Zein Nanoparticles Containing Arginine-Based Surfactants: Physicochemical Characterization and Effect on the Biological Properties
Abstract
1. Introduction
2. Results and Discussion
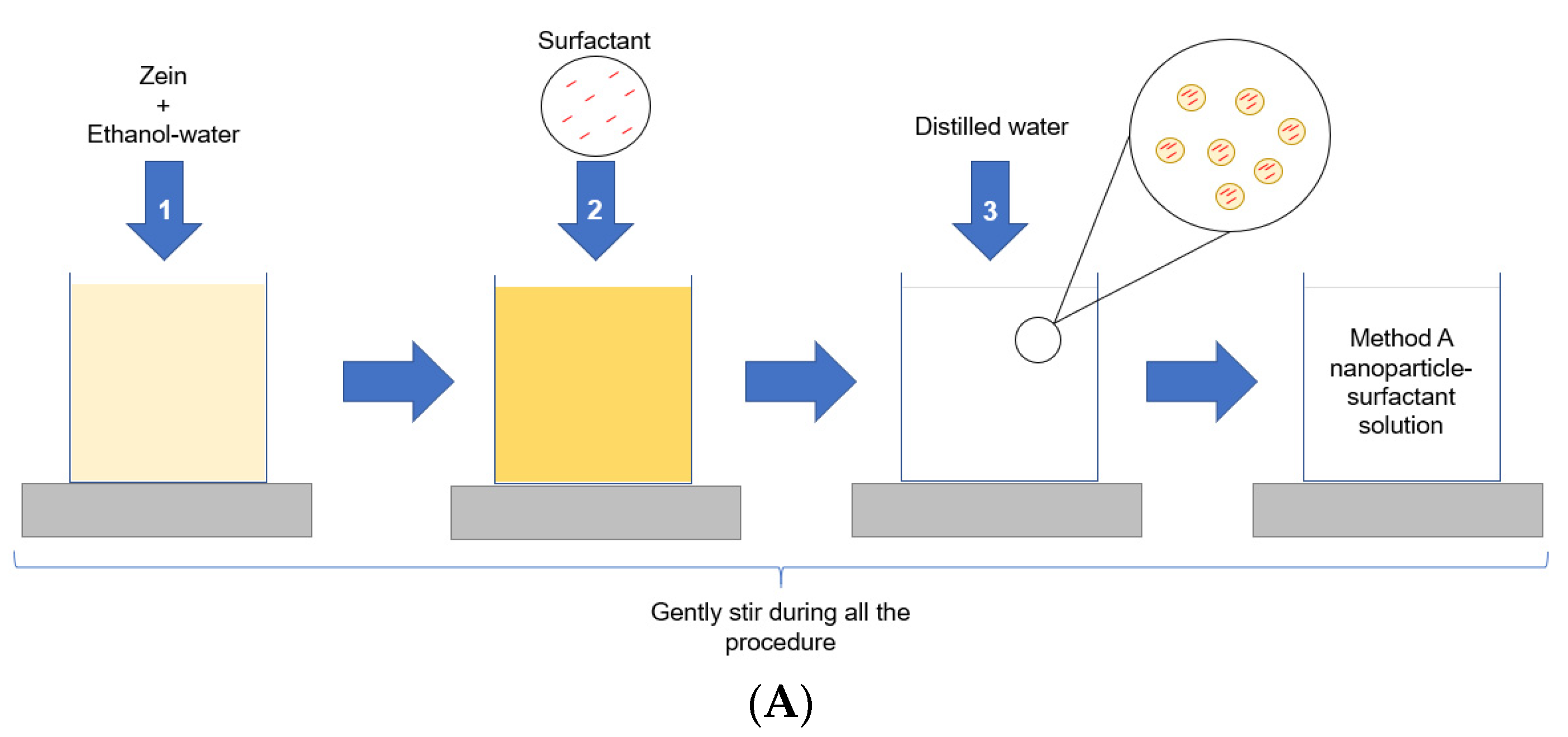
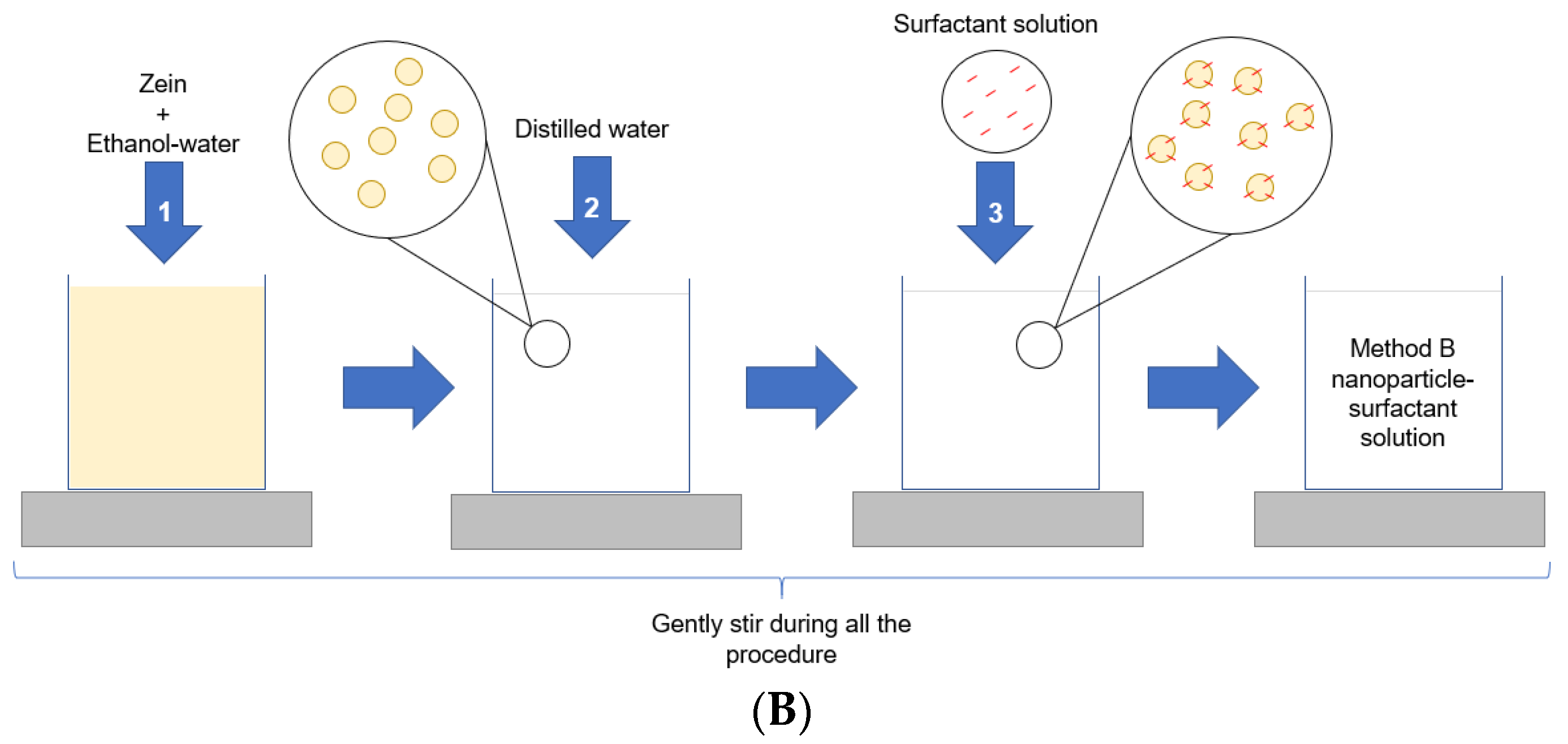
2.1. Nanoparticles Formation and Characterization
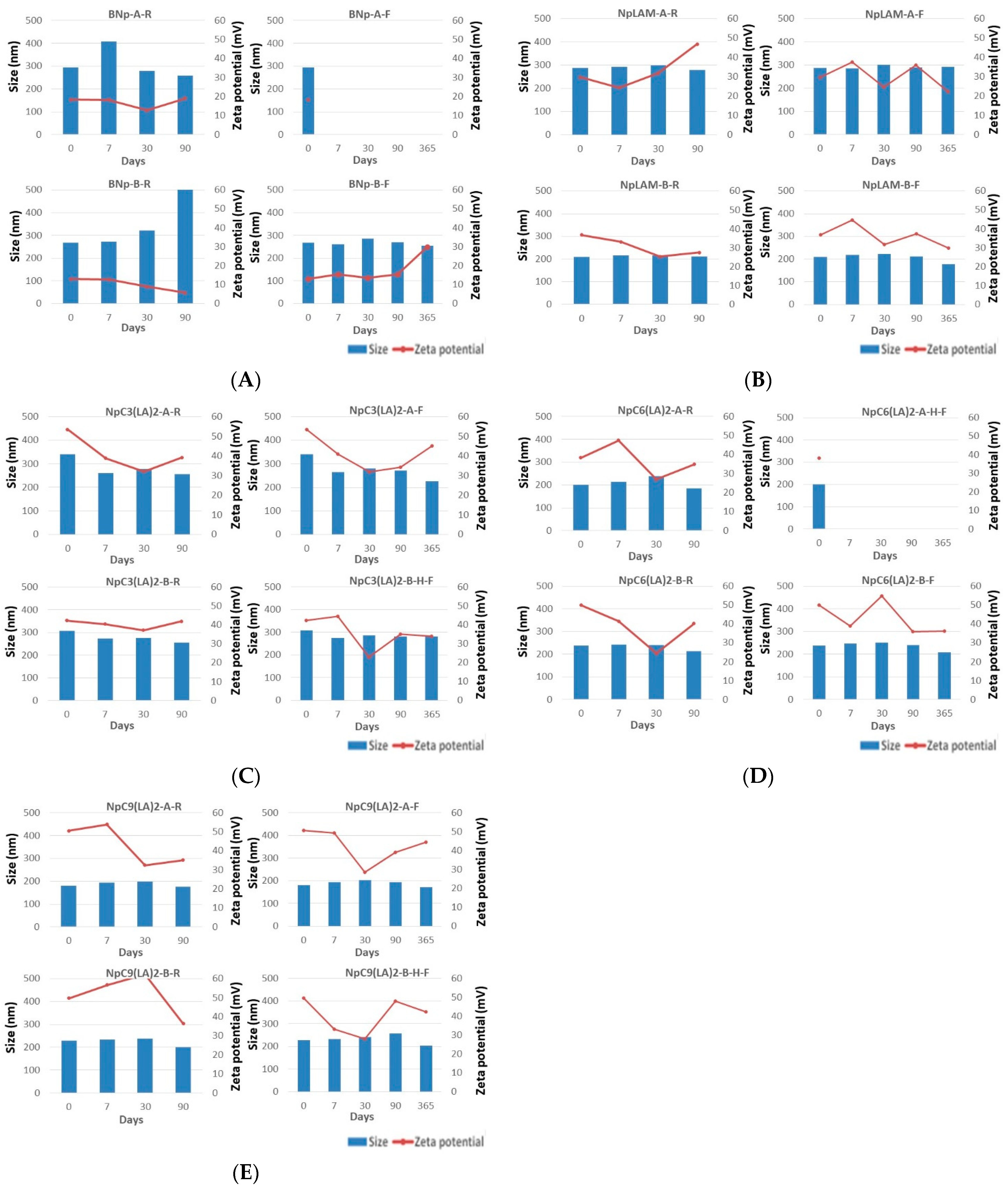
2.2. Blank Zein Nanoparticles
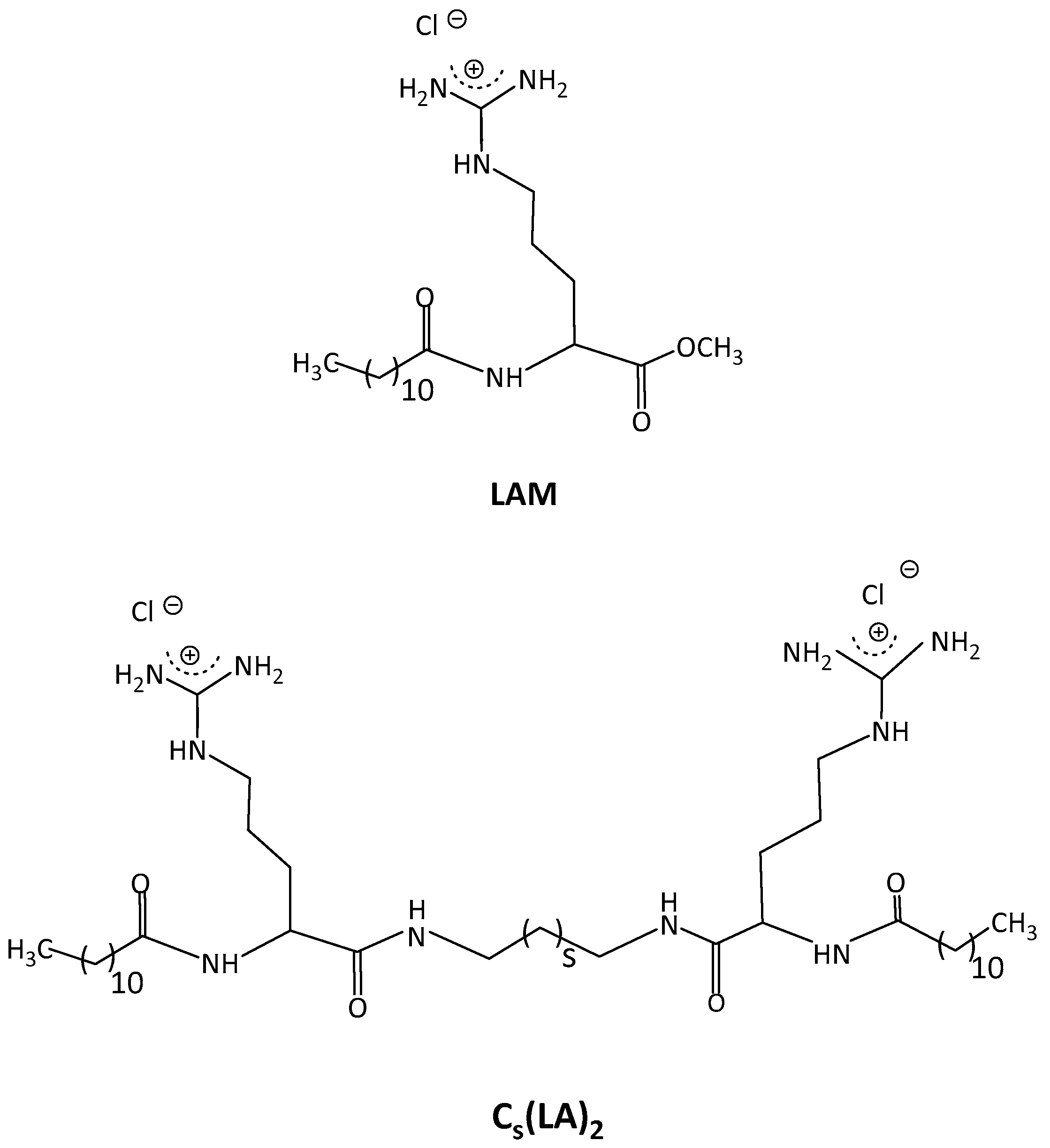
2.3. LAM-Loaded Zein Nanoparticles
2.4. C3(LA)2-Loaded Zein Nanoparticles
2.5. C6(LA)2-Loaded Zein Nanoparticles
2.6. C9(LA)2-Loaded Zein Nanoparticles
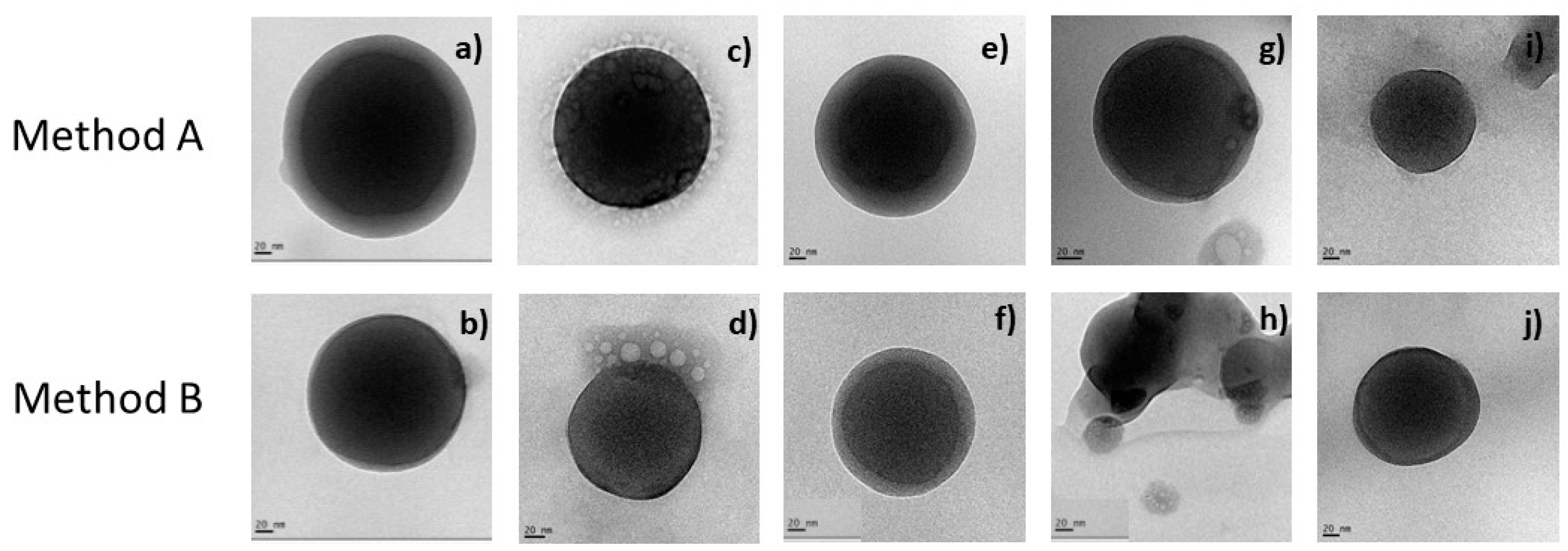
2.7. TEM Morphological Appreciation
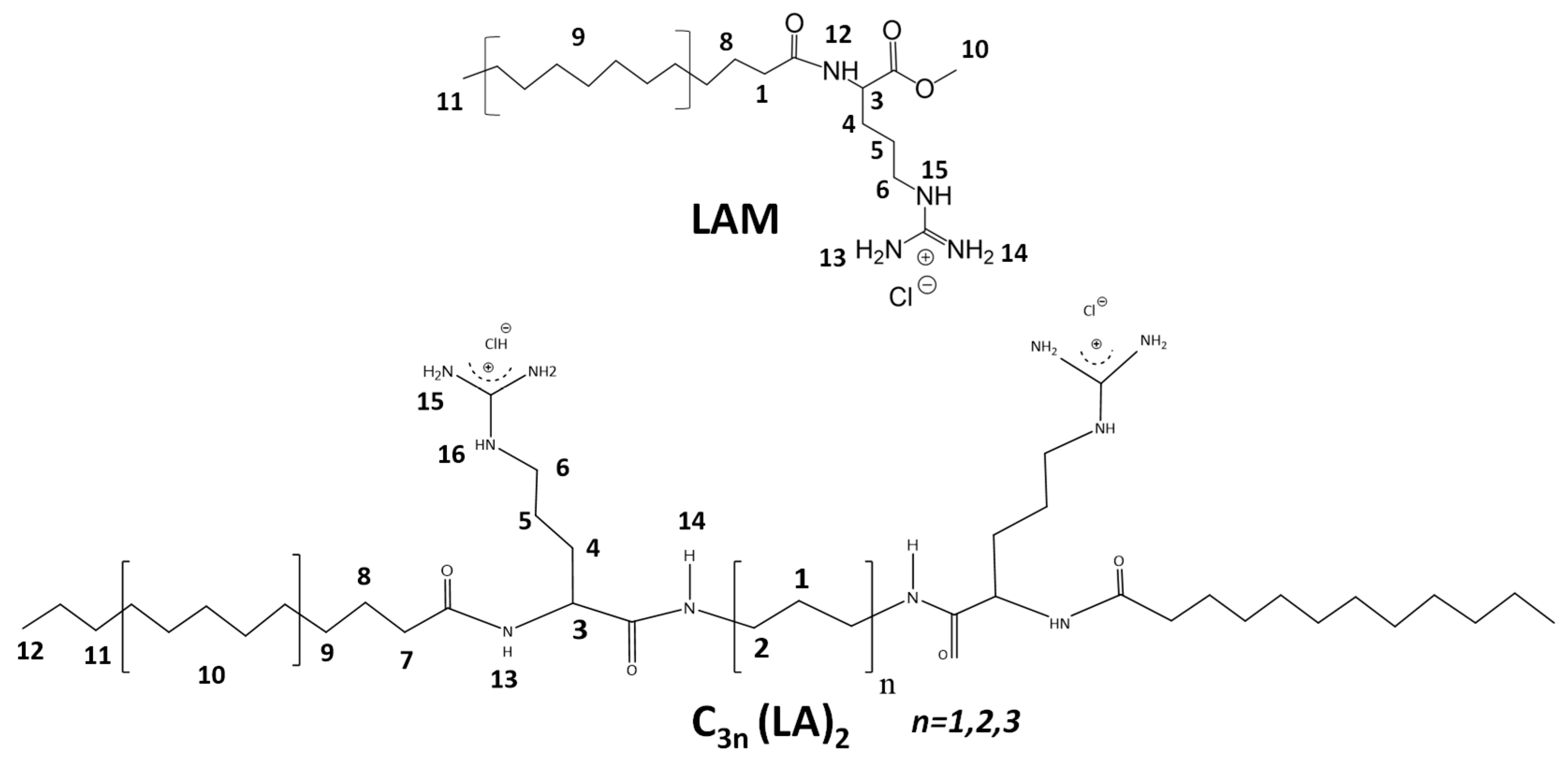
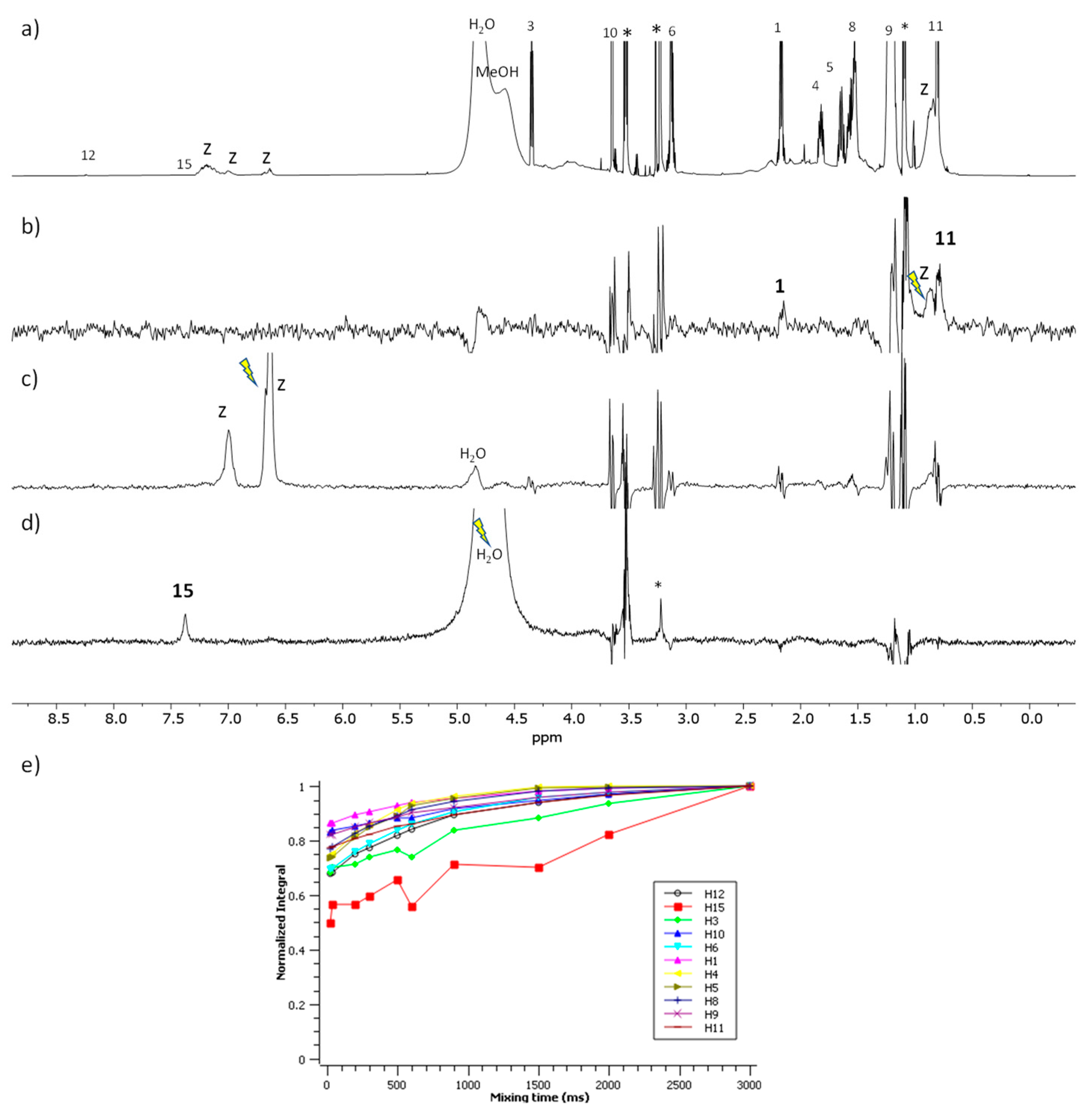
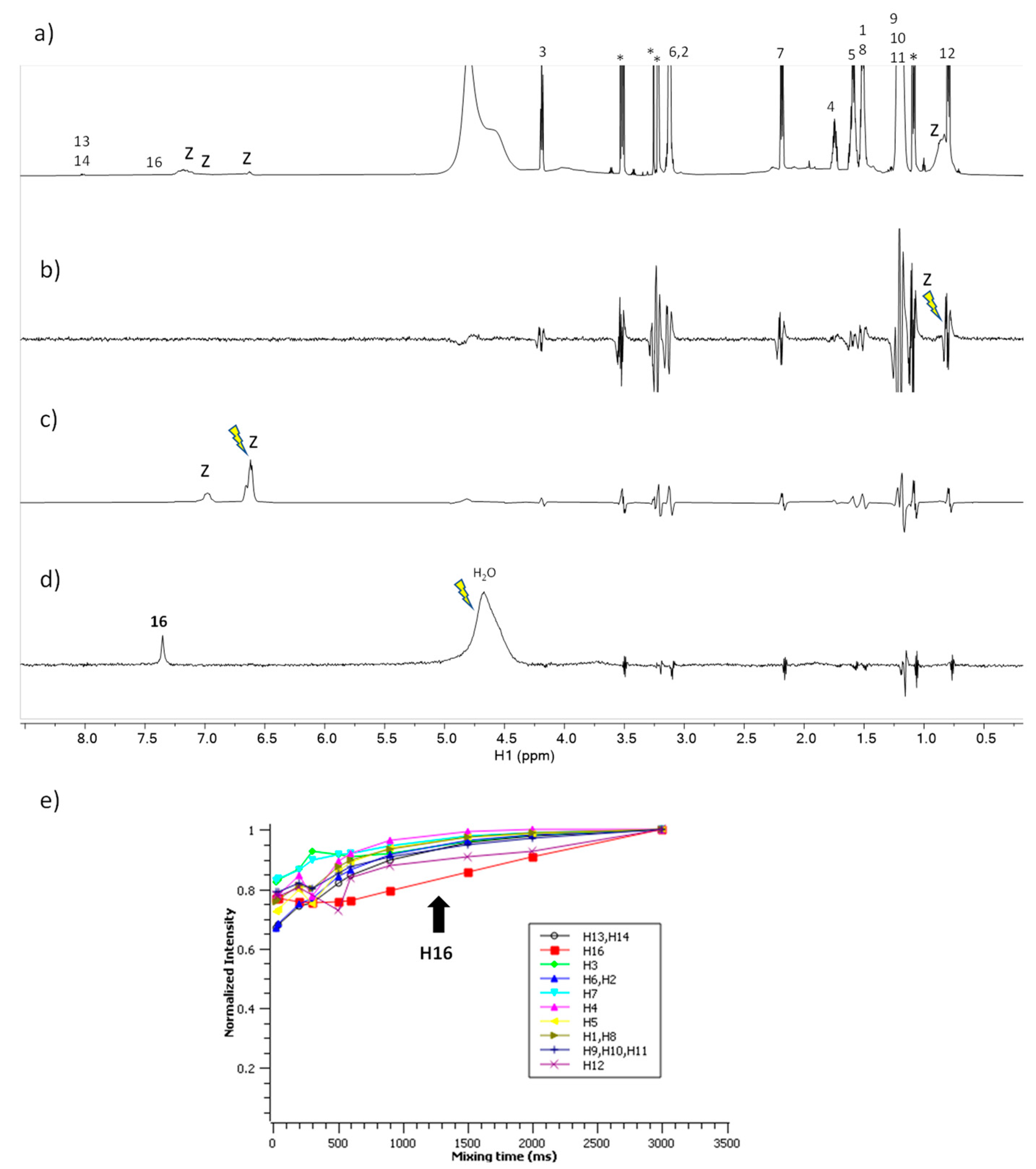
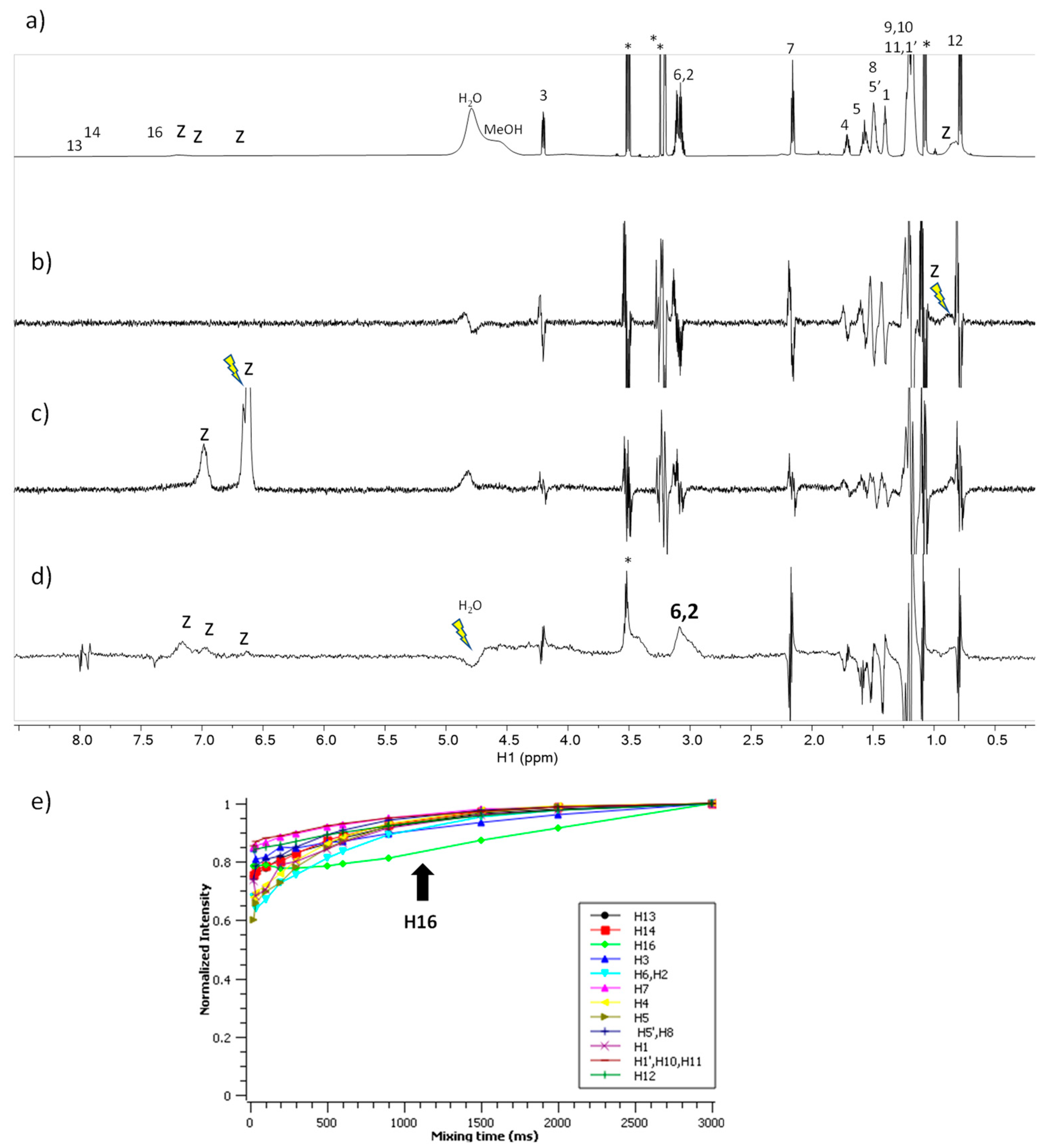
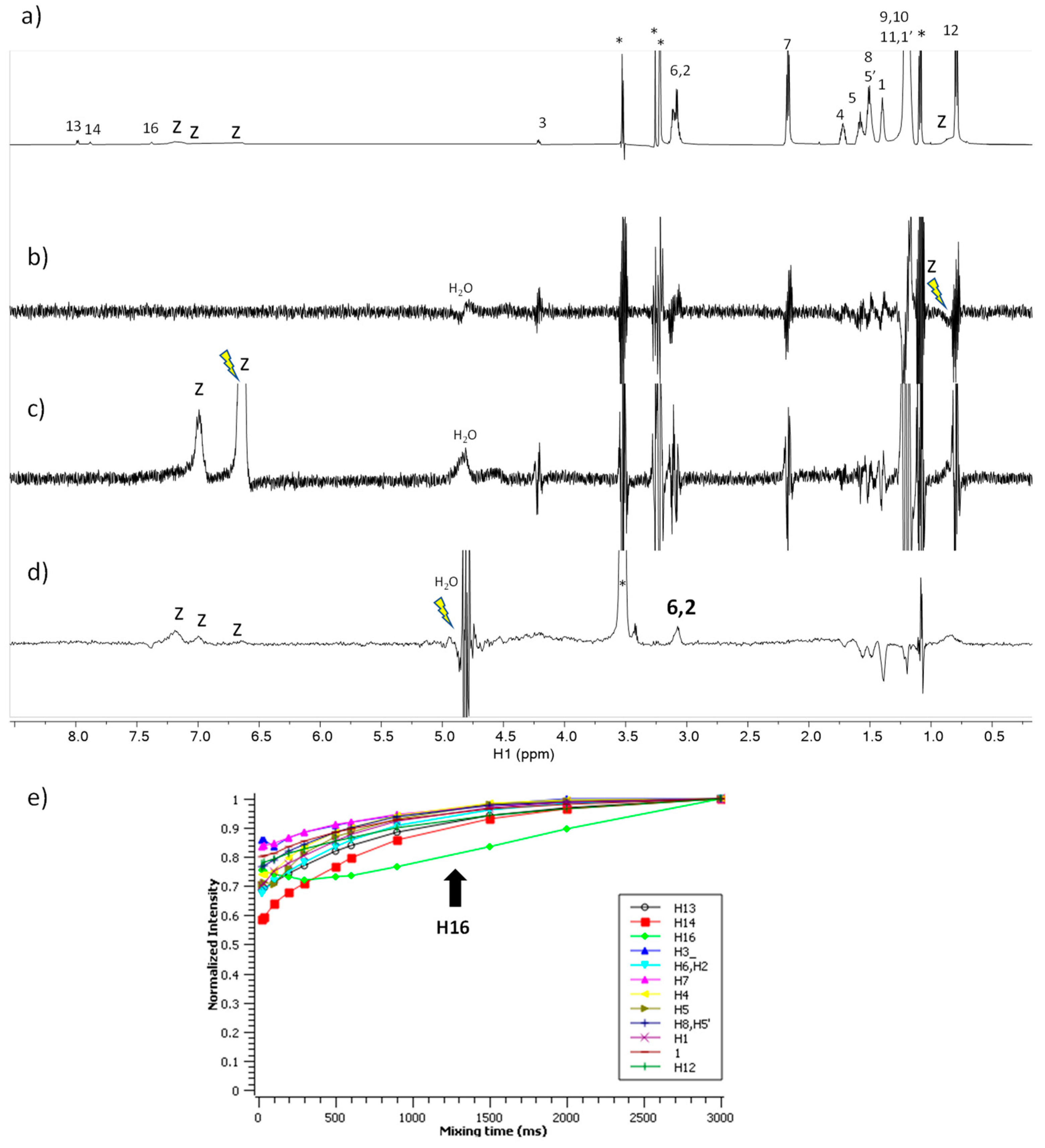
2.8. NMR Studies
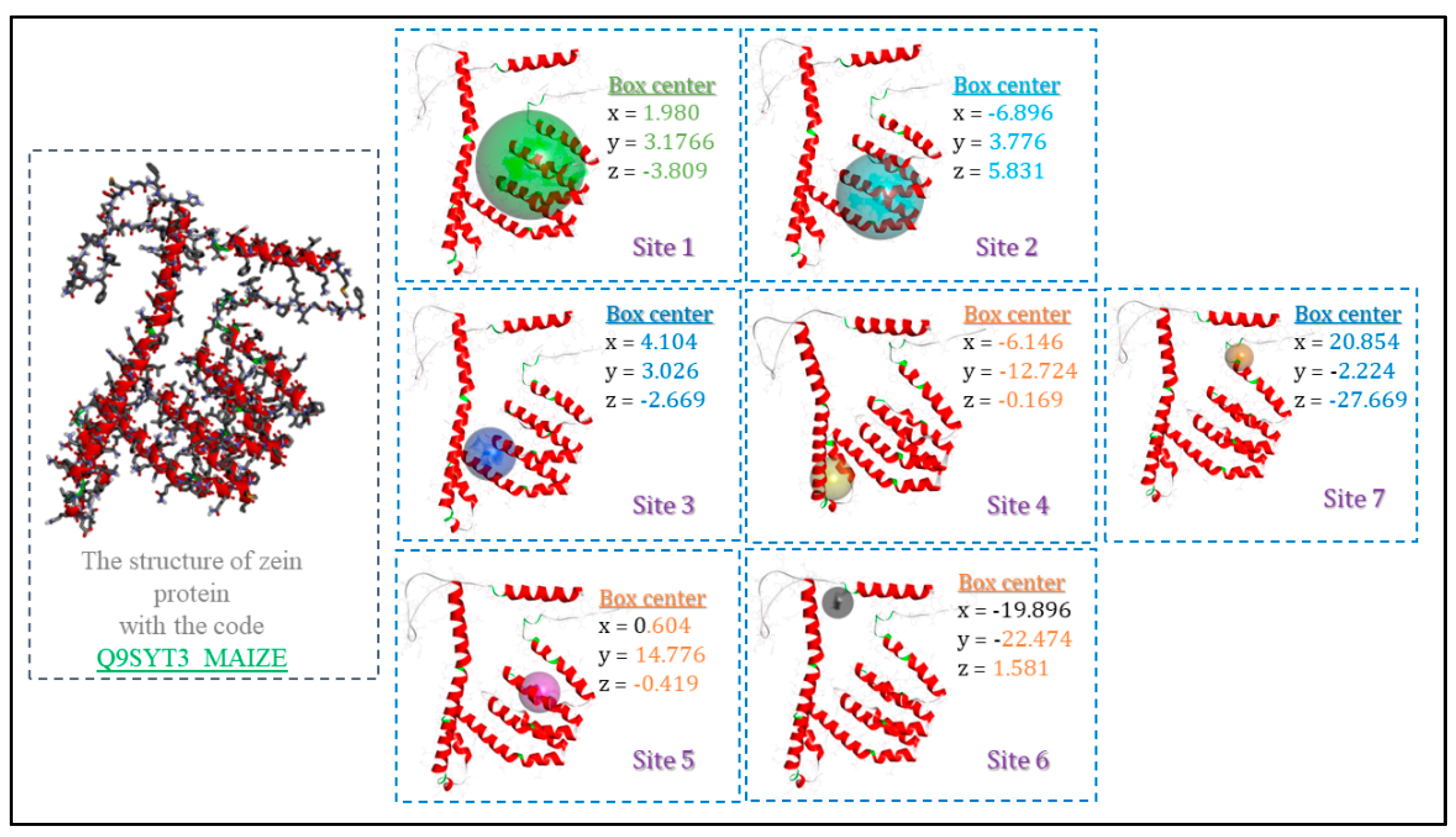
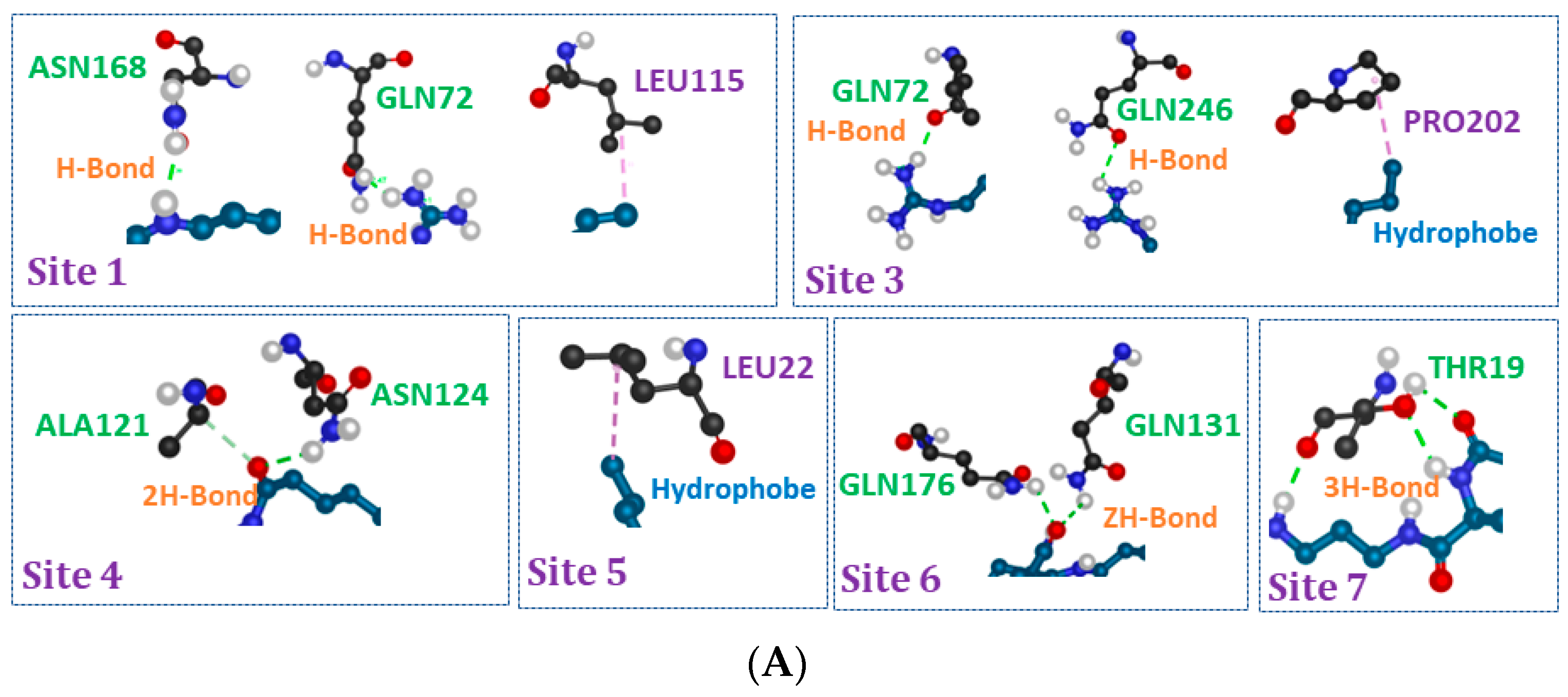
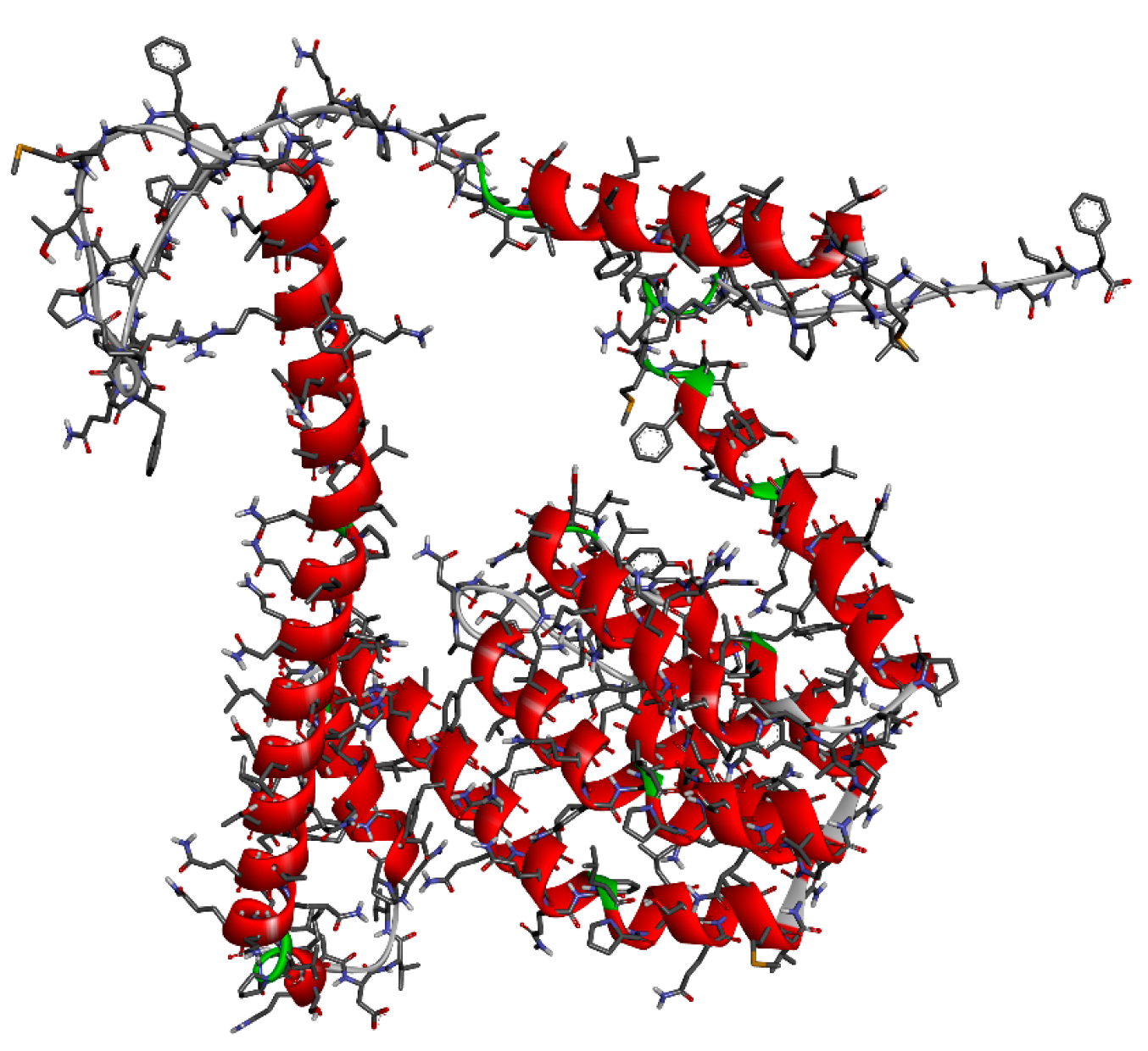
2.9. Molecular Docking Studies
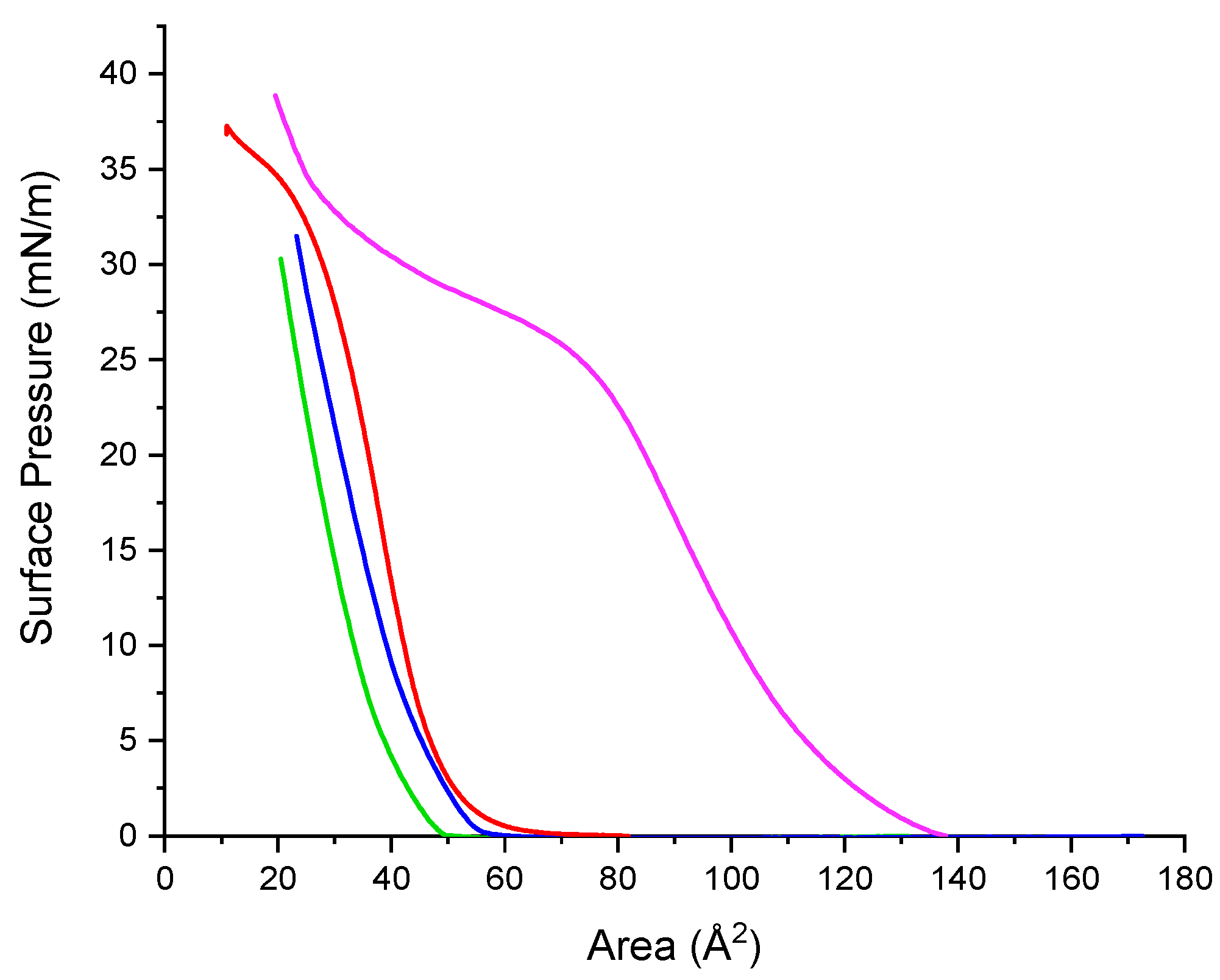
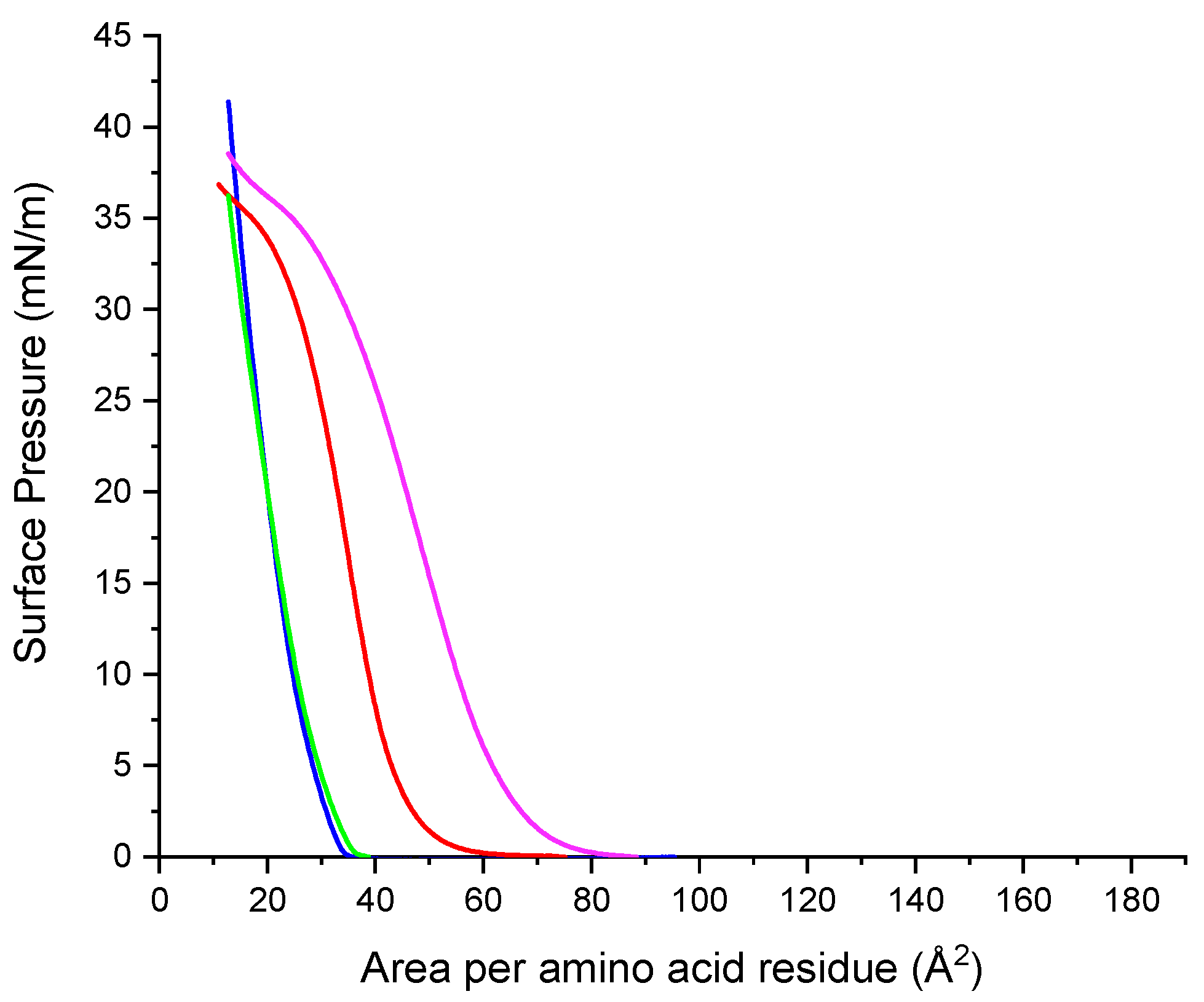
2.10. Surface Pressure/Area Isotherms
2.10.1. Single Component Systems
2.10.2. Mixed Systems
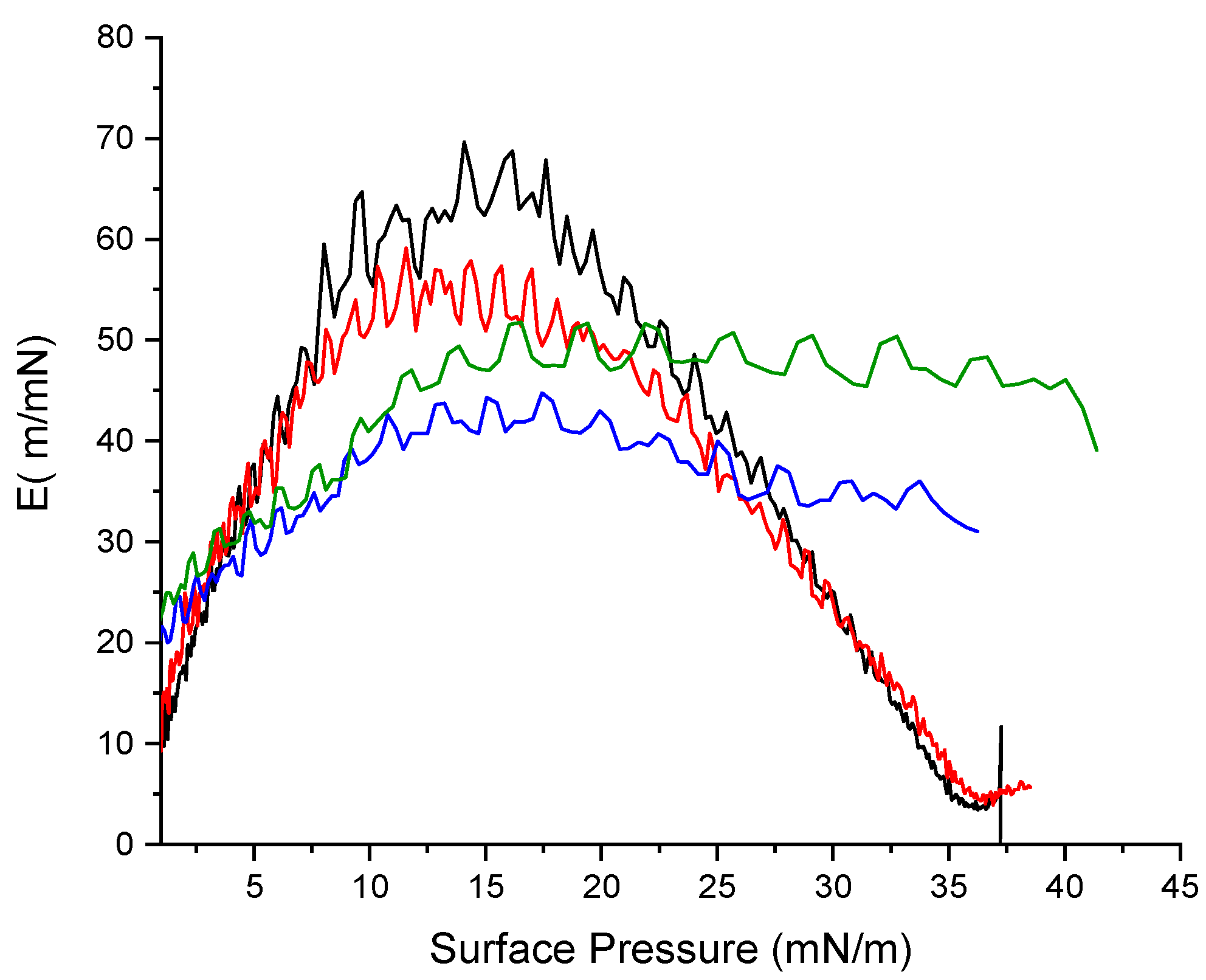
2.11. Mechanical properties
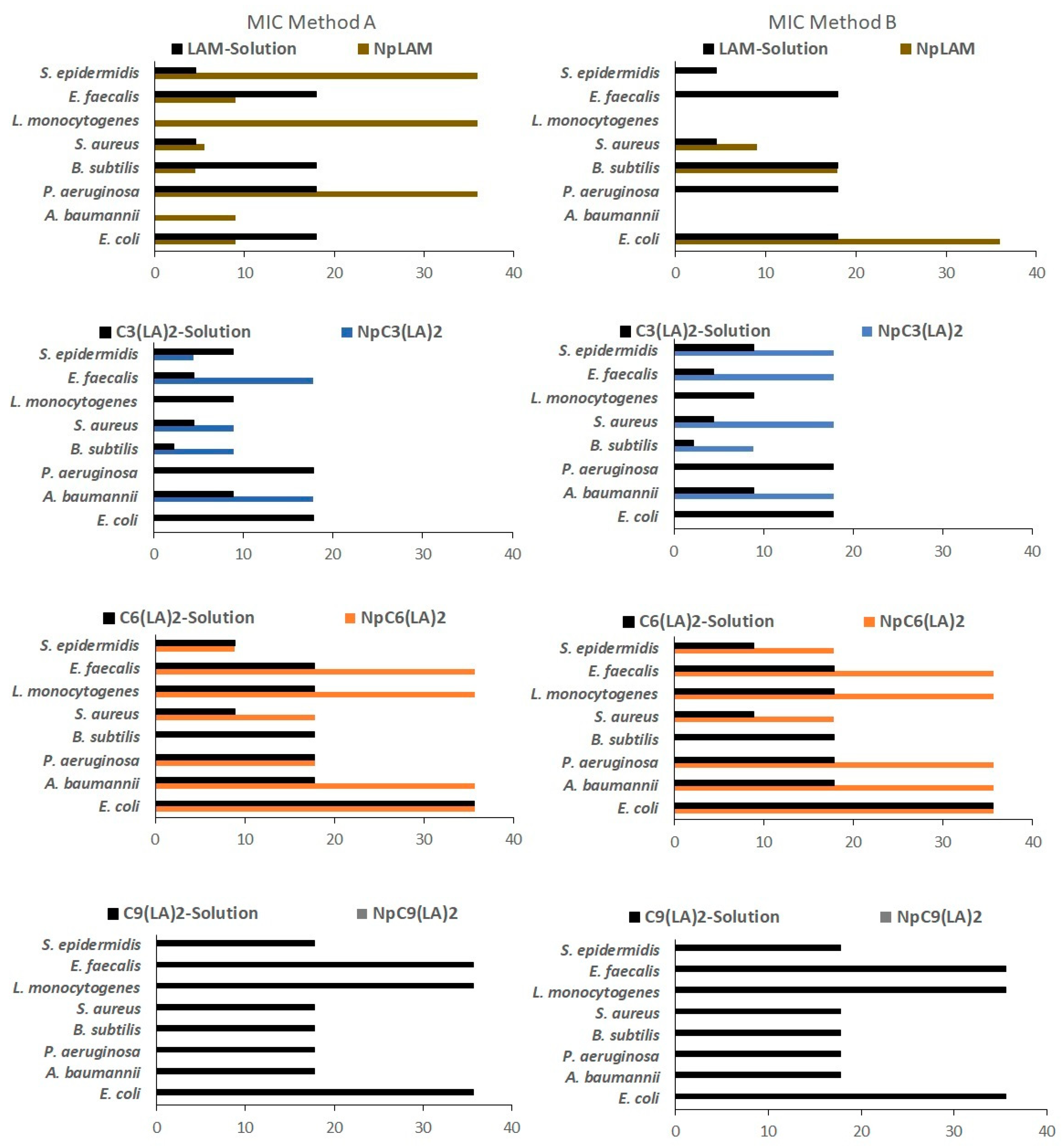
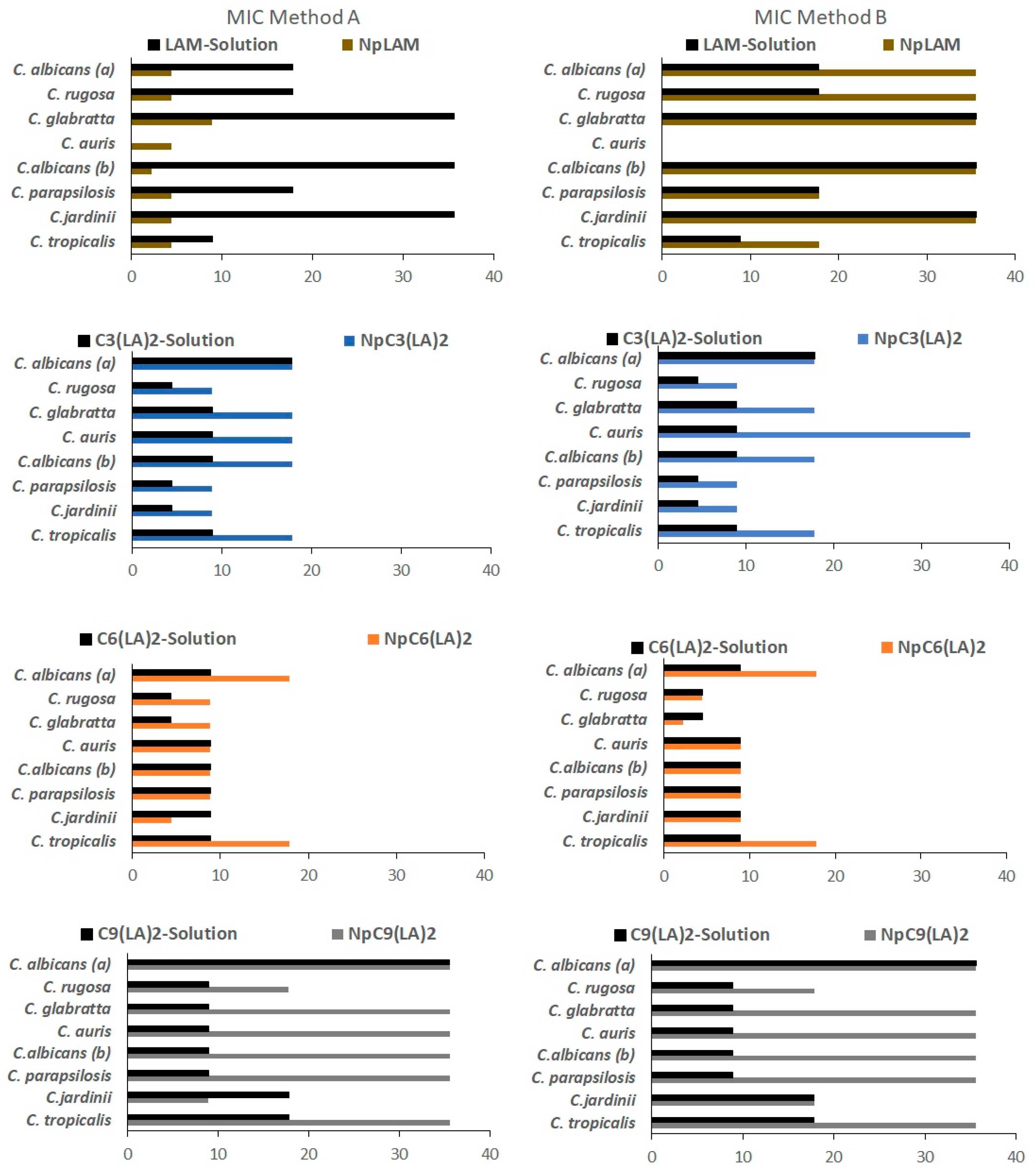
2.12. Antimicrobial Activity
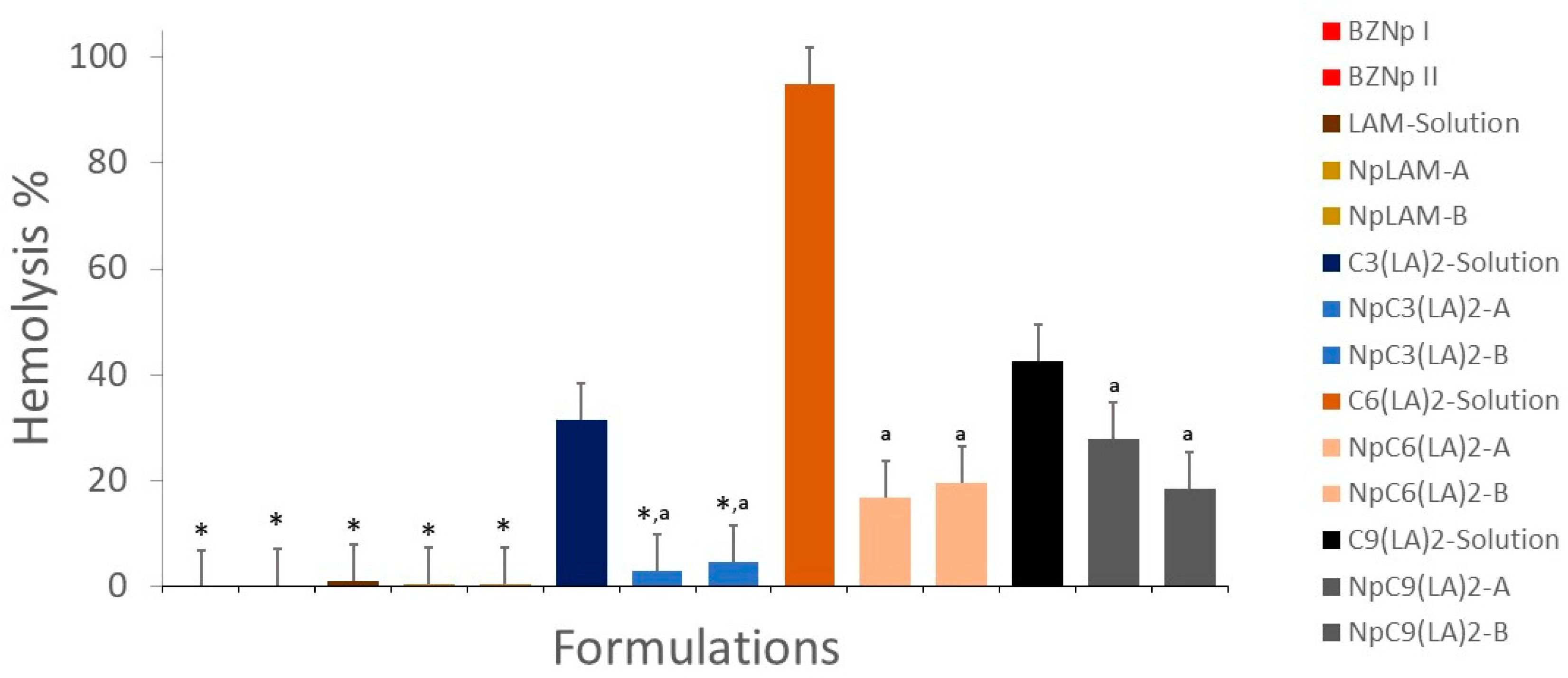
2.13. Hemolytic Activity
3. Materials and Methods
3.1. Materials
3.2. Nanoparticles Preparation
3.3. Nanoparticles Characterization and Stability
3.4. NMR Spectroscopy
3.5. Molecular Docking Studies
3.6. Surface Pressure-Area Isotherms
3.7. Antimicrobial Activity
3.7.1. Microorganisms and Culture Conditions
3.7.2. Minimum Inhibitory and Minimum Bactericide/Fungicide Concentrations
3.7.3. Hemolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino acid-based surfactants: New antimicrobial agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Pinazo, A.; Pons, R.; Infante, M. Gemini surfactants from natural amino acids. Adv. Colloid Interface Sci. 2014, 205, 134–155. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Barnes, R.H.; Karatzas, K.A.; Edwards-Gayle, C.J.; Greco, F.; Hamley, I.W.; Rambo, R.; Seitsonen, J.; Ruokolainen, J. Arginine-Containing Surfactant-Like Peptides: Interaction with Lipid Membranes and Antimicrobial Activity. Biomacromolecules 2018, 19, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Morán, M.C.; Pons, R. Aggregation behavior, antibacterial activity and biocompatibility of catanionic assemblies based on amino acid-derived surfactants. Int. J. Mol. Sci. 2020, 21, 8912. [Google Scholar] [CrossRef]
- King, A.T.; Davey, M.R.; Mellor, I.R.; Mulligan, B.J.; Lowe, K.C. Surfactant effects on yeast cells. Enzym. Microb. Technol. 1991, 13, 148–153. [Google Scholar] [CrossRef]
- Pérez, L.; Garcia, M.T.; Ribosa, I.; Vinardell, M.P.; Manresa, A.; Infante, M.R. Biological properties of arginine-based gemini cationic surfactants. Environ. Toxicol. Chem. 2002, 21, 1279–1285. [Google Scholar] [CrossRef]
- Li, M.; Yu, M. Development of a nanoparticle delivery system based on zein/polysaccharide complexes. J. Food Sci. 2020, 85, 4108–4117. [Google Scholar] [CrossRef]
- Wang, X.; Fan, M. Interaction behaviors and structural characteristics of zein/NaTC nanoparticles. RSC Adv. 2019, 9, 5748–5755. [Google Scholar] [CrossRef]
- Deo, N.; Jockusch, S.; Turro, N.J.; Somasundaran, P. Surfactant interactions with zein protein. Langmuir 2003, 19, 5083–5088. [Google Scholar] [CrossRef]
- Sousa, F.F.O.; Luzardo-Álvarez, A.; Pérez-Estévéz, A.; Seoane-Prado, R.; Blanco-Méndez, J. Sponges containing tetracycline loaded-PLGA-zein microparticles as a periodontal controlled release device. J. Drug Deliv. Sci. Technol. 2020, 59, 101858. [Google Scholar] [CrossRef]
- Araujo, J.T.C.; Martin-Pastor, M.; Pérez, L.; Pinazo, A.; Sousa, F.F.O. Development of anacardic acid-loaded zein nanoparticles: Physical chemical characterization, stability and antimicrobial improvement. J. Mol. Liq. 2021, 332, 115808. [Google Scholar] [CrossRef]
- Tavares, W.S.; Tavares-Júnior, A.G.; Otero-Espinar, F.J.; Martín-Pastor, M.; Sousa, F.F.O. Design of ellagic acid-loaded chitosan/zein films for wound bandaging. J. Drug Deliv. Sci. Technol. 2020, 59, 101903. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Tavano, L.; Infante, M.R.; Abo Riya, M.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Pérez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter. 2013, 9, 306–319. [Google Scholar] [CrossRef]
- Sánchez, L.; Martínez, V.; Rosa Infante, M.; Mitjans, M.; Pilar Vinardell, M. Hemolysis and antihemolysis induced by amino acid-based surfactants. Toxicol. Lett. 2007, 169, 177–184. [Google Scholar] [CrossRef]
- Shalel, S.; Streichman, S.; Marmur, A. The mechanism of hemolysis by surfactants: Effect of solution composition. J. Colloid Interface Sci. 2002, 252, 66–76. [Google Scholar] [CrossRef]
- Tavares, W.S.; Pena, G.R.; Martin-Pastor, M.; Sousa, F.F.O. Design and characterization of ellagic acid-loaded zein nanoparticles and their effect on the antioxidant and antibacterial activities. J. Mol. Liq. 2021, 341, 116915. [Google Scholar] [CrossRef]
- Hughes, J.M.; Budd, P.M.; Grieve, A.; Dutta, P.; Tiede, K.; Lewis, J. Highly monodisperse, lanthanide-containing polystyrene nanoparticles as potential standard reference materials for environmental “nano” fate analysis. J. Appl. Polym. Sci. 2015, 132, 42061. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Angulo, J.; Enríquez-Navas, P.M.; Nieto, P.M. Ligand-Receptor Binding Affinities from Saturation Transfer Difference (STD) NMR Spectroscopy: The Binding Isotherm of STD Initial Growth Rates. Chem.—A Eur. J. 2010, 16, 7803–7812. [Google Scholar] [CrossRef]
- Ludwig, C.; Michiels, P.J.; Wu, X.; Kavanagh, K.L.; Pilka, E.; Jansson, A.; Oppermann, U.; Günther, U.L. SALMON: Solvent accessibility, ligand binding, and mapping of ligand orientation by NMR spectroscopy. J. Med. Chem. 2008, 51, 1–3. [Google Scholar] [CrossRef]
- Podkorytov, I.S.; Skrynnikov, N.R. Transient NOE-exchange-relay experiment: Application to ligand-protein binding under slow exchange conditions. J. Magn. Reson. 2007, 187, 44–51. [Google Scholar] [CrossRef]
- Zana, R.; Talmon, Y. Dependence of aggregate morphology on structure of dimeric surfactants. Nature 1993, 362, 228–230. [Google Scholar] [CrossRef]
- Franke, M.E.; Rehage, H. Synthesis and characterisation of carboxy amide-bonded pyridinium Gemini surfactants: Influence of the nature of the spacer group and counterions on the aggregation behaviour. Tenside Surfactants Deterg. 2022, 59, 111–121. [Google Scholar] [CrossRef]
- Diamant, H.; Andelman, D. Dimeric Surfactants: Spacer Chain Conformation and Specific Area at the Air/Water Interface. Langmuir 1994, 10, 2910–2916. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Rosen, M.J.; Infante, M.R. Surface activity properties at equilibrium of novel gemini cationic amphiphilic compounds from arginine, bis(Args). Langmuir 1998, 14, 2307–2315. [Google Scholar] [CrossRef]
- Chen, Z.; Penfold, J.; Li, P.; Doutch, J.; Fan, Y.; Wang, Y. Effects of length and hydrophilicity/hydrophobicity of diamines on self-assembly of diamine/SDS gemini-like surfactants. Soft Matter. 2017, 13, 8980–8989. [Google Scholar] [CrossRef]
- Sousa, F.F.O.; Luzardo-Álvarez, A.; Blanco-Méndez, J.; Otero-Espinar, F.J.; Martín-Pastor, M.; Sández Macho, I. Use of 1H NMR STD, WaterLOGSY, and Langmuir monolayer techniques for characterization of drug-zein protein complexes. Eur. J. Pharm. Biopharm. 2013, 85, 790–798. [Google Scholar] [CrossRef]
- Rowell, R.L. Physical Chemistry of Surfaces, 6th ed.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 208, p. 582. [Google Scholar] [CrossRef]
- Kumar, B.; Suresh, K.A.; Gupta, S.K.; Kumar, S. Stress-strain relation in the collapse of Langmuir monolayer of a dimer of disk shaped moiety. J. Chem. Phys. 2010, 133, 044701. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Chen, Z.; Pan, D.; Cheng, Y.; Liu, Z.; Lin, Z.; Guan, X. Investigation of antibacterial activity and related mechanism of a series of nano-Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Perez, L.; Hafidi, Z.; Pinazo, A.; García, M.T.; Martín-Pastor, M.; Sousa, F.F.O.d. Zein Nanoparticles Containing Arginine-Phenylalanine-Based Surfactants: Stability, Antimicrobial and Hemolytic Activity. Nanomaterials 2023, 13, 200. [Google Scholar] [CrossRef]
- Weihs, D.; Danino, D.; Pinazo-Gassol, A.; Perez, L.; Franses, E.I.; Talmon, Y. Self-aggregation in dimeric arginine-based cationic surfactants solutions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 73–78. [Google Scholar] [CrossRef]
- Pérez, L.; Torres, J.L.; Manresa, A.; Solans, C. Infante MAR. Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, bis(args). Langmuir 1996, 12, 5296–5301. [Google Scholar] [CrossRef]
- ICH Expert Working Group. ICH Guideline Q1C Stability Testing for New Dosage Forms. In International Conference on Harmonization; ICH: Geneva, Switzerland, 1996. [Google Scholar]
- ICH Expert Working Group. ICH Guideline Q1A(R2) Stability Testing of New Drug Substances and Products. In International Conference on Harmonization; ICH: Geneva, Switzerland, 2003. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Song, R.; Llaca, V.; Linton, E.; Messing, J. Sequence, regulation, and evolution of the maize 22-kD α zein gene family. Genome Res. 2001, 11, 1817–1825. [Google Scholar] [CrossRef]
- Bietz, S.; Urbaczek, S.; Schulz, B.; Rarey, M. Protoss: A holistic approach to predict tautomers and protonation states in protein-ligand complexes. J. Cheminform. 2014, 6, 1–12. [Google Scholar] [CrossRef]
- Kerwin, S.M. ChemBioOffice Ultra 2010 suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef]
- Lozano, N.; Perez, L.; Pons, R.; Luque-Ortega, J.R.; Fernández-Reyes, M.; Rivas, L.; Pinazo, A. Interaction studies of diacyl glycerol arginine-based surfactants with DPPC and DMPC monolayers, relation with antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 196–203. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI standards M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Pape, W.J.; Pfannenbecker, U.; Hoppe, U. Validation of the red blood cell test system as in vitro assay for the rapid screening of irritation potential of surfactants. Mol. Toxicol. 1987, 1, 525–536. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.; Sentís, A.; Hafidi, Z.; Pinazo, A.; García, M.T.; Martín-Pastor, M.; de Sousa, F.F.O. Zein Nanoparticles Containing Arginine-Based Surfactants: Physicochemical Characterization and Effect on the Biological Properties. Int. J. Mol. Sci. 2023, 24, 2568. https://doi.org/10.3390/ijms24032568
Pérez L, Sentís A, Hafidi Z, Pinazo A, García MT, Martín-Pastor M, de Sousa FFO. Zein Nanoparticles Containing Arginine-Based Surfactants: Physicochemical Characterization and Effect on the Biological Properties. International Journal of Molecular Sciences. 2023; 24(3):2568. https://doi.org/10.3390/ijms24032568
Chicago/Turabian StylePérez, Lourdes, Adrià Sentís, Zakaria Hafidi, Aurora Pinazo, Maria Teresa García, Manuel Martín-Pastor, and Francisco Fábio Oliveira de Sousa. 2023. "Zein Nanoparticles Containing Arginine-Based Surfactants: Physicochemical Characterization and Effect on the Biological Properties" International Journal of Molecular Sciences 24, no. 3: 2568. https://doi.org/10.3390/ijms24032568
APA StylePérez, L., Sentís, A., Hafidi, Z., Pinazo, A., García, M. T., Martín-Pastor, M., & de Sousa, F. F. O. (2023). Zein Nanoparticles Containing Arginine-Based Surfactants: Physicochemical Characterization and Effect on the Biological Properties. International Journal of Molecular Sciences, 24(3), 2568. https://doi.org/10.3390/ijms24032568







