New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella
Abstract
1. Introduction
2. Results
2.1. Whole-Exome Sequencing (WES) Identifies Pathogenic Mutations in DNHD1 in MMAF Individuals
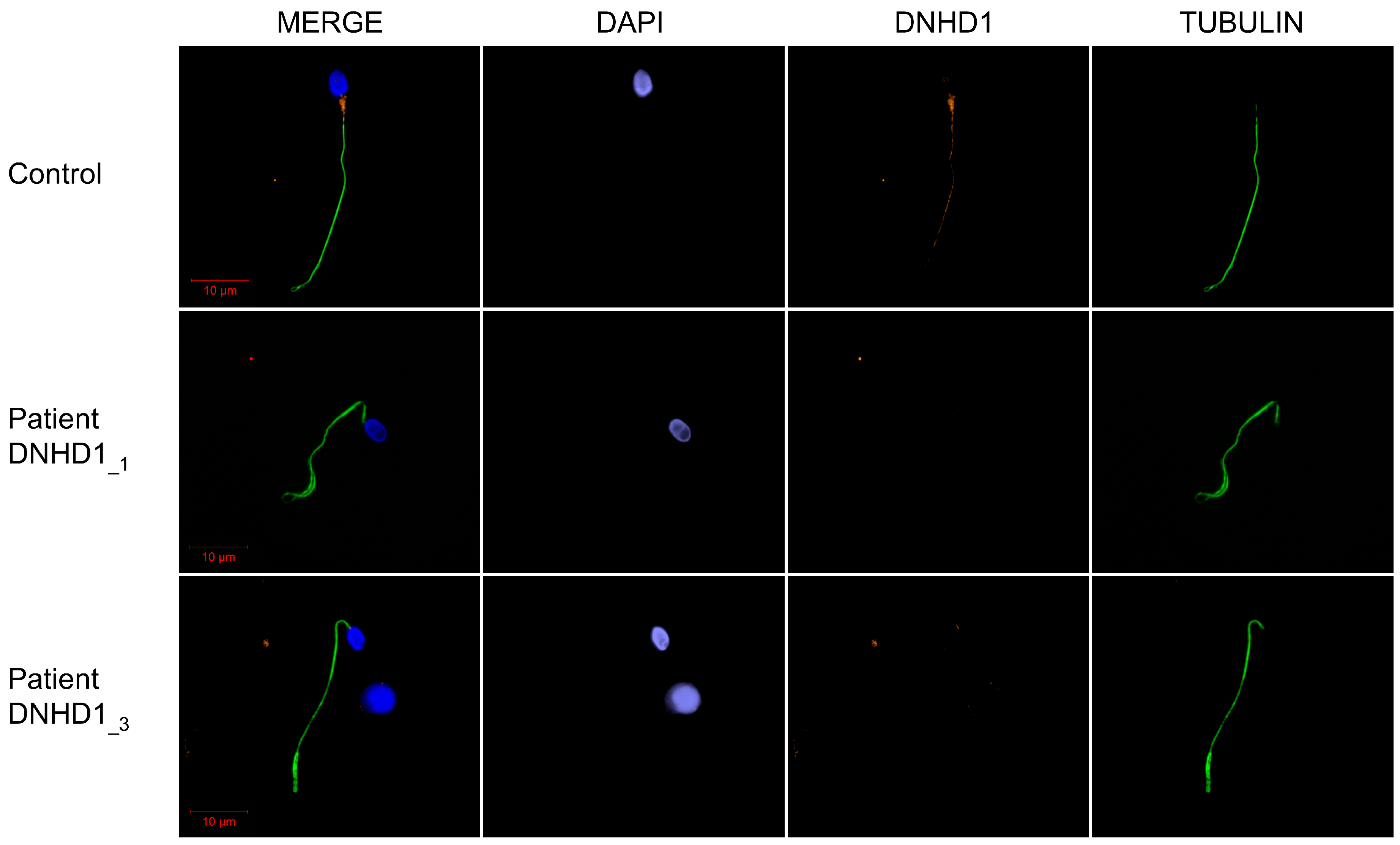
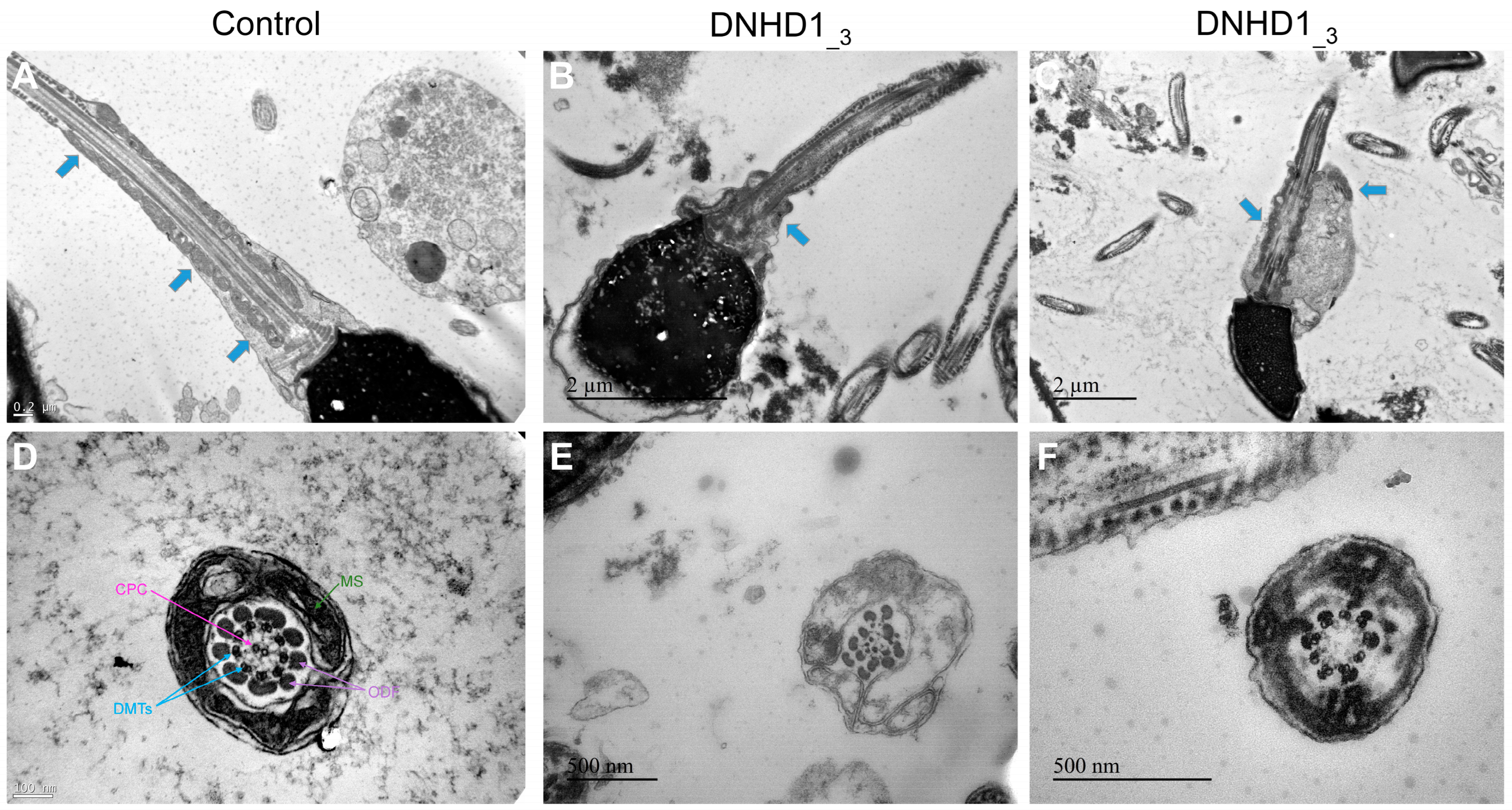
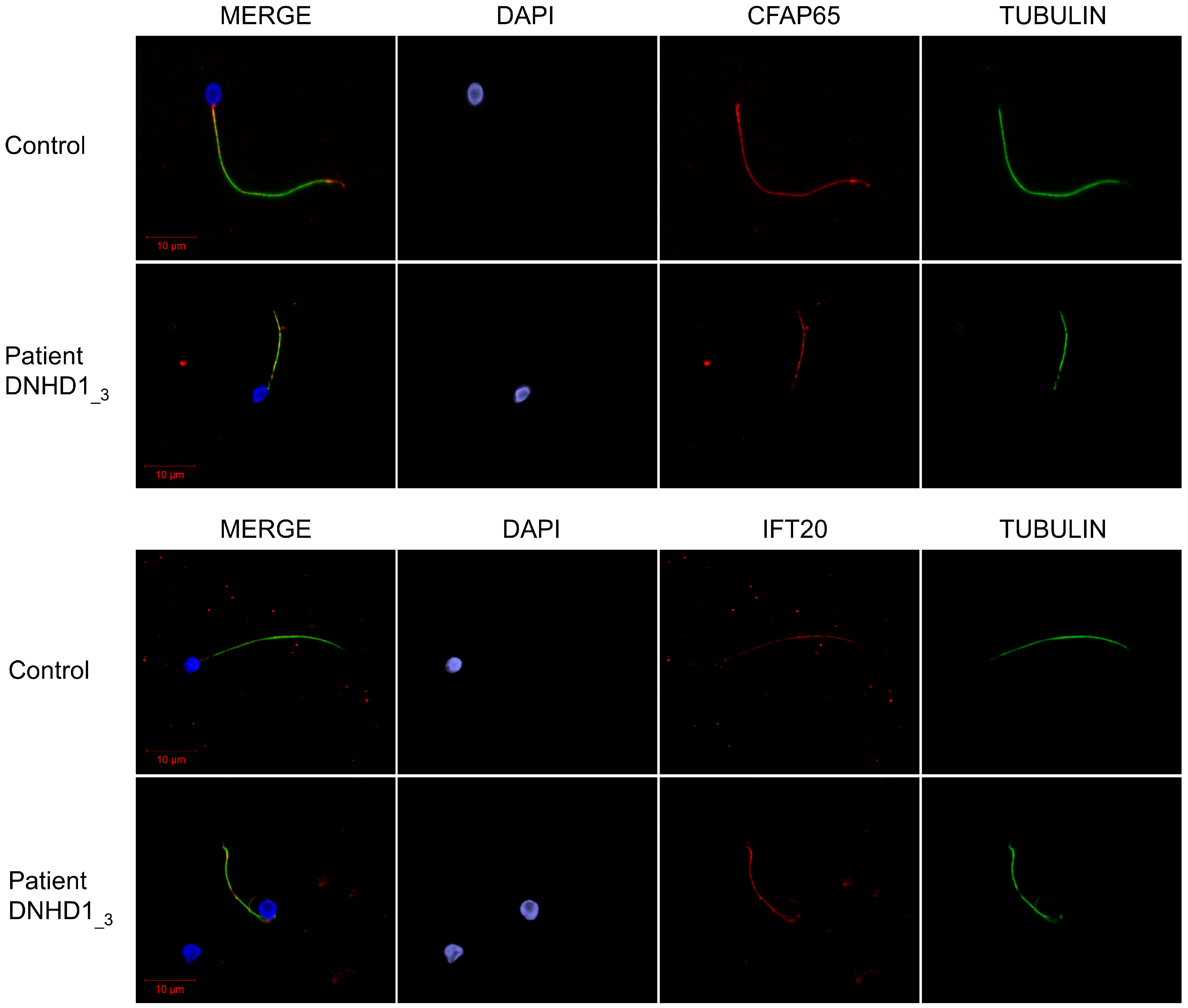
2.2. DNHD1 Variants Lead to a Severe Axonemal Disorganization of the Sperm Flagellum but Does Not Seem to Affect the Intra-Flagellar Transport (IFT)
3. Discussion
4. Materials and Methods
4.1. Human Subjects and Controls
4.2. Sanger Sequencing
4.3. Immunostaining in Human Sperm Cells
4.4. Transmission Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of Male Infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.J.; Riera-Escamilla, A.; Wyrwoll, M.J.; Salas-Huetos, A.; Xavier, M.J.; Nagirnaja, L.; Friedrich, C.; Conrad, D.F.; Aston, K.I.; Krausz, C.; et al. A Systematic Review of the Validated Monogenic Causes of Human Male Infertility: 2020 Update and a Discussion of Emerging Gene-Disease Relationships. Hum. Reprod. Update 2021, 28, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Salas-Huetos, A.; Oud, M.S.; Aston, K.I.; Veltman, J.A. Disease Gene Discovery in Male Infertility: Past, Present and Future. Hum. Genet. 2021, 140, 7–19. [Google Scholar] [CrossRef]
- Touré, A.; Martinez, G.; Kherraf, Z.-E.; Cazin, C.; Beurois, J.; Arnoult, C.; Ray, P.F.; Coutton, C. The Genetic Architecture of Morphological Abnormalities of the Sperm Tail. Hum. Genet. 2021, 140, 21–42. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Shen, L.; Zheng, A.; Meng, Q.; Li, H.; Yang, S. Clinical Detection, Diagnosis and Treatment of Morphological Abnormalities of Sperm Flagella: A Review of Literature. Front. Genet. 2022, 13, 1034951. [Google Scholar] [CrossRef]
- Lorès, P.; Coutton, C.; El Khouri, E.; Stouvenel, L.; Givelet, M.; Thomas, L.; Rode, B.; Schmitt, A.; Louis, B.; Sakheli, Z.; et al. Homozygous Missense Mutation L673P in Adenylate Kinase 7 (AK7) Leads to Primary Male Infertility and Multiple Morphological Anomalies of the Flagella but Not to Primary Ciliary Dyskinesia. Hum. Mol. Genet. 2018, 27, 1196–1211. [Google Scholar] [CrossRef]
- Coutton, C.; Martinez, G.; Kherraf, Z.-E.; Amiri-Yekta, A.; Boguenet, M.; Saut, A.; He, X.; Zhang, F.; Cristou-Kent, M.; Escoffier, J.; et al. Bi-Allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice. Am. J. Hum. Genet. 2019, 104, 331–340. [Google Scholar] [CrossRef]
- Coutton, C.; Vargas, A.S.; Amiri-Yekta, A.; Kherraf, Z.-E.; Ben Mustapha, S.F.; Le Tanno, P.; Wambergue-Legrand, C.; Karaouzène, T.; Martinez, G.; Crouzy, S.; et al. Mutations in CFAP43 and CFAP44 Cause Male Infertility and Flagellum Defects in Trypanosoma and Human. Nat. Commun. 2018, 9, 686. [Google Scholar] [CrossRef]
- Li, W.; Wu, H.; Li, F.; Tian, S.; Kherraf, Z.-E.; Zhang, J.; Ni, X.; Lv, M.; Liu, C.; Tan, Q.; et al. Biallelic Mutations in CFAP65 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella in Humans and Mice. J. Med. Genet. 2020, 57, 89–95. [Google Scholar] [CrossRef]
- Dong, F.N.; Amiri-Yekta, A.; Martinez, G.; Saut, A.; Tek, J.; Stouvenel, L.; Lorès, P.; Karaouzène, T.; Thierry-Mieg, N.; Satre, V.; et al. Absence of CFAP69 Causes Male Infertility Due to Multiple Morphological Abnormalities of the Flagella in Human and Mouse. Am. J. Hum. Genet. 2018, 102, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Beurois, J.; Martinez, G.; Cazin, C.; Kherraf, Z.-E.; Amiri-Yekta, A.; Thierry-Mieg, N.; Bidart, M.; Petre, G.; Satre, V.; Brouillet, S.; et al. CFAP70 Mutations Lead to Male Infertility Due to Severe Astheno-Teratozoospermia. A Case Report. Hum. Reprod. 2019, 34, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Beurois, J.; Dacheux, D.; Cazin, C.; Bidart, M.; Kherraf, Z.-E.; Robinson, D.R.; Satre, V.; Le Gac, G.; Ka, C.; et al. Biallelic Variants in MAATS1 Encoding CFAP91, a Calmodulin-Associated and Spoke-Associated Complex Protein, Cause Severe Astheno-Teratozoospermia and Male Infertility. J. Med. Genet. 2020, 57, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Ben Khelifa, M.; Coutton, C.; Zouari, R.; Karaouzène, T.; Rendu, J.; Bidart, M.; Yassine, S.; Pierre, V.; Delaroche, J.; Hennebicq, S.; et al. Mutations in DNAH1, Which Encodes an Inner Arm Heavy Chain Dynein, Lead to Male Infertility from Multiple Morphological Abnormalities of the Sperm Flagella. Am. J. Hum. Genet. 2014, 94, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Yekta, A.; Coutton, C.; Kherraf, Z.-E.; Karaouzène, T.; Le Tanno, P.; Sanati, M.H.; Sabbaghian, M.; Almadani, N.; Sadighi Gilani, M.A.; Hosseini, S.H.; et al. Whole-Exome Sequencing of Familial Cases of Multiple Morphological Abnormalities of the Sperm Flagella (MMAF) Reveals New DNAH1 Mutations. Hum. Reprod. 2016, 31, 2872–2880. [Google Scholar] [CrossRef]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-Allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef]
- Martinez, G.; Kherraf, Z.-E.; Zouari, R.; Fourati Ben Mustapha, S.; Saut, A.; Pernet-Gallay, K.; Bertrand, A.; Bidart, M.; Hograindleur, J.P.; Amiri-Yekta, A.; et al. Whole-Exome Sequencing Identifies Mutations in FSIP2 as a Recurrent Cause of Multiple Morphological Abnormalities of the Sperm Flagella. Hum. Reprod. 2018, 33, 1973–1984. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, F.; Li, F.; Jiang, X.; Yang, Y.; Li, X.; Li, W.; Wang, X.; Cheng, J.; Liu, M.; et al. Loss-of-Function Mutations in QRICH2 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Nat. Commun. 2019, 10, 433. [Google Scholar] [CrossRef]
- Liu, C.; Lv, M.; He, X.; Zhu, Y.; Amiri-Yekta, A.; Li, W.; Wu, H.; Kherraf, Z.-E.; Liu, W.; Zhang, J.; et al. Homozygous Mutations in SPEF2 Induce Multiple Morphological Abnormalities of the Sperm Flagella and Male Infertility. J. Med. Genet. 2020, 57, 31–37. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Yang, S.; Zouari, R.; Wang, J.; Wu, H.; Kherraf, Z.-E.; Liu, C.; Coutton, C.; Zhao, R.; et al. Bi-Allelic Mutations in TTC21A Induce Asthenoteratospermia in Humans and Mice. Am. J. Hum. Genet. 2019, 104, 738–748. [Google Scholar] [CrossRef]
- Lorès, P.; Dacheux, D.; Kherraf, Z.-E.; Nsota Mbango, J.-F.; Coutton, C.; Stouvenel, L.; Ialy-Radio, C.; Amiri-Yekta, A.; Whitfield, M.; Schmitt, A.; et al. Mutations in TTC29, Encoding an Evolutionarily Conserved Axonemal Protein, Result in Asthenozoospermia and Male Infertility. Am. J. Hum. Genet. 2019, 105, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Kherraf, Z.-E.; Amiri-Yekta, A.; Dacheux, D.; Karaouzène, T.; Coutton, C.; Christou-Kent, M.; Martinez, G.; Landrein, N.; Le Tanno, P.; Fourati Ben Mustapha, S.; et al. A Homozygous Ancestral SVA-Insertion-Mediated Deletion in WDR66 Induces Multiple Morphological Abnormalities of the Sperm Flagellum and Male Infertility. Am. J. Hum. Genet. 2018, 103, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Lorès, P.; Kheraff, Z.-E.; Amiri-Yekta, A.; Whitfield, M.; Daneshipour, A.; Stouvenel, L.; Cazin, C.; Cavarocchi, E.; Coutton, C.; Llabador, M.-A.; et al. A Missense Mutation in IFT74, Encoding for an Essential Component for Intraflagellar Transport of Tubulin, Causes Asthenozoospermia and Male Infertility without Clinical Signs of Bardet-Biedl Syndrome. Hum. Genet. 2021, 140, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Kherraf, Z.E.; Sun, S.; Zhang, X.; Cazin, C.; Coutton, C.; Zouari, R.; Zhao, S.; Hu, F.; et al. CFAP61 Is Required for Sperm Flagellum Formation and Male Fertility in Human and Mouse. Development 2021, 148, dev199805. [Google Scholar] [CrossRef]
- Liu, C.; Tu, C.; Wang, L.; Wu, H.; Houston, B.J.; Mastrorosa, F.K.; Zhang, W.; Shen, Y.; Wang, J.; Tian, S.; et al. Deleterious Variants in X-Linked CFAP47 Induce Asthenoteratozoospermia and Primary Male Infertility. Am. J. Hum. Genet. 2021, 108, 309–323. [Google Scholar] [CrossRef]
- Cong, J.; Wang, X.; Amiri-Yekta, A.; Wang, L.; Kherraf, Z.-E.; Liu, C.; Cazin, C.; Tang, S.; Hosseini, S.H.; Tian, S.; et al. Homozygous Mutations in CCDC34 Cause Male Infertility with Oligoasthenoteratozoospermia in Humans and Mice. J. Med. Genet. 2022, 59, 710–718. [Google Scholar] [CrossRef]
- Shen, Q.; Martinez, G.; Liu, H.; Beurois, J.; Wu, H.; Amiri-Yekta, A.; Liang, D.; Kherraf, Z.-E.; Bidart, M.; Cazin, C.; et al. Bi-Allelic Truncating Variants in CFAP206 Cause Male Infertility in Human and Mouse. Hum. Genet. 2021, 140, 1367–1377. [Google Scholar] [CrossRef]
- Priyanka, P.P.; Yenugu, S. Coiled-Coil Domain-Containing (CCDC) Proteins: Functional Roles in General and Male Reproductive Physiology. Reprod. Sci. 2021, 28, 2725–2734. [Google Scholar] [CrossRef]
- Tan, C.; Meng, L.; Lv, M.; He, X.; Sha, Y.; Tang, D.; Tan, Y.; Hu, T.; He, W.; Tu, C.; et al. Bi-Allelic Variants in DNHD1 Cause Flagellar Axoneme Defects and Asthenoteratozoospermia in Humans and Mice. Am. J. Hum. Genet. 2022, 109, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tu, C.; Nie, H.; Meng, L.; Li, Y.; Yuan, S.; Zhang, Q.; Du, J.; Wang, J.; Gong, F.; et al. Biallelic Mutations in CFAP65 Lead to Severe Asthenoteratospermia Due to Acrosome Hypoplasia and Flagellum Malformations. J. Med. Genet. 2019, 56, 750–757. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lamb, D.J. The Biology of Infertility: Research Advances and Clinical Challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Meienberg, J.; Bruggmann, R.; Oexle, K.; Matyas, G. Clinical Sequencing: Is WGS the Better WES? Hum. Genet. 2016, 135, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Coutton, C.; Escoffier, J.; Martinez, G.; Arnoult, C.; Ray, P.F. Teratozoospermia: Spotlight on the Main Genetic Actors in the Human. Hum. Reprod. Update 2015, 21, 455–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Jia, Y.; Xiong, C.; Meng, T.; Guan, H.; Xia, W.; Ding, M.; Yuchi, M. Variability in the Morphologic Assessment of Human Sperm: Use of the Strict Criteria Recommended by the World Health Organization in 2010. Fertil. Steril. 2014, 110, 945–949. [Google Scholar] [CrossRef] [PubMed]

| DNHD1_1 | DNHD1_2 | DNHD1_3 | Normal Range | |
|---|---|---|---|---|
| Specimen characteristics | ||||
| Abstinence duration | nt | 7 | 5 | 2–7 |
| Volume (mL) | 3.2 | 4 | 3.25 | 1.5–7 |
| pH | 8.1 | 7.9 | 7.9 | 7.2–8.0 |
| Viscosity | normal | normal | normal | - |
| Numeration | ||||
| Sperm count (106/mL) | 0.3 | 40 | 1.54 | 15–200 |
| Total numeration | 0.97 | 160 | 4.65 | >39 |
| Round cells (106/mL) | 4 | nt | 2.25 | <1 |
| Polynuclear (106/mL) | <1 | nt | <1 | <1 |
| Motility | ||||
| Motile sperm (%) | 20 | 0 | 10.5 | >40 |
| Progressive sperm (%) | 10 | 0 | 3.5 | >32 |
| Non-progressive sperm (%) | 10 | 0 | 6.5 | >8 |
| Immotile sperm (%) | 60 | 100 | 89.5 | <60 |
| Other tests | ||||
| Viability (% alive) | nt | 79 | 73 | >58 |
| Fructose, seminal (mg/dL) | nt | 300 | nt | 120–450 |
| Morphology | ||||
| Normal morphology (%) | 9 | 0 | 4 | >4 |
| Abnormal morphology (%) | 91 | 100 | 96 | <96 |
| Multiple anomalies index | 1.51 | nt | 2.3 | - |
| Head anomaly | 32 | 10 | 96 | - |
| Elongated | 0 | nt | 0 | - |
| Thinned | 0 | nt | 8 | - |
| Microcephalic | 1 | nt | 12 | - |
| Macrocephalic | 1 | nt | 1 | - |
| Duplicated | 0 | nt | 6 | - |
| Abnormal base | 0 | 10 | 47 | - |
| Malformed acrosome | 30 | nt | 89 | - |
| Intermediate piece anomaly | 3 | nt | 15 | - |
| Cytoplasmic residue | 1 | nt | 1 | - |
| Thin | 0 | nt | 5 | - |
| Angulation | 2 | nt | 9 | - |
| Flagellum anomaly | 18 | 90 | 43 | - |
| Absent | 7 | nt | 0 | - |
| Short | 3 | 90 | 0 | - |
| Irregular size | 0 | nt | 2 | - |
| Coiled | 5 | nt | 39 | - |
| Multiple | 1 | nt | 2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, G.; Barbotin, A.-L.; Cazin, C.; Wehbe, Z.; Boursier, A.; Amiri-Yekta, A.; Daneshipour, A.; Hosseini, S.-H.; Rives, N.; Feraille, A.; et al. New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella. Int. J. Mol. Sci. 2023, 24, 2559. https://doi.org/10.3390/ijms24032559
Martinez G, Barbotin A-L, Cazin C, Wehbe Z, Boursier A, Amiri-Yekta A, Daneshipour A, Hosseini S-H, Rives N, Feraille A, et al. New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella. International Journal of Molecular Sciences. 2023; 24(3):2559. https://doi.org/10.3390/ijms24032559
Chicago/Turabian StyleMartinez, Guillaume, Anne-Laure Barbotin, Caroline Cazin, Zeina Wehbe, Angèle Boursier, Amir Amiri-Yekta, Abbas Daneshipour, Seyedeh-Hanieh Hosseini, Nathalie Rives, Aurélie Feraille, and et al. 2023. "New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella" International Journal of Molecular Sciences 24, no. 3: 2559. https://doi.org/10.3390/ijms24032559
APA StyleMartinez, G., Barbotin, A.-L., Cazin, C., Wehbe, Z., Boursier, A., Amiri-Yekta, A., Daneshipour, A., Hosseini, S.-H., Rives, N., Feraille, A., Thierry-Mieg, N., Bidart, M., Satre, V., Arnoult, C., Ray, P. F., Kherraf, Z.-E., & Coutton, C. (2023). New Mutations in DNHD1 Cause Multiple Morphological Abnormalities of the Sperm Flagella. International Journal of Molecular Sciences, 24(3), 2559. https://doi.org/10.3390/ijms24032559







