Abstract
Stomata are microscopic pores on the plant epidermis that serve as a major passage for the gas and water exchange between a plant and the atmosphere. The formation of stomata requires a series of cell division and cell-fate transitions and some key regulators including transcription factors and peptides. Monocots have different stomatal patterning and a specific subsidiary cell formation process compared with dicots. Cell-to-cell symplastic trafficking mediated by plasmodesmata (PD) allows molecules including proteins, RNAs and hormones to function in neighboring cells by moving through the channels. During stomatal developmental process, the intercellular communication between stomata complex and adjacent epidermal cells are finely controlled at different stages. Thus, the stomata cells are isolated or connected with others to facilitate their formation or movement. In the review, we summarize the main regulation mechanism underlying stomata development in both dicots and monocots and especially the specific regulation of subsidiary cell formation in monocots. We aim to highlight the important role of symplastic connection modulation during stomata development, including the status of PD presence at different cell–cell interfaces and the function of relevant mobile factors in both dicots and monocots.
1. Introduction
Stomata are cell complexes on the plant epidermis for gas and water exchange between plants and environment [1]. The dynamic change of the pore size controls the loss of water and promotes the absorption of carbon dioxide in plants, enabling them to function as essential regulators of photosynthesis and transpiration [2], as well as growth and development [3,4]. Stomata are also one of the channels for pathogens to invade plants [5], and plays an important role in plant responses to abiotic and biotic stresses [6,7,8,9,10].
The stomata of dicot are usually composed of a pair of kidney-shaped guard cells that are produced through a series of asymmetric and symmetric cell divisions, which also modulate the overall density and pattern of stomata [11]. In most dicot leaves, stomata appear to be randomly distributed on the epidermis of dicotyledons but with ‘one-cell spacing rule’, in which two stomata are separated by at least one intervening nonstomatal epidermal cell [12]. In contrast, the stomata of monocot are kidney- or dumbbell-shaped and arranged in parallel with uniform distribution [13,14,15]. In addition, a significant feature of stomata in monocot is the presence of one or more pairs of subsidiary cells flanking the guard cells that are different in form, size or arrangement compared with regular epidermal pavement cells [16]. Subsidiary cells (SCs) provide mechanical support or act as a reservoir for water and ions supporting the movement and function of guard cells (GCs) [17].
Apoplast and symplast transport are two ways of intercellular communication in plants [11,18,19]. In the apoplast pathway, molecules are transported from the extracellular matrix to the cytoplasm, or as ligands to target receptors in the extracellular layer. In contrast, the symplast pathway is carried out through nano channels, named plasmodesmata (PD) [20,21,22]. Symplastic communication via PD are essential for a wide range of plant biological activities, including organ formation, tissue patterning and stress responses [23,24,25,26,27,28]. PD are channels composed of plasma membrane (PM) and endoplasmic reticulum (ER), which provides cytoplasmic and membrane continuity between adjacent cells for the trafficking of metabolites and signaling molecules [27,29,30,31]. Defects in PD structure and connectivity limit the intercellular transport of sugars, ions, transcription factors and a series of RNA molecules including messenger RNA (mRNAs), short interfering RNA (siRNA), microRNA (miRNAs), trans acting siRNAs (tasiRNAs), and also the hormone distribution [32,33,34,35,36,37].
Based on the inner structure, PD are classified into two types, simple and branched (X-, Y- and V-shaped) [38]. According to the presence or absence of cytoplasmic sleeve, it can be divided into type I (no) and type II (yes). Simple PD have higher permeability than branched PD in mature leaves, while type I PD exhibit higher permeability than type II PD in young tissues [39]. At present, many relative studies focusing on the plasmodesmal structure and components have been reported [40,41,42]. The PD aperture is mediated by the change of flanking cell walls, mainly by the turnover of synthesis/degradation of callose. Callose is a β-1,3-linked homopolymer of glucose with a minority of β-1,6-branches synthesized with UDP-glucose as substrate [43]. Callose levels are regulated by two antagonistic enzymes: callose synthase (CalS) or glucan synthase-like (GSL) for Arabidopsis only and β-1,3-glucanases, which produce and break down callose, respectively [44,45].
In this review, we briefly summarize the conserved and distinct regulation mechanisms underlying stomata development in dicots and monocots and the specific regulation of SCs formation in monocots. Then, we focus on the comparison of the modulation of symplastic communication during stomatal development in both dicots and monocots based on recent studies. Although there are not too many cases in this field at present, our review would be of help for further deep investigation.
2. A Brief Summary of Key Transcription Factors Regulating Stomatal Development
The orderly development of stomatal complex is regulated by many pathways, including a series of transcription factors, signal peptides and mitogen-activated protein kinase (MAPK) signal pathway [46]. Among them, basic Helix-Loop-Helix (bHLH) family transcription factors play an important role. SPEECHLESS (SPCH), MUTE and FAMA belong to the same subgroup showing certain similar structure but different function [47]; INDUCER OF COLD EXPRESSION(ICE1)/SCREAM (SCRM) and SCRM2 have functional redundancy (Figure 1). Experiments showed that they function with SPCH, MUTE and FAMA in heterodimers with a dose effect [47]. SPCH acts on the initial stage of stomatal cell formation, mainly affecting the transformation from protodermal cell (PDCs) to meristemoid mother cells (MMCs), which undergo self-renewal and differentiate to meristemoids. The SPCH mutation in Arabidopsis significantly reduced the stomatal index, whereas overexpression of SPCH induces extra cell divisions in epidermal cells that do not normally divide [48]. Recent studies have revealed the signal molecules SOL1 and SOL2 are the transcriptional targets of SPCH and they function as negative regulators on fate transitions of the stomatal lineage, and these two genes are enriched in stomatal precursors. TSO1, a paralog of SOL1 and SOL2, acts in opposition to them during this process [49]. AtMUTE is required for the transition from M to guard mother cell (GMC), it also ensure the symmetrical division of GMC into GC by inducing cyclin (CYCD5; 1) and other cell cycle factors [50]. Loss-of-function mutations of MUTE lead to the developmental progress getting stuck in a continuous self-renewing stage [51]. Almost all epidermal cells were converted into stomatal precursors in 35S::MUTE plants [14]. FAMA mediates the symmetrical division of GMC to generate GC. FAMA is only expressed in GMCs and early GCs. GMC division in the atfama mutant is blocked and GMCs cannot differentiate into GCs [3]. The overexpression of FAMA will induce other mesophyll cells to gain the stomata fate. In addition, FAMA directly inhibit CYCD7; 1 to prevent excessive division of GCs [52]. Two MYB transcription factors FLP and MYB88 have functional redundancy with FAMA, which are responsible for GMC division regulation. The stomatal phenotype of the double mutant flp myb88 is consistent with the fama mutant [53].
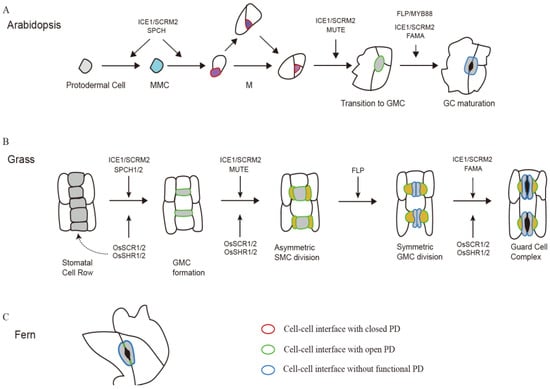
Figure 1.
Schematic diagrams showing key regulators and symplastic connections during stomatal development in Arabidopsis, Grass and Fern. (A) Stomatal development in Arabidopsis. A protodermal cell converts to the stomatal lineage when it becomes a meristemoid mother cell MMC, then the MMC undergoes an asymmetric division to produce a smaller meristemoid (purple) and a larger stomatal lineage ground cell (SLGC; white). The meristemoid (M) undergoes additional self-renewing asymmetric divisions or differentiates into a guard mother cell (GMC; light gray). The GMC divides symmetrically to form a pair of guard cells (GCs; gray). Five bHLH transcription factors, SPCH, MUTE, FAMA and their heterodimeric partners ICE1 and SCRM2 regulate fate transitions during stomatal development. SPCH and ICE1/SCREAM2 mediate both entry and [54] amplifying asymmetric divisions, while MUTE and ICE1/SCREAM2 mediate the transition from M to GMC fate. FAMA, FOUR LIPS (FLP)/MYB88, and ICE1/SCREAM2 control the GMC symmetric division and GC maturation. Plasmodesmata (PD) were observed in ventral walls between sister guard cells and, most importantly, between immature GCs and epidermal cells, but degenerated until GC became mature. However, the PD are supposed to be closed to block the intercellular movement of transcription factor SPCH before GCs formed. (B) Stomatal development in grasses. The developmental process of stomata in grasses can be divided into five steps: (1) specification of the stomatal file, (2) GMC formation, (3) asymmetric division of subsidiary mother cells (SMCs) to generate SCs, (4) GMC symmetric division to form two immature GCs, (5) maturation of two dumbbell-shaped GCs flanked by two triangle-shaped SCs. SPCH has been duplicated and both homologues act to induce asymmetric divisions and promote the acquisition of stomatal lineage identity, in which SPCH2 plays a predominant role. The grass MUTE has a unique character to move from GMCs into SMC where it functions to promote the asymmetric division of SMCs to form SCs. FAMA and FLP regulate the symmetric division of GMCs and the maturation of GCs. Like the Arabidopsis counterparts, grass ICE1/2 proteins form heterodimers with SPCH1/2, MUTE, or FAMA, respectively. In rice, SCARECROW (SCR)1/2 and SHORTROOT (SHR)1/2, have been shown to act together to regulate the initiation of stomatal lineage cells, formation of SCs, and maturation of GCs. Before the complete maturation of stomata complex, there are functional PD connecting GMCs with surrounding cells. However, PD are just present between ordinary epidermal cells and SCs when mature GCs formation, but not GC-GC and GC-SC interfaces. (C) Stomata of fern. A few long and narrow PD are present between two GC cells in fern, allowing the fluorescent dyes slowly move from guard cell to another, but the electrical conductance of the PD is not high enough to provoke membrane potential changes.
Compared with Arabidopsis, some transcription factors of monocots that play key roles in stomatal development are somewhat different in functions. According to previous studies, three grass species-maize, rice and Brachypodium distachyon-have only one orthologue of Arabidopsis MUTE and FAMA, but two of SPCH [55]. The protein sequences of these transcription factor genes are highly conserved between these two typical angiosperms. Lots of functional differences of these TFs between grass and Arabidopsis have been well studied. The AtSPCH protein is highly regulated by phosphorylation, but the phosphorylation regulatory domain is not conserved in BdSPCH1/2. Overexpression of AtSPCH induced excessive epidermal cell divisions, and 35S::OsSPCH1 showed normal stomata phenotype, but 35S::OsSPCH2 can promote cell division in the pavement cells [48,56]. The bdspch1 single-mutants showed a 20% reduction in stomatal numbers, while the loss of BdSPCH2 would lead to an 80% reduction [57]. SPCH1/2 of grass plays a role in promoting stomatal development, and OsSPCH2 and BdSPCH2 play a more important role during this process than OsSPCH1 and BdSPCH1, respectively. The function of grass SPCH is not completely equivalent to AtSPCH. Grass MUTE regulates stomatal development but in a divergent way to AtMUTE. MUTE in Arabidopsis control the cell fate transition from M to GMC, and from GMC to paired GCs [50]. BdMUTE appears to function in the recruitment and establishment of SC identity, and it expresses not only in GMCs and subsidiary mother cells (SMCs), but also in young GCs and SCs. Moreover, it can travel to and accumulates in the adjacent neighbor cells through PD [58]. OsMUTE is also mobile but it is induced restrictively in GMCs and SMCs, and it controls GC shape by positively regulating OsFAMA expression [59]. Loss of OsMUTE or ZmMUTE led to more severe consequences for GMC identity compared with mutant of BdMUTE [59]. The loss-of-function mutants of ZmMUTE failed to recruit SCs and resulted in longitudinal division of GMC [60]. However, the loss-of-function mutants of BdMUTE showed almost 70% normal GMC division, which is totally different from loss-of-function mutants of OsMUTE and ZmMUTE that completely lack stomata [58]. Overexpression of AtMUTE and ZmMUTE in Arabidopsis led to other epidermal cells developing into stomata [56], but Ubip:YFP-BdMUTE showed the stomata phenotype such as 35S::SPCH [59]. These differences in the role of MUTE might be due to variances in the protein structure or interacting partners. OsFAMA controls the cell fate transition of GMCs and SMCs, but not cell division that AtFAMA does in Arabidopsis [55]. Ectopic AtFAMA expression induced the conversion of epidermal cells into GC-like cells, but overexpression of OsFAMA usually resulted in the formation of box-shaped GCs because OsFAMA only regulates GC differentiation without the function of GC morphogenesis. osfama showed stomata lacking one SC, which indicates OsFAMA contributes to the recruitments of SCs [55,59]. When transforming ProFAMA:OsFAMA into osfama, it can only restore the phenotype of GCs differentiation rather than the cluster stomata [59,61].These evidence suggest that the function of FAMA is relatively conserved between monocots and dicots, and the grass FAMAs probably have lost their ability to regulate GMC fate. In contrast, the roles of MUTE and SPCH are discrepant to some extent.
In addition to the previously mentioned more ordered arrangement of stomata in monocots, a pair of dumbbell-shaped GCs flanked by two triangular SCs is another key character. The dynamic open and closure of GC are engaged by ions (K+, Cl−), which are absorbed and stored in SCs when stomata are closed and transported back to GCs until stomata open. SCs are thus acting as dynamic reservoirs of K+ and Cl−, facilitating quick and efficient modulation of stomatal aperture by GCs to control water loss. Many experiments indicate that SCs play a key role in enhancing drought tolerance. SCs are formed by an asymmetric division, with cell polarization. The cell polarization is controlled by the BRICK (BRK)–PANGLOSS (PAN)–RHO FAMILY GTPASE (ROP) pathway and other intrinsic or extrinsic cues [62]. This pathway consists of several plasma membrane-associated proteins, all of them are polarized within SMCs. BRK1 belongs SCAR/WAVE regulatory complex, which activates the actin-nucleating ARP2/3 complex and is required for PAN polarization, further promotes branched actin networks [63,64]. PAN1 and PAN2 are two catalytically inactive leucine-rich repeat receptor-like kinases (LRRKs) [65]. PAN2 acts upstream because it is required for the polarized accumulation of PAN1 and PAN2 interacts with itself for its own localization [66]. Type I Rho family GTPases (ROPs) 2 and 9 function together with PAN1 in the polarization of asymmetric SMC divisions. The polarized localization of ROPs depends on PAN1, loss of ROP2/9 causes abnormal SMC division polarity. When these proteins are polarized accumulation at the GMC/SMC contact sites, then the actin patch will form and the nucleus of SMC will migrate toward the GMC [56]. The actin filaments in SMC may provide tracks for polarized organelle movement or facilitate endo/exocytosis at the GMC/SMC interface [67,68,69]. PAN1 and PAN2 are two downstream targets of BZU2/ZmMUTE as ZmMUTE directly binds to the promoters of PAN1 and PAN2 [60].
3. Peptide Signaling and Environmental Factors in Stomatal Development
In Arabidopsis, two stomata are separated by at least one intervening epidermal cell when they are produced, known as the “one cell spacing rule”, which guarantees the suitable open and closure of stomata [70]. This process is performed by small peptides, ligand receptors, and MAPKs. Secreted small peptides encoded by epidermal patterning factor-like (EPFL) family genes including EPF1, EPF2, STOMAGEN/EPFL9 and CHALLAH (chal)/EPFL6 signal inhibit or promote stomatal development in a way of intercellular communication [71,72,73]. EPF1 and EPF2 are specifically expressed in the stomatal lineage cells. They are perceived by a receptor complex consisting of a receptor-like protein, TOO MANY MOUTHS (TMM), and ERECTA family (ERf) receptor kinases, followed by the activation of a MAPK module [74]. The MAPK phosphorylated and destabilized the transcription factor SPCH, ultimately led to a reduction in stomatal number and density [75]. The “one cell spacing rule” was broken in epf1 and epf2 mutants, which showed clustered stomata and appeared similar to the SPCH overexpression plants [72]. Protein phosphatase 2A(PP2A) functioned as a upstream regulator of SPCH by promoting its protein stability, whereas SPCH can directly binds to the PP2A-A subunits in vitro [76]. EPFL9 is a positive regulator of stomatal development, which is coexpressed with EPF1 and STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1) [77]. EPFL9 acted independently of EPF1, EPF2 and SDD1, and EPFL9 competes with EPF1 and EPF2 for binding to the ERf-TMM complex [78,79]. EPFL6, expressed only in hypocotyls and stems, was able to suppress stomatal formation and functioned as a suppressor of the TMM [80]. Exogenous application of bioactive BdEPF2 also inhibited stomata initiation, and meanwhile antagonized with BdSTOMAGEN [81]. These results indicate EPF peptides have relatively conserved function in different species. In addition, the CLE peptide family also regulates stomatal patterning. CLAVATA3/ESR-RELATED 9/10 (CLE9/10) functions differently in different plant tissues. CLE9/10, negatively regulates the division of MMCs in leaves, were recognized by the receptor kinase HAESA-LIKE1 (HSL1) and result in the phosphorylation and destabilization of SPCH [82].
Apart from the key genetic regulators, environmental factors, including light, carbon dioxide (CO2) levels and pathogen attack, as well as endogenous signals, such as abscisic acid (ABA) and apoplastic reactive oxygen species (ROS) also affect the development and movement of stomata [83,84]. Dark-grown wild-type Arabidopsis plants showed only a few mature stomata on the cotyledons. Many of them are retained in the stomatal precursor stage or form stomatal clusters occasionally [85,86]. In Arabidopsis, the expression of STOMAGEN, SPCH, MUTE, FAMA, EPF2, and TMM can be induced by light [87], and the maturation of stomata complex is disrupted in some photoreceptor mutants such as phyB, phyA, and cry1cry2 [88]. In rice, the developed leaves of phyB1 or phyB2 mutant displayed larger epidermal cells than wild-type leaves, resulting in the reduction in stomatal density and smaller stomata [89]. The expression of ZmPIF1 in rice-enhanced tolerance to drought stress, because it plays an important role in the ABA-mediated regulation of stomatal closure [90]. These studies suggest the light-signaling components between grasses and Arabidopsis have relatively conserved function. CO2 functions as a main substrate for photosynthesis, which can be uptaken into the plant body via stomata. CO2 can also meditate both stomatal development and movements. In the Arabidopsis GCs, CO2 can be catalyzed to HCO3– and protons by two carbonic anhydrases of βCA1 and βCA4 [91]. In the grass, disruption of ZmβCA1, ZmβCA2 and OsβCA1 led to an increase in stomatal conductance and decreased sensitivity to high CO2 concentrations [92,93]. However, impaired stomatal development in Arabidopsis βca1 βca4 double mutants was not found in grasses [91].
Phytohormones function in regulating not only various aspects of development and plant growth, but also stomatal patterning. Exogenous ABA application repressed the entrance of stomatal-lineage development rather than the asymmetric division [94]. Disruption of ABA biosynthesis led to disturbed stomatal numbers [95]. The overexpression of ZmWRKY79 in Arabidopsis led to enhanced drought tolerance by inducing the expression of ABA biosynthetic related genes to promote stomatal closure and lateral roots growth [96]. Exogenous treatment with methyljasmonate (MeJA) negatively regulates stomatal development in the cotyledon of Arabidopsis [97]. JA-biosynthetic mutants (jar1) and signaling-related mutants (myc2myc3myc4) exhibited increased stomatal clusters in Arabidopsis cotyledons. MYC2/3/4, the key components of JA signal pathway, suppressed the expression of SPCH, MUTE, and FAMA [98]. In rice, OsJAZ9 regulated potassium homeostasis and stomatal density to enhance the water-deficit stress, and the overexpression of OsJAZ9 led to the enhanced JA level and reduced stomata density [99].
Long-distance signaling also plays an important role in the adaptation to severe environmental conditions of plants. CLAVATA3/EMBRYO-SURROUNDING REGION related25 (CLE25) peptide is an example of long-distance signaling molecule modulating stomatal closure. It was synthesized in roots under water-deficient condition and transported via vasculature to leaves, and then perceived by the receptors BARELY ANY MERISTEM1 (BAM1) and BAM3 to regulate ABA synthesis and stomatal aperture eventually [100].
Callose accumulation is spatiotemporally participated in numerous plant developmental processes and environmental stress responses, such as cold [101], heat and heavy metals [102,103]. Callose is deposited in the cell wall, PD and sieve pores and can prevent the further diffusion of the pathogen and metals into the plant tissue, and maintain the homeostasis of cellular water [104]. Previous studies showed that limited synthesis or degradation reduced the sensitivity of stomata to light in the fern Asplenium nidus [105]. In the young stomata of angiosperms, callose appears in the young ventral wall and might act on the formation of stomata. However, in the mature stomata complex of angiosperm, callose seems to be implicated in stomatal movement [106]. To date, direct evidence showing stress-induced excess callose production directly affects stomatal formation or function is still lacking.
4. Symplastic Communication during Stomatal Development
Multiple studies over the past few years have underscored the crucial role of PD in plant development by governing movement of biological macromolecules that determine cell fate. Some regulators of stomatal formation possessing the ability to traffic through PD have been revealed (Table 1). miRNAs play a key role in stomatal development, miRNA-deficient mutants, such as dcl1, hen1 and ago1, display abnormal in stomatal density and patterning. The MADS box gene AGAMOUS-LIKE16 (AGL16), expressed in GCs, could be cleavage by miR824, which is expressed in GMCs, meristemoids and stomatal-lineage ground cells [61]. Expression of a miR824-resistant AGL16 mRNA, but not the wild-type AGL16 mRNA induced, excessive satellite meristemoid lineages. In contrast, plants overexpressing miR824 decreased the incidence of high-order stomatal complexes. miR824 was supposed to be able to transport through PD [107]. Indeed, several screens for mutants defective in stomata development have identified some genes affecting PD transport. miR399 acts as a long-distance signal, it negatively regulates the ubiquitin-conjugating E2 enzyme PHO2 and contributes to the regulation of stomatal development and phosphate homeostasis [108]. SPCH, EPF2, and TMM transcripts are up-regulated in 35S::MIR399b plants. The SPCH protein is restricted to MMCs and meristemoids but altered plasmodesmatal permeability led to ectopic location of AtSPCH [109]. chorus is a weak recessive allele of GSL8, which encodes a callose synthase [110]. SPCH-YFP readily diffuses from cell to cell via PD in the chorus mutant [24]. Stomatal clusters were formed in this mutant due to the excessive range of SPCH movement caused by increased plasmodesmal permeability in the mutants. KOBITO1 is a putative glycosyltransferase-like protein previously implicated in cellulose biosynthesis, the kob1-3 mutation leads to the formation of stomata clusters in the erl1 erl2 background, but it is normal in the wild type background [111]. However, the other mutants defective in cellulose biosynthesis do not show kobito1-like phenotypes [112], suggesting KOBITO1 may function in another, as yet unknown pathway restricting PD movement. These findings support the idea that callose-mediated restriction of PD permeability is important to stomatal patterning (Figure 1).

Table 1.
Experimental verified mobile factors involved in stomatal development in grasses and Arabidopsis.
BdMUTE encodes a bHLH transcription factor associated with GMC identity, which is an ortholog of the stomatal regulator AtMUTE [58]. The specification of lateral SCs by BdMUTE appears due to its acquisition of cell-to-cell mobility from GMCs to SMCs [58] (Figure 2). This behavior is similar to that of ZmMUTE/BZU2, which is required for not only SMC differentiation but also GC division [60]. When expressed in rice, the fluorescence of YFP-BdMUTE, YFP-ZmMUTE and YFP-OsMUTE driven by GMC-specific promoter of OsMUTE were first detected in the early GMCs, then appeared in the SMCs, indicating their similar mobility [60]. In addition, both YFP-BdMUTE and YFP-ZmMUTE expressed in Arabidopsis with the GMC-specific AtMUTE promoter were visible in the neighbor cells of GMC [60] (Figure 2). In contrast, the OsMUTEp:YFP-AtMUTE expressed in rice was not detected in SMCs, and the pBdMUTE::AtMUTE-YFP fluorescence was restricted to GMCs and GCs in Brachypodium, a similar behavior like that of AtMUTE in Arabidopsis [58,60] (Figure 2). In addition, the pBdMUTE::AtMUTE-YFP failed to rescue the SC and GMC defects in the BdMUTE loss-of-function mutant [58].These results imply the potential divergent characteristic and function of MUTE proteins in different species (Figure 2).
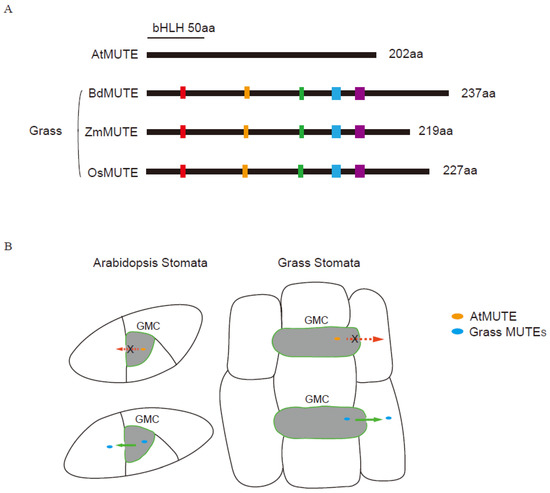
Figure 2.
Schematic diagrams showing the mobility of homologous MUTE proteins in Arabidopsis and grasses. (A) Schematic diagram of potential residues controlling the mobility of MUTE proteins. MUTE orthologs of the representative grass species Brachypodium (BdMUTE–Bradi1g18400), maize (ZmMUTE-GRMZM2G417164) and rice (OsMUTE, LOC_Os05g51820) were aligned with the representative eudicot species Arabidopsis (AtMUTE, At3g06120). Colored-boxes represent the potential motifs responsible for mobility regulation. (B) Mobility of MUTE orthologues in grass and Arabidopsis. In Arabidopsis, YFP-BdMUTE/ZmMUTE/OsMUTE driven by the GMC-specific AtMUTE promoter was visible in the neighboring cells, unlike GMC-only expression of AtMUTE-YFP (Left panel). AtMUTE-YFP expressed in grass stomata driven by the promoter of grass MUTE is restricted in GMC and does not move to the neighboring cells, whereas BdMUTE, ZmMUTE, and OsMUTE can move from the GMC to the adjacent SMC (Right panel). GMC (guard mother cell), dark gray. Grass MUTE proteins, blue oval. AtMUTE protein, orange oval. Red/Green arrows indicate the MUTE proteins cannot/can move through plasmodesmata, respectively.
MUTEs are small proteins, either those symplastic connections allow for its passive transport, or certain domains within BdMUTE define its mobility (Figure 2). MUTE proteins from different species have a variable C-terminal region, whereas the N-terminal region is relatively conserved [60]. Wang et al. generated truncated MUTE proteins lacking 30 amino acids in C-terminal (indicated byΔC) to investigate their mobility. The fluorescence of AtMUTEp:YFP-ZmMUTE-ΔC and AtMUTEp:YFP-BdMUTE-ΔC were only detected in the GMCs, which is similar to the pattern of AtMUTEp:AtMUTE-YFP. YFP-ZmMUTE-ΔC and YFP-BdMUTE-ΔC are only located in the early GMCs, and not in the SMCs and young SCs when expressed by OsMUTE promoter in rice [60]. Based on these assays, 30 amino acids of the C-terminus were thought to be necessary for the mobility of ZmMUTE and BdMUTE.
The GRAS transcription factor SCARECROW (SCR) and SHORTROOT(SHR) are notable regulators involved in multiple development processes in plants [113]. The SHR/SCR module functions in endodermis development of Arabidopsis and maize roots, but regulate different cell type in leaf between Arabidopsis and maize [114]. The ectopic expression of ZmSHR1 in rice bundle sheath cells resulted in ultra-stomatal rows between veins (Figure 1). It was supposed that SHR might act as a mobile signal originated from vein precursor cells and executed together SCR to establish the GC lineage [54,115], and OsSCR/OsSHR thus regulates the initiation of stomatal lineage cells and the formation of SCs.
The double mutant osshr1 osshr2 showed abnormal stomata. Stomata with anomalous SCs and stomatal density decreased in osscr1 mutant; however, mutation of OsSCR2 showed a normal stomatal phenotype [59]. In addition, OsSPCH and OsMUTE might activate OsSCR by directly binding to its promoter. This suggests that the grass SHR/SCR module not only controls vein development in maize [54,115,116], but also participates in the development of stomata complex in rice [54].The permeability of PD is regulated not only by callose but also their architecture. PLM encodes a putative enzyme involved in the biosynthesis of sphingolipids with very-long-chain fatty acid. Loss of PLM enhances plasmodesmatal permeability by altering the inner structure of cytoplasmic sleeve in PD [117]. Arabinogalactan proteins (AGPs) are a gene family of extracellular proteoglycans. Hyp O-galactosyltransferase (HPGT) encodes a critical enzyme that functions in AGP biosynthesis in Arabidopsis. The reduction in the formation of functional AGPs in hpgt1,2,3 triple mutant led to two or more stomata clusters. This phenomenon is ascribed to the change of PD structure with increased highly permeable complex branched PD, which may increase PD permeability [118].
5. PD Distribution at Different Cell-to-Cell Interfaces
Guard cell opening and closing are engaged by ions (K+, Cl−) that function as a rapid response signal [119,120], the pumping or exclusion of ions changes the osmotic potential, resulting in changing the turgor pressure. Thus, stomata should be rather sensitive and require a safe and controllable connection with surrounding cells. Usually, the mature guard cells are not connected with neighboring epidermal cells by functional plasmodesmata in most seed plants, this situation may be able to maintain their different osmotic potential and may impel the ions traffic intercellularly in a transmembrane manner. However, plasmodesmata were observed in ventral walls between sister guard cells and, most importantly, between GCs and epidermal cells in both Vicia faba, Allium cepa, Beta vulgaris diand tobacco [41,121]. Microinjection experiments indicated that fluorescent dye injected into the GMCs and immature GCs of A. cepa and Commelina communis L. spread rapidly into surrounding cells, but no diffusion happened when injected into mature GCs or SCs [79]. However, one exception is that fluorescent dyes can move from one guard cell to another in fern stomata [122], which is consistent with the observation of PD between two GCs [119]. This symplastic connection between guard cells in fern suggests a potential distinct regulation mechanism for stomatal movement. Even so, hyperpolarizing pulses applied to one guard cell will not provoke Ca2+ signal transport to another, suggesting the electrical conductance of the plasmodesmata between stomata of fern is limited [122]. One more case is that PD are present between ordinary epidermal cells and SCs, but not between GCs and SCs and two GCs in maize, but the fluorescent dye BCECF [(20,70-bis-(2-carboxyethyl)-5(and6) carboxyflurescein] can move between two GC of maize unexpectedly in an unknown way [123]. Thus, SCs are not isolated but connected to adjacent epidermal cells, which is different from GCs. Overall, the present of functional plasmodesma within the stomatal complex may differ somewhat between different cell types and plant species.
6. Organelle-Nucleus-Plasmodesmata Signaling (ONPS) and Stomata Movement
PD allows cell-to-cell movement of various metabolites and signaling molecules and therefore play critical roles in responding to environmental signals and stresses. In addition to sugars and mobile proteins, hormones can move from cell to cell through PD. Gibberellic acid (GA) and ABA were found to be transported via PD in Chara vulgaris and P. patens, respectively, and the diffusion of auxin via PD is essential for the hypocotyl phototropism and lateral root development [124,125,126]. Furthermore, the level of plasmodesmatal callose is regulated by hormones. ABA regulate the expression of PD proteins involved in callose synthesis/ degradation, for instance CalSs, PLASMODESMATA-LOCALIZED PROTEINS (PDLPs) and β-1, 3 glucanases to modulate PD permeability and molecular trafficking [127]. Signaling originating from other organelles (such as the chloroplasts and mitochondria) functions in influencing PD formation and function. These signals act in the nucleus to alter the expression of genetic pathways that control both plasmodesmatal permeability and density. This pathway was named ONPS. SAL1(nucleotide phosphatase)-PAP(3′-phosphoadenosine-5′-phosphate) retrograde pathway serves as a second messenger and interacts with ABA signaling to regulate stomatal closure in Arabidopsis [128]. It is thus possible that PD participates in this process, but more direct evidence is required to support this speculation.
7. Perspectives
Stomata are essential for plant development; mutations blocking stomatal formation are lethal [48,51,57,129]. Striking differences in stomatal patterning are noted in comparisons of monocot and eudicot leaves. Namely, stomata appear to be randomly distributed across the epidermis of eudicot leaves, whereas monocot stomata are arranged in evenly distributed, parallel rows [13,14,15]. At the proximal base of the growing leaf blade, stomatal precursor cells arise in cell files with higher rates of cell division relative to flanking cell files. Importantly, this proliferation phase is much more abbreviated compared with that in Arabidopsis, and another major distinction is that grasses lack a self-renewing meristemoid phase. Every cell in the precursor cell file undergoes one asymmetric division in the same orientation (i.e., parallel to the marginal axis of leaves) to produce a smaller GMC and a larger inter-stomatal sister cell. In grasses, cells in the flanking files become polarized in response to cues from the GMC and divide asymmetrically to become SMCs [130]. During these different patterning stages of stomatal development in dicot and monocot, both conserved and distinct regulation mechanisms are uncovered. For example, the MUTE and FAMA transcription factors play important roles in both dicot and grasses, while PAN1 and PAN2 only function in SC formation in monocots. Notably, ZmMUTE is involved in the regulation of both GC and SC development in maize [60]. Whether other mobile factors, except MUTE, are involved in the SC formation is still elusive. Moreover, whether the PD allow bi-directional movement between GMCs and SCs is also unknown. It is, thus, possible that there are also feedback signals from SMCs to GMCs, which need further investigation.
Symplastic trafficking between neighboring cells mediated by PD is one of the efficient ways for the short-distance transport of mobile molecules to function non-anonymously. Based on the present studies in stomatal development, it is speculated that the GC differentiation, either in dicots or monocots, requires a relative isolated cellular environment with blocked symplastic communication between GC and neighboring epidermal cells. This may on the one hand protect GCs from disturbing by exterior signals, and prevent the specific factors function at specific stages trafficking outside. However, this situation changes when SCs in grasses start differentiation, which need signals transported from GCs to activate the following steps. Symplastic communication between these two cell layers is required. Thus, the changes of symplastic trafficking and PD permeability are dynamically dependent on the stomata developmental stages. The underlying regulation mechanism remains unclear to date. Studies on more plant species would provide more clues.
Author Contributions
D.Y., J.L. (Jie Liu) and D.L. conceptualized the topic; Y.C., M.H., D.L., J.L. (Jinxin Liu), J.L. (Jie Liu) and D.Y. wrote the manuscript; Y.C. created the figure. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Project of the Higher Education Institutions of Henan Province (23A180008) and the Scientific Research Foundation for Advanced Talents of Henan University (S22001Y).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Biol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Bergmann, D.C. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 2006, 18, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.A.; Beerling, D.J.; Franks, P.J. Stomata: Key players in the earth system, past and present. Curr. Opin. Plant Biol. 2010, 13, 232–239. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Stomata and pathogens: Warfare at the gates. Plant Signal. Behav. 2009, 4, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Assmann, S.M.; Jegla, T. Guard cell sensory systems: Recent insights on stomatal responses to light, abscisic acid, and CO(2). Curr. Opin. Plant Biol. 2016, 33, 157–167. [Google Scholar] [CrossRef]
- Guimarães, R.L.; Stotz, H.U. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004, 136, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Allègre, M.; Daire, X.; Héloir, M.C.; Trouvelot, S.; Mercier, L.; Adrian, M.; Pugin, A. Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol. 2007, 173, 832–840. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Torres, P.S.; Vojnov, A.A. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol. 2009, 149, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, D.C. Integrating signals in stomatal development. Curr. Opin. Plant Biol. 2004, 7, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kagan, M.L.; Sachs, T. Development of immature stomata: Evidence for epigenetic selection of a spacing pattern. Dev. Biol. 1991, 146, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Torii, K.U. Lineage-specific stem cells, signals and asymmetries during stomatal development. Development 2016, 143, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.R.; Bergmann, D.C. Transcriptional control of cell fate in the stomatal lineage. Curr. Opin. Plant Biol. 2016, 29, 1–8. [Google Scholar] [CrossRef]
- Hepworth, C.; Caine, R.S.; Harrison, E.L.; Sloan, J.; Gray, J.E. Stomatal development: Focusing on the grasses. Curr. Opin. Plant Biol. 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Chaffey, N. Esau’s Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. 3rd edn. Ann. Bot. 2007, 99, 785–786. [Google Scholar] [CrossRef]
- Gray, A.; Liu, L.; Facette, M. Flanking Support: How Subsidiary Cells Contribute to Stomatal Form and Function. Front. Plant Sci. 2020, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Blilou, I. Cell-to-Cell Communication During Plant-Pathogen Interaction. Mol. Plant Microbe Interact. 2022, 35, 98–108. [Google Scholar] [CrossRef]
- Heinlein, M. Plasmodesmata: Channels for Viruses on the Move. In Plasmodesmata: Methods and Protocols; Heinlein, M., Ed.; Springer: New York, NY, USA, 2015; pp. 25–52. [Google Scholar] [CrossRef]
- Baluska, F.; Volkmann, D.; Barlow, P.W. Eukaryotic cells and their cell bodies: Cell Theory revised. Ann. Bot. 2004, 94, 9–32. [Google Scholar] [CrossRef]
- Kragler, F. Plasmodesmata: Intercellular tunnels facilitating transport of macromolecules in plants. Cell Tissue Res. 2013, 352, 49–58. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Scholthof, K.-B.G. Plant Immune Responses Against Viruses: How Does a Virus Cause Disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, M.; Lin, Q.; Wang, M.; Niu, X.; Cheng, J.; Xu, M.; Qin, Y.; Liao, X.; Xu, J.; et al. Symplastic communication in the root cap directs auxin distribution to modulate root development. J. Integr. Plant Biol. 2022, 64, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gallagher, K.L. Mobile protein signals in plant development. Curr. Opin. Plant Biol. 2011, 14, 563–570. [Google Scholar] [CrossRef]
- Wu, S.; Gallagher, K.L. Transcription factors on the move. Curr. Opin. Plant Biol. 2012, 15, 645–651. [Google Scholar] [CrossRef]
- Faulkner, C. Plasmodesmata and the symplast. Curr. Biol. 2018, 28, R1374–R1378. [Google Scholar] [CrossRef] [PubMed]
- Otero, S.; Helariutta, Y.; Benitez-Alfonso, Y. Symplastic communication in organ formation and tissue patterning. Curr. Opin. Plant Biol. 2016, 29, 21–28. [Google Scholar] [CrossRef]
- Azim, M.F.; Burch-Smith, T.M. Organelles-nucleus-plasmodesmata signaling (ONPS): An update on its roles in plant physiology, metabolism and stress responses. Curr. Opin. Plant Biol. 2020, 58, 48–59. [Google Scholar] [CrossRef]
- Pankratenko, A.V.; Atabekova, A.K.; Morozov, S.Y.; Solovyev, A.G. Membrane Contacts in Plasmodesmata: Structural Components and Their Functions. Biochemistry 2020, 85, 531–544. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Burch-Smith, T.M. Review: Plant-pathogen interactions through the plasmodesma prism. Plant Sci. 2019, 279, 70–80. [Google Scholar] [CrossRef]
- Heo, J.O.; Roszak, P.; Furuta, K.M.; Helariutta, Y. Phloem development: Current knowledge and future perspectives. Am. J. Bot. 2014, 101, 1393–1402. [Google Scholar] [CrossRef]
- Han, X.; Kumar, D.; Chen, H.; Wu, S.; Kim, J.Y. Transcription factor-mediated cell-to-cell signalling in plants. J. Exp. Bot. 2014, 65, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Kawade, K.; Tanimoto, H. Mobility of signaling molecules: The key to deciphering plant organogenesis. J. Plant Res. 2015, 128, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.L.; Sozzani, R.; Lee, C.M. Intercellular protein movement: Deciphering the language of development. Annu. Rev. Cell Dev. Biol. 2014, 30, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Zalepa-King, L.; Citovsky, V. A plasmodesmal glycosyltransferase-like protein. PLoS ONE 2013, 8, e58025. [Google Scholar] [CrossRef]
- Nazim Uddin, M.; Kim, J.Y. Intercellular and systemic spread of RNA and RNAi in plants. Wiley Interdiscip. Rev. RNA 2013, 4, 279–293. [Google Scholar] [CrossRef]
- Gao, C.; Liu, X.; De Storme, N.; Jensen, K.H.; Xu, Q.; Yang, J.; Liu, X.; Chen, S.; Martens, H.J.; Schulz, A.; et al. Directionality of Plasmodesmata-Mediated Transport in Arabidopsis Leaves Supports Auxin Channeling. Curr. Biol. 2020, 30, 1970–1977.e1974. [Google Scholar] [CrossRef]
- Xu, M.; Cho, E.; Burch-Smith, T.M.; Zambryski, P.C. Plasmodesmata formation and cell-to-cell transport are reduced in decreased size exclusion limit 1 during embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 5098–5103. [Google Scholar] [CrossRef]
- Nicolas, W.J.; Grison, M.S.; Trépout, S.; Gaston, A.; Fouché, M.; Cordelières, F.P.; Oparka, K.; Tilsner, J.; Brocard, L.; Bayer, E.M. Architecture and permeability of post-cytokinesis plasmodesmata lacking cytoplasmic sleeves. Nat. Plants 2017, 3, 17082. [Google Scholar] [CrossRef]
- Leijon, F.; Melzer, M.; Zhou, Q.; Srivastava, V.; Bulone, V. Proteomic Analysis of Plasmodesmata From Populus Cell Suspension Cultures in Relation With Callose Biosynthesis. Front. Plant. Sci. 2018, 9, 1681. [Google Scholar] [CrossRef]
- Fernandez-Calvino, L.; Faulkner, C.; Walshaw, J.; Saalbach, G.; Bayer, E.; Benitez-Alfonso, Y.; Maule, A. Arabidopsis plasmodesmal proteome. PLoS ONE 2011, 6, e18880. [Google Scholar] [CrossRef]
- Grison, M.S.; Brocard, L.; Fouillen, L.; Nicolas, W.; Wewer, V.; Dörmann, P.; Nacir, H.; Benitez-Alfonso, Y.; Claverol, S.; Germain, V.; et al. Specific membrane lipid composition is important for plasmodesmata function in Arabidopsis. Plant Cell 2015, 27, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhang, Z.; Olson, J.M.; Verma, D.P. A novel UDP-glucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 2001, 13, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Hong, Z.; Chatterjee, J.; Kim, S.; Verma, D.P.S. Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 2008, 229, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Doxey, A.C.; Yaish, M.W.; Moffatt, B.A.; Griffith, M.; McConkey, B.J. Functional divergence in the Arabidopsis beta-1,3-glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol. Biol. Evol. 2007, 24, 1045–1055. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.J.; Kim, M.H.; Kwak, J.M. MAPK Cascades in Guard Cell Signal Transduction. Front. Plant Sci. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef]
- MacAlister, C.A.; Ohashi-Ito, K.; Bergmann, D.C. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 2007, 445, 537–540. [Google Scholar] [CrossRef]
- Simmons, A.R.; Davies, K.A.; Wang, W.; Wang, W.; Liu, Z.; Bergmann, D.C. SOL1 and SOL2 regulate fate transition and cell divisions in the Arabidopsis stomatal lineage. Development 2019, 146, dev171066. [Google Scholar] [CrossRef]
- Han, S.K.; Qi, X.; Sugihara, K.; Dang, J.H.; Endo, T.A.; Miller, K.L.; Kim, E.D.; Miura, T.; Torii, K.U. MUTE Directly Orchestrates Cell-State Switch and the Single Symmetric Division to Create Stomata. Dev. Cell. 2018, 45, 303–315.e305. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Sloan, D.B.; Bogenschutz, N.L.; Torii, K.U. Termination of asymmetric cell division and differentiation of stomata. Nature 2007, 445, 501–505. [Google Scholar] [CrossRef]
- Weimer, A.K.; Matos, J.L.; Sharma, N.; Patell, F.; Murray, J.A.H.; Dewitte, W.; Bergmann, D.C. Lineage- and stage-specific expressed CYCD7;1 coordinates the single symmetric division that creates stomatal guard cells. Development 2018, 145, dev160671. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.B.; Nadeau, J.A.; Lucas, J.; Lee, E.K.; Nakagawa, T.; Zhao, L.; Geisler, M.; Sack, F.D. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. Plant Cell 2005, 17, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.L.; Sedelnikova, O.V.; Walker, B.J.; Westhoff, P.; Langdale, J.A. SHORTROOT-Mediated Increase in Stomatal Density Has No Impact on Photosynthetic Efficiency. Plant Physiol. 2018, 176, 757–772. [Google Scholar] [CrossRef]
- Liu, T.; Ohashi-Ito, K.; Bergmann, D.C. Orthologs of Arabidopsis thaliana stomatal bHLH genes and regulation of stomatal development in grasses. Development 2009, 136, 2265–2276. [Google Scholar] [CrossRef]
- Humphries, J.A.; Vejlupkova, Z.; Luo, A.; Meeley, R.B.; Sylvester, A.W.; Fowler, J.E.; Smith, L.G. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 2011, 23, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Raissig, M.T.; Abrash, E.; Bettadapur, A.; Vogel, J.P.; Bergmann, D.C. Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc. Natl. Acad. Sci. USA 2016, 113, 8326–8331. [Google Scholar] [CrossRef]
- Raissig, M.T.; Matos, J.L.; Anleu Gil, M.X.; Kornfeld, A.; Bettadapur, A.; Abrash, E.; Allison, H.R.; Badgley, G.; Vogel, J.P.; Berry, J.A.; et al. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 2017, 355, 1215–1218. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Yu, Q.; Zhou, W.; Gou, X.; Li, J.; Hou, S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Qiao, X.; Guo, J.; Li, Z.; Zhou, Y.; Bai, S.; Gao, Z.; Wang, D.; Wang, P.; et al. BZU2/ZmMUTE controls symmetrical division of guard mother cell and specifies neighbor cell fate in maize. PLoS Genet. 2019, 15, e1008377. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Liljegren, S.J.; Pelaz, S.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; Vergara-Silva, F.; Yanofsky, M.F. MADS-box gene evolution beyond flowers: Expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000, 24, 457–466. [Google Scholar] [CrossRef]
- Nan, Q.; Char, S.N.; Yang, B.; Bennett, E.J.; Yang, B.; Facette, M.R. Polarly localized WPR proteins interact with PAN receptors and the actin cytoskeleton during maize stomatal development. Plant Cell 2023, 35, 469–487. [Google Scholar] [CrossRef]
- Facette, M.R.; Park, Y.; Sutimantanapi, D.; Luo, A.; Cartwright, H.N.; Yang, B.; Bennett, E.J.; Sylvester, A.W.; Smith, L.G. The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat. Plants 2015, 1, 14024. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; Smith, L.G. A small, novel protein highly conserved in plants and animals promotes the polarized growth and division of maize leaf epidermal cells. Curr. Biol. 2002, 12, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Facette, M.R.; Smith, L.G. Division polarity in developing stomata. Curr. Opin. Plant Biol. 2012, 15, 585–592. [Google Scholar] [CrossRef]
- Zhang, X.; Facette, M.; Humphries, J.A.; Shen, Z.; Park, Y.; Sutimantanapi, D.; Sylvester, A.W.; Briggs, S.P.; Smith, L.G. Identification of PAN2 by quantitative proteomics as a leucine-rich repeat-receptor-like kinase acting upstream of PAN1 to polarize cell division in maize. Plant Cell 2012, 24, 4577–4589. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Bezanilla, M. Actin and microtubule cross talk mediates persistent polarized growth. J. Cell Biol. 2018, 217, 3531–3544. [Google Scholar] [CrossRef] [PubMed]
- Hadley, R.; Hable, W.E.; Kropf, D.L. Polarization of the endomembrane system is an early event in fucoid zygote development. BMC Plant Biol. 2006, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Kimata, Y.; Higaki, T.; Kawashima, T.; Kurihara, D.; Sato, Y.; Yamada, T.; Hasezawa, S.; Berger, F.; Higashiyama, T.; Ueda, M. Cytoskeleton dynamics control the first asymmetric cell division in Arabidopsis zygote. Proc. Natl. Acad. Sci. USA 2016, 113, 14157–14162. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.G.; Torii, K.U. Take a deep breath: Peptide signalling in stomatal patterning and differentiation. J. Exp. Bot. 2013, 64, 5243–5251. [Google Scholar] [CrossRef]
- Hara, K.; Yokoo, T.; Kajita, R.; Onishi, T.; Yahata, S.; Peterson, K.M.; Torii, K.U.; Kakimoto, T. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol. 2009, 50, 1019–1031. [Google Scholar] [CrossRef]
- Hunt, L.; Gray, J.E. The Signaling Peptide EPF2 Controls Asymmetric Cell Divisions during Stomatal Development. Curr. Biol. 2009, 19, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kajita, R.; Miyazaki, A.; Hokoyama, M.; Nakamura-Miura, T.; Mizuno, S.; Masuda, Y.; Irie, K.; Tanaka, Y.; Takada, S.; et al. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 2010, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jewaria, P.K.; Hara, T.; Tanaka, H.; Kondo, T.; Betsuyaku, S.; Sawa, S.; Sakagami, Y.; Aimoto, S.; Kakimoto, T. Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant Cell Physiol 2013, 54, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Lampard, G.R.; Macalister, C.A.; Bergmann, D.C. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 2008, 322, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Guo, X.; Zhang, Y.; Wang, L.; Xu, T.; DeLong, A.; Dong, J. Protein phosphatase 2A promotes stomatal development by stabilizing SPEECHLESS in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 13127–13137. [Google Scholar] [CrossRef]
- Hunt, L.; Bailey, K.J.; Gray, J.E. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 2010, 186, 609–614. [Google Scholar] [CrossRef]
- Qi, X.; Han, S.K.; Dang, J.H.; Garrick, J.M.; Ito, M.; Hofstetter, A.K.; Torii, K.U. Autocrine regulation of stomatal differentiation potential by EPF1 and ERECTA-LIKE1 ligand-receptor signaling. Elife 2017, 6, e24102. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, L.; Han, Z.; Yang, X.; Liu, W.; Li, E.; Chang, J.; Qi, Y.; Shpak, E.D.; Chai, J. A receptor-like protein acts as a specificity switch for the regulation of stomatal development. Genes Dev. 2017, 31, 927–938. [Google Scholar] [CrossRef]
- Abrash, E.B.; Davies, K.A.; Bergmann, D.C. Generation of signaling specificity in Arabidopsis by spatially restricted buffering of ligand-receptor interactions. Plant Cell 2011, 23, 2864–2879. [Google Scholar] [CrossRef]
- Jangra, R.; Brunetti, S.C.; Wang, X.; Kaushik, P.; Gulick, P.J.; Foroud, N.A.; Wang, S.; Lee, J.S. Duplicated antagonistic EPF peptides optimize grass stomatal initiation. Development 2021, 148, dev.199780. [Google Scholar] [CrossRef]
- Qian, P.; Song, W.; Yokoo, T.; Minobe, A.; Wang, G.; Ishida, T.; Sawa, S.; Chai, J.; Kakimoto, T. The CLE9/10 secretory peptide regulates stomatal and vascular development through distinct receptors. Nat. Plants 2018, 4, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Zhou, M.; Ge, Z.; Zhang, F.; Foyer, C.H.; Yuan, X.; Xie, Y. The coordination of guard-cell autonomous ABA synthesis and DES1 function in situ regulates plant water deficit responses. J. Adv. Res. 2021, 27, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, M.; Hoecker, U. Auxin—A novel regulator of stomata differentiation. Trends Plant Sci. 2014, 19, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.H.; Park, C.M. Light Inhibits COP1-Mediated Degradation of ICE Transcription Factors to Induce Stomatal Development in Arabidopsis. Plant Cell 2017, 29, 2817–2830. [Google Scholar] [CrossRef]
- Hronková, M.; Wiesnerová, D.; Šimková, M.; Skůpa, P.; Dewitte, W.; Vráblová, M.; Zažímalová, E.; Šantrůček, J. Light-induced STOMAGEN-mediated stomatal development in Arabidopsis leaves. J. Exp. Bot. 2015, 66, 4621–4630. [Google Scholar] [CrossRef]
- Kang, C.Y.; Lian, H.L.; Wang, F.F.; Huang, J.R.; Yang, H.Q. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 2009, 21, 2624–2641. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Zhou, J.; Chen, F.; Wang, B.; Xie, X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Zhang, M.; Jiang, W.; Ren, X.; Liang, E.; Zhang, D.; Zhang, C.; Xiao, N.; Li, Y.; et al. A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol. J. 2018, 16, 1375–1387. [Google Scholar] [CrossRef]
- Engineer, C.B.; Ghassemian, M.; Anderson, J.C.; Peck, S.C.; Hu, H.; Schroeder, J.I. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature 2014, 513, 246–250. [Google Scholar] [CrossRef]
- Kolbe, A.R.; Brutnell, T.P.; Cousins, A.B.; Studer, A.J. Carbonic Anhydrase Mutants in Zea mays Have Altered Stomatal Responses to Environmental Signals. Plant Physiol. 2018, 177, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, H.; Wu, J.; Fan, X.; Li, X.; Lin, Y. Absence of OsβCA1 causes a CO(2) deficit and affects leaf photosynthesis and the stomatal response to CO(2) in rice. Plant J. 2017, 90, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.C.; Caine, R.S.; Fleming, A.J.; Gray, J.E. Origins and Evolution of Stomatal Development. Plant Physiol. 2017, 174, 624–638. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nose, T.; Jikumaru, Y.; Kamiya, Y. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 2013, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Zhou, L.; Wang, Y.; Zhang, G.; Chen, X. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, Y.; Zhang, G.; Jiang, Y.; Chen, X.; Yu, D. Jasmonate Negatively Regulates Stomatal Development in Arabidopsis Cotyledons. Plant Physiol. 2018, 176, 2871–2885. [Google Scholar] [CrossRef]
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Fromm, J.; Hajirezaei, M.R.; Becker, V.K.; Lautner, S. Electrical signaling along the phloem and its physiological responses in the maize leaf. Front. Plant Sci. 2013, 4, 239. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, S.; Cui, Y.; Yan, D. Heat Stress Reduces Root Meristem Size via Induction of Plasmodesmal Callose Accumulation Inhibiting Phloem Unloading in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 2063. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Citovsky, V. Identification of an interactor of cadmium ion-induced glycine-rich protein involved in regulation of callose levels in plant vasculature. Proc. Natl. Acad. Sci. USA 2005, 102, 12089–12094. [Google Scholar] [CrossRef] [PubMed]
- Piršelová, B.; Matušíková, I. Callose: The plant cell wall polysaccharide with multiple biological functions. Acta Physiol. Plant. 2012, 35, 635–644. [Google Scholar] [CrossRef]
- Apostolakos, P.; Livanos, P.; Nikolakopoulou, T.L.; Galatis, B. Callose implication in stomatal opening and closure in the fern Asplenium nidus. New Phytol. 2010, 186, 623–635. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Sotiriou, P.; Nikolakopoulou, T.L.; Galatis, B.; Apostolakos, P. Callose and homogalacturonan epitope distribution in stomatal complexes of Zea mays and Vigna sinensis. Protoplasma 2020, 257, 141–156. [Google Scholar] [CrossRef]
- Kutter, C.; Schöb, H.; Stadler, M.; Meins, F.; Si-Ammour, A. MicroRNA-mediated regulation of stomatal development in Arabidopsis. Plant Cell 2007, 19, 2417–2429. [Google Scholar] [CrossRef]
- Zhu, J.; Park, J.H.; Lee, S.; Lee, J.H.; Hwang, D.; Kwak, J.M.; Kim, Y.J. Regulation of stomatal development by stomatal lineage miRNAs. Proc. Natl. Acad. Sci. USA 2020, 117, 6237–6245. [Google Scholar] [CrossRef]
- Guseman, J.M.; Lee, J.S.; Bogenschutz, N.L.; Peterson, K.M.; Virata, R.E.; Xie, B.; Kanaoka, M.M.; Hong, Z.; Torii, K.U. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis CHORUS (GLUCAN SYNTHASE-LIKE 8). Development 2010, 137, 1731–1741. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Liu, L.; Lee, E.; Han, X.; Rim, Y.; Chu, H.; Kim, S.-W.; Sack, F.D.; Kim, J.-Y. The Arabidopsis callose synthase gene gsl8 is required for cytokinesis and cell patterning. Plant Physiol. 2009, 150, 105–113. [Google Scholar] [CrossRef]
- Shpak, E.D.; McAbee, J.M.; Pillitteri, L.J.; Torii, K.U. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 2005, 309, 290–293. [Google Scholar] [CrossRef]
- Kong, D.; Karve, R.; Willet, A.; Chen, M.K.; Oden, J.; Shpak, E.D. Regulation of plasmodesmatal permeability and stomatal patterning by the glycosyltransferase-like protein KOBITO1. Plant Physiol. 2012, 159, 156–168. [Google Scholar] [CrossRef]
- Nakajima, K.; Sena, G.; Nawy, T.; Benfey, P.N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 2001, 413, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.D.G.; Zhang, D.; Raissig, M.T. Form, development and function of grass stomata. Plant J. 2020, 101, 780–799. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L.; Anderson, A.; Price, S.; Withee, J.R.; Gallagher, K.L.; Turgeon, R. Short-root1 plays a role in the development of vascular tissue and kranz anatomy in maize leaves. Mol. Plant 2014, 7, 1388–1392. [Google Scholar] [CrossRef]
- Hughes, T.E.; Sedelnikova, O.V.; Wu, H.; Becraft, P.W.; Langdale, J.A. Redundant SCARECROW genes pattern distinct cell layers in roots and leaves of maize. Development 2019, 146, dev.177543. [Google Scholar] [CrossRef]
- Yan, D.; Yadav, S.R.; Paterlini, A.; Nicolas, W.J.; Petit, J.D.; Brocard, L.; Belevich, I.; Grison, M.S.; Vaten, A.; Karami, L.; et al. Sphingolipid biosynthesis modulates plasmodesmal ultrastructure and phloem unloading. Nat. Plants 2019, 5, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Okawa, R.; Hayashi, Y.; Yamashita, Y.; Matsubayashi, Y.; Ogawa-Ohnishi, M. Arabinogalactan protein polysaccharide chains are required for normal biogenesis of plasmodesmata. Plant J. 2022. [Google Scholar] [CrossRef]
- Terry, B.R.; Robards, A.W. Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 1987, 171, 145–157. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y. Symplastic intercellular transport from a developmental perspective. J. Exp. Bot. 2014, 65, 1857–1863. [Google Scholar] [CrossRef]
- Willmer, C.M.; Sexton, R. Stomata and plasmodesmata. Protoplasma 1979, 100, 113–124. [Google Scholar] [CrossRef]
- Voss, L.J.; McAdam, S.A.M.; Knoblauch, M.; Rathje, J.M.; Brodribb, T.; Hedrich, R.; Roelfsema, M.R.G. Guard cells in fern stomata are connected by plasmodesmata, but control cytosolic Ca(2+) levels autonomously. New Phytol. 2018, 219, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Mumm, P.; Wolf, T.; Fromm, J.; Roelfsema, M.R.; Marten, I. Cell type-specific regulation of ion channels within the maize stomatal complex. Plant Cell Physiol. 2011, 52, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hyun, T.K.; Zhang, M.; Kumar, R.; Koh, E.J.; Kang, B.H.; Lucas, W.J.; Kim, J.Y. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev. Cell 2014, 28, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.; Wang, X.; Hill, K.; Yoo, B.C.; Caplan, J.; Nedo, A.; Tran, T.; Bennett, M.J.; Lee, J.Y. Auxin-dependent control of a plasmodesmal regulator creates a negative feedback loop modulating lateral root emergence. Nat. Commun. 2020, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Alfonso, Y.; Faulkner, C.; Pendle, A.; Miyashima, S.; Helariutta, Y.; Maule, A. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell 2013, 26, 136–147. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y. The Role of Abscisic Acid in the Regulation of Plasmodesmata and Symplastic Intercellular Transport. Plant Cell Physiol. 2019, 60, 713–714. [Google Scholar] [CrossRef]
- Pornsiriwong, W.; Estavillo, G.M.; Chan, K.X.; Tee, E.E.; Ganguly, D.; Crisp, P.A.; Phua, S.Y.; Zhao, C.; Qiu, J.; Park, J.; et al. A chloroplast retrograde signal, 3′-phosphoadenosine 5′-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. Elife 2017, 6, e23361. [Google Scholar] [CrossRef]
- MacAlister, C.A.; Bergmann, D.C. Sequence and function of basic helix-loop-helix proteins required for stomatal development in Arabidopsis are deeply conserved in land plants. Evol. Dev. 2011, 13, 182–192. [Google Scholar] [CrossRef]
- Iino, M.; Ogawa, T.; Zeiger, E. Kinetic properties of the blue-light response of stomata. Proc. Natl. Acad. Sci. USA 1985, 82, 8019–8023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).