Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex
Abstract
1. Introduction
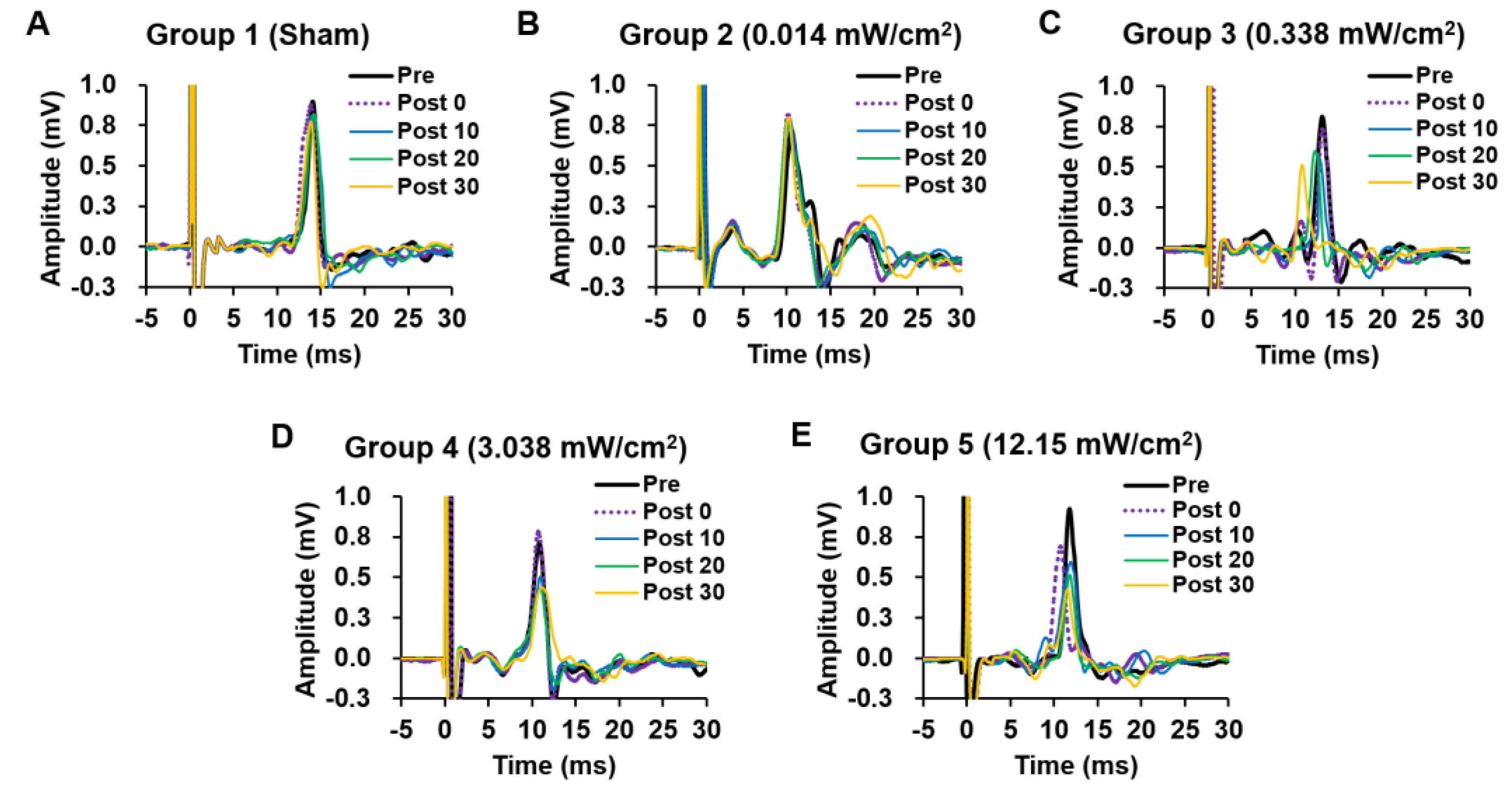
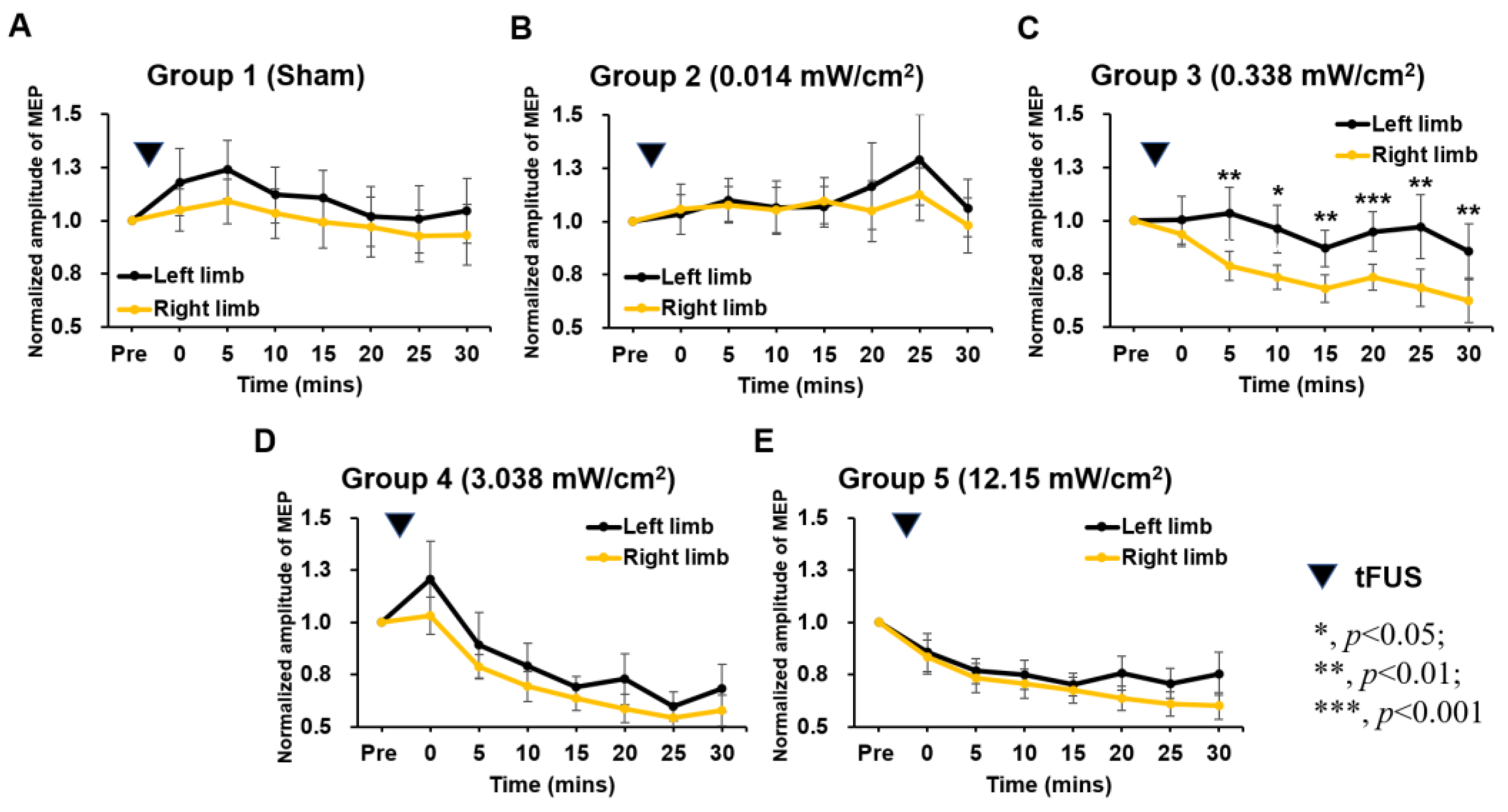
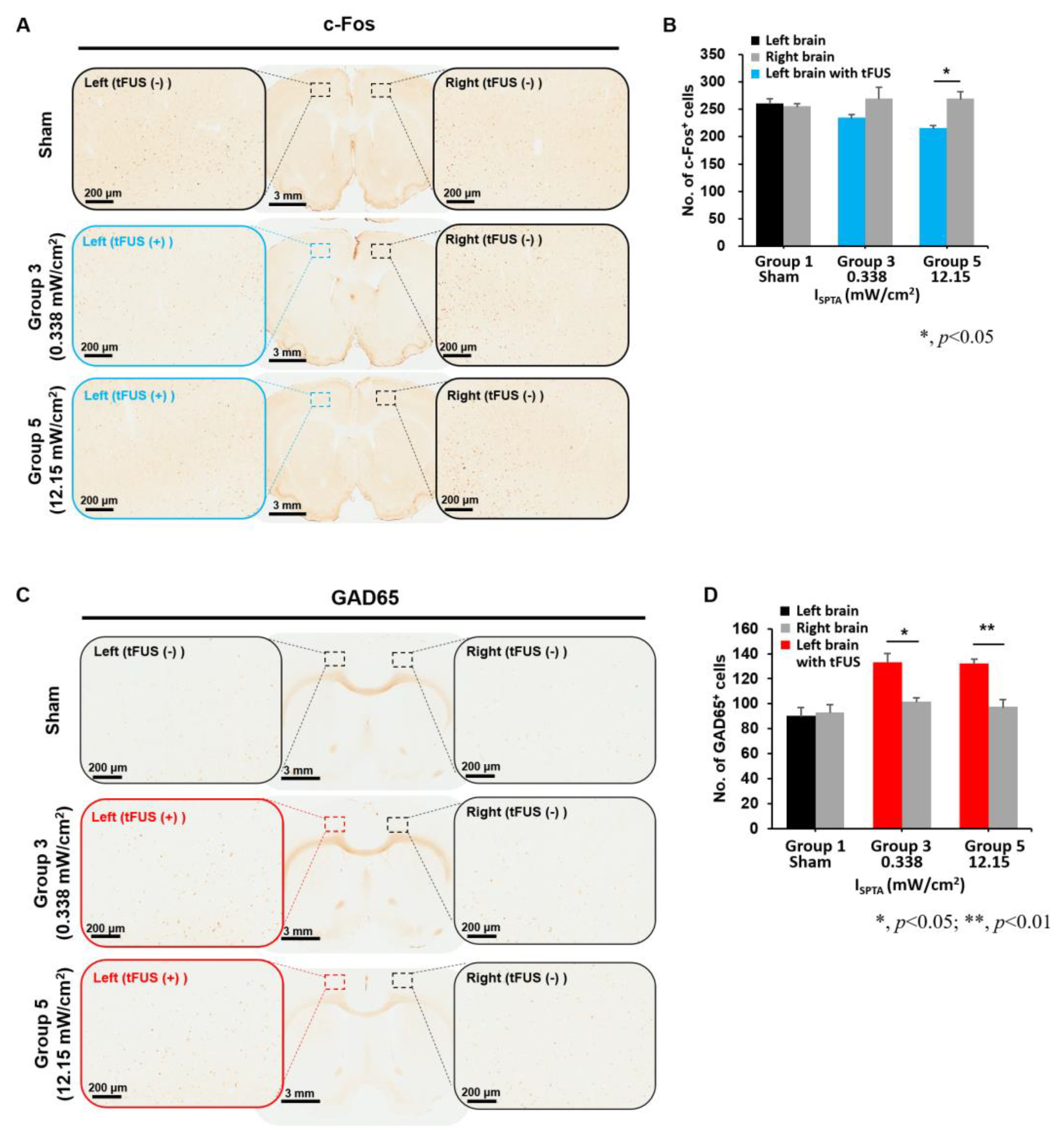
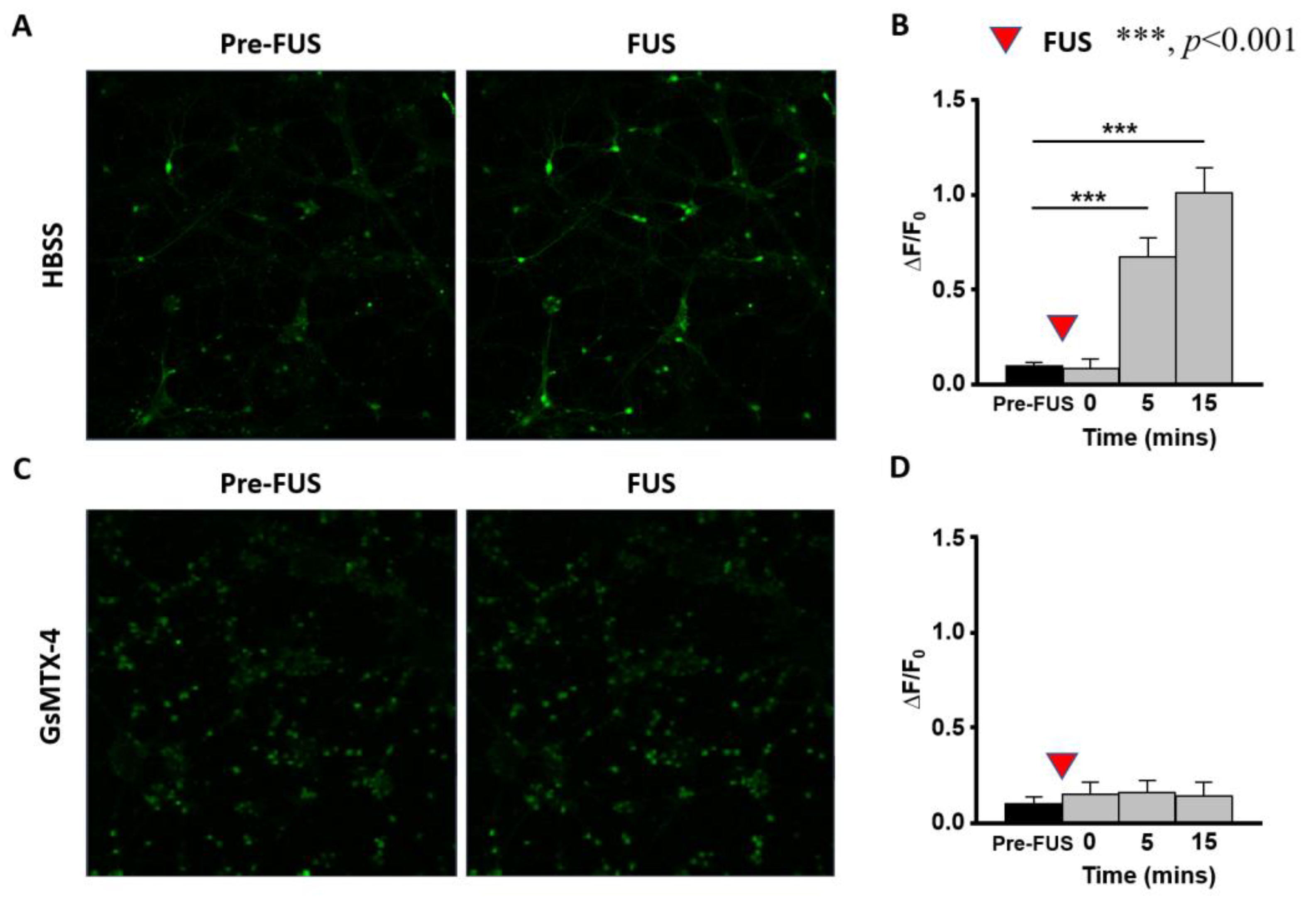
2. Results
2.1. The Changes in Motor-Evoked Potentials (MEPs) before and after Weak Sonication
2.2. Histological Examinations
2.3. Primary Neuron Cells with Weak Sonication
3. Discussion
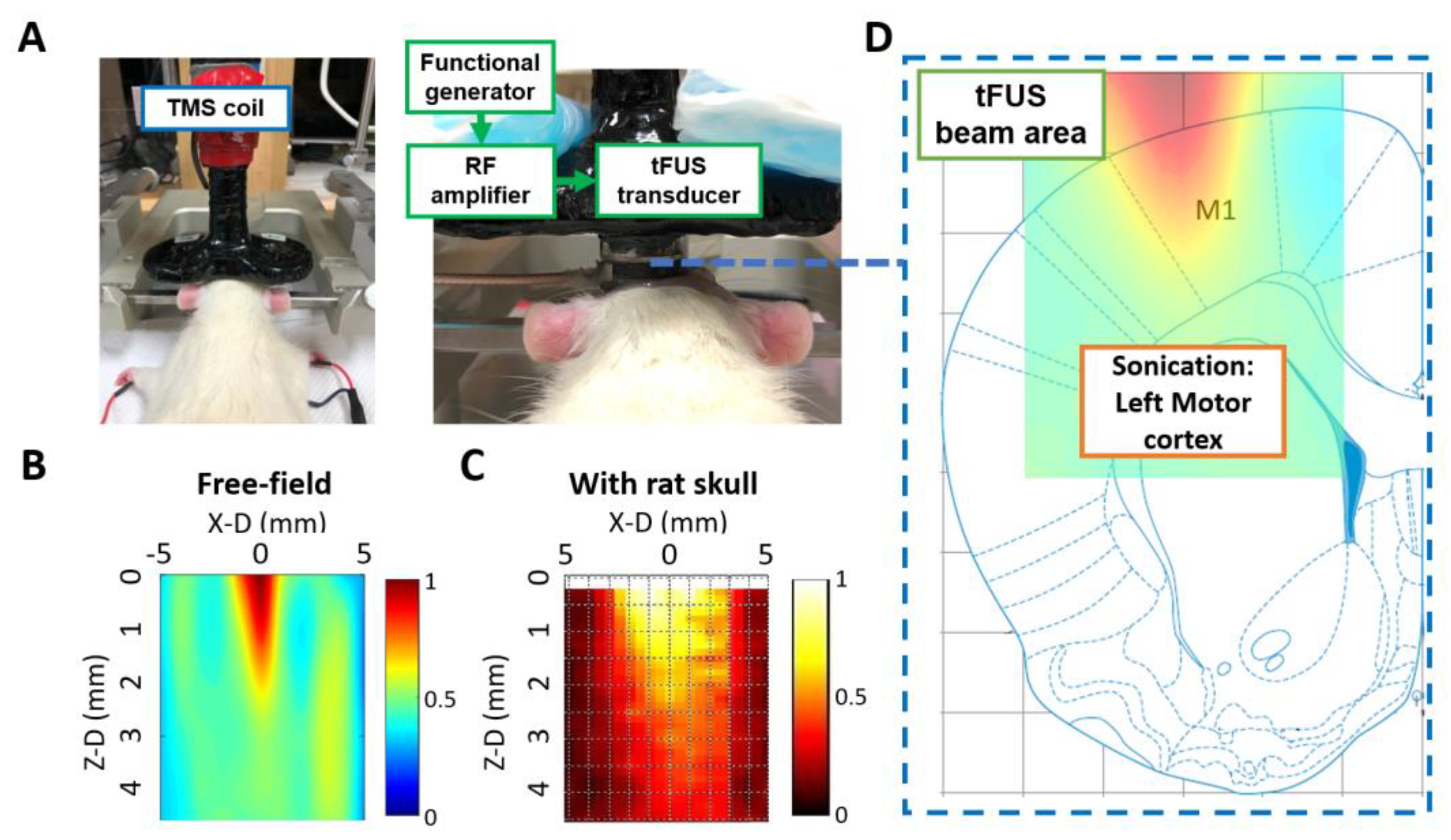
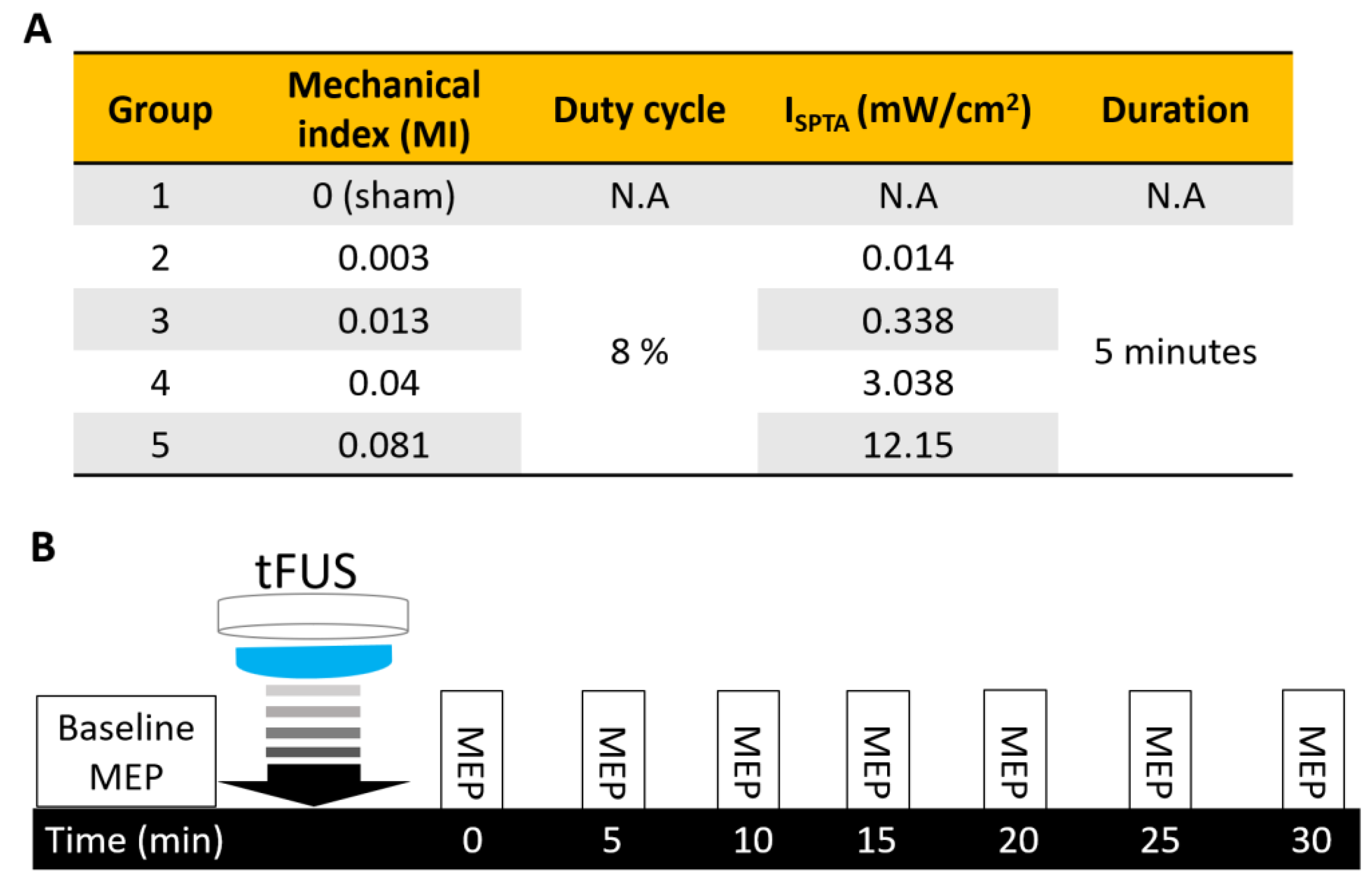
4. Materials and Methods
4.1. Animals and Preparations
4.2. tFUS Setup and Study Design
4.3. Recordings and TMS Assessments
4.4. Histological and Immunohistochemistry Examinations
4.5. Primary Neuron Cell Culture and Real-Time Calcium Signal following Weak-Intensity Sonication
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, M.D.; Lim, H.H.; Netoff, T.I.; Connolly, A.T.; Johnson, N.; Roy, A.; Holt, A.; Lim, K.O.; Carey, J.R.; Vitek, J.L.; et al. Neuromodulation for brain disorders: Challenges and opportunities. IEEE. Trans. Biomed. Eng. 2013, 60, 610–624. [Google Scholar] [CrossRef]
- Parpura, V.; Silva, G.A.; Tass, P.A.; Bennet, K.E.; Meyyappan, M.; Koehne, J.; Lee, K.H.; Andrews, R.J. Neuromodulation: Selected approaches and challenges. J. Neurochem. 2013, 124, 436–453. [Google Scholar] [CrossRef]
- Muresanu, D.F. Neuromodulation with pleiotropic and multimodal drugs–future approaches to treatment of neurological disorders. Acta Neurochir Suppl. 2010, 106, 291–294. [Google Scholar]
- Darmani, G.; Bergmann, T.O.; Butts Pauly, K.; Caskey, C.F.; de Lecea, L.; Fomenko, A.; Fouragnan, E.; Legon, W.; Murphy, K.R.; Nandi, T.; et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 2022, 135, 51–73. [Google Scholar] [CrossRef]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef]
- Bormann, N.L.; Trapp, N.T.; Narayanan, N.S.; Boes, A.D. Developing Precision Invasive Neuromodulation for Psychiatry. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 201–209. [Google Scholar] [CrossRef]
- Rossi, S.; Santarnecchi, E.; Valenza, G.; Ulivelli, M. The heart side of brain neuromodulation. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Cleary, R.T.; Bucholz, R. Neuromodulation Approaches in Parkinson’s Disease Using Deep Brain Stimulation and Transcranial Magnetic Stimulation. J. Geriatr. Psychiatry Neurol. 2021, 34, 301–309. [Google Scholar] [CrossRef]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef]
- Dietz, N.; Neimat, J. Neuromodulation: Deep Brain Stimulation for Treatment of Dystonia. Neurosurg. Clin. N. Am. 2019, 30, 161–168. [Google Scholar] [CrossRef]
- Yan, H.; Ren, L.; Yu, T. Deep brain stimulation of the subthalamic nucleus for epilepsy. Acta. Neurol. Scand. 2022, 146, 798–804. [Google Scholar] [CrossRef]
- Baur, D.; Galevska, D.; Hussain, S.; Cohen, L.G.; Ziemann, U.; Zrenner, C. Induction of LTD-like corticospinal plasticity by low-frequency rTMS depends on pre-stimulus phase of sensorimotor mu-rhythm. Brain Stimul. 2020, 13, 1580–1587. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Lu, M.K.; Antal, A.; Classen, J.; Nitsche, M.; Ziemann, U.; Ridding, M.; Hamada, M.; Ugawa, Y.; Jaberzadeh, S.; et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. 2017, 128, 2318–2329. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; Cheeran, B.; Rio-Rodriguez, D.; Fernandez-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; Fernandez-Del-Olmo, M.; Costantini, A.; Gonzalez-Henriquez, J.J.; Cheeran, B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin. Neurophysiol. 2015, 126, 2342–2347. [Google Scholar] [CrossRef]
- Darrow, D.P. Focused Ultrasound for Neuromodulation. Neurotherapeutics 2019, 16, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.K.; Ai, L.; Bansal, P.; Legon, W. Numerical evaluation of the skull for human neuromodulation with transcranial focused ultrasound. J. Neural Eng. 2017, 14, 066012. [Google Scholar] [CrossRef]
- di Biase, L.; Falato, E.; Di Lazzaro, V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front. Neurol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Tyler, W.J.; Lani, S.W.; Hwang, G.M. Ultrasonic modulation of neural circuit activity. Curr. Opin. Neurobiol. 2018, 50, 222–231. [Google Scholar] [CrossRef]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 2008, 3, e3511. [Google Scholar] [CrossRef] [PubMed]
- Dallapiazza, R.F.; Timbie, K.F.; Holmberg, S.; Gatesman, J.; Lopes, M.B.; Price, R.J.; Miller, G.W.; Elias, W.J. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J. Neurosurg. 2018, 128, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.S.; Yoo, S.S. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef]
- Fini, M.; Tyler, W.J. Transcranial focused ultrasound: A new tool for non-invasive neuromodulation. Int. Rev. Psychiatry 2017, 29, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Bansal, P.; Tyshynsky, R.; Ai, L.; Mueller, J.K. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Sci. Rep. 2018, 8, 10007. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.C.; Sanguinetti, J.L.; Badran, B.W.; Yu, A.B.; Klein, E.P.; Abbott, C.C.; Hansberger, J.T.; Clark, V.P. Increased Excitability Induced in the Primary Motor Cortex by Transcranial Ultrasound Stimulation. Front. Neurol. 2018, 9, 1007. [Google Scholar] [CrossRef]
- Baek, H.; Pahk, K.J.; Kim, H. A review of low-intensity focused ultrasound for neuromodulation. Biomed Eng. Lett. 2017, 7, 135–142. [Google Scholar] [CrossRef]
- Blackmore, J.; Shrivastava, S.; Sallet, J.; Butler, C.R.; Cleveland, R.O. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol. 2019, 45, 1509–1536. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Wang, Z.; Li, X.; Yuan, Y. Neuromodulation Effects of Ultrasound Stimulation under Different Parameters on Mouse Motor Cortex. IEEE Trans. Biomed. Eng. 2020, 67, 291–297. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Liu, M.; Shoham, S. Cortical hemodynamic responses induced by low-intensity transcranial ultrasound stimulation of mouse cortex. NeuroImage 2020, 211, 116597. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, W.; Kunes, J.; Yoon, K.; Lee, J.E.; Foley, L.; Kowsari, K.; Yoo, S.S. Transcranial focused ultrasound modulates cortical and thalamic motor activity in awake sheep. Sci. Rep. 2021, 11, 19274. [Google Scholar] [CrossRef]
- Lim, J.; Tai, H.H.; Liao, W.H.; Chu, Y.C.; Hao, C.M.; Huang, Y.C.; Lee, C.H.; Lin, S.S.; Hsu, S.; Chien, Y.C.; et al. ASIC1a is required for neuronal activation via low-intensity ultrasound stimulation in mouse brain. eLife 2021, 10, e61660. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Chu, Y.C.; Tai, H.H.; Chien, A.; Huang, S.S.; Chen, C.C.; Wang, J.L. Auditory independent low-intensity ultrasound stimulation of mouse brain is associated with neuronal ERK phosphorylation and an increase of Tbr2 marked neuroprogenitors. Biochem. Biophys. Res. Commun. 2022, 613, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, A.; Muller, P.A.; Vahabzadeh-Hagh, A.M.; Navarro, X.; Lopez-Vales, R.; Pascual-Leone, A.; Jensen, F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin. Neurophysiol. 2010, 121, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Marufa, S.A.; Hsieh, T.H.; Liou, J.C.; Chen, H.Y.; Peng, C.W. Neuromodulatory effects of repetitive transcranial magnetic stimulation on neural plasticity and motor functions in rats with an incomplete spinal cord injury: A preliminary study. PLoS ONE 2021, 16, e0252965. [Google Scholar] [CrossRef]
- Plaksin, M.; Kimmel, E.; Shoham, S. Cell-Type-Selective Effects of Intramembrane Cavitation as a Unifying Theoretical Framework for Ultrasonic Neuromodulation. eNeuro 2016, 3, ENEURO.0136-15.2016. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef]
- Chen, S.G.; Tsai, C.H.; Lin, C.J.; Lee, C.C.; Yu, H.Y.; Hsieh, T.H.; Liu, H.L. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. 2020, 13, 35–46. [Google Scholar] [CrossRef]
- Lescrauwaet, E.; Vonck, K.; Sprengers, M.; Raedt, R.; Klooster, D.; Carrette, E.; Boon, P. Recent Advances in the Use of Focused Ultrasound as a Treatment for Epilepsy. Front. Neurosci. 2022, 16, 886584. [Google Scholar] [CrossRef]
- Yu, K.; Niu, X.; He, B. Neuromodulation Management of Chronic Neuropathic Pain in The Central Nervous system. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Trippe, J.; Mix, A.; Aydin-Abidin, S.; Funke, K.; Benali, A. theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp. Brain Res. 2009, 199, 411–421. [Google Scholar] [CrossRef]
- Yoo, S.; Mittelstein, D.R.; Hurt, R.C.; Lacroix, J.; Shapiro, M.G. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat. Commun. 2022, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Kala, S.; Guo, J.; Xian, Q.; Zhu, J.; Zhu, T.; Hou, X.; Wong, K.F.; Yang, M.; Wang, H.; et al. Targeted Neurostimulation in Mouse Brains with Non-invasive Ultrasound. Cell Rep. 2020, 32, 108033. [Google Scholar] [CrossRef]
- Chu, Y.C.; Lim, J.; Chien, A.; Chen, C.C.; Wang, J.L. Activation of Mechanosensitive Ion Channels by Ultrasound. Ultrasound Med. Biol. 2022, 48, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, Z.; Xu, E.; Zhou, J.; Yan, F. Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles. Ultrason. Sonochem. 2021, 73, 105494. [Google Scholar] [CrossRef]
- Verhagen, L.; Gallea, C.; Folloni, D.; Constans, C.; Jensen, D.E.; Ahnine, H.; Roumazeilles, L.; Santin, M.; Ahmed, B.; Lehericy, S.; et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. eLife 2019, 8, e40541. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, W.; Lee, J.E.; Xu, L.; Croce, P.; Foley, L.; Yoo, S.S. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE 2019, 14, e0224311. [Google Scholar] [CrossRef]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused ultrasound modulates region-specific brain activity. NeuroImage 2011, 56, 1267–1275. [Google Scholar] [CrossRef]
- Chu, P.C.; Yu, H.Y.; Lee, C.C.; Fisher, R.; Liu, H.L. Pulsed-Focused Ultrasound Provides Long-Term Suppression of Epileptiform Bursts in the Kainic Acid-Induced Epilepsy Rat Model. Neurotherapeutics 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Clennell, B.; Steward, T.G.J.; Gialeli, A.; Cordero-Llana, O.; Whitcomb, D.J. Focused Ultrasound Stimulation as a Neuromodulatory Tool for Parkinson’s Disease: A Scoping Review. Brain Sci. 2022, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chou, C.C.; Hsiao, F.J.; Chen, Y.H.; Lin, C.F.; Chen, C.J.; Peng, S.J.; Liu, H.L.; Yu, H.Y. Pilot study of focused ultrasound for drug-resistant epilepsy. Epilepsia 2021, 63, 162–175. [Google Scholar] [CrossRef]
- Guo, H.; Hamilton, M., 2nd; Offutt, S.J.; Gloeckner, C.D.; Li, T.; Kim, Y.; Legon, W.; Alford, J.K.; Lim, H.H. Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 2018, 98, 1020–1030. [Google Scholar] [CrossRef]
- Mohammadjavadi, M.; Ye, P.P.; Xia, A.; Brown, J.; Popelka, G.; Pauly, K.B. Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain Stimul. 2019, 12, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Madhavan, S. Non-invasive brain stimulation in the modulation of cerebral blood flow after stroke: A systematic review of Transcranial Doppler studies. Clin. Neurophysiol. 2018, 129, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Huang, Y.Z.; Rotenberg, A.; Pascual-Leone, A.; Chiang, Y.H.; Wang, J.Y.; Chen, J.J. Functional Dopaminergic Neurons in Substantia Nigra are Required for Transcranial Magnetic Stimulation-Induced Motor Plasticity. Cereb. Cortex 2015, 25, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A.; Muller, A.; Hynynen, K. Ultrasound insertion loss of rat parietal bone appears to be proportional to animal mass at submegahertz frequencies. Ultrasound Med. Biol. 2011, 37, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Fomenko, A.; Chen, K.S.; Nankoo, J.F.; Saravanamuttu, J.; Wang, Y.; El-Baba, M.; Xia, X.; Seerala, S.S.; Hynynen, K.; Lozano, A.M.; et al. Systematic examination of low-intensity ultrasound parameters on human motor cortex excitability and behavior. eLife 2020, 9, e54497. [Google Scholar] [CrossRef]
- Gersner, R.; Kravetz, E.; Feil, J.; Pell, G.; Zangen, A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J. Neurosci. 2011, 31, 7521–7526. [Google Scholar] [CrossRef]
- Vahabzadeh-Hagh, A.M.; Muller, P.A.; Pascual-Leone, A.; Jensen, F.E.; Rotenberg, A. Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J. Neurophysiol. 2011, 105, 615–624. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kang, J.W.; Lai, J.H.; Huang, Y.Z.; Rotenberg, A.; Chen, K.Y.; Wang, J.Y.; Chan, S.Y.; Chen, S.C.; Chiang, Y.H.; et al. Relationship of mechanical impact magnitude to neurologic dysfunction severity in a rat traumatic brain injury model. PLoS ONE 2017, 12, e0178186. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chang, M.Y.; Liu, H.H.; He, X.K.; Chan, S.Y.; Huang, Y.Z.; Peng, C.W.; Chang, P.K.; Pan, C.Y.; Hsieh, T.H. Cortical Electrical Stimulation Ameliorates Traumatic Brain Injury-Induced Sensorimotor and Cognitive Deficits in Rats. Front. Neural. Circuits. 2021, 15, 693073. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Hsieh, T.H.; Chen, K.Y.; Wu, J.C.; Hoffer, B.J.; Greig, N.H.; Li, Y.; Lai, J.H.; Chang, C.F.; Lin, J.W.; et al. Glucose-Dependent Insulinotropic Polypeptide Ameliorates Mild Traumatic Brain Injury-Induced Cognitive and Sensorimotor Deficits and Neuroinflammation in Rats. J. Neurotrauma 2016, 33, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Bullitt, E. Expression of C-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; de Graaf, R.A.; Martin, D.L.; Battaglioli, G.; Behar, K.L. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J. Neurochem. 2006, 97, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.P.; Kao, L.S.; Liao, H.T.; Pan, C.Y. Reverse mode Na+/Ca2+ exchangers trigger the release of Ca2+ from intracellular Ca2+ stores in cultured rat embryonic cortical neurons. Brain Res. 2008, 1201, 41–51. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, P.-C.; Huang, C.-S.; Chang, P.-K.; Chen, R.-S.; Chen, K.-T.; Hsieh, T.-H.; Liu, H.-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. https://doi.org/10.3390/ijms24032578
Chu P-C, Huang C-S, Chang P-K, Chen R-S, Chen K-T, Hsieh T-H, Liu H-L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. International Journal of Molecular Sciences. 2023; 24(3):2578. https://doi.org/10.3390/ijms24032578
Chicago/Turabian StyleChu, Po-Chun, Chen-Syuan Huang, Pi-Kai Chang, Rou-Shayn Chen, Ko-Ting Chen, Tsung-Hsun Hsieh, and Hao-Li Liu. 2023. "Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex" International Journal of Molecular Sciences 24, no. 3: 2578. https://doi.org/10.3390/ijms24032578
APA StyleChu, P.-C., Huang, C.-S., Chang, P.-K., Chen, R.-S., Chen, K.-T., Hsieh, T.-H., & Liu, H.-L. (2023). Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. International Journal of Molecular Sciences, 24(3), 2578. https://doi.org/10.3390/ijms24032578











