Mn-SOD, ApoE, ApoB100, PTP1C, GSK-3β | Atherosclerosis | Martinet W. et al., 2003 |
| Carotid plaque | Human | Microarray | 512 | Caspase-9; clived Gads; GIT1; HIF-1α; JAM-1; JNK; L-caldesmon; c-src; TNF-α; TOPO-II-α; TRAF4 | Plaque instability | Slevin M. et al., 2006 |
| Carotid plaque | Human | LC-MS/MS | 3082 | CD5; S100A12; CKB; CEMIP; endophilin-B1 | Plaque instability | Bao M.H. et al., 2021 |
| Carotid plaque | Human | LC-MS/MS | 1161 | LAMC1; LAMA5; LAMB2; HSPG2; NID1; AGRN; NID2; COL18A1; ELN; FBLN5; LTBP4; MFAP4; BCAM | Plaque instability | Vaisar T. et al., 2020 |
| Carotid plaque | Human | 2-DE/MALDI-TOF MS | 620 | Cathepsin D; LRG; transferrin; apoA-I; fibrinogen; α-1-antitrypsin; protein HC; SAP; HSP27; enolase 1 | Atherosclerosis | Duran M.C. et al., 2007 |
| Carotid plaque | Human | ELISA | 6 | visfatin, adiponectin, IL-6, lipocalin-2, resistin, TNFR2 | Plaque instability | Auguet T. et al., 2016 |
| Carotid plaque | Human | iTRAQ labeling/nanoLC-MS/MS | 162 | Defensin 1; apoE; clusterin; ZAG; ecSOD; Prdx2; CA1; Hsp70 | Atherosclerosis | Aragones G. et al., 2016 |
| Coronary plaque | Human | LC-MS/MS | 806 | SDF1-α; TGF-β; PEDF; MFG-E8; annexin I | Atherosclerosis | Bagnato C. et al., 2007 |
| Coronary plaque | Human | 2-DE/MALDI-TOF MS | n.a. | Vimentin, tropomyosin β-chain; actin; keratin; tubulinβ-chain; MAGP4; SAP; annexin5 | Atherosclerosis | Stakhneva E.M. et al., 2019 |
| THP-1 cells | Human | Antibody Microarray | 384 | LAR(PTP); AKAP 149; rabaptin-5; RanBP1; NM23-H1; SIPA; CDK-1; cell division cycle 27; CLIP-115; CLIP-115; proliferation antigen Ki-67; Rnase HI; TFII-I, Kalinin B1; CD36; COX-2; p47phox; caspase-1; caspase-6; caspase-8; TRADD | Atherosclerosis | Tuomisto T.T. et al., 2005 |
| THP-1 cells | Human | 2-DE/MALDI-TOF MS | 2500 | vimentin; MGA6; PP2A; β-1,3-galactosyltransferase; annexin I; ferritin; TFPI 2; HFR; rho GDI-α; rho GDI-β; piridoxal kinase | Atherosclerosis | Kang J.H. et al., 2006 |
| U937 cells | Human | 2-DE/LC-MS/MS | 1340 | nucleophosmin; serum albumin; serum protein 90K; Hsp70; fumarase; Prdx 1; transgelin 2; aldolase A; GAPDH; hnRNP; calreticulin precursor | Atherosclerosis | Yu Y.L. et al., 2003 |
| Monocyte-derived macrophages | Human | nanoLC-MS/MS | 5102 | MRC1; APOL2; APOL3, IDO1, GBP1, GBP5, CD274, STAT1, STAT2, WARS, TAP2; NEDD8 ultimate buster 1; CD38; HLA-DRA; HLA-DRB1; HLA-DR3; HLA-DPA1; HLA-DPB1; CD74; ALOX15; CRABP2; CD209; NAIP; CD163; FPR1; coagulation factor XIII; CD32 | Atherosclerosis | Court M. et al., 2017 |
| Monocyte-derived macrophages | Human | label-free LC-MSE | 132 | Rab3A; FABP4; TGM-2; HSP70; HSP90; actin, tubulin, myosin | Atherosclerosis | Eligini S. et al., 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eligini, S.; Gianazza, E.; Mallia, A.; Ghilardi, S.; Banfi, C. Macrophage Phenotyping in Atherosclerosis by Proteomics. Int. J. Mol. Sci. 2023, 24, 2613. https://doi.org/10.3390/ijms24032613
Eligini S, Gianazza E, Mallia A, Ghilardi S, Banfi C. Macrophage Phenotyping in Atherosclerosis by Proteomics. International Journal of Molecular Sciences. 2023; 24(3):2613. https://doi.org/10.3390/ijms24032613
Chicago/Turabian StyleEligini, Sonia, Erica Gianazza, Alice Mallia, Stefania Ghilardi, and Cristina Banfi. 2023. "Macrophage Phenotyping in Atherosclerosis by Proteomics" International Journal of Molecular Sciences 24, no. 3: 2613. https://doi.org/10.3390/ijms24032613
APA StyleEligini, S., Gianazza, E., Mallia, A., Ghilardi, S., & Banfi, C. (2023). Macrophage Phenotyping in Atherosclerosis by Proteomics. International Journal of Molecular Sciences, 24(3), 2613. https://doi.org/10.3390/ijms24032613







